Abstract
Sterculic acid is a cyclopropene fatty acid with numerous biological activities. In this study we demonstrate that sterculic acid is a potent inhibitor of endoplasmic reticulum (ER) stress and related inflammation caused by 7-ketocholesterol (7KCh). 7KCh is a highly toxic oxysterol suspected in the pathogenesis of various age-related diseases such as atherosclerosis, Alzheimer’s disease and age-related macular degeneration. Sterculic acid demonstrated to be 5–10 times more effective than other anti-inflammatory fatty acids at inhibiting 7KCh-mediated inflammatory responses in cultured cells. In vivo, sterculic acid was effective at inhibiting the formation of choroidal neovascularization (CNV) in the laser-injury rat model. Our data suggests that sterculic acid may be useful in treating CNV in certain forms of age-related macular degeneration.
Supplementary Key words: Long-chain fatty acids, oxysterol, UPR, angiogenesis
INTRODUCTION
Accumulation and subsequent oxidation of lipoprotein deposits is a major feature in the development of chronic inflammation in aging diseases like age-related macular degeneration (AMD) [1, 2] and cardiovascular disease (CVD) [3–5]. 7-ketocholesterol (7KCh) is one of the major components of oxidized low-density lipoprotein (oxLDL) deposits and is mainly responsible for the cytotoxicity associated with oxLDL [6–9]. Many studies have shown that 7KCh activates endoplasmic reticulum (ER) stress [10–14], and induces several angiogenic and inflammatory cytokines [15–17], as well as causing cell death in different cell types [2–4, 6, 7, 15, 18]. Hence, 7KCh found in oxidized lipid plaques is suspected of inducing chronic inflammation and subsequent stress to the surrounding tissues [2–6, 15, 18].
Several in vivo and in vitro studies have demonstrated that polyunsaturated fatty acids (PUFAs), particularly omega-3s (ω-3) and their metabolites, exhibit anti-inflammatory properties induced by various inflammatory cytokines [19, 20]. Diets rich in PUFAs have been shown to reduce the risk of AMD (21–23) and CVD [24, 25] which are diseases in which oxLDL is suspected in their pathogenesis [1–5, 18]. There is also ample evidence suggesting that prolonged ER stress and the subsequent inflammatory responses are involved in the pathogenesis of atherosclerosis [10, 26]. 7KCh [11] and oxLDL [12] are known to induce ER stress by affecting intracellular calcium levels [13, 14]. Since 7KCh is one of the major toxic and inflammatory components in oxLDL deposits [2, 6–8, 18], the therapeutic effects of certain fatty acids may be related to their antagonistic effects on 7KCh-mediated ER stress and inflammation.
In this study, using ARPE-19 cultured cells as an in vitro model, we demonstrated that various fatty acids are capable of inhibiting 7KCh-mediated cell death, ER stress, and inflammation. The most potent of the fatty acid tested was sterculic acid which is a ω-9 cyclopropene fatty acid originally found in the seeds of plant Sterculia foetida [27]. This plant is suspected of having numerous medicinal properties [28, 29]. Moreover, sterculic acid was found to effectively inhibit the formation of choroidal neovascularization (CNV) in the rat laser-induced injury model [30, 31]. We show evidence that 7KCh may play a role in the CNV formation in this model.
MATERIALS AND METHODS
Treatments to culture cells
APRE-19 cell were used in these experiments for two important reasons: 1) ARPE-19 cells are derived from human RPE and express RPE-derived proteins; 2) ARPE-19 cells grow more slowly and are less sensitive to 7KCh than other similar RPE-derived cell lines (D407, RPEJ cells). This slower growth and increased resistance to 7KCh provide a more stable system which increased reproducibility between experiments.
ARPE-19 cells (American-Type Culture Collection, Manassas, VA) were grown in DMEM/F-12 medium (Mediatech, Manassas, VA) containing 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Water soluble 7KCh solution was prepared in hydroxypropyl-β-cyclodextrins (HPBCD) as previously described [17]. Stock solutions of fatty acids (Biofine, Vancouver, Canada; Matreya, Pleasant Gap, PA; Acros, Morris Plains, NJ; and Sigma-Aldrich, St. Louis, MO) were dissolved in either dimethyl sulfoxide (DMSO) or ethanol. Three different concentrations of 7KCh were used. In experiments where protection from 7KCh-mediated cell death was examined, 12 μM 7KCh was used. This is roughly the LD50 for 7KCh in ARPE-19 cells. In experiments where 7KCh-mediated inflammatory responses were measured at the transcriptional levels (mRNA), 8 μM 7KCh was used. In 24 h this concentrations does not cause any significant cell death. In experiments where 7KCh-mediated inflammatory responses were measured at the translational level (protein), 6 μM was used. Measurable protein responses required 48 h and a lower dose of 7KCh was required to prevent cell death.
The effect of the fatty acids against 7KCh-mediated cell death was examined 24 h after incubating cells in serum-free medium containing 12 μM 7KCh and fatty acids at 0.1, 0.5, 1, 5 and 10 μM concentrations. The cell viability was determined by dehydrogenase activity using Cell Counting Kit-8 (Dojindo, Gaithersburg, MD). The effects of the fatty acids against 7KCh-mediated inflammation and ER stress were examined by incubating cells in 1 μM fatty acids with either 8 μM 7KCh (24 h for quantitative real-time PCR (qRT-PCR), 24 and 48 h for immunoblot), or 6 μM 7KCh for 48 h (for ELISA).
In order to test the anti-inflammation effect of fatty acids against TNF-α (Roche, Basel, Switzerland), ARPE-19 cells were treated with 2 ng/ml of TNF-α for 24 h in serum-free medium with or without 1 μM sterculic acid. The mRNA expressions of cytokines were then examined using qRT-PCR.
Quantitative Real-time PCR
The RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA). The reverse transcription was performed with reagents and kits from Invitrogen. Quantification of mRNA expression was performed using the Taqman gene expression assays (Applied Biosystems, Foster City, CA) and the following primers (VEGFa, Hs00173626_m1; IL-1β, Hs01555413_m1; IL-6, Hs00174131_m1; IL-8, Hs00174103_m1; IκBα, Hs00153283_m1; GRP78, Hs99999174_m1; CHOP, Hs01090850_m1; TNF-α, Hs00174128_m1; TGF-β1, Hs00998133_m1; GAPD, 4352934e). GAPD expression was used as an endogenous standard. All qRT-PCR experiments were measured by an ABI 7500 Real-Time PCR Instrument (Applied Biosystems).
