Abstract
Pancreatic β-cells arise from Ngn3+ endocrine progenitors within the trunk epithelium of the embryonic pancreas. The emergence of endocrine cells requires E-cadherin downregulation, but the crucial steps that elicit such are not clear, yet probably important for ultimately being able to efficiently generate β-cells de novo from stem cells. Grg3 (groucho-related gene 3, also known as Tle3), encodes a member of the Groucho/TLE family of co-repressors and its function in various cell contexts is mediated by recruitment to target genes by different transcription factors. Grg proteins broadly regulate the progression of progenitor cells to differentiated cell types, but specific developmental mechanisms have not been clear. We find that Grg3 is expressed in most β-cells and a subset of other endocrine cell types in the pancreas. Grg3 is highly expressed in Ngn3+ endocrine progenitor descendants just after transient Ngn3 expression. Grg3-null embryos die at E14.5, which is associated with placental defects, so we explanted E12.5 pancreata to allow endocrine differentiation to occur in culture. Grg3 knockout explants displayed a drastic decrease in the differentiation of all endocrine cell types owing to defects in the delamination of early endocrine progenitors from the trunk epithelium. We find that Grg3 normally suppresses E-cadherin gene expression, thereby allowing delamination of endocrine cells from the trunk epithelium and revealing how this transcriptional co-repressor modulates this crucial step of β-cell development.
Keywords: Grg3, Tle3, β-Cell, Endocrine, Pancreas, Delamination, E-cadherin, Mouse
INTRODUCTION
There has been much emphasis on creating insulin-producing, pancreatic β-cells in culture from ES and iPS cells, drawing on the concepts of developmental biology (Spence and Wells, 2007; Zaret, 2008). Signaling pathways elicit specific cell fate choices, and gene regulation by transcription factors determines the correct step-wise progression towards β-cell differentiation. Although transcription factors have been well described, little is understood about how the co-factors recruited by these transcription factors are involved in the differentiation of a pancreatic progenitor cell towards the β-cell lineage. Yet, it is well established that transcriptional co-activators and co-repressors are crucial effectors of gene regulation.
The embryonic pancreas first forms as ventral and dorsal endodermal buds that later fuse to form the mature organ. Within the buds, Pdx1+ multipotent pancreas progenitors are born and have the capacity to become the endocrine, acinar and duct cell types (Gu et al., 2002). The progenitors undergo branching morphogenesis (Villasenor et al., 2010), whereby tips cells extend into the surrounding mesenchyme and leave behind trunk cells (Zhou et al., 2007). The tip cells, which express Ptf1a and Cpa1, are thought to be multipotent, being able to give rise to acinar, duct and endocrine cells until around E14, when they commit to acinar differentiation (Zhou et al., 2007). Trunk cells are bipotent, giving rise to duct and endocrine cells (Solar et al., 2009). Within these bipotent trunk progenitors, some cells will transiently express Ngn3, allocating them to an endocrine fate.
It has been reported that Ngn3 expression in the bipotent trunk progenitors initiates a cascade of E-cadherin suppression and delamination of endocrine progenitors from the trunk epithelium, in a process similar to an epithelial-mesenchymal transition (Cole et al., 2009; Gouzi et al., 2011; Rukstalis and Habener, 2007). However, the regulatory factors involved in this step are unknown. The delaminated cells ultimately differentiate into the one of the five hormone-expressing endocrine cell types. Transcription factors such as Nkx2.2, Nkx6.1 and Pdx1 have been shown to be essential for proper endocrine cell development (Ahlgren et al., 1998; Sander et al., 2000; Sussel et al., 1998), whereas other factors, such Hnf6 and Hnf1β, promote a duct fate (Pierreux et al., 2006; Solar et al., 2009; Zhang et al., 2009).
Members of the Groucho/Grg/TLE family of co-repressors are recruited to DNA by transcription factors and repress associated genes by recruiting HDACs (Chen et al., 1999; Gasperowicz and Otto, 2005; Jennings and Ish-Horowicz, 2008) and causing a closed conformation change that excludes other factors from the local chromatin (Sekiya and Zaret, 2007). Recently, we showed that two family members, Grg1 and Grg3 (also known as Tle1 and Tle3), are highly expressed in the multipotent ventral endoderm, but become extinguished as the liver gene program initiates (Santisteban et al., 2010). Grg3 was found to repress liver genes such as albumin in the endoderm, restraining liver specification until the Grg3 protein was extinguished. Conversely, Grg3 is highly expressed in the pancreas during embryonic development (Doyle et al., 2007; Hoffman et al., 2008). RNA in situ analysis in the pancreas demonstrated that Grg3 is expressed at much higher levels than other Grg family members, Grg1, Grg2 and Grg4 (Doyle et al., 2007). Grg3 has an overlapping expression pattern with Nkx2.2 and is expressed in α-cells, but not in amylase+ exocrine tissue (Doyle et al., 2007). Grg3 interacts with Nkx2.2 in a pancreas cell line, suggesting that Grg3 may help to facilitate the Nkx2.2-mediated repression during α- and β-cell differentiation (Doyle et al., 2007). However, Grg3 binds additional transcription factors (Arce et al., 2009; Brantjes et al., 2001; Cinnamon and Paroush, 2008; Jennings et al., 2006; Jimenez et al., 1997; Nagel et al., 2005) and thus might have a broader role than that of Nkx2.2 in the context of endocrine cell differentiation.
We now find that Grg3 protein is highly expressed in the endocrine compartment of the embryonic pancreas, immediately succeeding Ngn3 expression, and its expression persists in β-cells while being retained in only a subset of other endocrine cell types. Given these findings, we sought to determine whether Grg3 was required for the early delamination and differentiation of endocrine cells from endocrine progenitors, using Grg3 knockout embryos and pancreatic explants. Furthermore, we sought to determine whether Grg3 suppressed E-cadherin gene expression to promote delamination of endocrine cells from the trunk epithelium. These studies provide insights into the roles of a transcriptional co-repressor in β-cell development and how the factor controls endocrine progenitor cell emergence.
