Abstract
Purpose
The exact pathogenesis of diverticular disease of the sigmoid colon is not well established. However, the hypothesis that a low-fibre diet may result in diverticulosis and a high-fibre diet will prevent symptoms or complications of diverticular disease is widely accepted. The aim of this review is to assess whether a high-fibre diet can improve symptoms and/or prevent complications of diverticular disease of the sigmoid colon and/or prevent recurrent diverticulitis after a primary episode.
Methods
Clinical studies were eligible for inclusion if they assessed the treatment of diverticular disease or the prevention of recurrent diverticulitis with a high-fibre diet. The following exclusion criteria were used for study selection: studies without comparison of the patient group with a control group.
Results
No studies concerning prevention of recurrent diverticulitis with a high-fibre diet met our inclusion criteria. Three randomised controlled trials (RCT) and one case–control study were included in this systematic review. One RCT of moderate quality showed no difference in the primary endpoints. A second RCT of moderate quality and the case–control study found a significant difference in favour of a high-fibre diet in the treatment of symptomatic diverticular disease. The third RCT of moderate quality found a significant difference in favour of methylcellulose (fibre supplement). This study also showed a placebo effect.
Conclusion
High-quality evidence for a high-fibre diet in the treatment of diverticular disease is lacking, and most recommendations are based on inconsistent level 2 and mostly level 3 evidence. Nevertheless, high-fibre diet is still recommended in several guidelines.
Keywords: High-fibre dietary therapy, Diverticular disease, Sigmoid colon
Introduction
Diverticulosis of the colon is an acquired condition resulting from herniation of the mucosa through defects in the muscle layer of the bowel wall. The condition is rare in developing countries but common in Western and industrialized countries. Approximately 60% of humans over age 60 living in westernized countries will develop colonic diverticula [1]. In the great majority of cases, the condition is asymptomatic with only 10% to 25% of affected individuals developing symptoms [2, 3]. Symptomatic diverticular disease results in an annual hospitalization rate of 130,000 in the USA [4].
Autopsy series from 1920 to 1940 identified diverticulosis present in 2% to 10% of individuals [5, 6]. In later autopsy studies, the presence of diverticulosis increased up to 20–50% [7–11]. This sharp rise in the incidence of diverticulosis has been largely attributed to dietary changes, mainly in the decline of dietary fibre intake from cereal grains [6]. Burkitt and Painter first proposed the initial hypothesis of a fibre deficiency as the aetiology of diverticular disease in 1971. While Burkitt was in Uganda, he found that diverticulitis was virtually nonexistent in rural Africa. He investigated fibre intake, stool weight and transit time, by comparing a rural Ugandan population to an English population. The Ugandan population had a lower average transit time and a lower stool volume compared to the English population who had a diet with higher refined sugar and lower dietary fibre [12].
The hypothesis is that decreased dietary fibre intake results in decreased intestinal contents and smaller size of the lumen. This in turn results in the transmission of muscular contraction pressure to the wall of the colon rather than to the contents of the lumen. The result of increased pressure on the wall is the formation of diverticula at the weakest point in the wall, namely the sites of penetration by blood vessels, called vasa recta [12]. This systematic review summarizes the results of studies investigating high-fibre diet in the treatment of diverticular disease and discusses the evidence and the aetiology of diverticular disease.
Methods
Published guidelines
A Medline search was performed to identify guidelines issued by professional organisations on the conservative treatment of diverticulitis and recommendations on the use of high-fibre diet. A Google search was used to identify guidelines not published in Medline indexed journals. We evaluated (a) whether or not high-fibre diet will prevent symptomatic diverticular disease, (b) whether or not high-fibre diet is mandatory in treatment of symptomatic diverticular disease and (c) whether or not high-fibre diet will prevent recurrence. The evidence supporting the recommendations was noted, specifically references to original research dealing with high-fibre diet in symptomatic diverticular disease. Only practice parameters and guidelines published by professional organisations between 1999 and 2010 were included.
