Abstract
Rationale
Mammalian heart has minimal regenerative capacity. In response to mechanical or pathological stress, the heart undergoes cardiac remodeling. Pressure and volume overload in the heart cause increased size (hypertrophic growth) of cardiomyocytes. Whereas the regulatory pathways that activate cardiac hypertrophy have been well established, the molecular events that inhibit or repress cardiac hypertrophy are less known.
Objective
To identify and investigate novel regulators that modulate cardiac hypertrophy.
Methods and Results
Here, we report the identification, characterization and functional examination of CIP, a novel cardiac Isl1-interacting protein. CIP was identified from a bioinformatic search for novel cardiac-expressed genes in mouse embryonic hearts. CIP encodes a nuclear protein without recognizable motifs. Northern blotting, in situ hybridization and reporter gene tracing demonstrated that CIP is highly expressed in cardiomyocytes of developing and adult hearts. Yeast-two-hybrid screening identified Isl1, a LIM/homeodomain transcription factor essential for the specification of cardiac progenitor cells in the second heart field, as a co-factor of CIP. CIP directly interacted with Isl1 and we mapped the domains of these two proteins which mediate their interaction. We show that CIP represses the transcriptional activity of Isl1 in the activation of the MEF2C enhancer. The expression of CIP was dramatically reduced in hypertrophic cardiomyocytes. Most importantly, overexpression of CIP repressed agonist-induced cardiomyocyte hypertrophy.
Conclusions
Our studies therefore identify CIP a novel regulator of cardiac hypertrophy.
Keywords: Isl1-interacting protein, transcription factor, cardiac development, cardiomyocyte hypertrophy
INTRODUCTION
Complex genetic pathways intimately regulate heart development and function1. Much of our current understanding of how cardiac gene expression is controlled is at the level of transcriptional regulation, in which multiple tissue-specific transcription factors have been implicated in the control of gene expression during cardiomyocyte differentiation2. Those include Nkx2.5, a homeobox protein3-5, MEF2C, a member of the myocyte enhance factor-2 (MEF2) family of MADS-box transcription factors6-11, and GATA4, a GATA family zinc finger protein12, 13. Some of the well-characterized cardiac genes regulated by these cardiac transcription factors include cardiac-specific atrial natriuretic factor (ANF) and cardiac α-actin13, 14.
Two distinct sources of cardiac precursor cells from the “primary” and “secondary heart fields” participate in heart formation15-17. Whereas the primary heart field is essential for the formation of the initial heart tube, additional cardiac precursor cells recruited from the secondary heart field contribute to the future right ventricle and outflow tract18, 19. Loss-of-function studies have defined genes whose activity is required in secondary heart field progenitors for morphogenesis of outflow tract and right ventricle18, 20. Among the genes with key function in secondary heart field is Isl1, a LIM/homeodomain transcription factor21-24.
One of the major responses of the heart to biomechanical stress and pathological stimuli is to undergo cardiac hypertrophy, an increase in the thickness of the cardiac ventricular wall. At a cellular level, cardiac hypertrophy is defined as an increase of cardiomyocyte size25, 26. Initially, cardiac hypertrophy is an adaptive response that maintains cardiac output in the face of increased workload. However, chronic activation of hypertrophic pathways is associated with adverse consequences that may lead to heart failure and sudden death27.
Cardiac hypertrophy occurs in response to a variety of mechanical, hemodynamic, hormonal, and pathologic stimuli28-30. Numerous studies have demonstrated that many signaling pathways and transcriptional networks that normally regulate different aspects of cell proliferation, differentiation and survival are also involved in the induction of cardiac hypertrophy1, 29, 31. Hypertrophic growth involves control of cardiomyocyte gene expression at multiple molecular levels. Key regulators of gene expression in cardiac myocytes such as members of the MEF2 and GATA families are involved in the control of gene expression during cardiomyocyte hypertrophy. Epigenetic regulation of gene expression, including histone modification by histone acetyltransferases (HATs) and histone deacetylases (HDACs) to remodel chromatin, has been proved another mean of hypertrophic regulation 32, 33. Cardiac hypertrophy is also accompanied by reactivation of a set of cardiac fetal genes, including those that encode atrial natriuretic factor (ANF), B-type natriuretic peptide (BNP), and βMHC26. Reactivation of these fetal genes suggests that molecular pathways that control heart development are redeployed to regulate hypertrophic growth34. The pathophysiology of cardiac hypertrophy has been extensively studied for decades and much is known about signaling pathways that activate cardiac hypertrophy26, 30. However, relatively less is known about how cardiac hypertrophy is repressed.
We previously identified myocardin as a SAP (SAF-A/B, Acinus, PIAS) domain transcriptional regulator35, 36. Myocardin is expressed in both cardiomyocytes and a subset of vascular and visceral smooth muscle cell types35, 36. Myocardin does not to bind DNA directly, but forms a stable ternary complex with SRF bound to DNA37, 38. As a transcriptional cofactor of SRF, myocardin potently transactivates CArG box-containing reporter genes36, 39, 40. Genetic studies revealed that myocardin and MRTFs play critical roles in a variety of biological processes, including vascular smooth muscle development, aortic vessel patterning, mammary myoepithelium formation and others41-43. In this study, we report the identification and characterization of a novel cardiac-specific nuclear protein, named CIP. CIP interacts with the LIM/homeodomain transcription factor Isl1. We showed that the expression of CIP was repressed in hypertrophic hearts, and most importantly, CIP represses cardiomyocyte hypertrophy.
RESULTS
Identification of CIP, a novel gene from mouse hearts
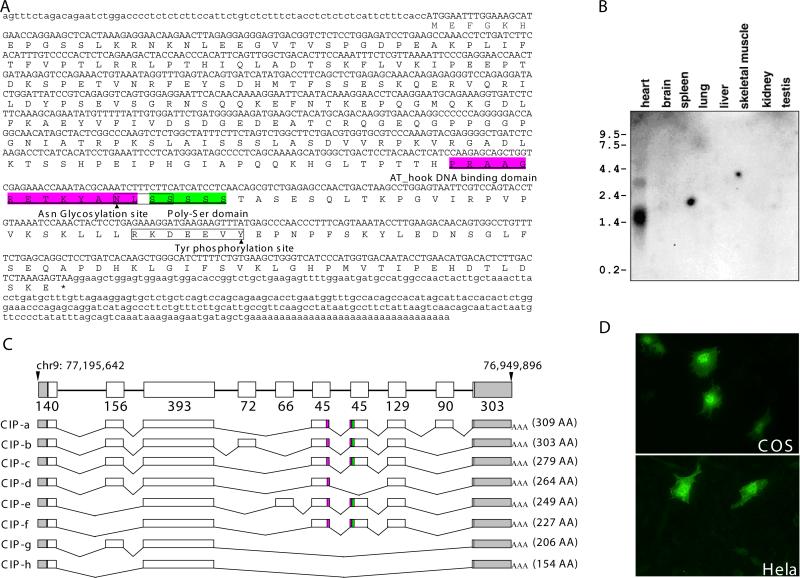
We searched expressed sequence tag (EST) databases for novel sequences present in cardiac cDNA libraries. Previously, this approach succeeded in identifying myocardin as a novel cardiac- and smooth muscle-specific transcription co-factor for serum response factor (SRF)36. An additional candidate EST sequence recovered in the search (GenBank Accession Number AA919489), contained a short 273 nucleotide tag that was derived from a mouse embryonic day 13 (E13.5) heart cDNA library. Using this sequence as a probe to screen a mouse embryonic heart cDNA library, we identified a larger cDNA sequence of 1336 nucleotides encoding a putative protein of 309 amino acids, which we name CIP (Cardiac Isl1-interesting Protein) (Fig. 1A). Genbank Database search indicated that this cDNA clone is similar to RIKEN cDNA clone 2310046A06Rik, and is a novel, alternative slicing-produced isoform of a recently reported protein called MLIP (Muscle-enriched A-type lamin interacting protein) 44. In addition to the putative lamin-A interaction domain in its N-terminus, CIP contains an AT-hook DNA binding domain in its C-terminus (aa 212-224), suggesting that CIP could function as a DNA-binding transcriptional regulator (Figs. 1A, C; Online Figure I). Furthermore, the CIP protein contains a putative asparagine (Asn) glycosylation site (aa 223-226), a poly serine stretch (aa 225-229) and a tyrosine (Tyr) phosphorylation site (aa 254-260) (Fig. 1A). Northern blot analysis using an adult mouse multiple tissue blot demonstrated that the expression of CIP is restricted to the heart. Two major transcripts, ~1.4K and ~3.4K nucleotides, respectively, were detected in the heart, likely represent products of alternative splicing (Fig. 1B). The cDNA sequence we have cloned represents the ~1.4 kb transcript, whereas the sequences of the ~3.4 kb remain to be identified.