ELISA assays
The levels of secreted VEGF, IL-6, IL-8, and TNF-α in conditioned medium of ARPE-19 cell cultures were measured 48 h after treatments of 6 μM 7KCh and 1 μM fatty acids using the Quantkine ELISA kits (R&D systems, Minneapolis, MN). The protein levels were measured in triplicates. The ELISA results were quantified using an Envision multilable plate reader (PerkinElmer, Covina, CA).
Immunoblots
Lysate of ARPE-19 cells was prepared using MPER buffer solution (Thermo Fisher, Waltham, MA) containing Complete Protease Inhibitor Cocktail (Roche). Proteins were separated by SDS-PAGE in 10% Bis-Tris gels (Invitrogen) and each lane was loaded with 10 μg of protein lysate. The gels were blotted on to nitrocellulose membranes (Invitrogen). The blots were incubated with primary antibodies for CHOP (1:1000, Cell Signaling, Danvers, MA), GRP78 (1:1000, Cell Signaling), or GAPDH (1:2000, Abcam, Cambridge, MA) at 4°C overnight. The blots were washed then further incubated with anti-rabbit IgG, HRP-conjugated antibodies (1:2000, Cell Signaling) at room temperature for 1 h. The blots were again washed and incubated with Chemiluminescent Substrate (Thermo Fisher) then visualized using Kodak X-Ray films (Carestream Health, Rochester, NY).
Animals
Eight weeks old male Brown Norway rats weighing approximately 150 grams were purchased from Charles River Laboratories (Rockville, MD). All animal procedures were approved by the National Eye Institute’s Animal Care and Use Committee and all animals were treated in accordance with the National Institutes of Health guidelines for Animal Care and Use.
Analysis of 7KCh levels in photocoagulated tissues by LCMS
Rats were anesthetized and prepared as previously described [31]. To evaluate the level of 7KCh in tissue after photocoagulation, 8 or 32 laser burns were made in each retina surrounding the optic nerve. The burns were made using a OcuLight® 532 nm laser system (Iridex, Mountain View CA) with a 5.4 mm contact fundus laser lens (Ocular Instruments, Bellevue WA), for 0.1 second duration at 80–90 mW to generate a 50 μm spot size. The eyes were removed at 6, 24 and 48 h post laser treatment. The neural retina (NR) and the RPE/choroid (PEC) were harvested and snap-frozen in dry ice. Eyes with no laser treatment were used as controls. To each sample 100 nmoles of deuterated cholesterol (D7-Ch, Cambridge Isotope, Andover, MA) were added as an internal control and then lyophilized. This value was used because it is approximately the amount of free cholesterol found in two rat retinas.
To avoid autooxidation and the extraction of unwanted lipids, the dried retinal tissues were extracted with 1 ml of dry ethanol containing 1 mM EDTA (Digene/Qiagen). The tissues were homogenized using a tissue grinder (Bio-Gen PRO 200, PRO Scientific, Oxford, CT) and the insoluble material was removed by centrifugation. The dissolved materials were placed in HPLC vials and dried under a nitrogen stream. The lipids were redissolved in 100 μl of ethanol and analyzed by HPLC without further manipulation. The solid residue was further extracted with methanol/chloroform [32] but no additional free 7KCh, cholesterol (Ch) or 7-ketocholesterol fatty acid esters (7KFAEs) were detected.
The 7KCh, Ch and the 7KFAEs were separated by HPLC, identified and quantified by MS as previously described [17]. The only significant variation was the HPLC method. An Agilent 1200 series HPLC (Santa Clara CA) equipped with a capillary pump, a column heater and an autosampler was used. A Varian (Agilent) XRs C8 column (2×100 mm) running a binary gradient at 0.1 ml/min was used to separate the 7KCh, Ch and 7KFAEs. The initial conditions were 25% water, 75% acetonitrile 0.2% formic acid and the gradient was completed in 10 min reaching 100% methanol, 0.1% formic acid. The 100% methanol flow was sustained for an additional 25 min then the column was re-equilibrated to initial condition for an additional 10 min. The HPLC column was heated to 60°C.
7KCh, and Ch were quantified by peak area integration of the total ion current (TIC) for their corresponding ions. For 7KCh and deuterated 7KCh (D7-7K) the quantification was performed using the M+H+ ions of m/z 401 and m/z 408, respectively. For Ch and deuterated cholesterol (D7-Ch) the TIC for the M-OH+ ions of m/z 369 and m/z 376 were used, respectively. Masslynx software (Waters, Milford, MA) was used to analyze the data. Standard curves were prepared for each of the compounds of interest, 7KCh, D7-7K, Ch, and D7-Ch. The formation of D7-7K during the extraction process was essentially nil for most samples. In the cases where D7-7K was formed it was subtracted from the 7KCh amount based on % formation. However, this subtraction made no significant difference to the values reported. The levels of 7KCh were reported as pmol 7KCh per nmol of Ch. The 7KFAEs, although detectable in some samples as a prominent m/z 383 ions (M-FA+), were not quantified.
Laser-induced choroidal neovascularization (CNV) model for testing sterculic acid
To evaluate the in vivo antagonist effect of sterculic acid to 7KCh, four laser burns (50 μm spot size, 0.1 second duration, 80–90 mW) were made in each eye surrounding the optic nerve. Laser breakage of Bruch’s membrane was observed by the formation of a bubble. The laser-induced CNV lesions were evaluated 7 days after laser treatment.
Intravitreal injections of sterculic and oleic acid
The intravitreal injection was performed at 24 or 48 h after laser treatment. After anesthesia and pupil dilation, a 33G needle attached to a Hamilton syringe was used to pierce the sclera at the level of the pars plana. The needle was introduced parallel to the retina to avoid damaging the lens. Sterilized 10% DMSO/PBS containing 1 mM sterculic acid or 1 mM oleic acid (1 μl) was injected into the vitreous cavity. This was followed by topically applied Neomycin and polymyxin B sulfates and bacitracin zinc ophthalmic ointments USP (Bausch & Lomb, Madison, NJ). Seven days after laser treatment, all animals were euthanized for CNV lesion evaluation. Oleic acid was used as control in these experiments because stearic acid tended to precipitate out of solution in 10% DMSO.
Topical Delivery of sterculic and oleic acid
Sterculic acid and oleic acid were dissolved in 10% DMSO/PBS at neutral pH and used directly as eye drop solutions. Immediately after laser treatment, rats were administered with solutions containing sterilized 10% DMSO/PBS, 1 mM sterculic acid, or 1 mM oleic acid one drop (50 μl)/day; or with solutions containing sterilized 10% DMSO/PBS, 0.1 mM, 1 mM, or 10 mM sterculic acid three drops/day, for 6 consecutive days. Afterwards, all animals were euthanized for CNV lesion evaluation.