Literature search
Two authors (CU, LD) independently performed a literature search to identify studies investigating the effectiveness of a high-fibre diet in human subjects proven of diverticular disease. In our search, we have also included the articles that compared use of fibre supplement such as methylcellulosis or psyllium seed husks with placebo. We searched MEDLINE databases for papers published between January 1966 and January 2011, using the following keywords: (((“Diverticulitis, Colonic” [MeSH] OR “Diverticulitis, Colonic/diet therapy” [MeSH])) OR “Diverticulum, Colon” [MeSH]) AND “Dietary Fiber/therapeutic use” [MeSH]. EMBASE database was searched with the following terms: diverticulosis and high-fibre diet. CINAHL database was also checked for relevant studies with the following keywords: (MM “Diverticulum, Colon”) AND (MM “Dietary Fibre”) or (MM “Diet Therapy”)). The Cochrane Database of Systematic Reviews was searched with the following words: Diverticular disease and fibre diet.
The “related articles” function in PubMed and reference lists of retrieved articles were also used to identify articles not found in the original search. Clinical studies published in English were included. No unpublished data or abstracts were included.
Validity assessment
After identifying relevant titles, all abstracts were read and eligible articles were retrieved. A manual cross-reference search of the bibliographies of relevant articles was performed to identify other studies not found in the search. Only clinical studies published in English were included. No unpublished data were included. A full search strategy is available at request. Two authors independently assessed the methodological quality of the articles using the Jadad score and the checklist of the Cochrane collaboration. The Jadad score is a well-known instrument assigning a numerical score between 0 and 5 to each study, reflecting its quality (0 indicating poor quality and 5 high quality) [13].
Selection
Diverticular disease comprises a wide spectrum of conditions; in order to be able to reliably compare the data, uncomplicated diverticular disease was defined as symptomatic disease associated with colonic diverticula. This is associated with mild symptoms, usually abdominal pain and/or change in bowel habit, but without clinical features of inflammation. Diverticulosis is asymptomatic colonic diverticula. Diverticulitis is complicated diverticular disease with severe clinical symptoms and evidence of inflammation. Complicated diverticulitis is perforation, abscess, fistula, bleeding or stricture/obstruction, usually needing surgical, percutaneous or, in case of bleeding, endoscopic intervention.
Inclusion and exclusion criteria
Types of studies
Clinical studies were eligible for inclusion if they assessed the treatment of symptomatic diverticular disease or the prevention of recurrent diverticulitis with a high-fibre diet. The only exclusion criterion used for study selection was studies without comparison of the patient group with a control group.
Types of participants
Patients of 18 years or older diagnosed with uncomplicated diverticular disease or an episode of acute diverticulitis were included. The diagnosis had to be confirmed by barium enema, colonoscopy, ultrasonography or computed tomography (CT).
Types of interventions
Studies that compare high-fibre intake vs low-fibre intake were searched. Also studies on the effect of different kinds of fibres, soluble or insoluble, were included.
Types of outcome measures
Primary endpoint parameters for inclusion were the occurrence of complicated diverticular disease (e.g. diverticulitis, abscess, perforation) or recurrent diverticulitis after a primary episode of diverticulitis. Secondary endpoints were severity of symptoms and pain by diverticular disease, morbidity and mortality.
Results
Published guidelines
Guidelines are summarized in Table 1. A total of four guidelines were identified after searching Medline. The American College of Gastroenterology [14], the European Association for Endoscopic Surgery [18] and The American Society of Colon and Rectal Surgeons [21] published guidelines concerning the treatment of diverticular disease of the sigmoid colon. A further search using Google identified one other guideline by the World Gastroenterology Organization [24]. All guidelines recommend the use of high-fibre diet.
Table 1.
Organisations with advises concerning fibres
| Organisation | Year | Fibres recommended preventing diverticular disease | Original research cited | Fibres recommended in treatment of symptomatic diverticular disease | Original research cited | Fibres recommended in preventing recurrence of diverticulitis | Original research cited |
|---|---|---|---|---|---|---|---|
| American College of Gastroenterology [14] | 1999 | Yes | Aldoori et al. [15] | Yes | Brodribb [16], Ornstein et al. [17] | Not mentioned | |
| European Association for Endoscopic Surgery [18] | 1999 | Yes | Brodribb and Humphreys [19], Gear et al. [20] | Yes | Brodribb [16] | Yes | None |
| American Society of Colon and Rectal Surgeons [21] | 2006 | Not mentioned | Not mentioned | Yes | Larson et al. [22], Painter [23] | ||
| World Gastroenterology Organization [24] | 2007 | Yes | Painter and Burkitt [6] Talbot [25] | Yes | Nair and Mayberry [26], Aldoori et al. [15] | Not mentioned |
Systematic review
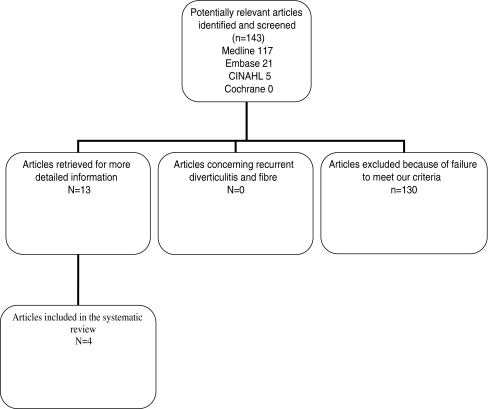
The first search resulted in a combined total of 195 articles. After reviewing the abstracts, 13 studies addressed the use of high-fibre diet specifically in colonic diverticular disease and four met our inclusion criteria (Fig. 1). In Table 2, the nine excluded studies are summarized with the reason of exclusion.