MATERIALS AND METHODS
Immunofluorescence
Immunofluorescence (IF) was performed on 4% paraformaldehyde (PFA) fixed tissues, embedded in OCT and frozen sectioned. Sections were probed with the primary antibodies rabbit-α-Grg3 (Santisteban et al., 2010), rabbit-α-Grg1 (Santisteban et al., 2010), rabbit α-Aes (Abcam), guinea pig-α-insulin (Abcam), mouse-α-insulin [Beta Cell Biology Consortium (BCBC)], mouse-α-glucagon (BCBC), mouse-α-somatostatin (BCBC), guinea pig-α-pancreatic peptide (Millipore), rabbit-α-ghrelin (BCBC), mouse-α-Nkx6.1 (BCBC), mouse-α-Pdx1 (BCBC), rabbit-α-Hes1 (a gift from Nadean Brown, University of Cincinnati, Cincinnati, OH, USA), mouse-α-Ngn3 (BCBC), chicken-α-GFP (Abcam), rabbit-α-amylase (Sigma), rabbit-α-Muc1 (Abcam), rat-α-E-cadherin (Invitrogen), guinea pig-α-Hnf6 (BCBC), rabbit-α-pH3 (histone H3-phospho S10, Abcam), rabbit-α-cleaved-Caspase3 (Cell Signaling) and chicken-α-βgal (Abcam). Primary antibodies were detected with Alexa Fluor-conjugated secondary antibodies (Invitrogen) and TSA kits (Invitrogen and Perkin Elmer).
For Xgal/Muc1 and Xgal/E-cadherin co-stained sections, lacZ-positive sections (Grg3+/– and Grg3–/–) and lacZ-negative (Grg3+/+, data not shown) were stained with Xgal solution overnight at 37°C, washed and re-fixed with 4% PFA for 15 minutes. Sections were then subsequently stained for Muc1 or E-cadherin.
Mice
All animal studies were performed with IACUC approval. Ngn3 linage trace experiments were performed by crossing the Ngn3-Cre transgenic mouse (Gu et al., 2002) line to the Rosa26RYFP reporter mouse line (Srinivas et al., 2001). Grg3+/– mice will be described elsewhere (M.G., C. Surmann-Schmitt, Y. Hamada, F.O. and J.C.C., unpublished). Briefly, a cassette containing βgal reporter gene (lacZ) and neomycin resistance gene (NeoR), was introduced into the EcoRI site on exon1 of Grg3 gene.
Pancreas explants
Grg3+/+, Grg3+/– and Grg3–/– embryos were harvested at E12.5 and their pancreata were dissected (supplementary material Fig. S2A). Pancreata were then cultured at the air-liquid interphase on Nuclepore Track-Etch Membranes (Whatman, 110414) floating on DMEM (Gibco, 11995) with 10% FBS and pen-strep. Media was replaced after 2 days. Explants were then harvested after 4 days, fixed in 4% PFA and embedded in OCT compound for further sectioning and analysis.
Cell area and number quantitation
To analyze hormone (insulin, glucagon, SS)-expressing cell areas relative to Pdx1+ pancreatic epithelium area from IF stained sections, images were analyzed with ImageJ software. In detail, hormone and Pdx1 IF images from the same co-stained section were independently set to grayscale, and a threshold was set to only highlight the relevant pixels to be counted. Next, total pixel area was counted and documented for both the hormone area and Pdx1 area on each section. Hormone area was divided by Pdx1 area to obtain a final relative quantitation of hormone area based on explant size. Data are presented as percentage hormone-positive area compared with Grg3+/+ hormone-positive area.
To analyze PP and Ghrelin cell numbers, total numbers of hormone-positive cells were counted per section divided by the number of sections counted per sample to obtain a mean number of hormone-positive cells per section. Data are presented as number of hormone-positive cells.
Quantitation of proliferation and apoptosis
To analyze E14.5 pancreas proliferation, the total number of pH3+/E-cadherin+ cells was divided by the total number of E-cadherin+ cells to obtain a ratio of pH3 cells/E-cadherin cells. To analyze liver proliferation, the number of pH3+ hepatoblasts/40× field where compared. To analyze explant proliferation and apoptosis (cleaved-caspase 3), we performed the same analysis using ImageJ as above but normalized pH3 and cleaved-caspase 3 area to DAPI area. pH3 data are presented as percentage proliferation compared with Grg3+/+ or Grg3+/–; cleaved-caspase 3 data are presented as the ratio of cleaved-caspase 3 area over DAPI area.
Lentivirus production and infection
Grg3 cDNA was cloned into pAcGFP1-C1 (Clontech) expression plasmid to create a GFP-Grg3 fusion construct. GFP-Grg3 and GFP were then independently cloned into the pWPT lentivirus expression plasmid. To make the lentivirus, lentiviral packaging plasmids (psPAX2 and pMD.G) and the pWPT plasmid (GFP-Grg3 or GFP) were transfected into 293T cells, as described (Sekiya and Zaret, 2007; Zufferey et al., 1997). Lentivirus containing media from the transfected 293T cells was passed through a 0.45 μm filter and added to βTC-6 cells and cultured for 4 days. Infection was verified by GFP expression.
RT-qPCR
After 4 days in culture, βTC-6 cells were harvested for RNA with an RNeasy Mini Kit (Qiagen). RT-qPCR was performed using AgPath-ID One-Step RT-PCR Kit (Ambion) along with Taqman Gene Expression Assays (Applied Biosystems): Gapdh (Mm99999915_g1), E-cadherin (Mm01247357_m1), Hnf6 (Mm00839394_m1), Hnf1β (Mm00447458_m1) and Grg3 (Mm00437097_m1). Reactions were run on a 7900HT Fast Real-Time PCR System (Applied Biosystems) and quantitated on the RQ Manager Software (Applied Biosystems) using the ΔΔCT method.
RESULTS
Grg3 protein is initially expressed in all pancreatic endocrine cell types but then resolves primarily to β-cells
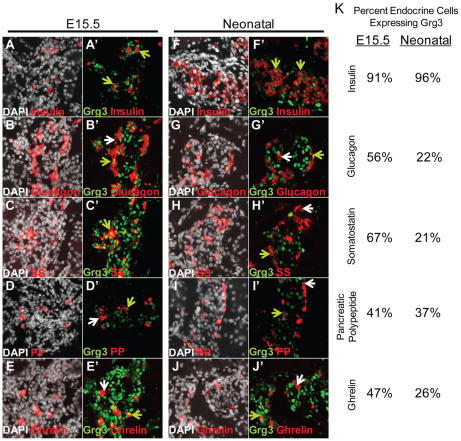
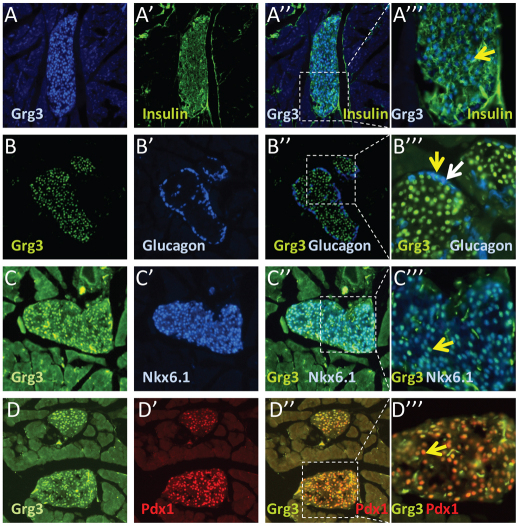
Using a specific antibody (Santisteban et al., 2010), we detected nuclear Grg3 expression in nearly all β-cells at embryonic day 15.5 (E15.5) and in neonates (Fig. 1A′,F′ green arrows, 1K). However, only 41-67% of other endocrine cell types (α, SS, PP, ghrelin) at E15.5 express Grg3 (Fig. 1B′-E′,G′-J′ green versus white arrows, 1K). Interestingly, the percentage of non-β-endocrine Grg3-positive cells decreased to 21-37% at neonatal timepoints (Fig. 1K). Furthermore, in 7-week-old adult mouse islets, Grg3 is expressed in virtually all insulin+ β-cells (Fig. 2A″′, yellow arrow) but only in a few glucagon+ α-cells (Fig. 2B″′, yellow versus white arrow). Grg3 is co-expressed with transcription factors Pdx1 and Nkx6.1, which are abundant in β-cells (Fig. 2C″′,D″′, yellow arrows). These data suggest that Grg3 may be important for the early differentiation of all endocrine cell types, particularly the β-cell.
