Abstract
Objectives
The purpose of this study is to analyze the potential benefits of aerobic exercises at different intensities in the management of pre menstrual syndrome.
Methods
The study design is quasi-experimental; sixty-one female subjects were randomly allocated into three groups, Group A (mild intensity), Group B (moderate intensity) and Group C (severe intensity) and the intervention were given for 6 weeks. The study setting was general community settings. The outcome measures were menstrual symptom questionnaire, VO2 max, forced vital capacity (FVC), maximum voluntary ventilation (MVV) and lipid profile (HDL, LDL, TGL).
Results
There is significant decrease in menstrual symptoms in both Groups B and C. However, Group C improved with increased rate of perceived exertion. LDL levels did not change significantly but HDL, TGL, VO2 max, FVC, and MVV improved significantly in Groups B and C, but remains significantly unchanged in Group A.
Conclusions
This study encourages the employment of regular, moderate intensity aerobic exercise as a potential intervention for pre menstrual syndrome.
Keywords: Aerobic exercise, Pre menstrual syndrome, Metabolic changes, Menstrual symptoms, Quality of life
Introduction
Pre menstrual syndrome (PMS) is an array of predictable physical, cognitive, affective and behavioral symptoms that occur cyclically during the luteal phase of the menstrual cycle [1]. Evidence suggests that PMS is not a single condition but a set of interrelated symptom complexes with multiple genotypes, phenotypes or subtypes and several different patho physiologic events that begin with ovulation [2, 3]. Premenstrual symptoms occur in 90% of all women of reproductive age and about 10% are diagnosed as having premenstrual dysphoric disorder [4]. Over 200 premenstrual symptoms have been reported and individuals with underlying disorders such as affective or anxiety disorder may be particularly vulnerable to menstrual triggers but do not show symptomatology at other times because of factor which prevent them from being vulnerable outside of the premenstrual time frame [2, 3].
However, many conditions are also subject to menstrual magnification in which symptoms are triggered or exacerbated by the menstrual cycle. (e.g., include depressive disorders, seizures, migraine headache, allergies and asthma). Recognizing this phenomenon it is important in distinguishing PMS from chronic underlying conditions [5]. It is reported that behavior changes, criminal behavior, suicide attempts, absenteeism from work, hospital admissions and increased prone to accidents can be aggravated premenstrually [1]. Gannon et al. found that the length of time women had been exercising correlated significantly with lower levels of menstrual symptoms via correction of neuroendocrine abnormality [6]. Keye reported lower levels of anxiety in women who exercised regularly compared with non-exercisers [7] and Schwartz found that women runners reported a decrease in PMS [8]. In a cross sectional study, Timonon reported that of 748 female university students surveyed, 136 students of physical education department has fewer pre menstrual symptoms [9]. Coggen et al. reported that, if the training stimulus is adequate, the majority of the responses are independent of sex and age. This study was conducted to examine the impact of an aerobic exercise program at different intensities on, menstrual symptoms, metabolic control, aerobic capacity and respiratory endurance in girls with pre menstrual symptoms.
Design and Methods
Eighty-six female subjects volunteered to participate in the study from the general community of Puducherry and 61 of 86 met selection criteria. Informed written consent was obtained from all the selected subjects. Samples were divided into Group A (n = 20), B (n = 20), and C (n = 21) using random group allocation software. Of the 61 recruited subjects, two did not complete the program, Group B = 1, C = 1. Six subjects did not have lipid profile investigation Group A = 1, B = 2, and C = 3. Menstrual symptom questionnaires (MSQ) was handed out to the subjects and instructed to fill the questionnaires during three separate occasions (menstrually, premenstrually, intermenstrually). The reliability of the questionnaire is 0.88. A prospective chart of Borg scale was given to the patients for identifying the rate of perceived exertion from day 1 to end of exercise session (Figs. 1, 2, 3, and 4).