Fig. 1.
Flowchart
Table 2.
Relevant excluded studies
| Article | Year | Study design | Number and type of participants | Type(s) of treatment | Results | Reason(s) for exclusion |
|---|---|---|---|---|---|---|
| Painter et al. [27] | 1972 | Prospective interventional study | 70 unselected patients with diverticular disease proven by barium enema | High-residue, low-sugar diet together with unprocessed bran for an average of 22 months | Symptoms in 88.6% of patients relieved or abolished | No control group |
| None of the 62 patients who took the diet needed surgery | ||||||
| Plumley and Francis [28] | 1973 | Prospective interventional (partly cross-over) study | 48 patients with the “spastic colon” type of diverticulosis proven by barium enema | HFC (OR normal crispbread) for at least 2 months | Of 42 patients suffering pain 69% were controlled satisfactorily on HFC | Only 14 patients took part in the cross-over trial (HFC → normal crispbread) of which the design was unclear and no results were mentioned |
| Of the total of 98 presenting symptoms 73% were controlled satisfactorily on HFC | ||||||
| 5 patients required surgery | ||||||
| Brodribb and Humphreys [19] | 1976 | Prospective interventional study | 40 patients with diverticular disease proven by barium enema | Wheat bran 24 g/day for at least 6 months | 60% of all symptoms were abolished, and a further 28% were relieved | No control group |
| Taylor and Duthie [29] | 1976 | Cross-over randomised trial | 20 patients with symptomatic diverticular disease (confirmed by barium enema) | HRD or Normacol plus an antispasmodic or bran tablets for 2 periods of 1 month | All patients experienced some improvement with each treatment: 20% of patients was symptom-free after HRD, 40% after Normacol and 60% after bran tablets | No high vs low-fibre diet but vs supplements |
| 8 included patients were recently diagnosed with acute diverticulitis, 2 were surgically drained | ||||||
| Devroede et al. [30] | 1977 | Randomised trial | 80 diverticular disease patients | Six treatments (high-residue, low-residue or no specific diet, with placebo or Metamucil) | With Metamucil a significant reduction of symptoms (p < 0.025) and more patients without symptoms (p < 0.05) | Only abstract |
| No significant differences between diet treatments | Radiological confirmation of diagnosis not mentioned | |||||
| Eastwood et al. [31] | 1978 | Non-randomised prospective study | 31 patients with diverticular disease proven by barium enema | Bran or ispaghula (Fybogel) or lactulose for 4 weeks period | All agents equally alleviated symptoms | Non-randomised |
| No high vs low-fibre diet compared but different fibre supplements and a laxative | ||||||
| Ewerth et al. [32] | 1980 | Double-blind cross-over randomised trial | 9 patients with constipation as well as diverticuli on barium enema | Vi-Siblin (6 g ×2) OR placebo (lactose 6 g ×2) during 2 periods of 8 weeks with 4-week interval | Less symptoms with Vi-Siblin (2 vs 16) | Only 9 patients included |
| Significant improvement of constipation with Vi-Siblin (p < 0.05) | Selection bias: constipation as initial complaint, diverticuli possibly accidental non-causative finding | |||||
| Hyland and Taylor [33] | 1980 | Retrospective cohort study | 100 patients with acute diverticular disease | High-fibre diet | 91% of patients on a high-fibre diet reviewed 5 to 7 years after admission had remained symptom-free | It is uncertain whether the patients have diverticulitis or symptomatic diverticular disease |
| Smits et al. [34] | 1990 | Randomised trial | 43 patients with a confirmed diagnosis of diverticular disease | High-fibre diet (30–40 g daily) or lactulose (15 ml bd) for 12 weeks | Both treatments effective with respect to abdominal pain and bowel movement improvement, even some differences in favour of lactulose | No high vs low-fibre diet compared but high-fibre diet vs laxative |
HFC high-fibre crispbread, HRD high-roughage diet
Dietary fibre to prevent recurrence of diverticulitis
No dietary fibre study met the inclusion criteria of this systematic review question.