Fig. 1.
Grg3 is expressed in almost all β-cells and a subset of other non-β endocrine cells during development. (A-J′) E15.5 and neonatal pancreas sections were double stained for Grg3 and hormones for all five endocrine cell types and counterstained with DAPI. All insulin+ β-cells in view express Grg3 (A,A′,F,F′; green arrows). Grg3 is co-expressed with a subset of the four other non-β endocrine cell types (green arrows) as seen by glucagon (B,B′,G,G′), somatostatin (SS; C,H), pancreatic polypeptide (PP; D,D′,I,I′) and ghrelin (E,E′,J,J′) co-staining. White arrows indicate Grg3– endocrine cells. (K) Grg3 was expressed in most insulin+ β-cells at E15.5 (91%) and in neonates (96%). Only a subset of non-β endocrine cell types expresses Grg3 at E15.5 (41-67%) and at neonatal timepoints (21-37%).
Fig. 2.
Grg3 is highly expressed in the adult islet. (A-D″′) Seven-week-old adult islets were stained for Grg3 and insulin; essentially all insulin+ β-cells express Grg3 (A-A″′, yellow arrow). Islets were stained for Grg3 and glucagon; only a few glucagon+ α-cells express Grg3 (B-B″′). The white arrow indicates a typical Grg3-negative α-cell, and the yellow arrow indicates a rare Grg3-positive α-cell (B″′). Grg3 colocalizes with islet transcription factors Nkx6.1 (C-C″′, yellow arrow) and Pdx1 (D-D″′, yellow arrow).
We also analyzed the expression of other Grg family members in the embryonic pancreas. We found in published microarray experiments that Grg2 and Grg6, as well as Grg3, were enriched in endocrine cells, compared with the rest of the pancreas (Soyer et al., 2010). Furthermore, we stained E15.5 pancreata with Grg1 and Aes/Grg5 antibodies. We found that Grg1 was expressed in non-epithelial cells of the pancreas adjacent to exocrine and endocrine cells (supplementary material Fig. S1A-F). Aes/Grg5 was expressed in α- and β-endocrine cells as well as in Hnf6+ duct/trunk epithelium (supplementary material Fig. S1G-L). However, of the Grg proteins analyzed by immunofluorescence, only Grg3 appeared to be exclusive to the endocrine compartment during this time period (supplementary material Fig. S1M-R).
Grg3 is induced in endocrine cells immediately after Ngn3 expression
To determine precisely when and where Grg3 protein is first expressed in β-cell development, we analyzed early stages of pancreas organogenesis. At E12.5 we found a low level of Grg3 gene expression in the pancreatic epithelium, compared with the surrounding mesenchyme (supplementary material Fig. S2A, arrow), with only a few Pdx1+ epithelial cells and glucagon+ cells positive for Grg3 protein (supplementary material Fig. S2B, arrows). These findings differ slightly from previously published data in which Grg3 mRNA was found to be expressed relatively higher in the epithelium than the mesenchyme in the E12.5 pancreas (Doyle et al., 2007). The previous study did not analyze Grg3 protein at this developmental time point.
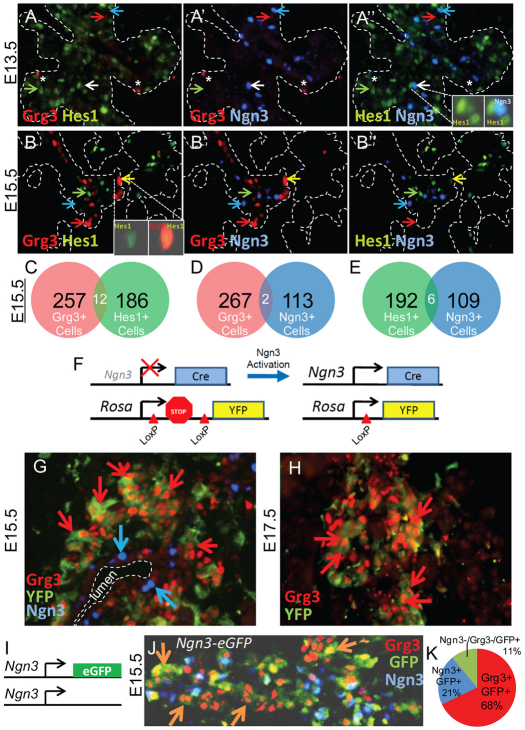
To determine whether Grg3 protein is expressed in Hes1+ multipotent pancreas progenitors and/or Ngn3+ endocrine progenitors, we triple-stained E13.5 and E15.5 pancreata with Grg3, Hes1 and Ngn3 antibodies (Fig. 3A,B). We found that Grg3 protein was expressed in very few cells at E13.5 (Fig. 3A, red arrow) and the detectable Grg3 did not co-express with Hes1 (Fig. 3A, green arrow) or Ngn3 (Fig. 3A, blue arrow). However, after the onset of the secondary transition at E15.5, Grg3 was highly expressed in many cells (Fig. 3B, red arrow). Interestingly, Grg3 (red arrow) is rarely co-expressed with Hes1 (green arrow) and Ngn3 (blue arrow) at E15.5 (Fig. 3B-D), suggesting that Grg3 does not play a role in Hes1-mediated Notch signaling. After extensive studies, we found Grg3 and Ngn3 were largely mutually exclusive, except for a few cells (Fig. 3B, see quantitation in 3D). The yellow arrow indicates a rare Grg3+/Hes1+ cell (Fig. 3B, inset). Similarly, Hes1 and Ngn3 are rarely co-expressed at E13.5 and E15.5 (Fig. 3A,B,E), consistent with Hes1 repressing Ngn3, as shown previously (Apelqvist et al., 1999; Jensen et al., 2000; Lee et al., 2001). The white arrow indicates a rare Hes1+/Ngn3+ cell (Fig. 3A, inset). The presence of rare co-expressing cells suggests that these cells are transitioning between expression of these proteins.