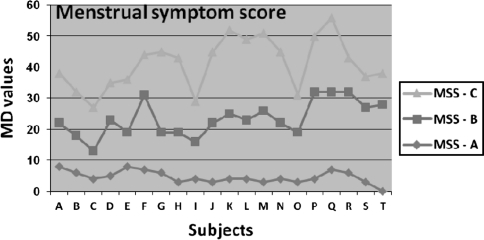
Fig. 1.
Mean difference scores of three groups. A-T subjects; MSS Menstrual symptom score; MD Mean difference
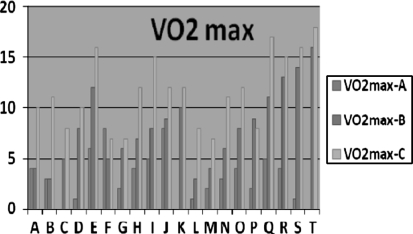
Fig. 2.
Mean difference scores of three groups
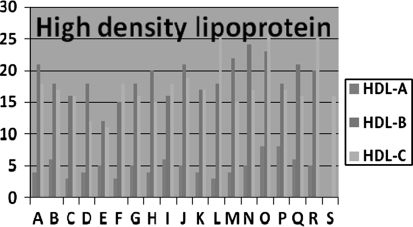
Fig. 3.
Mean difference scores of three groups
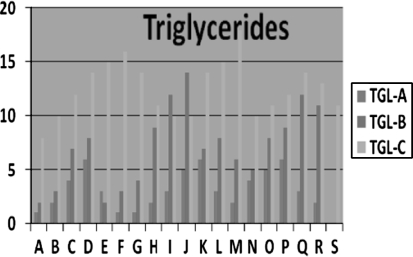
Fig. 4.
Mean difference scores of three groups
Subjects were included as per American college of obstetrics and gynecology diagnostic criteria for menstrual symptoms Table 1, with age between 15 and 25 years. Participants were excluded if they have known cardiovascular disorder, thyroid disease, and renal impairment, subjects under medication for any systemic disease, non menstruating girls and pregnant mothers. The mode of exercise chosen for the intervention was aerobic and the training protocol was framed using FIIT’s principle for all the recruited subjects. Intensity was calculated using Karovenen formula as per the aerobic guidelines according to American College of Sport Medicine (ACSM). Group A—mild intensity 35–60% of HRmax, Group B—moderate intensity 60–80% of HRmax and Group C—high intensity 80–90% of HRmax. (HR max = 220—age of the subject). The aerobic exercise includes (1) warm up phase (2) upper body exercise (3) lower body exercise (4) cool down phase. All the exercises were demonstrated and the study group was instructed to continue the exercise at home regularly (4 days/week) and report the changes or discomfort noticed during/after the exercise session. The tools used in this study were (i) spirometer (RMS Helios 401), transducer, disposable cuff, cotton, measuring tape, stop watch, questionnaire copies, and aerobic exercise charts.
Table 1.
ACOG diagnostic criteria for PMS
| Affective symptoms | Somatic symptoms |
|---|---|
| Depression | Breast tenderness |
| Angry outbursts | Abdominal bloating |
| Irritability | Headache |
| Anxiety | Swelling of extremities |
| Confusion | |
| Social withdrawal |
American College of Obstetrics and Gynecology ACOG April 2000
Diagnosis made if there is a report of at least one of these affective and somatic symptoms in the three prior menstrual cycles during the 5 days before the onset of menses
The symptoms must resolve within 4 days of onset of menses and not recur until after day 12 of the cycle
The symptoms must be present in at least two cycles during prospective recording
The symptoms must adversely affect social or work related activities
Outcome Measures
(1) Menstrual symptom questionnaire (MSQ) is a self-reported instrument, five point Likert scale (Severe/Moderate/Mild/Hardly/No problem). The questions framed as “In relation to your menstrual periods, to what extent the following issues have been problem for you? It consists of eight domains under which 46 symptoms were grouped. The domains in the questionnaire are (i) pain (ii) concentration (iii) behavioral change (iv) autonomic reactions (v) water retention (vi) negative effect (vii) arousal (viii) control. (2) VO2max was calculated using 3 min Step test, (VO2max = 65.81—(0.1847 × Step test pulse beats/min). Subjects were asked to perform each stepping cycle of a four-step cadence, “up–up–down–down” for 3 min. After completion, pulse rate was measured. Recovery heart rate was converted to beats per minute and compared with predicted VO2 max [10]. Forced vital capacity (FVC) and maximum voluntary ventilation (MVV) were analyzed using computerized spirometry (RMS Helios 401). Proper usage of transducer was demonstrated and nose clips were given to the subjects to avoid errors, the results were tabulated and data interpreted. Lipid profile (HDL, LDL and TGL) has been taken as another indicator of metabolic control to analyse the effects of aerobic exercise in girls with PMS.