Figure 1. Identification of the CIP gene.
(A) Nucleotide sequences and deduced amino acid sequences. Nucleotides for open reading frame (ORF) are showed in uppercase; Nucleotides for both 5’ and 3’ untranslated regions (UTRs) are showed in lowercase. The corresponding amino acids (in single letter code) are shown below. Amino acids underlined indicate the putative AT_hook domain and poly-Ser domain. The predicted amino acid modifications are also indicated and their recognition motifs are shown in boxes.
(B) Northern blotting analysis of CIP expression using RNAs of different tissues from adult mice.
(C) Gene structure and alternative splicing of the mouse CIP gene. Empty boxes mark ORF, gray boxes indicate UTR. The color boxes in alternative splicing variants indicate putative domains shown in Figure 1A.
(D) CIP is a nuclear protein. Immunochemistry detecting the nuclear localization of the FLAG-CIP fusion proteins in transfected COS and Hela cells.
The Cip gene contains at least eight exons and spans over 245 kb on mouse chromosome 9. Cip is evolutionary conserved from fish to human (Online Figure II). Sequencing of Cip RT-PCR products identified complex alternative splicing (Fig. 1C). The functional significance of those isoforms is currently not clear.
Next, we examined the subcellular location of the CIP protein. We transiently overexpressed Flag-CIP fusion proteins in COS7 and Hela cells. Immunochemistry assays revealed that the Flag-CIP fusion proteins are primarily located in the nuclei of transfected cells. We also observed a weaker distribution of the Flag-CIP proteins in the cytoplasm (Fig. 1D).
CIP expression is restricted to cardiomyocytes of developing hearts
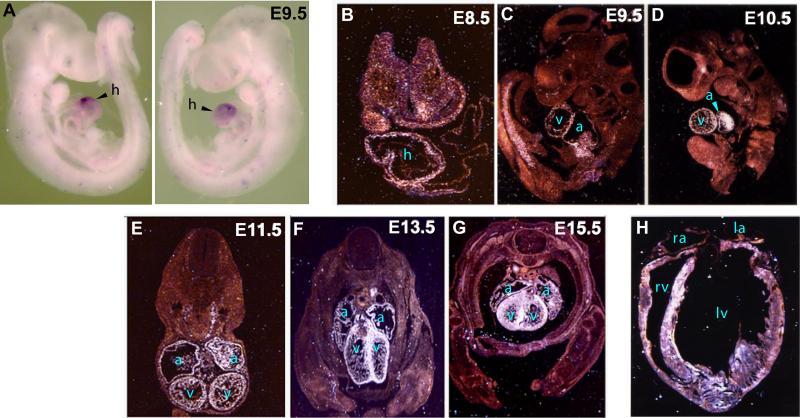
To define the expression pattern of Cip during development, in situ hybridization was performed using an antisense probe to the 3’ UTR of the mouse Cip transcript. Whole mount in situ hybridization first demonstrated that CIP expression is restricted to the heart of embryonic day (E9.5) mouse embryos, with highest expression level detected in the ventricle (Fig. 2A). In situ hybridization on tissue sections of staged mouse embryos revealed that Cip expression was first detected in the heart of E8.5 mouse embryos (Fig. 2B). Cip continued to be restricted to the heart from E9.5 to E15.5 (Fig. 2C, D, E, F, G). An apparently positive signal was also detected in the truck of E9.5 embryo, which might represent transient expression of CIP in presomitic mesoderm (Fig. 2C). From E11.5-E15.5, Cip expression appeared to be higher in the ventricles of embryonic hearts (Fig. 2E, F, G). Myocardial Cip expression continued in the adult heart (Fig. 2H).
Figure 2. Expression of CIP gene in embryonic and adult mouse tissues.
(A) Whole mount in situ hybridization detecting CIP transcript expression in E9.5 mouse embryos. In situ hybridization detecting CIP transcript expression on sections of staged mouse embryos (B-G) and in an adult mouse heart (H). (B, E, F, G) transverse sections; (C, D, H) sagittal sections. h: heart; a: atrium; la: left atrium; lv: left ventricle; ra: right atrium; rv: right ventricle; v: ventricle.
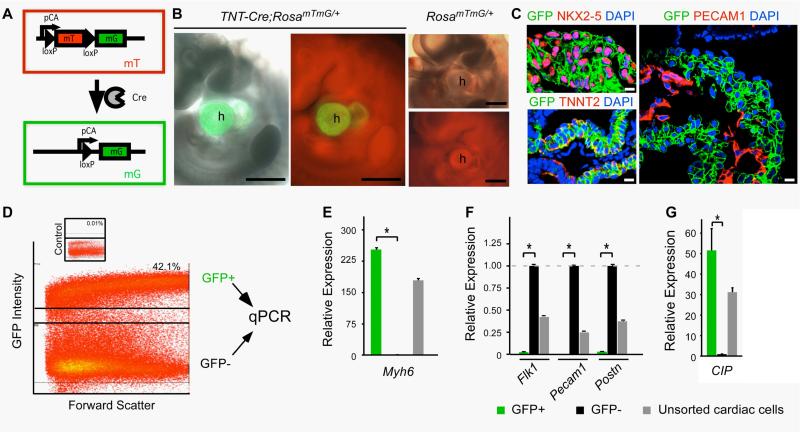
Since the heart is constituted of cardiomyocyte, cardiac fibroblast, smooth muscle cell, endothelial cell and epicardial cell, we performed additional experiments to determine exactly in which cell type/lineage the CIP is expressed. First, we utilized a genetic approach in which the Rosa-mT-mG reporter line was used to trace the expression of CIP in different cell types. The Rosa-mT-mG mice possess loxP sites on both sides of a membrane-targeted tdTomato (mT) cassette and express strong red fluorescence in all tissues and cell types (Fig. 3A, B). The presence of Cre will lead to the deletion of the mT cassette and the activation of the downstream membrane-targeted EGFP (mG) cassette (Fig. 3A). The membrane-targeted EGFP can be utilized as a marker for FACS sorting (Fig. 3D). To label and sort out the cardiomyocytes, the Rosa-mT-mG mice were bred with the cTNT-Cre mice, in which the Cre recombinase is driven by the cardiomyocyte-specific cardiac troponin T promoter (Fig. 3B). Immunochemistry confirmed the labeling of cardiomyocyte in Rosa-mT-mG/ cTNT-Cre embryos (Fig. 3C). Hearts were dissected out from E10.5 Rosa-mT-mG/cTNT-Cre embryos and digested. EGFP positive cardiomyocytes and EGFP negative non-cardiomyocytes were separated by FACS sorting (Fig. 3D). Quantitative RT-PCR detected high expression level of Myh6 (α-myosin heavy chain) and CIP in EGFP-positive cell sample but not in EGFP-negative cell sample, while endothelial markers Flk1, Pecam1 and fibrotic tissue marker Postn were highly expressed in EGFP-negative cell sample but not EGFP-positive cell sample (Fig. 3E, F, G), n = 3. These data, together with the results of in situ hybridization, suggested that CIP is expressed in cardiomyocytes of developing hearts.