CNV lesion volume evaluation
Animals were euthanized by CO2 exposure. The rat eyes were enucleated and flat-mounted as previously described [31]. Neovessels were visualized by labeling the endothelial cells in the RPE/choroid flat mounts using Alexa Fluor 568-isolectin IB4 (Invitrogen). Multiplane z-stacks of the neovessles were collected with an epifluorescent microscope (Zeiss ApoTome, Thornwood, NY). The neovessel volume was determined using high-performance 3D imaging software (Volocity; Perkin Elmer) as previously described [31].
Statistical analysis
Statistical comparisons between groups were performed using two-tailed Student’s t-test. We consider the result as significant when p < 0.05.
RESULTS
Effect of fatty acids on 7KCh-mediated inflammation and cell death
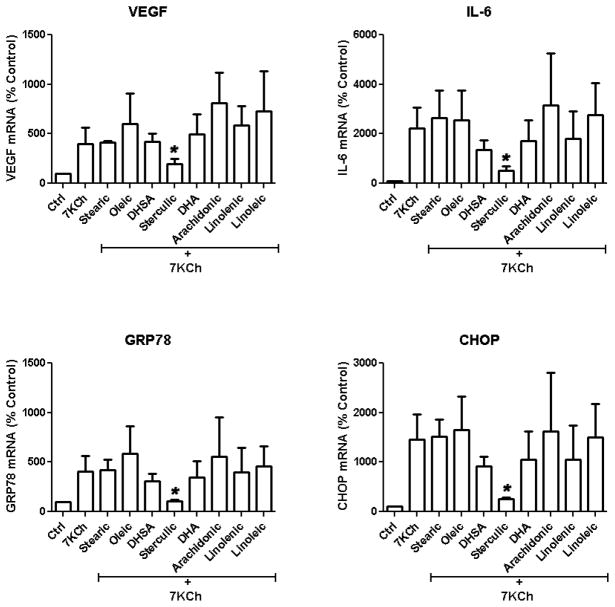
Unsaturated fatty acids have been previously shown to have anti-inflammatory effects [19, 20] and to provide beneficial therapeutical effects by protecting against AMD [21–23] and CVD [24, 25]. We started by testing several different types of fatty acids including stearic acid (18:0), ω-3s (18:3 and 22:6), ω-6s (18:2 and 20:4), and ω-9s (18:1, 19:0, 19:1) for their ability to antagonize the 7KCh-mediated induction of inflammatory cytokines and ER stress markers at 1 μM concentration. ARPE-19 cells were treated with 8 μM 7KCh for 24 h and the mRNA expression of vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), the 78 kDa glucose-regulated protein (GRP78), and CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) were measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) (Fig. 1). At 1 μM only sterculic acid demonstrated a significant inhibition of the 7KCh-mediated mRNA induction. 7KCh increased the expression of VEGF, IL-6, GRP78, and CHOP mRNA, 4-, 22-, 4-, and 15-fold, respectively. Simultaneous treatment with 7KCh and sterculic acid significantly reduced the mRNA expressions of VEGF, IL-6, GRP78 and CHOP close to basal levels. Treatments with 1 μM dihydrosterculic acid (DHSA) (19:0), docosahexanoic (DHA) (22:6), and α-linolenic acid (18:3) were somewhat effective at reducing IL-6, GRP78 and CHOP but essentially ineffective at reducing VEGF. The other fatty acids including stearic (18:0), oleic (18:1), linoleic (18:2) and arachidonic (20:4) were either ineffective or enhanced the 7KCh-mediated inflammatory responses.
Figure 1. Effects of fatty acids on 7KCh-mediated inflammation and ER stress.
ARPE-19 cells were treated simultaneously with 8 μM 7KCh and 1 μM of various fatty acids (see labels on figure). The mRNA expressions (mean ± s.d., n = 3) of inflammatory cytokines (VEGF and IL-6) as well as the ER stress markers (GRP78 and CHOP) in response to the treatments presented were determined by qRT-PCR. *p < 0.05 comparing to 7KCh, two-tailed Student’s t-test.
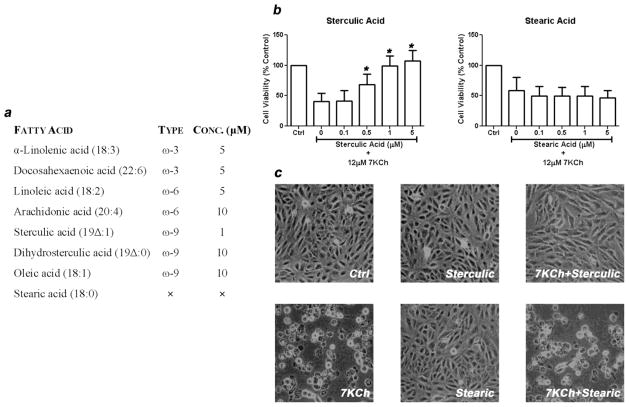
In order to determine if the other fatty acids were effective at higher concentrations ARPE-19 cells were treated with 12 μM 7KCh (LD50) with increasing concentrations of fatty acids (0.1, 0.5, 1, 5, and 10 μM) for 24 h (Fig. 2) and cell viability was measured. The minimal concentrations of these fatty acids required to maintain full cell viability is summarized in Fig. 2a. Sterculic acid at 0.5 μM started to show significant protective effect and at 1 μM completely offset the cell death induced by 7KCh (Fig. 2b). DHA, α-linolenic, and linoleic acid prevented 7KCh-mediated cell death at 5 μM but the rest of unsaturated fatty acids required 10 μM to be protective. Stearic acid (18:0) did not demonstrate any protective effect against 7KCh-mediated cell death. Again sterculic acid was the most effective compound. Fig. 2c shows representative images of the treated ARPE-19 cultured cells. These images demonstrate that 1 μM sterculic acid can fully protect the cells from a 12 μM dose of 7KCh while the stearic acid had no effect.
Figure 2. Effect of fatty acids on 7KCh-mediated cell death.