Fig. 3.
Grg3 is expressed in descendants of Ngn3+ endocrine progenitors. (A-B″) E13.5 and E15.5 pancreas sections were triple-stained for Grg3, Hes1 and Ngn3. Red arrows indicate Grg3+ cells that express neither Hes1 nor Ngn3; green arrows indicate Hes1+ cells that express neither Grg3 nor Ngn3; blue arrows indicate Ngn3+ cells that express neither Grg3 nor Hes1; yellow arrows indicate a rare Grg3/Hes1 double-positive cell; white arrows indicate a rare Hes1/Ngn3 double-positive cell (A,B). Asterisks indicate autofluorescing cells. (C-E) Grg3/Hes1, Grg3/Ngn3 and Hes1/Ngn3 double-positive cells were quantitated at E15.5, revealing that these factors are rarely co-expressed in the same cells. (F) Lineage-tracing strategy in which Cre recombinase is driven from the Ngn3 promoter deleting a LoxP flanked transcriptional ‘stop’ and activating a YFP reporter driven by the Rosa gene. (G) E15.5 pancreas sections from this cross were analyzed for Grg3, YFP and Ngn3. Ngn3+ cells near the trunk lumen (blue arrows) express neither YFP nor Grg3 (red arrows). However, Grg3 is highly expressed in YFP+ Ngn3 descendants. (H) At E17.5, Grg3 persists in YFP+ Ngn3 descendants (red arrows). (I,J) Analysis of E15.5 pancreas of Ngn3-eGFP (I) embryos reveal that Grg3 is expressed in Ngn3–/GFP+ newly differentiating endocrine cells (J, orange arrows). (K) Of 877 GFP+ cells counted, Grg3 is expressed in 68% of total GFP cells (86% of Ngn3–/GFP+ cells), Ngn3 is expressed in 21% total GFP cells, whereas Ngn3–/Grg3–/GFP+ cells represent 11%.
Ngn3 is transiently and briefly expressed in endocrine progenitors, and is required intrinsically for their subsequent differentiation into endocrine cells (Gradwohl et al., 2000; Gu et al., 2002; Miyatsuka et al., 2009). The relevant lineage-tracing experiments were performed by driving the expression of CRE recombinase under the control of the Ngn3 promoter, thus activating the expression of the reporter Rosa26RYFP. After the CRE-mediated deletion of a loxP flanked ‘stop’ sequence upstream of the reporter, YFP becomes expressed in all Ngn3+ descendant cells, even after Ngn3 expression is extinguished (Gu et al., 2002; Srinivas et al., 2001; Wang et al., 2010). We used this approach to determine whether Grg3 is expressed in the descendants of Ngn3+ progenitors (Fig. 3F). At E15.5, we found Ngn3 (Fig. 3G, blue arrow) associated with the trunk epithelium, but not co-expressed with the Ngn3-activated YFP reporter (Fig. 3G) or Grg3 (Fig. 3G, red arrow). The lack of YFP labeling of Ngn3+ cells reflects that Ngn3 is transiently expressed and shut off before the visibility of the YFP reporter (Miyatsuka et al., 2009). However, we did find that Grg3 is robustly expressed in YFP+ Ngn3 descendants at E15.5 and E17.5 timepoints (Fig. 3G,H, red arrows). Similarly, we analyzed pancreata from E15.5 embryos in which eGFP is targeted into the Ngn3 locus (Fig. 3I). In these embryos, eGFP activated in Ngn3+ endocrine progenitors persists in newly formed Ngn3– endocrine cells (Lee et al., 2002; White et al., 2008). We found that Grg3 marks the majority of Ngn3–/GFP+ cells (orange arrows, 86%), marking 68% of the total GFP+ cells (Fig. 3J,K) indicating that most endocrine cells express Grg3 as they delaminate and differentiate. These data indicate that endocrine cells express Grg3 just as Ngn3 expression ceases and that some endocrine cells will only express Grg3 transiently, as non-β-cell endocrine cell types are less likely to continue to express Grg3 during later pancreas development (Fig. 1K).
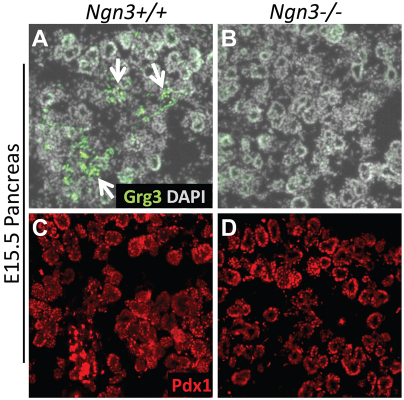
Furthermore, E15.5 Ngn3–/– pancreata that lack all endocrine cells (Gradwohl et al., 2000) fail to express Grg3 (Fig. 4A,B), although they remain positive for Pdx1 (Fig. 4C,D). These data are consistent with the above finding that Grg3 is expressed in differentiating endocrine cells (Figs 1, 2). We conclude that Grg3 is expressed throughout the endocrine lineage immediately after Ngn3 expression is extinguished, and Grg3 expression is dependent upon Ngn3.
Fig. 4.
Grg3 expression is lost in Ngn3–/– pancreas. (A,B) Grg3 protein is expressed in E15.5 Ngn3+/+ pancreata (A, arrows) but is lost in Ngn3–/– (B). (C,D) Positive control for antibody staining shows Pdx1 staining in the pancreas epithelium of Ngn3+/+ and Ngn3–/– pancreata.
Grg3 promotes α- and β-cell differentiation
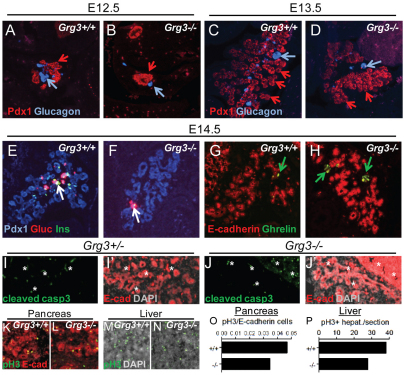
To address if Grg3 functions in promoting endocrine differentiation, we analyzed the pancreata of Grg3-null embryos. The Grg3–/– embryos are smaller than their heterozygous and wild-type littermates, and the vast majority of them die at E14.5, manifesting strong placental defects (M.G., C. Surmann-Schmitt, Y. Hamada, F.O. and J.C.C., unpublished). At E12.5 and E13.5, the Grg3–/– pancreata at these stages appeared smaller than Grg3+/+ littermates, probably owing to an overall decreased embryo size. However, Grg3–/– pancreata stained positive for Pdx1 (Fig. 5A-D, red arrows) and generated primary transition glucagon+ α-cells, although fewer than in Grg3+/+ littermates (Fig. 5C,D, blue arrows). The early embryonic lethality in Grg3–/– embryos precluded a quantitative analysis of the emergence of other endocrine lineages.