Figure 3. Quantitative RT-PCR analyses of CIP expression in cardiomyocytes of mouse embryos.
(A) Schematic of the RosamTmG/+ reporter system. In the presence of Cre recombinase, which deletes the mT cassette, the expression of membranous tomato red (mT) turns into membranous green fluorescence protein (mG).
(B) When the RosamTmG/+ reporter line was crossed with a cardiomyocyte specific Cre line (TNT-Cre), membranous GFP is detected in E10.5 heart (h) whereas there is no green signal in the heart of control littermate. Bar = 500 μm.
(C) Membranous GFP is detected in NKX2.5 positive and TNNT2 positive myocardium, but not the PECAM1 positive endocardium. Bar = 10 μm.
(D) FACS isolation of GFP positive and negative cell populations from TNT-Cre; RosamTmG/+ hearts. Insert shows that less than 0.01% GFP positive cells were found in the hearts of control littermates.
(E) GFP-positive population is highly enriched for cardiomyocyte specific marker Myh6, documented by quantitative RT-PCR assays.
(F) GFP-positive population expresses very low level of endothelial marker Flk1 or Pecam1, and fibrotic tissue marker Postn, indicating the specificity of cell sorting.
(G) CIP is highly expressed in the GFP-positive cardiomyocytes, but not in GFP-negative non-cardiomyocytes. Expression in GFP negative population is set as 1. * P<0.01, three embryos were used (n=3) for analysis in each group.
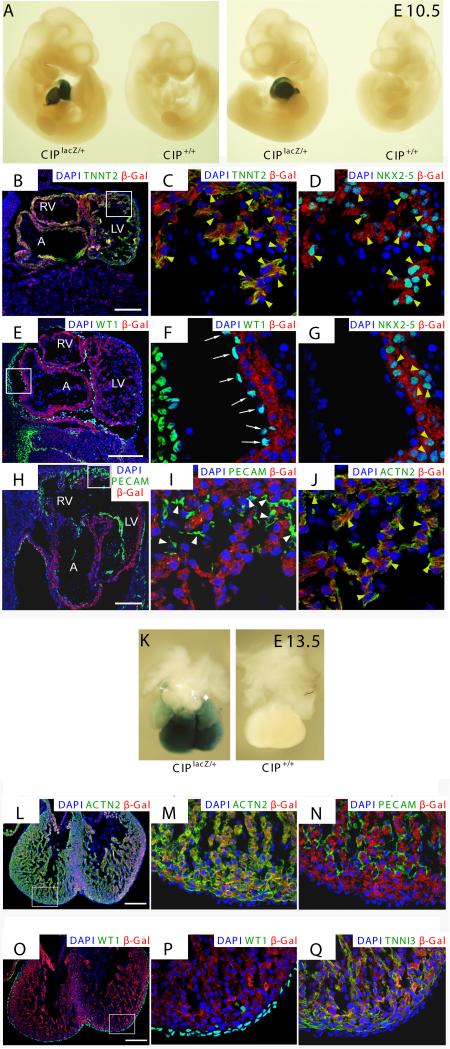
Next, we used a Cip gene trap mouse line in which a LacZ reporter gene was inserted into the Cip genomic locus (See Material and Methods for detail) to further map the expression of Cip in the heart (Online Figure III). LacZ reporter gene expression was restricted to the developing heart at E10.5 and E13.5, recapitulating endogenous expression pattern of Cip and highlighting the cardiac specific expression of this gene (Fig. 4A, E). Immunohistochemistry analyses clearly demonstrated that the expression of Cip, as marked by β-gal, overlapped with that of Nkx2-5, cardiac troponin T (TNNT2), and cardiac actin (ACTN2) in cardiomyocytes (Fig. 4B, C, D, G, J, yellow arrow heads). Cip expressing cells did not co-express WT1, a marker of epicardium, nor PECAM, a marker of endothelial cells (Fig. 4E, F, H, I, white arrows and arrow heads), n > 3. Together these studies clearly demonstrate that Cip is a specifically expressed in cardiomyocytes during embryogenesis. Interestingly, we detected CIP expression in the skeletal muscle of neonatal mice (Online Figure IV, middle panel). However, the expression of CIP in skeletal muscle appears to be transient, since we did not detect its expression in embryonic skeletal muscle (Online Figure IV, left panel) or in adult skeletal muscle tissue using the lacZ reporter (Online Figure IV, right panel) or by Northern blot analyses (Fig. 1B).
Figure 4. Characterization of CIP expression using a LacZ reporter gene in mouse embryos.
(A) Whole mount β-gal staining of mouse E10.5 embryos harboring a LacZ cassette inserted in the CIP locus (CIPlacZ/+), or the control (CIP+/+). Positive β-gal staining is only detected in the heart.
(B-J) Immunohistochemistry using indicated antibodies on tissue sections of E10.5 CIPlacZ/+ embryo. Note that CIP positive cells (marked by antibodies against the β-gal and is in red) overlay with Nkx2-5 (D, G), cardiac α-actin (ACTN2) (J) and cardiac troponin T (TNNT2) (B, C) positive cardiomyocytes (yellow arrowheads) but not that of WT1-positive epicardium (white arrows) (E, F) nor PECAM-positive fibroblasts (white arrowheads) (H, I). DAPI (blue) staining marks the nucleus.
(K) Whole mount β-gal staining of mouse E13.5 embryonic hearts harboring a LacZ cassette inserted in the CIP locus (CIPlacZ/+), or the control (CIP+/+).
(L-Q) Immunohistochemistry using indicated antibodies on tissue sections of E13.5 CIPlacZ/+ embryo. Note that CIP positive cells (marked by antibodies against the β-gal) overlay with cardiac α-actin (ACTN2) and cardiac troponin T (TNNT2) positive cardiomyocytes not that of WT1 positive epicardium, nor PECAM positive fibroblasts. DAPI staining marks the nucleus. (B, E, H, bar=100 μm; C, D, F, G, I, J, bar=10 μm; L, O, bar=200 μm, M, N, P, Q, bar=40 μm. Data are presented as representative images derived from >3 embryos. A, atrium; LV, left ventricle; RV, right ventricle.
CIP interacts with cardiac transcriptional factor Islet1
To identify putative CIP-interacting proteins, we performed yeast two-hybrid screening. The bait consisted of full-length CIP protein fused with the yeast GAL4 DNA binding domain. The bait construct did not autonomously activate transcription in yeast. We screened a mouse E10.5 embryonic cDNA prey library and identified more than 30 positive clones, one of which encoded Islet1 (ISL1), a LIM/homeodomain transcription factor essential for differentiation of second heart field cardiac progenitors23, 45.