(a) Minimum protective concentration of the various fatty acids to 7KCh-mediated cell death. ARPE-19 cells were treated with 12 μM 7KCh and cell viability was measured 24 h post treatment. Various concentrations of fatty acids were tested to determine the minimum concentration that provided protection from 7KCh cytotoxicity. The results were concluded after three independent experiments with quadruplicate measurements in each experiment. (b) ARPE-19 cells were treated with 12 μM 7KCh with or without 0.1–5 μM sterculic acid and stearic acid. Cell viability (mean ± s.d., n = 3) was measured 24 h after treatments. *p < 0.005 comparing to 7KCh-only treatment. (c) Representative images of changes in cell morphology in response to 12 μM 7KCh with or without 1 μM sterculic acid and stearic acid.
Antagonistic effects of sterculic acid to 7KCh-mediated induction of inflammatory cytokines and ER stress markers
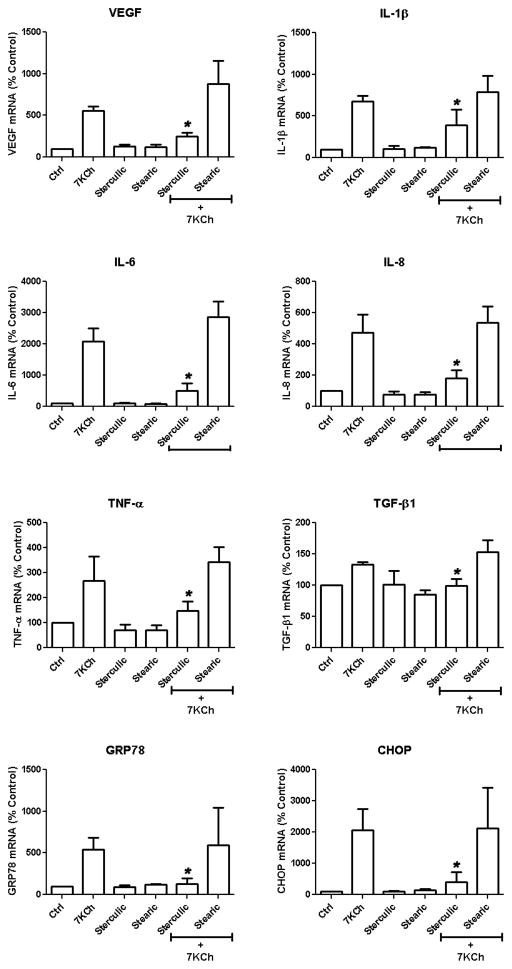
In order to get a more precise measure of the antagonistic effect of sterculic acid on the 7KCh-mediated inflammation, the mRNA expression of the cytokines VEGF, IL-1β, IL-6, IL-8, tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β1), and the ER stress markers GRP78 and CHOP were measured after treating ARPE-19 cells with 8 μM 7KCh with and without sterculic acid (Fig. 3). Stearic acid was used as a negative control. Sterculic acid significantly attenuated the induction of VEGF, (5.5 to 2.5-fold), IL-1β (6.7 to 3.9-fold), IL-6 (21 to 5.1-fold) IL-8 (4.7 to 1.8-fold), TNF-α (2.7 to 1.5-fold), TGF-β1 (1.3 to 1-fold) and the ER stress markers GRP78 (5.4 to 1.3-fold), and CHOP (12 to 4.1-fold). Stearic acid did not show any antagonistic effect but seemed to enhance the expression of VEGF (5.5 to 8.8-fold) and IL-6 (21 to 29-fold). Sterculic acid or stearic acid alone had no effect on the mRNA expression of these cytokines.
Figure 3. Effect of sterculic acid on the 7KCh-mediated mRNA induction of inflammatory cytokines and ER stress markers.
ARPE-19 cells were treated simultaneously with 8 μM 7KCh and 1 μM sterculic acid, 1 μM stearic acid, or the combinations of 7KCh with either sterculic acid or stearic acid (negative control). The mRNA expression (mean ± s.d., n = 4) of inflammatory cytokines (VEGF, IL-1β, IL-6, IL-8, TNFα, TGF-1β) and ER stress markers (GRP78 and CHOP) was measure by qRT-PCR 24 h after treatment. *p < 0.05 comparing to 7KCh, two-tailed Student’s t-test.
Antagonist effect of sterculic acid to 7KCh-mediated protein expression of cytokines and ER stress markers
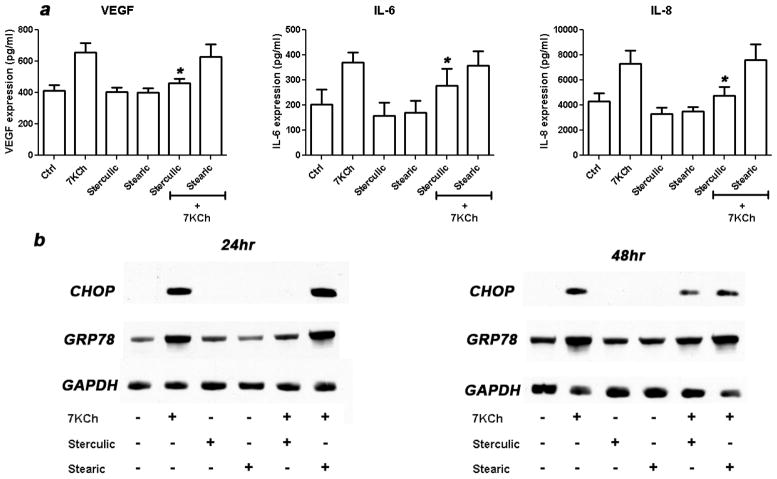
Since mRNA expression does not necessarily correlate with protein expression, we examined the secreted protein levels of VEGF, IL-6, IL-8, and the intracellular protein levels of CHOP and GRP78 after 7KCh treatment (Fig. 4). ARPE-19 cells were treated with 7KCh with or without sterculic and stearic acid. The conditioned media were collected and the secreted VEGF, IL-6 and IL-8 protein levels were measured by ELISA (Fig. 4a).
Figure 4. Effect of sterculic acid on the 7KCh-mediated protein induction of inflammatory cytokines and ER stress markers.
(a) ARPE-19 cells were treated simultaneously with 6 μM 7KCh and 1 μM sterculic acid, 1 μM stearic acid, or in combination. The secreted protein levels (mean ± s.d., n = 3) of VEGF, IL-6, and IL-8 were measured by ELISA 48 h after treatment. *p < 0.05 comparing to 7KCh, two-tailed Student’s t-test. (b) ARPE-19 cells were treated simultaneously with 8 μM 7KCh and 1 μM sterculic acid, 1 μM stearic acid, or in combination. The expression of GRP78 and CHOP were shown by immunoblotting 24 h and 48 h after treatment. GAPDH was used as an internal control for protein levels.