Data Analysis and Results
The data were collated in Microsoft Excel 2007. Analysis was undertaken using statistical package of social sciences windows version 16 (SPSS Inc., Chicago IL, USA) by a statistician who was blind to identity the three groups. Mean was used as the measure of central tendency and 95% of Confidence Interval was used to express the true population mean. Paired t test was used to find the significance of mean within group, while one-way ANOVA was used to find the significance of improvement between groups. Paired t test is also used to analyze correlation between VO2max and Borg scale scores for the three groups. The baseline characteristics of the subjects, in three groups were highly comparable the values are computed in Table 2, along with their P value and there is no significant difference both within and between the three groups.
Table 2.
Baseline characteristic of study subjects
| Baseline characteristics | Group A | P value | Group B | P value | Group C | P value | Total |
|---|---|---|---|---|---|---|---|
| Female (no.) | 20 | 20 | 21 | 61 | |||
| Age | |||||||
| Range | 17–21 | >0.05 | 17–21 | >0.05 | 17–21 | >0.05 | 17–21 |
| Mean | 18.68 | 18.4 | 18.95 | 18.677 | |||
| BMI | |||||||
| Range | 20–31.4 | >0.05 | 20–32 | >0.05 | 20–32.4 | >0.05 | 20–32.4 |
| Mean | 24.98 | 24.61 | 24.67 | 24.753 | |||
| RHR | |||||||
| Range | 62–86 | >0.05 | 70–84 | >0.05 | 68–82 | >0.05 | 62–86 |
| Mean | 73.84 | 75.6 | 74.6 | 74.69 | |||
| BP (systolic) | |||||||
| Range | 110–130 | >0.05 | 110–132 | >0.05 | 110–136 | >0.05 | 110–136 |
| Mean | 122.21 | 123.6 | 123 | 122.949 | |||
| BP (diastolic) | |||||||
| Range | 68–82 | >0.05 | 70.84 | >0.05 | 70–86 | >0.05 | 68–86 |
| Mean | 72.31 | 78 | 77.9 | 76.13 | |||
| MSS | |||||||
| Mean | 185.05 | >0.01 | 201.75 | >0.01 | 196.48 | >0.01 | 177.796 |
| SD | 19.23 | 20.82 | 21.98 | ||||
| VO2max | |||||||
| Mean | 24.89 | >0.01 | 23.70 | >0.01 | 24.15 | >0.01 | 24.37 |
| SD | 4.41 | 5.89 | 4.44 | ||||
| HDL | |||||||
| Mean | 33.06 | >0.01 | 32.11 | >0.01 | 28.37 | >0.01 | 31.127 |
| SD | 9.51 | 9.49 | 8.12 | ||||
| LDL | |||||||
| Mean | 112.78 | >0.01 | 114.61 | >0.01 | 111.95 | >0.01 | 113.090 |
| SD | 8.27 | 10.05 | 8.95 | ||||
| TGL | |||||||
| Mean | 86.39 | >0.01 | 87.44 | >0.01 | 93.16 | >0.01 | 81.327 |
| SD | 12.33 | 15.88 | 13.66 | ||||
| FVC | |||||||
| Mean | 0.2995 | >0.01 | 0.2710 | >0.01 | 0.2450 | >0.01 | 0.271 |
| SD | 0.1305 | 0.1195 | 0.1043 | ||||
| MVV | |||||||
| Mean | 31.63 | >0.01 | 31.30 | >0.01 | 32.15 | >0.01 | 31.694 |
| SD | 4.62 | 5.859 | 5.71 | ||||