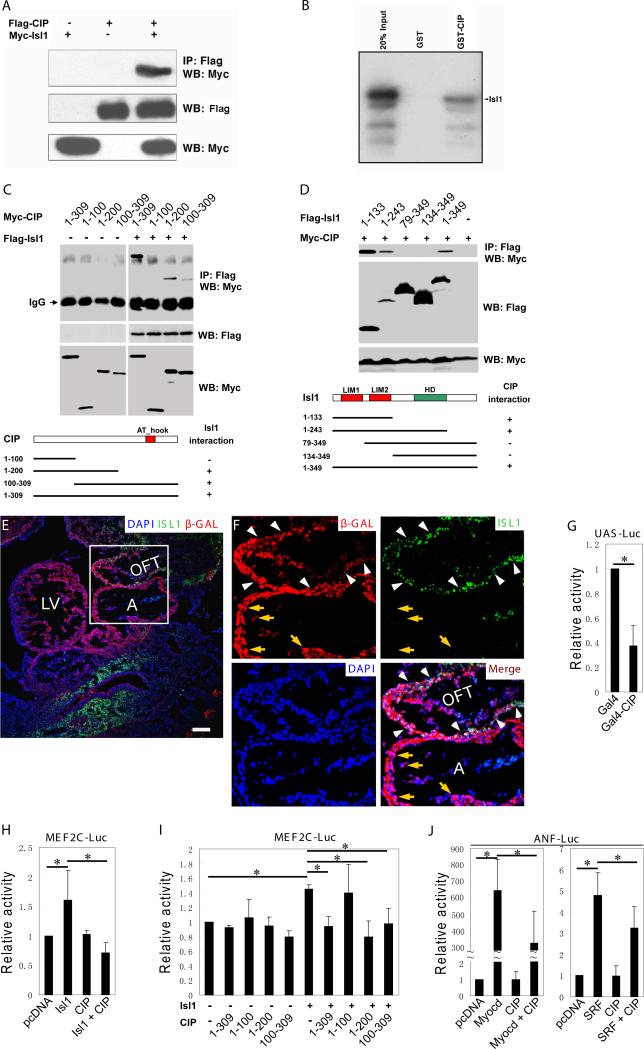
To confirm the interaction of CIP and ISL1, we performed co-immunoprecipitation (Co-IP) assays. HEK293T cells were transfected with expression plasmids for FLAG-CIP, Myc-ISL1, or both. FLAG immunoprecipitates of cells expressing both constructs contained ISL1 protein, as demonstrated by Western bloting with anti-Myc antibodies. As negative controls, ISL1 was not detected in FLAG immunoprecipitates of cells transfected with each construct alone (Fig. 5A). To further confirm direct interaction between CIP and ISL1, we performed in vitro GST-fusion protein pull down assays. ISL1 was 35S labeled in a cell-free translation system, and the labeled ISL1 protein was then incubated with either GST-CIP fusion protein or GST protein alone (to serve as a negative control). As expected, GST-CIP fusion protein, but not GST alone, bound ISL1 (Fig. 5B).
Figure 5. CIP interacts with Isl1 and represses its transcription activity.
(A) Co-immunoprecipitation assays showing the interaction between Flag-tagged CIP and Myc-tagged Isl1. HEK-293T cells were co-transfected Flag-CIP, Myc-Isl1, or both. Anti-Flag antibody was used to immunoprecipitate (IP) cell extracts and anti-Myc antibody was used in Western blot (WB) to detect Myc-Isl1 protein in the complex. 10% cell lysate was used as input to demonstrate the expression of tagged proteins (button two panels).
(B) In vitro GST-fusion protein pull down assays showing the direct interaction between CIP and Isl1. Isl1 protein was synthesized in vitro and labeled with S-35. GST-CIP protein, but not GST protein, pulled down S-35 labeled Isl1. 20% of labeled Isl1 protein was load as control (Input).
(C) Map the interaction domains of the CIP protein that mediate the interaction between Isl1 and CIP using co-immunoprecipitation assays. HEK-293T cells were co-transfected Flag-Isl1, Myc-CIP full-length (1-309) or indicated truncated mutants, or both. Anti-Flag antibody was used to immunoprecipitate (IP) cell extracts and anti-Myc antibody was used in Western blot (WB) to detect Myc-CIP protein in the complex. 10% cell lysate was used as input to demonstrate the expression of tagged proteins (lower two panels). The interaction domains are summarized in the bottom diagram.
(D) Map the interaction domains of Isl1 proteins which mediate the interaction between Isl1 and CIP using co-immunoprecipitation assays. HEK-293T cells were co-transfected Myc-CIP, Flag-Isl1 full-length (1-349) or indicated truncated mutants, or both. Anti-Flag antibody was used to immunoprecipitate (IP) cell extracts and anti-Myc antibody was used in Western blot (WB) to detect Myc-CIP protein in the complex. 10% cell lysate was used as input to demonstrate the expression of tagged proteins (lower two panels). The interaction domains are summarized in the bottom diagram.
(E) Isl1 and CIP are co-expressed in cardiomyocytes of outflow tract of mouse embryos. Immunohistochemistry to demonstrate that Isl1 (green) and β-gal positive CIP (red) expressing cells are co-located in the cardiomyocytes of outflow tract (OFT) of E10.5 mouse embryos (arrowheads). DAPI staining marks the nucleus. Bar = 50 μm. A, atrium; LV, left ventricle; OFT, outflow tract.
(F) Enlargement of boxed area in (E) to show that Isl1 and CIP expression is overlapped in the OFT cardiomyocytes (white arrowheads). The expression of Isl1 (green), CIP (as marked by β-gal positive cells, red) DAPI (blue) and merged images were shown. Isl1 and CIP positive cells were indicated by white arrowheads. Note that Isl1 is not expressed in the cardiomyocytes of atrium where CIP is expressed (yellow arrows). (G) CIP is a transcriptional repressor. HEK293T cells were transiently transfected with expression vectors encoding the CIP fused to GAL4 (1–147) and the pL8G5-luciferase reporter, which contains binding sites for the GAL4 DNA binding domain. Luciferase activity is expressed as relative activity in which the control was assigned a value of 1. Data represent the mean ± s.d. from at least three independent experiments in triplicate. *P<0.05.
(H) CIP represses the transcription activity of Isl1. MEF2C enhancer-luciferase reporter was co-transfected with indicated expression plasmids in HEK293T cells and luciferase activity was measured. Results were presented as relative luciferase activity in which the control was assigned a value of 1. Data represent the mean ± s.d. from at least three independent experiments in triplicate. *P<0.05.
(I) Define the domains of the CIP protein that mediate the repression of Isl1 transcription activity. MEF2C enhancer-luciferase reporter was co-transfected with indicated expression plasmids (full-length isl1, full-length or truncated CIP mutants) in HEK293T cells and luciferase activity was measured. Results were presented as relative luciferase activity in which the control was assigned a value of 1. Data represent the mean ± s.d. from at least three independent experiments in triplicate. *P<0.05.
(J) CIP represses myocardin- and SRF-mediated transactivation of the ANF-luciferase reporter. The ANF-luciferase reporter was co-transfected with indicated expression plasmids in HEK293T cells and luciferase activity was measured. Results were presented as relative luciferase activity in which the control was assigned a value of 1. Data represent the mean ± s.d. from at least three independent experiments in triplicate. *P<0.05.
Next, we attempted to map the domains of these two proteins that mediate their interaction. We made series of deletion mutants and tested their interaction using Co-IP assays. As shown in Fig. 5C, the interaction between CIP and Isl1 is readily detected in full-length (1-309) and both C- and N-terminal truncated mutants (1-200 and 100-309). However, further C-terminal deletion (1-100) abolished the interaction, suggesting that regions between aa 100 to 200 are required for CIP to interact wit Isl1 (Fig. 5C). Similarly, we examined domains in the Isl1 protein which are needed for the interaction with CIP. We found that aa 1-133 fragment is sufficient to mediate the interaction, whereas the aa 79-349 region failed to interact (Fig. 5D). These data indicate that the N-terminal region of the Isl1 protein is essential for its interaction with the CIP protein.
ISL1 is expressed in the cardiac progenitors and marks the second heart field. It was previously reported that ISL1 is not expressed in the cardiomyocytes of adult hearts23. We investigated whether CIP and ISL1 are co-expressed in mouse embryos. The expression of ISL1 was examined in Cip-lacZ reporter embryos. As shown in Fig. 5E and 5F, both LacZ and ISL1 were expressed in cardiomyocytes located in the outflow tract (OFT) of E10.5 mouse embryos (Fig. 5E, and 5F, white arrowheads). However, ISL1 is not expressed in the cardiomyocytes of atrium nor left ventricle, where CIP is expressed (Fig. 5F, yellow arrows). Given that ISL1 is expressed in cardiac progenitor cells of second heart field and newly formed cardiomyocytes in the outflow tracts23, these results confirmed that CIP and ISL1 are co-expressed in the cardiac progenitor cells of embryonic hearts.