Fig. 5.
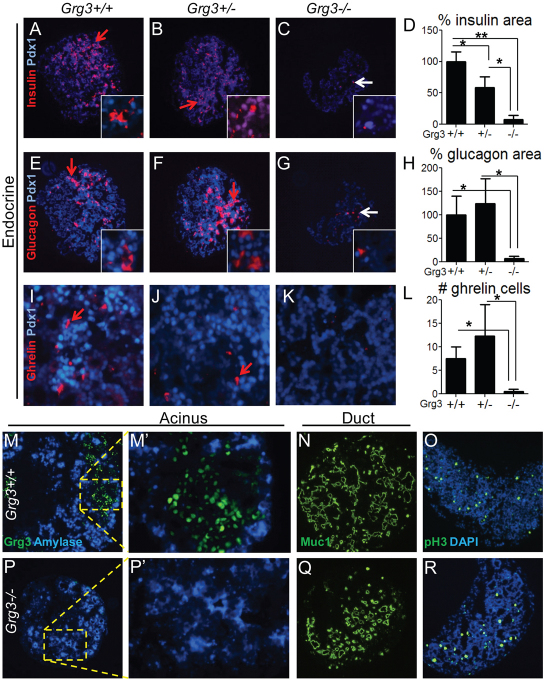
Grg3 promotes endocrine differentiation. (A-D) Grg3+/+ and Grg3–/– embryos at E12.5 (A,B) and E13.5 (C,D) both express Pdx1 (red arrows), but the Grg3–/– pancreas has fewer glucagon+ α-cells in the Grg3+/+ pancreas (blue arrows). (E,F) At E14.5, there is a drastic reduction of glucagon+ α-cells and insulin+ β-cells (white arrows) in the Grg3–/– pancreas compared with Grg3+/+. (G,H) A few small clusters of ghrelin+ cells are apparent in the Grg3–/– pancreas (H, green arrows), compared with single cells found in Grg3+/+ (G, green arrow). (I-J′) No evidence of apoptosis was found in Grg3+/– and Grg3–/– pancreata, as determined by lack of cleaved-caspase 3 staining (I,J, data not shown). Asterisks indicate autofluorescing cells and E-cadherin (I,I′) marks the pancreatic epithelium. (K-N) Grg3+/+ and Grg3–/– pancreata and liver were analyzed for proliferation by phospho-histone 3 (pH3) and E-cadherin staining. (O) Calculating the ratio of pH3 to E-cadherin cells indicates that Grg3–/– pancreas proliferates to a lesser extent than Grg3+/+. (P) However, Grg3–/– liver also proliferates slower indicating a general systemic effect on proliferation.
However, we were able to analyze one rare live E14.5 Grg3–/– embryo. We triple-stained the Grg3–/– and Grg3+/+ littermates with insulin, glucagon and Pdx1 (Fig. 5E,F), and double-stained for ghrelin and E-cadherin (Fig. 5G,H). We found that the Grg3–/– pancreas robustly stained for Pdx1 but had dramatically decreased insulin+ β- and glucagon+ α-cells compared with Grg3+/+ (Fig. 5E,F, white arrows). Whereas Grg3+/+ pancreata contained a few dispersed ghrelin+ cells (Fig. 5G, green arrow), the Grg3–/– pancreas had small clusters of ghrelin+ cells (Fig. 5H, green arrow). We next analyzed cleaved-caspase 3 and found no evidence of apoptosis in Grg3+/– nor Grg3–/– pancreata (Fig. 5I,J). We also analyzed phosho-Histone3 (pH3) and found about a 25% decrease in pH3/E-cadherin staining in the Grg3–/– pancreas (Fig. 5K,L,O). However, a similar decrement was seen in the liver, which does not normally express Grg3, indicating a general systemic effect on growth in the Grg3–/– embryos (Fig. 5M,N,P).
We were also able to obtain one rare live E17.5 Grg3–/– embryo and analyzed the pancreas for insulin, glucagon and ghrelin to compare with a Grg3+/– littermate (supplementary material Fig. S3A-D). Insulin, glucagon and ghrelin were expressed in the Grg3–/– pancreas, with insulin and glucagon at markedly lower levels than in the control littermate (supplementary material Fig. S3A,B,E,F) but little effect was seen on ghrelin. We conclude that Grg3 is necessary for the timely and efficient induction of pancreatic β- and α-cells.
Grg3 promotes the differentiation of all endocrine cell types
Because most Grg3–/– embryos die before the differentiation of the secondary transition endocrine cells, we dissected out the pancreata from E12.5 Grg3+/+, Grg3+/– and Grg3–/– embryos and explanted them in culture for 4 days (supplementary material Fig. S4A-C). Control and Grg3–/– explants expanded and branched alike in culture (supplementary material Fig. S4B,C), as well as proliferated similarly, as seen by phospho-histone 3 staining (Fig. 5O,R; supplementary material Fig. S4D) and had similar amounts of cleaved-caspase 3 staining (supplementary material Fig. S4E). We found that control explants displayed all aspects of pancreas differentiation, such as the formation of endocrine (insulin, glucagon, PP, SS, ghrelin) (Fig. 6A,B,E,F,I,J; supplementary material Fig. S6A,B,E,F), acinar (amylase) and duct (Muc1) cells (Fig. 6M,N). By contrast, Grg3–/– explants lacked almost all endocrine cell types (Fig. 6C,D,G,H; supplementary material Fig. S5C,D,G,H), but still retained acinar and ductal differentiation (Fig. 6P,Q). The lack of staining with Grg3 antibody in Grg3–/– embryos confirms antibody specificity. Insulin, glucagon and somatostatin levels decreased by over 90% (Fig. 6D,H; supplementary material Fig. S5D) and PP cell number decreased by 86% in Grg3–/– explants (supplementary material Fig. S5H), recapitulating the in vivo results at E14.5. Ghrelin+ cells were virtually absent in Grg3–/– explants (Fig. 6K,L), which is in contrast to the ghrelin+ clusters found in the E14.5 Grg3–/– pancreas (Fig. 5H). Overall, these data indicate that Grg3 selectively promotes the initial differentiation of endocrine cells in the pancreas.
Fig. 6.