BMI body mass index, RHR resting heart rate, BP blood pressure, MSS menstrual symptom score, HDL high density lipoprotein, LDL low density lipoprotein, TGL triglycerides, FVC force vital capacity, MVV maximum voluntary ventilation
The physiological and biochemical variables at baseline and after 6 weeks for Group A, B, and C were shown in Table 3. After 6 weeks of aerobic training, menstrual symptoms scores were decreased significantly in the next menstrual periods in Group B and C, However, Group C improved with increased rate of perceived exertion. The analysis of variance (ANOVA) for the MSQ items reveal that girls who underwent regular, aerobic exercise showed significantly lower levels menstrual symptoms with (P < 0.05) in all three groups (n = 61). However in examination of each group separately, Group B and C (moderate intensity and severe intensity group) showed significantly decreased level of menstrual symptoms, when compared to Group A. Analysis of individual symptoms under particular domain reveals significant lower levels of pain and concentration in Group A, pain, concentration, negative effect and behavioral change in B and C, but autonomic reactions, water retention, control and arousal did not differ significantly after the intervention by comparing the mean difference values Table 4. Pain and concentration significantly decreased in all the three groups. Muscle stiffness, headache, cramps, backache, fatigue and general aches are the seven items under “Pain”. Insomnia, forgetfulness, confusion, lowered judgment, difficulty in concentrating, accidents, and lowered motor coordination are the eight items under “concentration”.
Table 3.
Outcome measures in three groups before and after intervention
| Group A | Group B | Group C | P value | ||||
|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | ||
| MMS | 185.05 ± 19.23 | 180.26 ± 18.46 | 201.75 ± 20.82 | 182.95 ± 19.91 | 196.48 ± 21.98 | 177.90 ± 20.11 | <0.01 |
| VO2 max | 24.89 ± 4.41 | 28.21 ± 4.47 | 23.70 ± 5.89 | 31.20 ± 7.06 | 24.15 ± 4.44 | 35.25 ± 4.61 | <0.01 |
| HDL | 33.06 ± 9.51 | 37.94 ± 9.97 | 32.11 ± 9.49 | 50.89 ± 10.73 | 28.37 ± 8.12 | 46.11 ± 8.41 | <0.05 |
| LDL | 112.78 ± 8.27 | 116.78 ± 8.31 | 114.61 ± 10.05 | 118.39 ± 9.72 | 111.95 ± 8.95 | 116.63 ± 9.87 | <0.05 |
| TGL | 86.39 ± 12.33 | 83.11 ± 11.94 | 87.44 ± 15.88 | 80.28 ± 15.67 | 93.16 ± 13.66 | 80.63 ± 14.35 | <0.05 |
| FVC | 0.2995 ± 0.1305 | 0.5205 ± 0.2244 | 0.2710 ± 0.1195 | 0.7525 ± 0.1883 | 0.2450 ± 0.1043 | 1.4265 ± 0.3724 | <0.01 |
| MVV | 31.63 ± 4.62 | 33.47 ± 4.75 | 31.30 ± 5.859 | 36.05 ± 5.84 | 32.15 ± 5.71 | 38.25 ± 6.03 | <0.01 |
Table 4.