Dietary fibre for symptomatic diverticular disease
Randomised controlled trials
Brodribb conducted the first randomised double-blind controlled trial of patients with symptomatic diverticular disease back in 1977 [16]. The diagnosis of symptomatic diverticular disease was based on a composite symptoms score in patients with radiological evidence of diverticular disease. This radiological evidence was not explained any further. The symptoms were collected using a detailed symptom questionnaire to be completed at the beginning of the study. The detailed symptomatic questionnaire was based on “dyspeptic” symptoms (nausea, vomiting, heartburn, eructation and abdominal distension), pain and symptoms of “bowel dysfunction” (passage of excessive wind per rectum, the need to strain, the presence of anal pain on defecation, the frequency of evacuation, the consistency of the motion, the presence of blood or mucus, the feeling of incomplete emptying of the rectum after defecation and the use of laxatives). Symptoms were scored 0–6 for frequency and severity on a standard descriptive scale, and the score for pain was doubled to give it similar weighting to the other two symptom groups. The 18 enrolled patients were randomly allocated either to a wheat crisp bread supplying 0.6 g of fibre daily or a bran crisp bread containing 6.7 g of fibre daily. The patients were followed for 3 months and were interviewed monthly to determine compliance and to have them complete the enrolment questionnaire again. There was a highly significant reduction in the mean overall symptom score for the nine patients in the high-fibre group compared to controls (Table 3). Although the high-fibre group experienced a significant decline in the pain score, there were no significant differences in the dyspeptic and bowel dysfunction scores. No complications were recorded.
Table 3.
Results of Stollman and Raskin RCT [14]
| High-fibre group (mean scores initial vs 3 months; n = 9) | Low-fibre group (mean scores initial vs 3 months; n = 9) | p value | |
|---|---|---|---|
| Total symptom score | 34.3 to 8.1 | 42.0 to 35.1 | <0.002 |
| Pain score | 11.1 to 1.1 | 12.7 to 10.2 | <0.02 |
| Dyspeptic dysfunction | 11.4 to 3.7 | 14.7 to 11.6 | ns |
| Bowel dysfunction | 11.7 to 3.3 | 14.9 to 13.3 | ns |
Symptom and pain scores were better with high-fibre diet
The second randomised, cross-over, double-blind controlled trial was published in 1981. The trial included 58 patients with symptomatic, uncomplicated, diagnosed diverticular disease [17]. Ninety-four patients were interviewed of whom 18 declined to enter the trial and a further 18 withdrew. Diagnosis was based on a barium enema showing more than six diverticula in the left colon. Patients were randomly allocated to three forms of dietary fibre supplement treatments. Each treatment was taken for 16 weeks, and all patients subsequently underwent the three treatment periods. The following treatment forms were conducted: bran period (fibre 6.99 g/daily), ispaghula period (fibre 9.04 g/daily) and the placebo period (2.34 g/daily). These were provided in identical unmarked packages and combined to make three apparently similar treatments. Treatment order was randomised and a month’s supplements given at each of 12 visits; neither physician nor patient knew the order of treatment or the cross-over dates. Compliance was assessed by asking patients to return unused packages at each visit. A qualified dietician assessed each patient’s basal fibre intake at the start and end of the trial using the dietary history method and standard food-table values. Outcomes were based on a monthly self-administered symptom questionnaire and measurement of a 7-day stool collection at the end of each treatment period. The symptoms were used to compute a pain score, a lower bowel symptom score and a total symptom score. Twenty-two men and 36 women completed the trial (median age 64 years, range 43–78 years). The mean basal intake of dietary fibre was 15.2 (SD 5.8) g daily. Compliance was good, a maximum of 3.5 days’ supplements being returned each month, and there was no measurable preference for a particular supplement. There were no significant differences in pain, lower bowel symptoms and total symptom scores (Table 4). However, there was significant improvement in symptoms of constipation with the bran crisp bread (8.8 ± SD 6.7) and ispaghula drink (6.9 ± SD 6.2) compared to the initial score (9.7 ± SD 8.2), p < 0.0001.