Sterculic acid significantly reduced the expression of VEGF (1.6 to 1.1-fold), IL-6 (1.8 to 1.4-fold) and IL-8 (1.7 to 1.1-fold). Stearic again had no measurable effect. The immunoblots of ER stress markers CHOP and GRP78 also showed a similar trend as the mRNA expression (Fig. 4b). Treatment of cells with 8 μM 7KCh for 24 h induced CHOP and GRP78 protein expression but these inductions were greatly inhibited by sterculic acid. Stearic acid again had no effect. The inhibitory effect of sterculic acid on CHOP and GRP78 protein expression seems to decrease 48 h post-treatment. Comparing to the vehicle-only control, the expression of CHOP and GRP78 did not change when the cells were treated with fatty acids alone. We failed to detect any secreted TNF-α protein expression (data not shown). These experiments clearly demonstrated that sterculic acid reduced the secreted levels of these proteins and followed a similar trend as the mRNA expression.
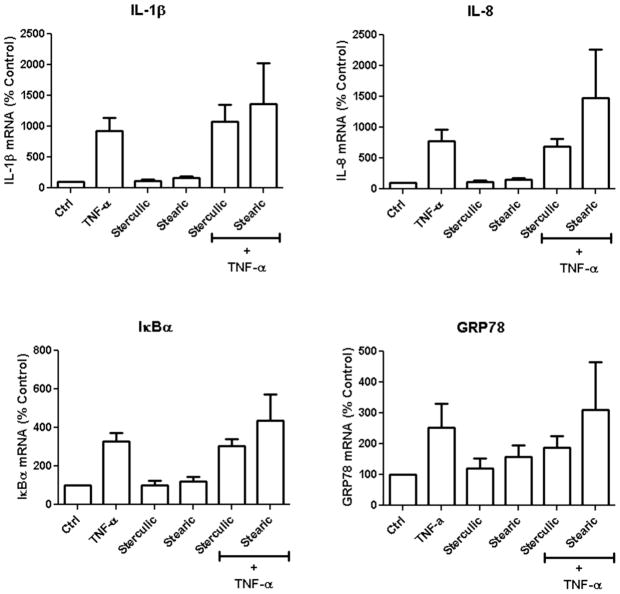
Sterculic acid is not antagonistic to TNF-α
TNF-α is a common pro-inflammatory cytokine that induces inflammation via several TNF-specific receptors [33] and thus induces some of the same cytokines and ER stress markers as 7KCh. In order to better understand the pharmacological properties of sterculic acid, mRNA expression of IL-1β, IL-8, IκBα, and GRP78 were measured in TNF-α treated ARPE-19 cells. IκBα (an inhibitor of NF-κB) is regulated by NF-κB and thus a good marker for NF-κB activation. ARPE-19 cells were incubated in 2 ng/ml TNF-α with or without 1 μM sterculic acid and stearic acid for 24 h (Fig. 5). The TNF-α treatment significantly induced the mRNA expressions of IL-1β (9-fold), IL-8 (8-fold), IκBα (3-fold), and GRP78 (2.5-fold) but not VEGF, IL-6, and CHOP (data not shown). Sterculic acid did not antagonize the TNF-α-mediated inductions of IL-1β, IL-8, or IκBα. However, it did have a small but measurable effect on GRP78 induction. Stearic acid seemed to slightly enhance the TNF-α induction of these inflammatory markers.
Figure 5. Effect of sterculic acid on TNFα-mediated mRNA induction of inflammatory cytokines and ER stress markers.
ARPE-19 cells were treated simultaneously with 2 ng/ml of TNFα, 1 μM sterculic acid, 1 μM stearic acid, or in combination for 24 h. The mRNA expression (mean ± s.d., n = 3) of IL-1β, IL-8, IκBα, and GRP78 was measured by qRT-PCR.
Laser-injury rat model has increased levels of 7KCh
The discovery that sterculic acid is an effective 7KCh antagonist in vitro prompted us to find a suitable in vivo model to test its effects. One model of particular interest was the laser-injury rat model in which laser burns induce CNV [30, 31]. This model is often used to study angiogenesis and to test anti-angiogenic drugs [34, 35]. However, the molecular mechanism(s) by which the laser injury induces CNV formation are not understood. We surmised that the laser burns not only break Bruch’s membrane but also photocoagulate lipoproteins and hemoglobin. This may cause the trapping of lipid-rich lipoproteins and membranes as well as the release Fe2+ from blood hemoglobin and damaged mitochondria. The cholesterol (Ch) and cholesterol esters (CEs) trapped in the photocoagulated lipid-rich deposits would then be amenable to autooxidation via a free-radical-mediated mechanism catalyzed by free Fe2+ [36]. This is a well-known mechanism for the formation of 7KCh from Ch.
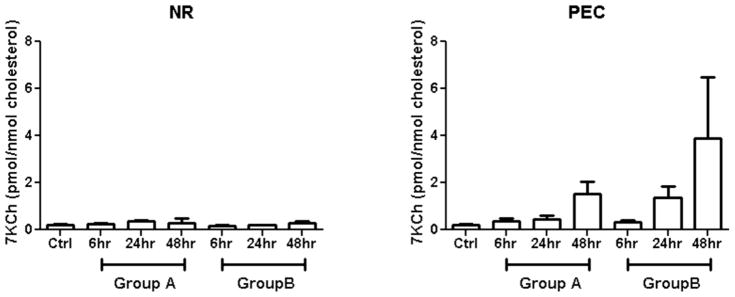
In order to determine whether 7KCh may be playing a role in the CNV formation in the rat laser-injury model, 7KCh in rat retina with or without laser burns was measured by liquid chromatography (HPLC)-mass spectroscopy (LCMS). Rats were divided into three groups, the control group which did not receive laser treatment; group A which received 8 laser burns per eye and group B which received 32 burns. Rats from both Group A and B were euthanized and enucleated 6, 24 and 48 h after treatment (Fig. 6). The neural retinas (NR) were dissected from the RPE/choroid (PEC) and the tissues from each rat were pooled. D7-Ch was added as an internal control to monitor autooxidation of cholesterol to 7KCh and other products. No saponification or any type of alkaline hydrolysis was used since this treatment destroys 7KCh and creates numerous artifacts [37, 38]. The levels of 7KCh (m/z 401 ion) were normalized to the levels of endogenous free cholesterol (m/z 369 ion). Autooxidation of Ch to 7KCh was monitored using the D7-Ch (m/z 376 ion) conversion to D7-7K (m/z 408 ion). Autooxidation under these conditions was found to be essentially nil and correction made no significant difference in the final values of 7KCh. A significant amount of 7KCh fatty acid esters (7KFAEs) was observed, but these molecules are fragmented by the APCi probe ionization generating a derivative ion 383 m/z ion representing the loss of the fatty acid moiety. Thus, no parental ion mass was observed. Without the parental ion mass the identification of the fatty acid moiety was impossible. The 7KFAEs can be identified and quantified more accurately using electron spray ionization but this was not further pursued in this study.