Change scores on 8 domains of menstrual symptom questionnaire in three study groups
| Variables | Groups | Pre test mean ± SD | Post test mean ± SD | P value |
|---|---|---|---|---|
| Pain | A | 28.32 ± 4.91 | 26.47 ± 4.86 | 0.000 |
| B | 28.10 ± 4.14 | 22.70 ± 4.46 | 0.000 | |
| C | 28.20 ± 4.35 | 17.85 ± 5.72 | 0.000 | |
| Concentration | A | 23.26 ± 4.29 | 21.74 ± 4.68 | 0.000 |
| B | 23.35 ± 4.76 | 18.85 ± 4.03 | 0.000 | |
| C | 31.85 ± 4.86 | 20.70 ± 6.15 | 0.000 | |
| Behavioral change | A | 15.84 ± 2.46 | 15.37 ± 2.73 | 0.046 |
| B | 31.75 ± 4.81 | 25.55 ± 3.89 | 0.000 | |
| C | 16.01 ± 2.51 | 8.30 ± 3.51 | 0.000 | |
| Autonomic reactions | A | 19.84 ± 3.15 | 18.95 ± 3.58 | 0.009 |
| B | 16.10 ± 2.22 | 13.25 ± 1.68 | 0.000 | |
| C | 16.15 ± 2.21 | 13.20 ± 2.97 | 0.000 | |
| Water retention | A | 15.37 ± 2.39 | 14.74 ± 2.68 | 0.010 |
| B | 15.35 ± 2.32 | 12.70 ± 2.34 | 0.000 | |
| C | 15.48 ± 2.33 | 7.55 ± 3.03 | 0.000 | |
| Negative effect | A | 31.89 ± 4.70 | 31.00 ± 5.10 | 0.132 |
| B | 31.95 ± 4.45 | 27.45 ± 4.33 | 0.000 | |
| C | 31.60 ± 4.56 | 21.65 ± 5.87 | 0.000 | |
| Arousal | A | 15.84 ± 2.57 | 15.42 ± 2.59 | 0.042 |
| B | 19.90 ± 3.08 | 16.60 ± 2.66 | 0.000 | |
| C | 19.70 ± 3.13 | 16.60 ± 3.27 | 0.000 | |
| Control | A | 31.63 ± 4.95 | 30.58 ± 4.90 | 0.006 |
| B | 14.75 ± 2.47 | 13.05 ± 2.35 | 0.000 | |
| C | 23.05 ± 4.24 | 18.95 ± 4.56 | 0.000 |
Low density lipoprotein did not change significantly in all the three groups. High density lipoprotein cholesterol and triglycerides improved significantly after 6 weeks in Group B and C, but did not change in Group A. No significant change in peak VO2 max in Group A, but increased in Group B and C. FVC, MMV significantly increased after 6 week in Group B and C, however no significant change was found in Group A. There is significantly high correlation between the VO2max and Borg scale scores in Group A mild intensity), B (moderate) and C (severe intensity) with r = 0.981, r = 0.812, and r = 0.844, respectively.