Table 4.
Results of Köhler et al. RCT [18]
| Initial score | Treatment period N = 58 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Bran | Ispaghula | Placebo | ||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Pain score | 22.6 | 27.3 | 15.2 | 16.9 | 19.5 | 18.4 | 17.5 | 15.6 |
| Lower bowel symptom score | 47.4 | 39.6 | 39.7 | 27.4 | 41.3 | 27.4 | 45.0 | 28.3 |
| General symptom score | 9.7 | 9.1 | 6.7 | 5.9 | 8.1 | 6.7 | 7.6 | 7.3 |
No significant differences were found between the active and placebo periods
The third randomised, partially cross-over, double-blind study was published in 1977 [35]. In this trial, 30 patients with radiologically confirmed diverticular disease were treated with two tablets of methylcellulose or placebo daily. The patients were followed for 3 months and were interviewed every 6 weeks to determine compliance and to have them complete the enrolment questionnaire again. Three patients dropped out and 27 patients were analysed. In the placebo group 11 patients and in the methylcellulose group 16 patients completed the 3-month period. Patients in the methylcellulose group showed more improvement than those in the placebo group. Symptom score decreased in 3 months from 19 ± SD 6 to 13 ± SD 4 (p < 0.01) in the methylcellulose group and from 21 ± SD 7 to 17 ± SD 9 (not significant) in the placebo group. Quality assessment of these three randomised controlled trials (RCT) is shown in detail in Table 5.
Table 5.
Quality assessment and study design of RCT
| Köhler et al. [18] | Stollman and Raskin [14] | Rafferty et al. [21] | Reference |
|---|---|---|---|
| RCT | RCT | RCT | Study design |
| High-fibre vs low-fibre diet | High-fibre vs low-fibre diet | Methylcellulose vs placebo | Intervention/comparison |
| 29/29 | 9/9 | 16/11 | Number intervention/number comparison |
| Yes | Yes | Yes | Randomisation? |
| Yes | Yes | Yes | Treatment allocation concealed? |
| No | No | No | Eligibility criteria specified? |
| Yes | Yes | Yes | Patient blinded |
| Yes | Yes | Yes | Outcome assessor blinded |
| Yes | Yes | Yes | Care provider blinded? |
| Not mentioned | Not mentioned | Not mentioned | Groups similar at baseline? |
| No | No | No | Follow-up? |
| Yes | Yes | Yes | Intention to treat? |
| 4 | 3 | 3 | Jadad score |
Case-controlled study
Leahy et al. evaluated 72 patients admitted to a UK hospital with symptoms of diverticular disease from 1972 to 1981 [36]. Fifty-six patients were treated non-operatively, of these 43 received advice concerning a high-fibre diet, containing a minimum of 25 g fibre daily, but only 31 patients complied, forming the high-fibre group. The 12 patients who failed to take additional fibre and the 13 patients who never received dietary advice (25 patients) formed the non-high-fibre group. There was a 72% compliance with recommendations. Those treated with fibre supplementation fared significantly better in developing fewer complications and required less surgery (p < 0.05). At the time of follow-up review, patients on a high-fibre diet reported significantly fewer symptoms (p < 0.05) (Table 6).
Table 6.
Results of the study of Leahy et al. [36]
| High-fibre group (n = 31) | Low-fibre group (n = 25) | |
|---|---|---|
| Surgery due to diverticular disease | 2 | 8 (p < 0.05) |
| Mortality | 7 | 2 |
| Mean follow-up (months) | 54 | 76 |
| Symptomatic at follow-up | 6 | 11 (p < 0.05) |
Significantly fewer symptoms and less surgery were found in the high-fibre group
Discussion
No comparative studies were found that assessed the effect of fibre diet in the recurrence rate of diverticulitis. The only three published randomised trials in patients with diverticular disease examining the effect of a high-fibre diet or fibre supplement showed inconsistent results. One RCT has found a significant reduction in pain and improvement of overall symptoms [16], while a second RCT has found no effect on pain and large bowel symptoms, except for reduction of constipation [17]. The third trial [35] showed a significant treatment effect of methylcellulose on symptoms. These trials have non-specific and subjective symptom outcomes. Their sample size is far too small to demonstrate a difference in objective outcomes such as the incidence of acute diverticulitis or other complications of diverticular disease (e.g. abscess, perforation, stenosis, fistula or bleeding).