Grg3 promotes endocrine differentiation in E12.5 pancreas explants. (A-C,E-G,I-K) Grg3+/+, Grg3+/– and Grg3–/– explants were stained for the endocrine markers/hormones insulin, glucagon and somatostatin. Red arrows indicate robust hormone expression in Grg3+/+ (A,E,I) and Grg3+/– (B,F,J) explants, and white arrows indicate the few remaining insulin+ β-cells (C) and glucagon+ α-cells in Grg3–/– explants (G). (D,H,L) The percentages of insulin+, glucagon+ and ghrelin+ cells are drastically and significantly reduced in Grg3–/– explants (*P<0.05, **P<0.005). Data are mean±s.e.m. (M,M′,N,P,P′,Q) Amylase (M,M′,P,P′) and Muc1 (N,Q) staining indicates that acinar and duct differentiation, respectively, occurs in both Grg3+/+ and Grg3–/– explants. Grg3 is expressed in a non-overlapping pattern with amylase in Grg3+/+ explants (M,M′) while Grg3–/– explants produce no Grg3 protein (P,P′). (O,R) pH3 staining indicates that control (Grg3+/+ and Grg3+/–) and Grg3–/– explants proliferate at similar rates (supplementary material Fig. S2D).
Grg3 is required for the delamination of endocrine cells from the trunk epithelium
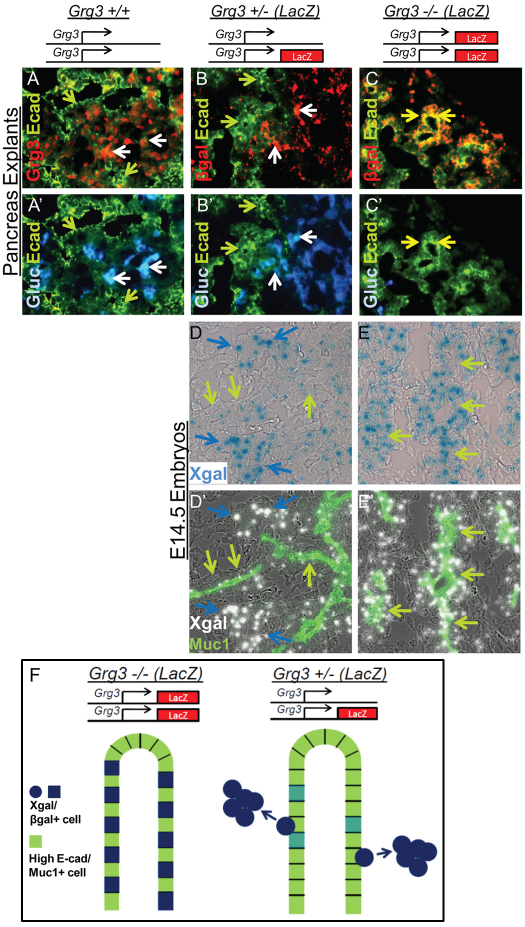
Recent studies show that Ngn3 initiates a cascade resulting in the downregulation of E-cadherin (Gouzi et al., 2011) and is required for endocrine delamination from the trunk epithelium (Beucher et al., 2011; Magenheim et al., 2011). However, the former study indicated that Ngn3 was not able to repress E-cadherin itself. We noticed that the Grg3 co-repressor (Fig. 7A,D, white and blue arrows) was highly expressed just outside the domains of the high E-cadherin/Muc1+ trunk epithelium (green arrows) in E14.5 embryos and explanted E12.5 pancreata (Fig. 7A,B,D, green arrows). In explants, Grg3 was expressed in regions of low E-cadherin along with glucagon+ α-cells (Fig. 7A, white arrows). In Grg3+/– explants, which express β-gal under the control of the Grg3 gene, we detected β-gal in a pattern like that of Grg3 (Fig. 7B). That is, β-gal was expressed along with glucagon (Fig. 7B, white arrows) in low E-cadherin regions, but not in high E-cadherin trunk epithelium (Fig. 7B, green arrows). These data indicate the faithfulness of the lacZ knock-in allele of Grg3.
Fig. 7.
Grg3 promotes the delamination of endocrine cells from the trunk epithelium. (A,A′) Grg3 is expressed outside the domain of high E-cadherin staining (green arrows) but is expressed in regions of relatively lower E-cadherin along with glucagon (white arrows) in Grg3+/+ explants. (B-C′) lacZ driven from the Grg3 locus is detected by β-gal staining. Similar to endogenous Grg3 expression (A,A′), β-gal is expressed in regions of low E-cadherin along with glucagon (white arrows) but absent from high E-cadherin epithelium (green arrows) in Grg3+/– explants (B,B′). (C,C′) Conversely, in Grg3–/– explants, cells that express lacZ (β-gal) but lack Grg3 are detected in the high E-cadherin epithelium (yellow arrows), indicating that Grg3-null cells fail to exit the trunk epithelium. (D-E′) E14.5 Grg3+/– and Grg3–/– pancreata were stained with Xgal and Muc1 to mark the trunk lumens. In the Grg3+/– pancreas, Xgal is expressed at low levels at Muc1+ trunks (green arrows) but is highly expressed in delaminating cells not associated with the trunks (blue arrows, D,D′). In the Grg3–/– pancreas, Xgal is highly expressed in Muc1+ trunk epithelium (green arrows, E,E′). (F) These data suggest that normally robust Grg3 expression promotes the delamination of cells from the E-cadherin+/Muc1+ trunk epithelium (bottom), and in the Grg3–/– mutant, pancreata cells that do not produce Grg3 protein fail to delaminate from the trunk epithelium (top).
In Grg3–/– explants, lacZ integration at the Grg3 locus causes β-gal to mark cells that would normally express Grg3, but fail to make the protein. In this way, β-gal can serve as an early tracer for cells that have failed to execute a Grg3-dependent process. In Grg3–/– explants, β-gal was found in high E-cadherin-expressing trunk epithelium (Fig. 7C, yellow arrows). Note the merge of red and green fluorescence into yellow in Grg3–/– explants (Fig. 7C, yellow arrows), compared with the lack of yellow seen in Grg3+/– explants (Fig. 7B). We also analyzed Grg3+/– and Grg3–/– pancreata at E14.5 in a similar manner as above, but using Muc1 staining and Xgal to detect β-gal. As stated above, cells not associated with Muc1+ trunk epithelium stained strongly with Xgal in Grg3+/– (blue arrows), and a few low level Xgal cells remained in the trunk epithelium (Fig. 7D, green arrows). However in the Grg3–/– pancreas, robust Xgal staining was apparent in the Muc1+ trunk epithelium (Fig. 7E, green arrows).