Discussion
Greene has described the symptoms of the premenstrual syndrome as of extraordinary diversity and relevant to every specialty. However, before any medical treatments are commenced, it is still important to confirm that lifestyle has been optimized. This study demonstrated that moderate and severe intensity aerobic exercise training over 6 weeks reduced menstrual symptoms in patients with PMS. However, severe intensity group improved with increased rate of perceived exertion during exercise. The impact of aerobic training at different intensities on PMS has not been fully investigated, but it is suggested that both intensity and duration of exercise are important for hormonal regulation and anti-oxidant adaptation. The onset of symptoms is gradual, there may be a fortnight of lethargy and depression, some 10 days of irritability, a gradual onset of vertigo during last week and finally a severe migraine attack on the last premenstrual day, progressed to more incapacitating symptoms. Maximum fluctuation in hormones occurs during the premenstrual/menstrual phases. Israel found a raised renal threshold for estrogens in patients with PMS and suggests that the cause was not high level of oestradiol in the blood but due to lack of progesterone to act as antagonist [11]. It is not only ovarian hormones which fluctuate, but that those of adrenal cortex (Aldosterone) also exhibit cyclical activity, which is increased in the week preceding menstruation. Aldosterone causes water and sodium retention, potassium depletion (electrolyte imbalance) and hypertension because of insufficient progesterone to act as aldosterone antagonists [12]. Aerobic exercise training acutely raises serum progesterone levels, a response prevented by prolonged fasting before exercise. Such increase in progesterone may be insufficient to substantially alter the menstrual cycle, but may provide positive benefit to alter mood and decrease stress via neurotransmitter systems (e.g., GABA, Serotonin) modulated by sex steroids [13]. The efficacy of aerobic exercise on pain may be related to release of endorphin, counteracting possible declines in endorphin levels in the luteal phase. Raised endorphin levels have been associated with significant reductions in depression. The disturbed ratio between mineralocorticoid (desoxycorticosterone, aldosterone, water and electrolyte balance) and estrogen on the one hand and progesterone and glucocorticoids (related to stress, anti-allergic, anti-inflammatory and carbohydrate metabolism) on the other hand would go a long way toward explaining the totality of the syndrome. The effects of exercise on hormonal secretions were described in Table 5. The reduction in menstrual symptoms by aerobic exercise training may be associated with improved insulin sensitivity and glycemic control. Reports indicate that the effect of insulin sensitivity of a single bout of aerobic exercise lasts for 24–72 h. Therefore the frequency of exercise session should be at least three times per week.
Table 5.
The effects of exercise on hormone output
| Host gland | Hormone | Exercise effects on hormone secretion |
|---|---|---|
| Anterior pituitary | Endorphins | Increase with increasing exercise |
| Growth hormone (GH) | ||
| Prolactin | ||
| Posterior pituitary | Vasopressin (ADH) | Increase with increasing exercise |
| Oxytocin | Increase with heavy exercise only | |
| Adrenal cortex | Cortisol | Increase with increasing exercise |
| Corticosterone | ||
| Aldosterone | ||
| Adrenal medulla | Epinephrine | Increase with increasing exercise |
| Norepinephrine | ||
| Thyroid | Thyroxine | Increase with increasing exercise |
| Triiodothyronine | ||
| Pancreas | Insulin | Decreases with exercise |
| Glucagon | Increases with exercises | |
| Ovaries | Estrogen | Increases with exercise |
| Progesterone | ||
| Kidney | Renin | Increase with increasing exercise |
High density lipoprotein and triglycerides improved significantly in Group B and C. However it is reported that improved glycemic control by aerobic training is always associated with reduction in serum malondialdehyde, a reliable measure of lipid peroxidation. Elosua et al. reported that aerobic training increased the activity of endogenous antioxidants, glutathione peroxidase and decreased low density lipoprotein concentration [14]. Kelly et al. reported in a meta analysis that effect of aerobic exercise in lipid profile was equivalent to improvement of all variables (HDL, LDL, triglycerides, and total cholesterol), but after conducting sensitivity analysis, only decrease in triglycerides remained statistically significant which in turn influence the hormonal function.