CIP is as transcription co-factor of Isl1 and regulates its transcriptional activity
Because CIP was localized to the nucleus, we asked whether it possessed transcriptional activity. We fused the full-length CIP protein to the DNA binding domain of yeast GAL4 protein. Interestingly, this fusion protein repressed the GAL4-dependent reporter when co-transfected into HEK293T cells (Fig. 5G). Similar, we observed that GAL4-CIP repressed the reporter in Hela and COS7 cells (data not shown).
Loss-of-function studies indicate that Isl1 is essential for cardiac development23. ISL1 activates the expression of the Mef2c gene by directly binding to its anterior heart field enhancer46. We tested whether CIP participates in regulation of the Mef2c anterior heart field enhancer. As expected, ISL1 constantly activated the Mef2c enhancer luciferase reporter, though its activity does not appear to be potent (Fig. 5I). CIP alone did not significantly affect Mef2c enhancer activity. However, co-expression of ISL1 (the full-length, 1-309) and CIP inhibited ISL1-mediated Mef2c enhancer luciferase activity (Fig. 5I). Further analyses, using different CIP deletion mutants, showed that the aa 1-200 and the aa 100-309 constructs (but not the aa 1-100) were able to repress Isl1-mediated activation of the Mef2c enhancer. These observations indicate that direct interaction of CIP and Isl1 is necessary for CIP to repress Isl1. Our results suggest that CIP is a transcription co-factor and represses the transcriptional activity of ISL1.
Next, we tested whether CIP represses the activity of other transcriptional regulators. Myocardin is a transcription factor specifically expressed in cardiac- and smooth muscle that potently stimulates ANF promoter activity in conjunction with the transcription factor SRF36. We found that CIP significantly repressed myocardin-mediated activation of the ANF-luciferase reporter (Fig. 5J). Similarly, CIP also repressed SRF-dependent activation of the ANF luciferase reporter (Fig. 5J). Together, our results indicated that CIP is a putative transcriptional repressor expressed in the heart.
The expression of CIP is dysregulated in hypertrophic hearts
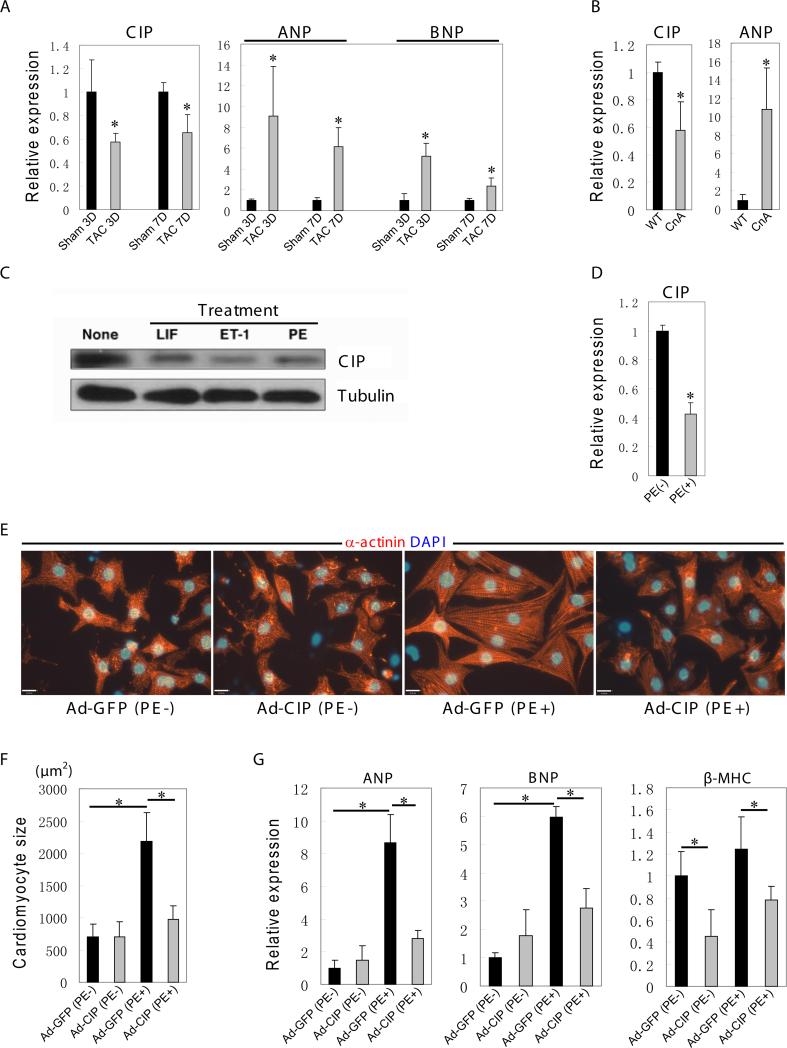
The heart undergoes cardiac remodeling in response to cardiac injury and/or stress. Increased pressure or volume overload in the heart results in cardiac hypertrophy, a process in which the size of cardiomyocytes increases without an increase in the number of cardiomyocytes. Interestingly, MEF2C was previously shown to be a key regulator of cardiac hypertrophy and dilated cardiomyopathy11, 47-50. Since CIP repressed the Mef2c enhancer activity, we hypothesized that CIP regulates cardiac hypertrophy. We first examined whether hypertrophic signals regulate cardiac CIP expression. We used a transverse aortic constriction to induce pressure overload and subsequent pathological cardiac hypertrophy. As expected ANP and BNP, well-known hypertrophy markers, were dramatically upregulated in Transverse Aortic Constriction (TAC) mice at day 3 (D3) and day 7 (D7) (Fig. 6A). In contrast, Cip transcripts were substantially reduced in TAC mice. Similarly, expression of CIP was reduced in a second well-characterized cardiac hypertrophy model27, in which transgenic expression of activated calcineurin stimulates dramatic cardiac hypertrophy (Fig. 6B).
Figure 6. CIP represses cardiomyocyte hypertrophy.
(A) Gene expression of CIP and hypertrophic marker, ANP and BNP, determined by qPCR in heart samples from Sham group and Transverse Aortic Constriction (TAC) group at two different time point (3 days and 7 days). n=4.
(B) Gene expression of CIP and hypertrophic marker ANP determined by qPCR in heart samples from 1 month old Myh6-calcineurin transgenic mice (CnA) and control littermates (WT). n=3.
(C) Western blotting analysis showing decreased expression of CIP proteins in neonatal rat cardiomyocytes, which were induced to develop hypertrophy by different hypertrophic agonists in vitro. None treated sample serves as a control. LIF, leukemia inhibitory factor; ET-1, Endothelin-1; PE, phenylephrine.
(D) Quantitative RT-PCR showing CIP expression in rat neonatal cardiomyocytes with or without phenylephrine (PE) treatment. *P<0.05.
(E) CIP represses PE-induced hypertrophy in neonatal cardiomyocytes. Representative images of cardiomyocytes infected with adenoviral-CIP (ad-CIP) or adenoviral-GFP (ad-GFP) (to serve as controls) and treated with phenylephrine (PE) (or without treatment to serve as controls). Anti α-actinin antibodies were used to mark cardiomyocytes (red). DAPI marks nucleus. Bar = 21 μm.
(F) Quantitative analysis of cardiomyocyte cell size. ~300 cells positive for α-actinin from each treatment were randomly chosen for surface area measurement. *P<0.05.
(G) Quantitative RT-PCR showing the downregulation of PE-induced expression of hypertrophic marker ANP, BNP and β-MHC when CIP was overexpressed in neonatal cardiomyocytes. n=4. *P<0.05. ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; β-MHC, β-myosin heavy chain.