From these data, we conclude that cells that failed to induce Grg3 in the Grg3–/– pancreas remain in the high E-cadherin/Muc1+ trunk epithelium, as opposed to the delaminating cells seen in Grg3+/– (and Grg3+/+) pancreata that induce Grg3 and do not remain in the high E-cadherin/Muc1+ trunk epithelium (Fig. 7F). Overall, these findings show that Grg3 is required for the delamination of endocrine cells from the trunk epithelium.
Grg3 suppresses the E-cadherin gene
The endocrine compartment has relatively lower levels of E-cadherin than the ductal epithelium during pancreatic development (Rukstalis and Habener, 2007). Co-staining with Grg3 and E-cadherin at E15.5 (Fig. 8A) and postnatal day 1 (Fig. 8B) indicate that Grg3 is expressed in endocrine cells with relatively low levels of E-cadherin, suggesting that Grg3 may play a role in reducing E-cadherin expression during endocrine differentiation and maturation. To determine directly whether Grg3 is able to repress E-cadherin, we evaluated the consequence of ectopic Grg3 expression in the mouse β-cell line βTC-6 (Fig. 8C-F). We found that βTC-6 cells express the trunk/duct-enriched genes E-cadherin, Hnf6 and Hnf1b (Fig. 8G), as well as Grg3 (data not shown). We introduced GFP-Grg3 and GFP alone as a control by lentivirus infection. As expected, GFP-Grg3 was found exclusively in the nuclei of the cells (Fig. 8D,F), whereas GFP was expressed in the nuclei and cytoplasm of cells (Fig. 8C,E). There appeared to be no overt change in cell morphology, growth or viability between GFP-Grg3 and GFP-infected cells. Notably, qRT-PCR analysis showed that ectopic expression of GFP-Grg3 fusion protein significantly suppressed E-cadherin expression, but not that of Hnf6 or Hnf1b, compared with GFP-infected cells (Fig. 8G). Most strikingly, immunofluorescence of individual infected cells indicated that only the cells that were successfully infected with GFP-Grg3 had low E-cadherin staining, whereas adjacent non-infected cells and GFP-infected control cells had higher levels of E-cadherin staining (Fig. 8H-K). Supporting this observation in vivo, E-cadherin was expressed much more strongly in the E17.5 Grg3–/– pancreas than in Grg3+/– pancreata (supplementary material Fig. 6). These data indicate that Grg3 normally suppresses E-cadherin, which allows for the accurately timed delamination of endocrine cells from the trunk epithelium.
Fig. 8.
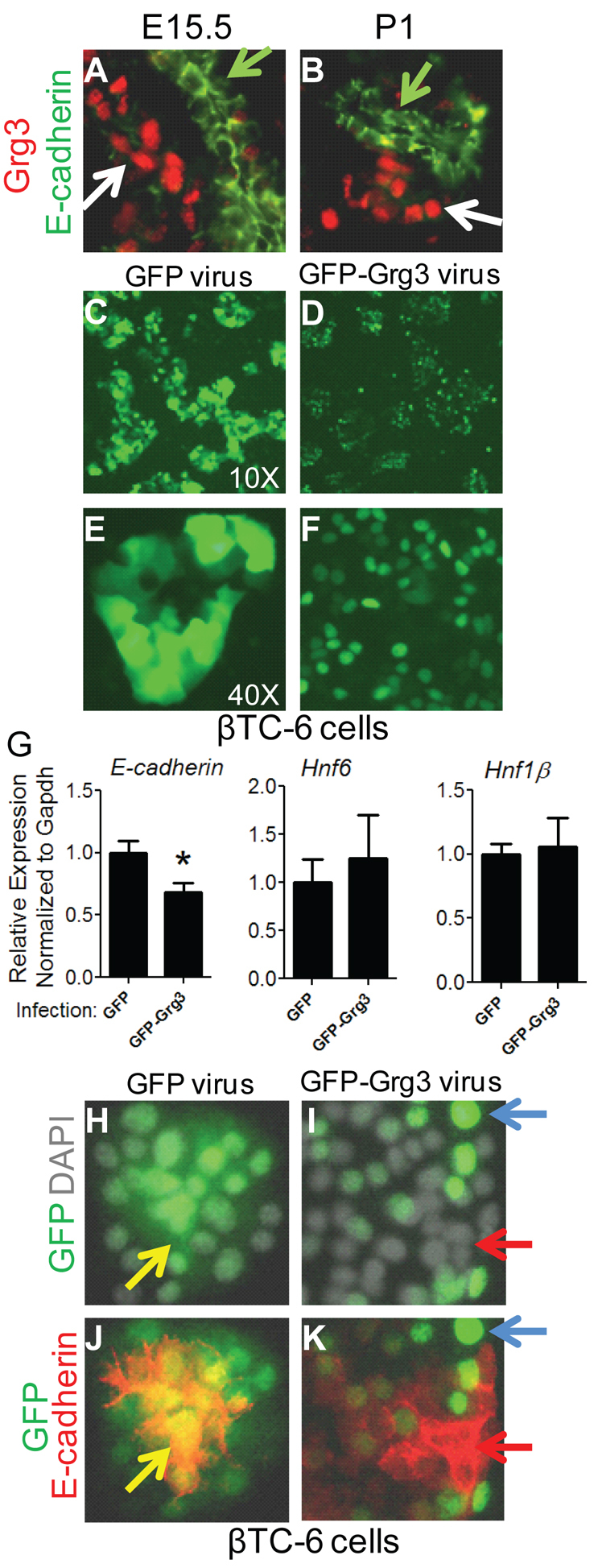
Grg3 suppresses E-cadherin expression. (A,B) Grg3 is highly expressed in low E-cadherin+ endocrine cells (white arrows) and not in high E-cadherin+ trunks/ducts (green arrows) at E15.5 and P1 (postnatal day 1). (C-F) GFP-Grg3 and Grg3 were infected by lentivirus into the βTC-6 mouse β-cell line. The GFP-Grg3 fusion protein was expressed exclusively in the nucleus (D,F), whereas GFP was found in both the cytoplasm and nucleus (C,E). (G) The ectopic expression of GFP-Grg3 significantly reduced E-cadherin expression (*P<0.05) but had no significant effect on Hnf6 and Hnf1β expression. Data are mean±s.e.m. (H-K) βTC-6 cells infected with GFP (H,J) and GFP-Grg3 (I,K) were stained for E-cadherin protein. Infected cells expressing control GFP expressed E-cadherin (J, yellow arrow), but cells successfully infected with GFP-Grg3 had greatly reduced E-cadherin staining (K, red arrow). Uninfected cells express higher levels of E-cadherin (K, blue arrow).