Results of VO2max, FVC, and MVV proved the beneficial effect of aerobic fitness of these menstruating girls. However, the VO2max positively correlated with Borg scale scores. The VO2 max does, provide important information on the capacity of the long-term energy system; in addition, this measure has significant physiological cardiovascular, neuromuscular adaptations which in turn regulate hormonal mechanism. Although increase in exercise duration can increase oxidative stress, it also simultaneously induces antioxidant systems. Long-term exercise training can reduce oxidative stress by enhancing antioxidant defence mechanisms. From this study we propose that moderate to severe intensity exercise training over 6 week or above is an effective intervention for reducing menstrual symptoms, and it also enhances the aerobic fitness in the menstruating girls. The aerobic exercises produce a characteristic “training effect”, in which the cardiovascular adaptations include reduced heart rate at rest and at submaximal workload, increased work capacity and increased maximal oxygen consumption (VO2 max). The theoretical utility of the study reveals that, if a person has to reach the maximum capacity for aerobic metabolism and adequate hormonal regulation for decrease in menstrual symptoms, it is achieved only by the sufficient dosage of aerobic exercise with appropriately framed protocol. Another finding of this study is that increase in HDL were associated with increase in maximum oxygen consumption (VO2max) in ml/kg/min r value 0.85, P < 0.05) and VO2 max positively correlated with rate of perceived exertion (Borg scores). Aerobic exercise may have psychological benefits like improved body image and self-efficacy, which improves self-esteem [15]. The results of this study encourage the incorporation of regular, moderate aerobic exercise as a potential intervention for PMS.
Acknowledgments
The authors thank our institute (M.T.P.G and R.I.H.S) for setting the stage for our performance and sincere thanks to the time, effort, and interest shown by the subjects of this study.
Appendix: Menstrual Symptom Questionnaire
In relation to your menstrual periods, to what extent the following issues have been a problem for you?
References
- 1.Braverman PK. Premenstrual syndrome and premenstrual dysphoric disorder. J Pediatr Adolesc Gynecol. 2007;20:3–12. doi: 10.1016/j.jpag.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Halbreich U. The diagnosis of premenstrual syndromes and premenstrual dysphoric disorder-clinical procedures and research perspectives. Gynecol Endocrinol. 2004;19:320. doi: 10.1080/0951590400018215. [DOI] [PubMed] [Google Scholar]
- 3.Indhusekar R, Usman SB, O’Brien S. Psychological aspects of premenstrual syndrome. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):207–220. doi: 10.1016/j.bpobgyn.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Ismail KHM, O’Brien S. Premenstrual syndrome. Curr Obstet Gynecol. 2005;15:25–30. doi: 10.1016/j.curobgyn.2004.09.003. [DOI] [Google Scholar]
- 5.Cronin L, Guyatt G, Griffith L, et al. Development of a health-related quality of life questionnaire (PCOSQ) for women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(6):1976–1987. doi: 10.1210/jc.83.6.1976. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetrics and Gynecology: ACOG practice bulletin: premenstrual syndrome. Washington, DC: ACOG; 2000. [PubMed]
- 7.Aganoff JA, Boyle GJ. Aerobic exercise, mood states and menstrual cycle symptoms. Humanities & social sciences papers; 1994. [DOI] [PubMed]
- 8.Randeva HS, Lewandowski KC, Drzewoski J, et al. Exercise decreases plasma total homocysteine in overweight young women with poly cystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(10):4496–4501. doi: 10.1210/jc.2001-012056. [DOI] [PubMed] [Google Scholar]
- 9.Steege JF, Blumenthal JA. The effects of aerobic exercise on premenstrual symptoms in middle-aged women: a preliminary study. J Psychosom Res. 1993;37(2):27–133. doi: 10.1016/0022-3999(93)90079-U. [DOI] [PubMed] [Google Scholar]
- 10.Mc Ardle WD, Margel JR, Delio DJ, et al. Specificity of run training on VO 2 max and hear rate responses. Med Sci Sport. 2006;6:16. [PubMed]
- 11.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 12.Kelly GA, Kelly KS, Tranz Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Obes (Lond) 2005;29(8):881–893. doi: 10.1038/sj.ijo.0802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassis GP, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. J metabol. 2005;54(11):1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Barnett JB, Woods MN, Lamon-Fava S, et al. Plasma lipids and lipoprotein levels during follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 2004;89:776–782. doi: 10.1210/jc.2003-030506. [DOI] [PubMed] [Google Scholar]
- 15.Rapkin AJ. New treatment approaches for premenstrual disorders. Am J Manag Care. 2005;11:480. [PubMed] [Google Scholar]