Other interventional studies that had an observational before–after design were not included in the present review because they lacked a control group. These types of studies have found a significant reduction in symptoms associated with diverticular disease with the use of a high-fibre diet [19, 27, 28]. Studies that compared a high-fibre diet with fibre supplements, such as lactulose or bran tablets, have found no advantage of one over the other [30, 34, 37]. A study comparing high-fibre, low-fibre, no specific diet, placebo and Metamucil showed that all treatment options gave reduction of symptoms. Metamucil showed the most significant reduction in symptoms. The authors suggest also a possible placebo effect of dietary therapy [30]. These studies are summarized in Table 2.
The initial hypothesis of Burkitt and Painter in 1971, which was based on a fibre deficiency as crucial factor in the aetiology of diverticular disease, has weaknesses in the evidence when one considers the populations under study. The increased lifespan of western populations throughout the twentieth century may parallel the increasing prevalence of diverticulosis. In the epidemiological studies, life expectancy has improved in the last 80 years in the Western world. On the African continent, life expectancy remains low. The World Health Organisation data report a life expectancy of 51 years for both South Africa and Kenya, the African countries from which necropsy data were referenced by Burkitt and Painter [38]. The prevalence of diverticular disease increases with age to up to 50–66% in patients older than 80 years [3]. The fibre hypothesis was studied in a large prospective follow-up study consists of a cohort of 51,529 male US health professionals, enrolled and interviewed in 1986 and interviewed about developing diverticular disease in 1990 and again in 1992 [39]. Fibre intake of individual participants has been measured before development of diverticular disease. In this cohort, fruit and vegetable intake, cellulose, hemicellulose and lignin are inversely associated with the risk of symptomatic diverticular disease. Insoluble fibre reduces the risk of diverticular disease by 37% (relative risk (RR) 0.63, 95% confidence interval (CI) 0.44 to 0.99) and cellulose by 48% (RR 0.52, 95% CI 0.36 to 0.75) [15]. Epidemiological and observational studies have been the two predominant approaches to illuminate the role of diet and lifestyle in the prevalence of diverticular disease [20, 40]. The epidemiological approach is currently confounded by the lack of available up-to-date data on prevalence in different populations.
Despite the lack of evidence, high-fibre diet as treatment for symptomatic diverticular disease, is been recommended in several guidelines [14, 18, 21, 24]. All of the evidence is based on two small sample size randomised clinical trials, with inconsistent results, or observational uncontrolled studies more than 20 years ago. Some of the references given in the several guidelines have not been discussed in this review because the inclusion criteria were not met or were expert opinion papers [19, 20, 22, 23, 25, 26]. In the case of high-fibre diet therapy to prevent recurrent diverticulitis, two guidelines [18, 21] advised high-fibre diet to prevent recurrence. None of the cited papers actually investigate or discuss this advise. One of the guidelines provides no evidence. All of the references given are discussed in this review or summarized in Table 2. Painter [23] gives a summary of his previous study [27] together with a review. Larson et al. [22] discuss surgical treatment vs medical treatment. The other references [25, 26] are reviews.
In present literature search, no study was found enrolling patients with acute diverticulitis. One study enrolled 100 patients with acute diverticular disease of which 25 patients had been operated to evaluate a high-fibre diet [33]. It is uncertain whether these patients have diverticulitis or symptomatic diverticular disease, but inclusion criteria suggest a mixed population. Ninety-one percent of patients on a high-fibre diet reviewed 5 to 7 years after admission had remained symptom-free. It was concluded that high-fibre diet may have a protective role and prevent further complications. This was essentially pre-CT scan era, and therefore, the accurate diagnosis of diverticulitis remains in question.