In vivo cardiac hypertrophy models are complex and encompass both direct effects of inducing stimuli and secondary effects of myocardial responses, including cardiac fibrosis and failure. To directly examine the effect of hypertrophic stimuli on cardiomyocyte Cip expression, we used the well-established neonatal rat cardiomyocyte hypertrophy model. We treated neonatal rat cardiomyocytes with a panel of hypertrophic agonists, and measured the effect on Cip expression. We found that the CIP protein level was significantly reduced in neonatal rat cardiomyocytes treated with the hypertrophic agonists leukemia inhibitory factor (LIF), endothelin (ET)-1 and phenylephrin (PE) (Fig. 6C). Quantitative RT-PCR analyses of PE-treated cardiomyocytes corroborated this finding and indicated that CIP expression is regulated at the transcriptional level (Fig. 6D). These results demonstrate that the expression of CIP is repressed in hypertrophic cardiomyocytes and hearts, in vitro and in vivo.
CIP represses cardiomyocyte hypertrophy
The above observation established an inverse correlation between the expression of CIP and cardiac hypertrophy and suggests that CIP could directly regulate cardiomyocyte hypertrophy. To test this hypothesis, we overexpressed CIP in cardiomyocytes by adenoviral gene transfer. Neonatal rat cardiomyocytes were isolated, cultured in vitro and treated with PE to induce hypertrophy as previously described51. PE treatment dramatically induced cardiomyocyte hypertrophy, as measured by increased cell surface area (Fig. 6E). Overexpression of CIP did not result in significant change to cardiomyocytes. However, CIP significantly attenuated PE-induced hypertrophy (Fig. 6E). Quantification of cardiomyocyte size confirmed the observation (Fig. 6F). At the level of gene expression, PE induced expression of hypertrophic marker genes ANP, BNP and β-MHC, as expected. PE-induced expression of hypertrophic marker genes was inhibited by CIP (Fig. 6G). Together, our results suggest that CIP negatively regulates cardiomyocyte hypertrophy.
DISCUSSION
In the present study, we identified CIP as a novel cardiac-specific expressed nuclear protein. We demonstrated that CIP expression is restricted to cardiomyocytes. We further characterized CIP as a transcription co-factor of LIM/homeodomain transcription factor ISL1. We found that CIP physically interacts with ISL1 and represses its transcriptional activity. Functionally, we established that CIP represses cardiomyocyte hypertrophy.
We identified ISL1 as a CIP interacting protein via unbiased yeast two-hybrid screening and subsequently confirmed their physical and functional interaction. Our data suggest that a direct interaction between these two proteins is needed for CIP to repress the transactivity of Isl1. Isl1 is expressed in the second heart field and is required for the morphogenesis of embryonic heart23. Interestingly, Isl1+ progenitor cells were shown multipotent and give rise to cardiac, smooth muscle and endothelial cell lineages22, 52. Given that CIP interacts with Isl1 and is expressed in cardiac progenitors, we would speculate that CIP might participate in the function of Isl1 in cell fate determination during early cardiac development. Despite its pivotal role in development, less is known about how Isl1 works at molecular level. Isl1 is a LIM/homeodomain transcription factor and it synergistically activates insulin gene transcription with BETA2 in pancreatic beta cells 53. Furthermore, Isl1 forms a complex with Jak1 and Stat3 and triggers the tyrosine phosphorylation of Jak1 and its kinase activity 54. Isl1 works upstream of the sonic hedgehog signaling and the MEF2C transcription factor in the heart21, 46. Our data showed that the transcriptional activity of Isl1, though not very strong when compared with many other transcription factors, was repressed by CIP. This suggests another layer of regulation of this transcription factor.
In addition to its unique cardiac expression pattern, several lines of evidences point CIP an important regulator of cardiac gene expression and cardiac hypertrophy. Intriguingly, our data showed the capability of CIP to repress Isl1-mediated activation of the MEF2C enhancer. It had been shown that MEF2C was upregulated during pathological hypertrophy50. Transgenic mice expressing a dominant-negative MEF2C displayed attenuated postnatal growth of myocardium48, 49. Given the critical role of MEF2C in cardiac development and hypertrophy, it is reasonable to predict that CIP is also involved in this process. Consistent with this view, we found that the expression level of CIP was consistently downregulated in cardiac hypertrophy models, both in vivo and in vitro. Most importantly, we provide evidence to demonstrate that overexpression of CIP partially represses agonist-induced cardiomyocyte hypertrophy. It remains to be determined, using genetic approaches, whether CIP participates in the regulation of cardiac gene expression during heart development and whether dysregulation of CIP expression will lead to cardiac pathophysiological condition or human cardiovascular disease. It will also be important to understand how CIP participates in the regulatory pathways of cardiac hypertrophy.
CIP is evolutionarily conserved in vertebrate. Its orthologs were found from fish to human, suggesting it has conserved biological function and may play an important role in cardiac development and disease. CIP appears to stand as a single member of this class of protein, and no homologous protein is encoded in the mouse genome. Recently, another splice variant in the locus of 2310046A06Rik was identified as a lamin interacting protein (MLIP)44. MLIP was shown to interact directly with Lamin A/C (LMNA), a nuclear envelope protein. Interestingly, LMNA is known to interact with many transcription factors/co-factors, including retinoblastoma transcriptional regulator (RB)55, germ cell-less (GCL)56, sterol response element binding protein (SREBP1)57, FOS58, and MOK259. However, the functional significance of such interaction remains to be fully understood. LMNA is essential for the function of heart and human patients with LMNA mutation often exhibit adult-onset dilated cardiomyopathy accompanied with conduction-system disease60. LMNA null mice display severe skeletal muscle atrophy and dilated cardiomyopathy, and die by 8 weeks61, 62. These observations suggest that the LMNA interacting protein CIP/MLIP could be part of the network that regulates gene expression in LMNA-related dilated cardiomyopathy. It will be interesting to understand how CIP/MLIP modulates the function of LMNA, and most importantly, whether CIP/MLIP is directly related to human cardiac and muscle diseases.
Our data demonstrate that CIP is expressed in the developing and adult heart. More specifically, we show that CIP expression is restricted to cardiomyocytes and cardiac progenitors during development. We have also observed transient CIP expression in the skeletal muscle. However, the level of CIP expression in skeletal muscle appears to be low, when compared with that of cardiac muscle. Moreover, skeletal muscle expression of CIP is not detected at all stages of embryos or postnatal mice. It will be interesting to investigate whether CIP plays a role in skeletal muscle development and function.
MATERIALS AND METHODS
Database Searching and Cloning of CIP
We screened for novel cardiac-specific genes in silico by performing a BLAST search with ESTs from mouse embryonic heart cDNA libraries in the database as described previously36. One of the cDNA sequences identified in this screen (Access number AA919489) corresponded to the 3’ untranslated region (3’-UTR) of the CIP transcript and was found in a cDNA library of E13.5 mouse embryonic hearts. This short cDNA fragment (273 nt) was used as a probe to screen a mouse embryonic heart cDNA library. Several cDNA clones were identified. Among them, the longest clone is 1336 nt in length. Polymerase chain reaction (PCR)-based cloning was further used to identify additional cDNA isoforms.
CIP reporter mice
The CIP reporter line with a retroviral gene trap cassette inserted into mouse genome chromosome 9 (between chr9: 77,046,001 and 77,046,002) was generated and obtained from Texas A&M Institute for Genomic Medicine (TIGM). The gene trap cassette containing the selectable marker β-geo, a functional fusion between the β-galactosidase and neomycin resistance gene, was inserted into the putative third intron of the CIP gene (Fig. S3).
In Situ Hybridization and Northern Analysis
Whole-mount and section in situ hybridization and Northern analyses were performed as described36, 63. The 273 nt cDNA fragment corresponding to the 3’-UTR of the CIP gene was used as a probe to perform both whole-mount and tissue section in situ hybridization on staged mouse embryos.