Figure 6. Levels of 7KCh in the laser treated rat neural retina and RPE/choroid.
Group A received 8 laser burns and group B received 32 burns. The levels of 7KCh (mean ± s.d., n = 3) were measured by LCMS and expressed as pmoles 7KCh/nmole Ch. After photocoagulation, the levels of 7KCh in neural retina (NR) did not change significantly with either increased laser burns or post-treatment time. However, the levels of 7KCh in RPE/choroid (PEC) increased greatly with both increasing laser burns and post-treatment time.
In all groups the levels of 7KCh in the neural retina (NR) varied little in response to post treatment time or to the number of laser burns. The levels were 0.2 – 0.45 pmol 7KCh/nmol Ch in the NR (Fig. 6a). In the retinal pigment epithelium/choroid tissue (PEC), the levels of 7KCh significantly increased in response to post-laser time and number of burns. Normal PEC (no laser burns) contained approximately 0.2 pmol 7KCh/nmol Ch but the 7KCh level increased 7-fold to 1.5 pmol/nmol with 8 laser burns in 48 h. In the PEC with 32 laser burns, the levels of 7KCh increased nearly 20-fold to 3.9 pmol/nmol Ch in 48 h (Fig. 6b). Since 7KCh is a potent inducer of VEGF [16, 17], this result suggests that 7KCh may be involved in the CNV induction in the rat laser-injury model.
Sterculic acid suppressed laser-induced CNV
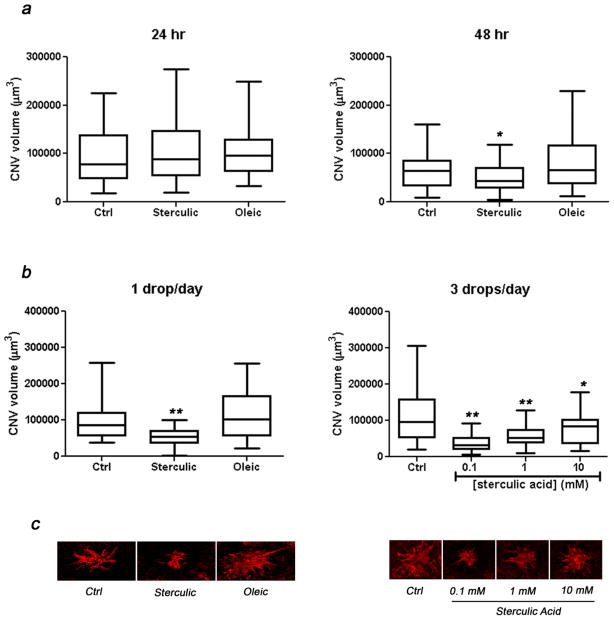
We examined whether sterculic acid could suppress the laser-induced CNV development by either single intravitreal injection or topical administration using eye drops. Oleic acid which is structurally similar to sterculic acid but unable to attenuate VEGF induction in vitro (Fig. 1) was used as negative control.
CNV volumes were measured after single intravitreal injections of sterculic acid (1 mM) administered 24 or 48 h after laser (Fig. 7a). The animals were then euthanized 7 days after the laser treatment. When the injections were performed 24 h post laser treatment, no statistically significant differences were found between vehicle-only control, sterculic acid, and oleic acid treatments. However, when sterculic acid was injected 48 h after laser treatment a significant reduction of CNV volume (35%) was detected when compared to controls. The injections of oleic acid did not change the CNV volumes. Stearic acid which was used as control in the in vitro experiments could not be used in these experiments due to its reduced solubility in 10% DMSO.
Figure 7. CNV suppression by sterculic acid.
(a) CNV volume in response to administration of sterculic acid via single intravitreal injection. Single injection was performed either 24 or 48 h after the laser treatment. The CNV volume of flat-mounted tissues was evaluated 7 days after laser treatments. The box-and-whisker plots (n = 45–51) represent the CNV volumes corresponding to these treatments. The box represents the central 50% data. The horizontal line inside the box corresponds to the median value of the group. The scale bars outside the boxes correspond to the minimum and maximum of data. *p < 0.05. Two-tailed Student’s t-test. (b) CNV volume in response to administration of sterculic acid via eye drop administrations. Immediately after laser treatment, eye drops of vehicle-only control, sterculic acid, or oleic acid were administered 1 drop/day or 3 drops/day for consecutive 6 days. The box-and-whisker plots (n = 38–50) represent the CNV volumes to these treatments. The box represents the central 50% data. The horizontal line inside the box corresponds to the median value of the group. The scale bars outside the boxes correspond to the minimum and maximum of data. *p < 0.005. **p < 0.0001, two-tailed Student’s t-test. (c) Representative flat-mount projections of laser induced CNV labeled with isolectin IB4 from confocal microscope Z-series in response to 1 drop/day or 3 drops/day of vehicle-only control, sterculic acid, or oleic acid.
We presumed that since sterculic acid is a low molecular weight lipophilic compound it may be able to reach the retina and choroid by topical administration. Hence we further tested the in vivo effect of sterculic acid by delivering it via eye drops (Fig. 7b). Application of 1 mM sterculic acid one drop/day for 6 consecutive days reduced CNV volume by 45% while oleic acid demonstrated no statistically significant effect. Increasing sterculic acid administration to 3 times a day with one drop per administration further suppressed the CNV volume by 67%, 46%, and 12% respectively with 0.1 mM, 1 mM, and 10 mM sterculic acid concentrations. No significant reduction in CNV formation was observed by increasing the frequency of administration of 1 mM sterculic from one (45%) to three drops/day (46%). Images of the lesions are shown in Fig. 7c.