New hypotheses have emerged which can be helpful in fully understanding the aetiology of diverticular disease. Diverticulosis may be ultimately proved to be a disease of ageing. Recent studies show increasing mitochondrial dysfunction in the ageing colonic epithelia, and these data correlate well with diverticulosis prevalence [41, 42]. Studies of mitochondrial deficiency or other age-associated changes in the colonic muscle might further illuminate the pathology of this condition. Another hypothesis in which environmental factors play a role is the colonic microflora in the disease process. Two studies emphasize the differences in microflora composition between high (Western)- and low (African/Asian)-risk populations [43, 44]. A direct comparison of the faecal and colonic mucosal flora between cases and controls might reveal differences associated with the altered luminal environment. The other way around, diverticulosis may also alter the colonic mucosal flora. New research is needed to distinguish these hypotheses. It seems probable that dietary and/or luminal environmental factors protect against diverticula formation, but solid evidence is lacking.
Conclusion
In conclusion, high-quality evidence for a high-fibre diet in the treatment of diverticular disease is lacking, and most recommendations are based on inconsistent level 2 and mostly level 3 evidence. Nevertheless, high-fibre diet is still recommended in several guidelines.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4:3–21. [PubMed] [Google Scholar]
- 2.Pohlman T. Diverticulitis. Gastroenterol Clin North Am. 1988;17:357–385. [PubMed] [Google Scholar]
- 3.Floch MH, Bina I. The natural history of diverticulitis: fact and theory. J Clin Gastroenterol. 2004;38:S2–S7. doi: 10.1097/01.mcg.0000124003.07433.ee. [DOI] [PubMed] [Google Scholar]
- 4.Munson KD, Hensien MA, Jacob LN, Robinson AM, Liston WA. Diverticulitis: a comprehensive follow-up. Dis Colon Rectum. 1996;39:318–324. doi: 10.1007/BF02049475. [DOI] [PubMed] [Google Scholar]
- 5.Mayo W, Wilson L, Griffin H. Acquired diverticulitis of the large intestine. Surg Gynecol Obstet. 1907;8:20. [Google Scholar]
- 6.Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450–454. doi: 10.1136/bmj.2.5759.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkitt DP, Clements JL, Jr, Eaton SB. Prevalence of diverticular disease, hiatus hernia, and pelvic phleboliths in black and white Americans. Lancet. 1985;2:880–881. doi: 10.1016/S0140-6736(85)90139-4. [DOI] [PubMed] [Google Scholar]
- 8.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stemmermann GN, Yatani R. Diverticulosis and polyps of the large intestine. A necropsy study of Hawaii Japanese. Cancer. 1973;31:1260–1270. doi: 10.1002/1097-0142(197305)31:5<1260::AID-CNCR2820310535>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Eastwood MA, Sanderson J, Pocock SJ, et al. Variation in the incidence of diverticular disease within the city of Edinburgh. Gut. 1977;18:571–574. doi: 10.1136/gut.18.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes LE. Postmortem survey of diverticular disease of the colon. Gut. 1969;10:336–351. doi: 10.1136/gut.10.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2:1408–1412. doi: 10.1016/S0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110–3121. doi: 10.1111/j.1572-0241.1999.01501.x. [DOI] [PubMed] [Google Scholar]
- 15.Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fibre types and symptomatic diverticular disease in men. J Nutr. 1998;128:714–719. doi: 10.1093/jn/128.4.714. [DOI] [PubMed] [Google Scholar]
- 16.Brodribb AJ. Treatment of symptomatic diverticular disease with a high fibre diet. Lancet. 1977;1:664–666. doi: 10.1016/S0140-6736(77)92112-2. [DOI] [PubMed] [Google Scholar]
- 17.Ornstein MH, Littlewood ER, Baird IM, et al. Are fibre supplements really necessary in diverticular disease of the colon? A controlled clinical trial. BMJ. 1981;282:1353–1356. doi: 10.1136/bmj.282.6273.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc. 1999;13:430–436. doi: 10.1007/s004649901007. [DOI] [PubMed] [Google Scholar]
- 19.Brodribb AJM, Humphreys DM. Diverticular disease: three studies. Br Med J. 1976;1:424–430. doi: 10.1136/bmj.1.6007.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gear JSS, Ware A, Fursdon P, Mann JI, Nolan DJ, Brodribb AJM, Vessey MP. Symptomless diverticular disease and intake of dietary fiber. Lancet. 1979;1:511–514. doi: 10.1016/S0140-6736(79)90942-5. [DOI] [PubMed] [Google Scholar]