Constructs, Cell Culture, and Luciferase Reporter Assays
Hela, COS7 and HEK293T cells were cultured in DMEM supplemented with 10% FBS in a 5% CO2 atmosphere at 37°C. Luciferase reporter constructs fused with the MEF2C enhancer were as reported and is a generous gift of Dr. Brian Black (University of California, San Francisco)46. Transfections were performed with either FuGENE6 (Roche) or Lipofectamine (Invitrogen) reagents according to manufacturers instruction. Unless otherwise indicated, 100 ng of reporter plasmid and 100 ng of each activator plasmid were applied. 48 hours after transfection, cell extracts were prepared and luciferase activity was determined. For luciferase assay, normalized luciferase expression from triplicate samples in 12-well plates relative to LacZ expression was calculated, and the results are expressed as fold activation over the value relative to the control (Luciferase reporter and empty pcDNA). The CIP adenoviral expression construct (Ad-CIP) contained full-length CIP cDNA and was constructed as previously described51.
Tissue dissociation and cell sorting of mouse embryos
The E10.5 embryonic hearts were isolated by microdissection and dissociated to single cells by collagenase digestion as previously described64. Isolated cells were FACS sorted into GFP positive and GFP negative populations. Sorted cells were collected into Trizol (Invitrogen) and frozen at -20°C for RNA isolation.
Immunochemistry and β-gal staining
Staged mouse embryos were dissected out, collected and fixed in 4% PFA at 4°C for 4 hours. After washing in PBS, embryos were treated in 15% and 30% sucrose for 2 hours each and embedded in OCT. About 5–8 μm cryostat sections were collected on positively charged slides. Sections were washed in PBS, blocked in 5% serum/PBS, and subjected to immunostaining. Antibody sources were as follows: GFP (Invitrogen); PECAM (BD Biosciences); Nkx2-5 (Santa Cruz); Wt1 (Santa Cruz); Cardiac Troponin T (Tnnt2) (generous gift of Dr. Jim Lin, University of Iowa); Cardiac Troponin I (Tnni3) (Santa Cruz); Actn2 (Sigma); Isl1 (DSHB, University of Iowa); β-galatosidase (MP Biomedical). Alexa-488 and 594 secondary antibodies (Invitrogen). Fluorescently stained slides were counterstained with DAPI and imaged with an FV1000 confocal microscope (Olympus). For β-gal staining, samples were stained with a solution containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-gal substrate at 37°C for 12 hours after fixation.
Quantitative RT-PCR and Western blot analyses
Total RNAs were isolated using Trizol Reagent (Invitrogen) from cells and tissue samples. For Quantitative RT-PCR, 2.0 μg RNA samples were reverse-transcribed to cDNA by using random hexamers and MMLV reverse transcriptase (Invitrogen) in 20 μl reaction system. In each analysis, 0.1 μl cDNA pool was used for quantitative PCR. For Western blot analyses, cell extractions were cleared by 10,000×g centrifugation for 10 min at 4°C. Samples were subsequently separated by SDS/PAGE and transferred to PVDF membranes that were incubated with 5% milk and Anti-FLAG (1:1,000, Sigma); Anti-Myc (1:1,000, Santa Cruz); β-tubulin (1:10,000, Sigma) overnight at 4°C and then washed three times with TBST buffer before adding secondary antibody. Polyclonal antibodies against the CIP protein were generated by immunizing rabbits with CIP proteins which were expressed and purified as GST-CIP fusion proteins in bacterial. Specific protein bands were visualized by using ECL (Invitrogen) reagents.
Coimmunoprecipitation Assays
COS7 cells were transiently transfected with plasmids encoding FLAG-tagged CIP and Myc-tagged Isl1 proteins with FuGENE6 (Roche). Cells were harvested 48 h after transfection in lysis buffer composed of 1X PBS containing 0.5% Triton X-100, 1 mM EDTA, 1 mM PMSF, 150 mM of NaCl and complete protease inhibitors (Roche). After a brief sonication and removal of cellular debris by centrifugation, FLAG-tagged CIP proteins were precipitated with anti-FLAG antibodies and protein A/G beads and associated proteins analyzed by Western blotting with anti-Myc antibodies.
In vitro GST Protein-Binding Assays
Plasmids encoding a GST fusion with CIP were transformed into BL21 plus cells (Stratagene). The cells were grown at 37°C in 2×YT medium to an optical density of 1.0. Isopropyl-β-D-thiogalactopyranoside (50 μM) was then added to the culture to induce protein expression. After being shaken at room temperature for 4 h, the cells were harvested and the GST protein was purified with glutathione beads. Isl1 proteins translated in vitro were labeled with 35S methionine by using a TNT T7 reticulocyte lysate system (Promega). Glutathione beads conjugated with GST fusion protein were incubated with 10 μl of TNT product at 4°C for 2 h in 500 μl of GST-binding buffer (20 mM Tris, pH 7.3/150 mM NaCl/0.5% Nonidet P-40/protease inhibitor/1 mM phenylmethylsulfonyl fluoride). The beads were washed three times with GST binding buffer. 50 μl of SDS loading buffer was then added to the beads. After boiling, 20 μl was loaded onto an SDS/PAGE gel and analyzed by autoradiography.
Cardiomyocyte culture
Neonatal rat cardiomyocytes were prepared as previously described51. Briefly, eighteen hours after plating, cells were changed into serum-free medium and infected with adenovirus (Ad-LacZ for control and Ad-CIP) at a multiplicity of infection (m.o.i.) of 25. 24 hours later, cells were treated with hypertrophic agents phenylephrine (PE). Cells were harvested 24 hours after PE treatment for RNA isolation or 48 hours after PE treatment for immunochemistry.
Supplementary Material
Novelty and Significance.
What Is Known?
Cardiac hypertrophy is one of the most common responses of the heart in response to mechanical stress and pathological stimuli
Chronic activation of pathological hypertrophy often leads to heart failure and sudden death
Several signaling pathways and transcriptional networks activate cardiac hypertrophy
Mechanisms underlying the repression of hypertrophy are not well understood.
What New Information Does This Article Contribute?
This study identified CIP (cardiac Isl1-interacting protein) as a novel cardiac-specific nuclear protein.
CIP is dynamically regulated in response to cardiac hypertrophy.
Overexpression of CIP represses cardiomyocyte hypertrophy.
Congestive heart failure is a common outcome of a variety of primary cardiovascular disease entities, including coronary artery disease, and hypertension,. Nevertheless, mechanisms underlying the progression of compensated hypertrophy to heart failure remain unclear.. Although the pathophysiology of cardiac hypertrophy has been extensively studied and much is known about signaling pathways that activate cardiac hypertrophy, processes leading to the repression of cardiac hypertrophy are less well understood. In this study, we identify CIP as a novel gene expressed in embryonic and adult hearts. Combinational approaches demonstrated that CIP expression is limited to cardiomyocytes, and that it is no expressed in other cell types (fibroblast, endothelial cells or vascular smooth muscle cells) of the heart. Our results show that CIP physically interacts with Isl1, a transcription factor essential for heart development and the fate of cardiac progenitors. Functionally, we found that CIP is a transcriptional repressor that represses agonist-induced cardiomyocyte hypertrophy and the expression of hypertrophic marker genes. These findings suggest that CIP may be a potential target in the development of future therapies for cardiac hypertrophy and heart failure.
ACKNOWLEDGEMENTS
We thank members of the Wang laboratory for advice and support.
Sources of Funding:
Work in Dr. Wang's laboratory was supported by the March of Dimes Birth Defect Foundation and National Institutes of Health. ZP Huang is a postdoctoral fellow and DZ Wang is an Established Investigator of the American Heart Association.