DISCUSSION
The most important contribution from this study is the discovery that sterculic acid is a potent antagonist of 7KCh-mediated inflammation and cytotoxicity. This naturally occurring fatty acid seems to antagonize all of the 7KCh-mediated inflammatory responses at relatively low concentrations (0.5–1 μM) in vitro (Fig. 1–4). Other fatty acids especially DHA (22:6), linoleic (18:2) and linolenic (18:3) which are known to have beneficial effects against CVD [19, 20] and AMD [21, 22] are 5–10 times less effective (Figs. 1, 2). Sterculic acid did not antagonize the inflammatory responses but did inhibited the ER stress induced by the receptor mediated TNF-α (Fig. 5). This suggests that the anti-inflammatory effect of this fatty acid may be ER specific or ER related. The presence of the cyclopropene group in the structure of sterculic acid seems to be critical to its pharmacological properties. This can be surmised from the complete lack of protection by stearic and the partial protection by DHSA (Fig. 1–2) which has a cyclopropane group at C-9 (without the double bond). Oleic which has a double bond at the C-9 carbon did protect from cytotoxicity at 10 μM concentration (Fig. 2a) but was unable to attenuate the inflammatory responses (Fig. 1).
It has been previously demonstrated that chronic ER stress and the subsequent inflammatory responses including VEGF induction via NF-κB and related pathways are associated with the pathogenesis of atherosclerosis [10, 26]. Chronic ER stress is also suspected in the CNV formation in exudative AMD [2, 39], and possibly the aging process in general [2, 10, 26, 40, 41]. Several studies have connected 7KCh with ER stress [11, 12] and we have previously demonstrated that 7KCh is a highly potent inducer of VEGF and other inflammatory cytokines via the non-conical or HIF-1 independent pathway [2, 17]. In this study we have demonstrated that sterculic acid significantly suppresses the induction of ER stress markers and several inflammatory cytokines induced by 7KCh (Fig. 3–4). Although the mechanism of action of sterculic acid and 7KCh remains to be elucidated, our results suggest it may work by suppressing the ER stress responses upstream of the unfolded protein response (UPR). This is supported by the results using TNF-α as an inflammatory agent (Fig. 5). Sterculic acid failed to attenuate the induction of IL-1β, and IL-8 as well as the NF-κB controlled feed-back regulator IκBα (Fig. 5). However, although the effects of TNF-α on ER stress are secondary [33], sterculic acid was able to attenuate the induction of GRP78 (Fig. 5). The precise mechanism of action of sterculic acid and 7KCh are currently under intense investigation by our group.
The effects of PUFAs on CNV formation have been previously observed by others [42]. Eicosapentaenoic acid (20:5), a ω-3 PUFA, has been shown to reduce laser-induced CNV volumes in mice when given as dietary supplement [42]. In this study we demonstrate that sterculic acid is highly effective at preventing CNV formation in the laser-injury rat model (Fig. 7). Sterculic acid was particularly effective when delivered topically (eye-drops) at low doses (0.1 mM) (Fig. 7b,c). However, the effectiveness of sterculic acid is difficult to explain without fully understanding the molecular mechanism by which the laser burns cause the CNV formation in this model.
We provided some insight into the plausible cause of the CNV formation by measuring 7KCh levels in relation to post-laser time and number of burns (Fig. 6). 7KCh is a potent VEGF inducer [16, 17] and its dramatic increase in the rat PEC after the laser burns suggest it may be responsible for the CNV formation. We propose that 7KCh is at least partially responsible for the CNV formation in this model and that this is the reason why sterculic acid is an effective anti-angiogenic agent. However, there is no direct scientific evidence connecting the7KCh increase to the CNV formation.
Other investigators examining the effects of oxidative injury to the brain found that the levels of Ch, 7KCh and 7β-hydroxycholesterol increased in the hyppocampus of rats that were injected with kianate (a glutamate analog) [43]. Kainate is known to cause excitotocixity by binding to glutamate receptors and causing an intracellular increase of Ca2+. In this model 7KCh and other oxysterols generated during the kainate treatment were suspected of continuing and further aggravating the neurotoxicity initially caused by kainate. The effect of kainate on 7KCh in the retina has not been previously investigated.
The role of 7KCh and other cholesterol oxides in the pathogenesis of aging diseases such as atherosclerosis [3–5, 18], AMD [1, 2] and Alzheimer’s disease [41] has been studied for many years but remains largely inconclusive and controversial [2]. Sterculic acid’s potent effect at antagonizing 7KCh-mediated responses and its suppression of CNV formation in the rat laser-injury model point to a potential therapeutical agent for the treatment of the aforementioned aging diseases. Our results suggest that sterculic acid may be a potential therapeutical agent for the treatment of CNV formation associated with “wet” forms of AMD. We envision these treatments in the form of eye-drops or ointments and given as supplements to the existing anti-VEGF therapy [44]. In addition we surmise that sterculic acid like other similar fatty acids may also be effective as a dietary supplement. Sterculic acid should enhance the effectiveness of present dietary supplements and fish oils that have proven beneficial to atherosclerosis and AMD [19–25].
Highlights.
Sterculic acid is a potent inhibitor of ER-stress induced by 7KCh.
7KCh is a potent inhibitor of SCD expression.
SCD inhibition by sterculic acid is unlikely related to anti-inflammatory effects.
The laser-injury rat angiogenesis model likely to be associated with 7KCh formation.
Mechanistic data supports 7KCh’s involvement in ER-stress related inflammation
Acknowledgments
The authors would like to thank Dr. Maria Campos at the NEI’s Biological Imaging Core facility for her assistance in imaging of the laser lesions. This work is supported by National Eye Institute Intramural Research Program.