- 21.Rafferty J, Shellito P, Hyman NH, Buie WD. Standards Committee of American Society of Colon and Rectal Surgeons. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006;49:939–944. doi: 10.1007/s10350-006-0578-2. [DOI] [PubMed] [Google Scholar]
- 22.Larson DM, Masters SS, Spiro HM. Medical and surgical therapy in diverticular disease: a comparative study. Gastroenterology. 1976;71:734–737. [PubMed] [Google Scholar]
- 23.Painter NS. Diverticular disease of the colon: the first of the Western diseases shown to be due to a deficiency of dietary fiber. S Afr Med J. 1982;61:1016–1020. [PubMed] [Google Scholar]
- 24.World Gastroenterology Organisation. http://www.worldgastroenterology.org. Accessed 26 Dec 2010
- 25.Talbot JM. Role of dietary fibre in diverticular disease and colon cancer. Fed Proc. 1981;40:2337–2342. [PubMed] [Google Scholar]
- 26.Nair P, Mayberry JF. Vegetarianism, dietary fibre and gastro-intestinal disease. Dig Dis. 1994;12:177–185. doi: 10.1159/000171451. [DOI] [PubMed] [Google Scholar]
- 27.Painter NS, Almeida AZ, Colebourne KW. Unprocessed bran in treatment of diverticular disease of the colon. Br Med J. 1972;2(5806):137–140. doi: 10.1136/bmj.2.5806.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plumley PF, Francis B. Dietary management of diverticular disease. J Am Diet Assoc. 1973;63(5):527–530. [PubMed] [Google Scholar]
- 29.Taylor I, Duthie HL. Bran tablets and diverticular disease. Br Med J. 1976;1(6016):988–990. doi: 10.1136/bmj.1.6016.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devroede G, Vobecky JS, Vobecky JM, et al. Medical management of diverticular disease: a random trial. Gastroenterology. 1977;72:A134. [Google Scholar]
- 31.Eastwood MA, Smith AN, Brydon WG, et al. Comparison of bran, ispaghula, and lactulose on colon function in diverticular disease. Gut. 1978;19:1144–1147. doi: 10.1136/gut.19.12.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewerth S, Ahlberg J, Holmström B, et al. Influence on symptoms and transit-time of Vi-SiblinR in diverticular disease. Acta Chir Scand Suppl. 1980;500:49–50. [PubMed] [Google Scholar]
- 33.Hyland JM, Taylor I. Does a high fibre diet prevent the complications of diverticular disease? Br J Surg. 1980;67:77–79. doi: 10.1002/bjs.1800670202. [DOI] [PubMed] [Google Scholar]
- 34.Smits BJ. Lactulose in the treatment of symptomatic diverticular disease: a comparative study with high fibre diet. BJCP. 1990;44:314–318. [PubMed] [Google Scholar]
- 35.Hodgson WJH. The placebo effect. Is it important in diverticular disease? Am J Gastroenterol. 1977;67:157–162. [PubMed] [Google Scholar]
- 36.Leahy AL, Ellis RM, Quill DS, et al. High fibre diet in symptomatic diverticular disease of the colon. Ann R Coll Surg Engl. 1985;67:173–174. [PMC free article] [PubMed] [Google Scholar]
- 37.Eastwood MA, Smith AN, Brydon WG, et al. Compared bran, ispaghula and lactulose on colon function in diverticular disease. Gut. 1978;19:1144–1147. doi: 10.1136/gut.19.12.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO (2008) World Health Organisation life expectancy statistics. http://www.who.int/infobase/report.aspx?rid=114&iso=KEN&ind=DIE
- 39.Aldoori WH. The protective role of dietary fibre in diverticular disease. Adv Exp Med Biol. 1997;427:291–308. doi: 10.1007/978-1-4615-5967-2_29. [DOI] [PubMed] [Google Scholar]
- 40.Manousos O, Day NE, Tzonou A, et al. Diet and other factors in the aetiology of diverticulosis: an epidemiological study in Greece. Gut. 1985;26:544–549. doi: 10.1136/gut.26.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor RW, Barron MJ, Borthwick GM, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arasaradnam RP, Greaves L, Commane D, et al. Novel preliminary findings of mtDNA mutations in colonic crypts of patients with diverticular disease. Gut. 2007;56(Suppl II):A146–A163. [Google Scholar]
- 43.Segal I, Walker AR, Wadee A. Persistent low prevalence of Western digestive diseases in Africa: confounding aetiological factors. Gut. 2001;48:730–732. doi: 10.1136/gut.48.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finegold SM, Attebery HR, Sutter VL. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27:1456–1469. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]