Non-standard Abbreviations and Acronyms
- ACTN2
cardiac actin
- ANF
atrial natriuretic factor
- BNP
brain natriuretic factor
- cDNA
complementary DNA
- CIP
cardiac Isl1-interacting protein
- Co-IP
co-immunoprecipitation
- E
embryonic
- EST
expressed sequence tag
- ET
endothelin
- FACS
fluorescence-activated cell sorting
- HATs
histone acetyltransferases
- HDACs
histone deacetylases
- IP
immunoprecipitation
- ISL1
Islet1
- LIF
leukemia inhibitory factor
- LMNA
Lamin A/C
- Luc
luciferase
- MEF2
Myocyte Enhancer Factor 2
- MYC
myosin heavy chain
- PE
phenylephrine
- qPCR
quantitative polymerase chain reaction
- TAC
transverse aortic constriction
- TNNT2
cardiac troponin T
- UTR
untranslated region
- WB
Western blot
Footnotes
Disclosures: The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10(5):467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 2.Musunuru K, Domian IJ, Chien KR. Stem cell models of cardiac development and disease. Annu Rev Cell Dev Biol. 26:667–687. doi: 10.1146/annurev-cellbio-100109-103948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A. 1993;90(17):8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9(13):1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 5.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119(3):969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276(5317):1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilly B, Zhao B, Ranganayakulu G, Paterson BM, Schulz RA, Olson EN. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267(5198):688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- 8.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120(5):1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 9.Creemers EE, Sutherland LB, Oh J, Barbosa AC, Olson EN. Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell. 2006;23(1):83–96. doi: 10.1016/j.molcel.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Naya FJ, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;11(6):683–688. doi: 10.1016/s0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 11.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Molkentin JD, Kalvakolanu DV, Markham BE. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Mol Cell Biol. 1994;14(7):4947–4957. doi: 10.1128/mcb.14.7.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterkin T, Gibson A, Loose M, Patient R. The roles of GATA-4, -5 and -6 in vertebrate heart development. Semin Cell Dev Biol. 2005;16(1):83–94. doi: 10.1016/j.semcdb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Burch JB. Regulation of GATA gene expression during vertebrate development. Semin Cell Dev Biol. 2005;16(1):71–81. doi: 10.1016/j.semcdb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 16.Harvey RP, Meilhac SM, Buckingham ME. Landmarks and lineages in the developing heart. Circ Res. 2009;104(11):1235–1237. doi: 10.1161/CIRCRESAHA.109.199729. [DOI] [PubMed] [Google Scholar]
- 17.Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18(4):210–216. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- 18.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18(1):67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development. Circ Res. 2009;104(8):933–942. doi: 10.1161/CIRCRESAHA.109.194464. [DOI] [PubMed] [Google Scholar]
- 20.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Bu L, Cai CL, Zhang X, Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol. 2006;295(2):756–763. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 22.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann T, Bodmer R, Pandur P. The Drosophila homolog of vertebrate Islet1 is a key component in early cardiogenesis. Development. 2009;136(2):317–326. doi: 10.1242/dev.022533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad F, Arad M, Musi N, He H, Wolf C, Branco D, Perez-Atayde AR, Stapleton D, Bali D, Xing Y, Tian R, Goodyear LJ, Berul CI, Ingwall JS, Seidman CE, Seidman JG. Increased alpha2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation. 2005;112(20):3140–3148. doi: 10.1161/CIRCULATIONAHA.105.550806. [DOI] [PubMed] [Google Scholar]
- 26.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 27.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104(4):557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 29.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 30.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 90(4):1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110(4):479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115(3):538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauschka SD. Myocardin. a novel potentiator of SRF-mediated transcription in cardiac muscle. Mol Cell. 2001;8(1):1–2. doi: 10.1016/s1097-2765(01)00297-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105(7):851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 37.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20(12):1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 38.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14(5):558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428(6979):185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100(12):7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100(16):9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci U S A. 2005;102(25):8916–8921. doi: 10.1073/pnas.0503741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh J, Richardson JA, Olson EN. Requirement of myocardin-related transcription factor-B for remodeling of branchial arch arteries and smooth muscle differentiation. Proc Natl Acad Sci U S A. 2005;102(42):15122–15127. doi: 10.1073/pnas.0507346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmady E, Deeke SA, Rabaa S, Kouri L, Kenney L, Stewart AF, Burgon PG. Identification of a novel muscle A-type lamin-interacting protein (MLIP). J Biol Chem. 286(22):19702–19713. doi: 10.1074/jbc.M110.165548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84(2):309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 46.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131(16):3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 47.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105(10):1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH, Molkentin JD. Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J Biol Chem. 2006;281(14):9152–9162. doi: 10.1074/jbc.M510217200. [DOI] [PubMed] [Google Scholar]
- 49.van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, Doevendans PA, Schneider MD, van Echteld CJ, De Windt LJ. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114(4):298–308. doi: 10.1161/CIRCULATIONAHA.105.608968. [DOI] [PubMed] [Google Scholar]
- 50.Kolodziejczyk SM, Wang L, Balazsi K, DeRepentigny Y, Kothary R, Megeney LA. MEF2 is upregulated during cardiac hypertrophy and is required for normal post-natal growth of the myocardium. Curr Biol. 1999;9(20):1203–1206. doi: 10.1016/S0960-9822(00)80027-5. [DOI] [PubMed] [Google Scholar]
- 51.Xing W, Zhang TC, Cao D, Wang Z, Antos CL, Li S, Wang Y, Olson EN, Wang DZ. Myocardin induces cardiomyocyte hypertrophy. Circ Res. 2006;98(8):1089–1097. doi: 10.1161/01.RES.0000218781.23144.3e. [DOI] [PubMed] [Google Scholar]
- 52.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Wang WP, Guo T, Yang JC, Chen P, Ma KT, Guan YF, Zhou CY. The LIM-homeodomain protein ISL1 activates insulin gene promoter directly through synergy with BETA2. J Mol Biol. 2009;392(3):566–577. doi: 10.1016/j.jmb.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 54.Hao A, Novotny-Diermayr V, Bian W, Lin B, Lim CP, Jing N, Cao X. The LIM/homeodomain protein Islet1 recruits Janus tyrosine kinases and signal transducer and activator of transcription 3 and stimulates their activities. Mol Biol Cell. 2005;16(4):1569–1583. doi: 10.1091/mbc.E04-08-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci U S A. 2004;101(26):9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura T, Ito C, Watanabe S, Takahashi T, Ikawa M, Yomogida K, Fujita Y, Ikeuchi M, Asada N, Matsumiya K, Okuyama A, Okabe M, Toshimori K, Nakano T. Mouse germ cell-less as an essential component for nuclear integrity. Mol Cell Biol. 2003;23(4):1304–1315. doi: 10.1128/MCB.23.4.1304-1315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11(7):769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- 58.Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O'Connor JE, Burke B, Andres V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20(3):307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dreuillet C, Tillit J, Kress M, Ernoult-Lange M. In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res. 2002;30(21):4634–4642. doi: 10.1093/nar/gkf587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sylvius N, Tesson F. Lamin A/C and cardiac diseases. Curr Opin Cardiol. 2006;21(3):159–165. doi: 10.1097/01.hco.0000221575.33501.58. [DOI] [PubMed] [Google Scholar]
- 61.Ostlund C, Bonne G, Schwartz K, Worman HJ. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J Cell Sci. 2001;114(Pt 24):4435–4445. doi: 10.1242/jcs.114.24.4435. [DOI] [PubMed] [Google Scholar]
- 62.Raharjo WH, Enarson P, Sullivan T, Stewart CL, Burke B. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J Cell Sci. 2001;114(Pt 24):4447–4457. doi: 10.1242/jcs.114.24.4447. [DOI] [PubMed] [Google Scholar]
- 63.Wang DZ, Reiter RS, Lin JL, Wang Q, Williams HS, Krob SL, Schultheiss TM, Evans S, Lin JJ. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development. 1999;126(6):1281–1294. doi: 10.1242/dev.126.6.1281. [DOI] [PubMed] [Google Scholar]
- 64.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.