Footnotes
AUTHOR CONTRIBUTIONS
J-D.H. performed the in vitro experiments and J.A. performed the in vivo experiments. J.W.L. and I.M.L. assisted with the in vitro and in vivo experiments. I.R.R. conceived, directed the project and performed the 7KCh LCMS analyses. J-D.H. and I.R.R. wrote the paper with assistance from J.A., J.W.L. and I.M.L.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez IR, Larrayoz IM. Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J Lipid Res. 2010:2847–2862. doi: 10.1194/jlr.R004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lordan S, Mackrill JJ, O’Brien NM. Oxysterols and mechanisms of apoptotic signaling: implications in the pathology of degenerative diseases. J Nutr Biochem. 2009:321–336. doi: 10.1016/j.jnutbio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Poli G, Sottero B, Gargiulo S, Leonarduzzi G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol Aspects Med. 2009:180–189. doi: 10.1016/j.mam.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Moore KJ, Tabas I. Macrophages and the pathogenesis of atherosclerosis. Cell. 2011:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes H, Mathews B, Lenz ML, Guyton JR. Cytotoxicity of oxidized LDL to porcine aortic smooth muscle cells is associated with the oxysterols 7-ketocholesterol and 7-hydroxycholesterol. Arterioscler Thromb. 1994:1177–1185. doi: 10.1161/01.atv.14.7.1177. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez IR, Alam S, Lee JW. Cytotoxicity of oxidized low-density lipoprotein in cultured RPE cells is dependent on the formation of 7-ketocholesterol. Invest Ophthalmol Vis Sci. 2004:2830–2837. doi: 10.1167/iovs.04-0075. [DOI] [PubMed] [Google Scholar]
- 8.Brown AJ, Leong SL, Dean RT, Jessup W. 7-Hydroperoxycholesterol and its products in oxidized low density lipoprotein and human atherosclerotic plaque. J Lipid Res. 1997:1730–1745. [PubMed] [Google Scholar]
- 9.Garcia-Cruset S, Carpenter KL, Guardiola F, Stein BK, Mitchinson MJ. Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic Res. 2001:31–41. doi: 10.1080/10715760100300571. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WH, Lee CS, Kwon K, Kwon YS, Kim SW, Goo TW, Kwon OY. 7-ketocholesterol induces endoplasmic reticulum stress in HT-29 cells. Z Naturforsch C. 2009:307–310. doi: 10.1515/znc-2009-3-425. [DOI] [PubMed] [Google Scholar]
- 12.Sanson M, Augé N, Vindis C, Muller C, Bando Y, Thiers JC, Marachet MA, Zarkovic K, Sawa Y, Salvayre R, Nègre-Salvayre A. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009:328–336. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 13.Saito E, Wachi H, Sato F, Seyama Y. 7-ketocholesterol, a major oxysterol, promotes pi-induced vascular calcification in cultured smooth muscle cells. J Atheroscler Thromb. 2008:130–137. doi: 10.5551/jat.e556. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki H, Watanabe F, Murano T, Miyashita Y, Shirai K. Vascular smooth muscle cell apoptosis induced by 7-ketocholesterol was mediated via Ca2+ and inhibited by the calcium channel blocker nifedipine. Metabolism. 2007:357–362. doi: 10.1016/j.metabol.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009:153–170. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Larrayoz IM, Huang JD, Lee JW, Pascual I, Rodriguez IR. 7-ketocholesterol-induced inflammation: involvement of multiple kinase signaling pathways via NFkappaB but independently of reactive oxygen species formation. Invest Ophthalmol Vis Sci. 2010:4942–4955. doi: 10.1167/iovs.09-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreira EF, Larrayoz IM, Lee JW, Rodriguez IR. 7-Ketocholesterol is present in lipid deposits in the primate retina: potential implication in the induction of VEGF and CNV formation. Invest Ophthalmol Vis Sci. 2009:523–532. doi: 10.1167/iovs.08-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sottero B, Gamba P, Gargiulo S, Leonarduzzi G, Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr Med Chem. 2009:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- 19.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 20.Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient intervention on arterial stiffness: a systematic review. Am J Clin Nutr. 2011:446–454. doi: 10.3945/ajcn.110.002725. [DOI] [PubMed] [Google Scholar]
- 21.SanGiovanni JP, Chew EY, Agrón E, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R, Sperduto RD. Age-Related Eye Disease Study Research Group. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol. 2008:826–833. doi: 10.1001/archopht.126.6.826. [DOI] [PubMed] [Google Scholar]
- 23.Swenor BK, Bressler S, Caulfield L, West SK. The impact of fish and shellfish consumption on age-related macular degeneration. Ophthalmology. 2010:2395–2401. doi: 10.1016/j.ophtha.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badimon L, Vilahur G, Padro T. Nutraceuticals and atherosclerosis: human trials. Cardiovasc Ther. 2010:202–215. doi: 10.1111/j.1755-5922.2010.00189.x. [DOI] [PubMed] [Google Scholar]
- 25.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006:3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma JP, Dasgupta S, Nath B, Aggarwal JS. Composition of the seed oil of sterculia foetida, Linn. J Am Oil Chem Soc. 1957:452–454. [Google Scholar]
- 28.Bao X, Katz S, Pollard M, Ohlrogge J. Carbocyclic fatty acids in plants: biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculiafoetida. Proc Natl Acad Sci U S A. 2002:7172–7177. doi: 10.1073/pnas.092152999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salun J. Cyclopropene derivatives and their diverse biological activities. Top Curr Chem. 2000:1–67. [Google Scholar]
- 30.Dobi ET, Puliafito CA, Destro M. A new model of experimental choroidal neovascularization in the rat. Arch Ophthalmol. 1989:264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- 31.Campos M, Amaral J, Becerra SP, Fariss RN. A novel imaging technique for experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006:5163–5170. doi: 10.1167/iovs.06-0156. [DOI] [PubMed] [Google Scholar]
- 32.Bligh EE, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 33.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 34.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith LL. Review of progress in sterol oxidations: 1987–1995. Lipids. 1996:453–487. doi: 10.1007/BF02522641. [DOI] [PubMed] [Google Scholar]
- 37.Park PW, Guardiola F, Park SH, Addis PB. Kinetic evaluation of 3-b-hydroxycholest-5-en-7-one (7-ketocholesterol) stability during saponification. J Am Oil Chem Soc. 1996:623–629. [Google Scholar]
- 38.Busch TP, King AJ. Stability of Cholesterol, 7-Ketocholesterol and β-Sitosterol during Saponification: Ramifications for Artifact Monitoring of Sterol Oxide Products. J Am Oil Chem Soc. 2010:955–962. doi: 10.1007/s11746-010-1572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salminen A, Kaarniranta K. NF-kappaB signaling in the aging process. J Clin Immunol. 2009:397–405. doi: 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 41.Nelson TJ, Alkon DL. Oxidation of cholesterol byamyloid precursor protein and beta-amyloid peptide. J Biol Chem. 2005:7377–7387. doi: 10.1074/jbc.M409071200. [DOI] [PubMed] [Google Scholar]
- 42.Koto T, Nagai N, Mochimaru H, Kurihara T, Izumi-Nagai K, Satofuka S, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Oike Y, Ishida S. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2007:4328–4334. doi: 10.1167/iovs.06-1148. [DOI] [PubMed] [Google Scholar]
- 43.Ong WY, Goh EW, Lu XR, Farooqui AA, Patel SC, Halliwell B. Increase in cholesterol and cholesterol oxidation products, and role of cholesterol oxidation products in kainate-induced neuronal injury. Brain Pathol. 2003:250–262. doi: 10.1111/j.1750-3639.2003.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campa C, Harding SP. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr Drug Targets. 2011:173–181. doi: 10.2174/138945011794182674. [DOI] [PubMed] [Google Scholar]