DISCUSSION
Grg3 as a step between Ngn3 expression and endocrine progenitor delamination
In this study, we have evaluated the temporal and spatial expression of Grg3 protein in the embryonic pancreas. We found that Grg3 protein is robustly expressed in newly differentiating endocrine cells as they delaminate from the trunk epithelium, just after transient Ngn3 expression, and that Ngn3 is required for Grg3 expression. We also used Grg3 knockout studies to show that Grg3 is required for delamination. In Grg3-null embryos, the early endocrine progenitors in the trunk epithelium of Grg3-null cells remain in the trunk epithelium. We further show that Grg3 promotes delamination by suppressing E-cadherin gene expression. It has recently been reported that downregulation of E-cadherin is sufficient to induce delamination from the trunk epithelium (Gouzi et al., 2011). Also in that study, it was determined that Ngn3 initiates a cascade of delamination, yet it was shown that Ngn3 was unable to repress E-cadherin directly (Gouzi et al., 2011) and other factors must be required for repression. It has also been recently shown that Ngn3 is required for delamination (Beucher et al., 2011; Magenheim et al., 2011). We thus place Grg3 between the Ngn3 transient expression step and E-cadherin downregulation step, to allow endocrine cell delamination.
Grg3 in early endocrine cells and adult islets
As Ngn3 is required for delamination (Beucher et al., 2011; Gouzi et al., 2011; Magenheim et al., 2011) and the developmental timing of Ngn3 expression impacts endocrine cell fate decisions (Johansson et al., 2007), the subsequent induction of Grg3 similarly modulates endocrine cell fate decisions as endocrine cells are born. Although Grg3 is expressed in β-cells and only a subset of non-β endocrine cells, the numbers of both α- and β-cells in the E14.5 Grg3–/– pancreas and all endocrine cells in Grg3–/– pancreatic explants are drastically reduced. This suggests that Grg3 must be expressed in all or most newly differentiating endocrine cells as they delaminate. This is also supported by data indicating that Grg3 is expressed in most early descendants of Ngn3+ progenitors.
In addition, our finding that Grg3 protein is expressed in neonatal and adult endocrine cells beyond the Ngn3 step suggests an independent role for endocrine cell maturation/maintenance. Our detailed analysis revealed that Grg3 was expressed in virtually all β-cells and a subset of other endocrine cell types. As development proceeds from E15.5 to neonates, Grg3 expression persists in β-cells, but the percentage of non-β-cells expressing Grg3 decreases (Fig. 1; Fig. 2A,B). This suggests that Grg3 plays a role during β-cell maturation to suppress non-β-cell identity in the β-cell. Indeed, a recent study suggests that Grg3 is in a repressive complex that precludes β-cells from converting to α-cells (Papizan et al., 2011). Studies using a Grg3 conditional knockout in the maturing β-cell will be needed to address this hypothesis further.
The lack of an apoptotic phenotype and the lack of a proliferative effect in vitro in Grg3-null embryos indicate that Grg3 autonomously functions in the pancreas to promote differentiation, and defects in endocrine differentiation in Grg3–/– pancreata are not due to systemic deficiencies in the embryo.
Grg3 as a general co-repressor of endocrine differentiation
Our data indicate that Grg3 is robustly induced immediately after Ngn3 transient expression in Ngn3+ descendants. Interestingly, microarray data from a previous study showed that Grg3 is enriched in FACS-isolated Ngn3-eYFP pancreatic cells, compared with non-YFP cells (Soyer et al., 2010). The eYFP is more stable than Ngn3 protein in this mouse line, allowing for the isolation of Ngn3+ cells as well as early Ngn3– descendants of Ngn3+ cells. We do not yet know how Ngn3 leads to Grg3 induction in early endocrine cells that have extinguished Ngn3 expression. However, multiple transcription factors involved in subsequent endocrine differentiation (Brantjes et al., 2001; Cinnamon and Paroush, 2008; Doyle et al., 2007; Gao et al., 2008; Jensen et al., 2000; Lee et al., 2005) could interact with Grg3 in this context.
Nkx2.2 has been shown to interact with Grg3 in a pancreatic cell line and to have overlapping expression during pancreas development (Doyle et al., 2007). Nkx2.2 is required for β-cell differentiation and the proper number of α and PP cells (Sussel et al., 1998), and in Nkx2.2–/– embryos, these cell types are replaced by ghrelin+ cells (Prado et al., 2004). Interestingly, a repressor form of Nkx2.2 that binds Grg3 introduced into the Nkx2.2–/– mouse is sufficient to rescue α-cells, partially rescue β-cells and partially restore ghrelin+ cells to normal levels (Doyle et al., 2007). The E14.5 Grg3–/– pancreas has decreased α- and β-cells but retains ghrelin+ clusters as similarly seen in Nkx2.2–/– embryos (Anderson et al., 2011; Prado et al., 2004). Furthermore, a recent study suggests that a main defect in Nkx2.2–/– pancreata is the failure of Tm4sf4 repression, which results in a migratory defect of endocrine progenitors (Anderson et al., 2011). It will be interesting to determine whether Grg3 collaborates with Nkx2.2 to repress Tm4sf4 to promote efficient delamination and differentiation of endocrine cells.
However, loss of Nkx2.2 only results in reduced β-, α- and PP cell numbers (Sussel et al., 1998), but loss of Grg3 also results in decreased SS cells and ghrelin+ cell reduction in explants. This suggests that Grg3 must also interact with other transcription factors to promote endocrine differentiation.
Drosophila Groucho interacts with E(spl)/Hes to repress genes required for proneural differentiation (Cinnamon and Paroush, 2008). The mouse homologue of E(spl), Hes1, has been demonstrated to promote the maintenance of multipotent pancreas progenitors while repressing endocrine differentiation (Jensen et al., 2000), but a Hes1-Grg3 interaction has not been demonstrated in the pancreas. We do not find that Grg3 is expressed in Hes1+ progenitors. This suggests that Grg3 does not play a role in mediating Hes1 repression during pancreatic organogenesis, consistent with the pro-endocrine function of Grg3.
FoxA2 is specifically required for α-cell differentiation during development and proper β-cell function (Lee et al., 2005), and FoxA1 and FoxA2 have redundant roles in activating Pdx1 expression and subsequently promoting the expansion of multipotent pancreas progenitors (Gao et al., 2008). Previous studies have shown that Grg3 interacts with FoxA proteins (Sekiya and Zaret, 2007). As FoxA2 is required for α-cell differentiation (Lee et al., 2005) and Grg3–/– pancreata have drastically decreased α-cell numbers, Grg3 could possibly interact with FoxA2 in this context. However, as all endocrine cell types are drastically decreased in Grg3–/– explants, Grg3 must again play a broader role in endocrine differentiation.
As Grg3 is expressed in endocrine cells as they first differentiate from the bipotential trunk/duct epithelium and Grg3 expression persists in β-cells through development into adulthood, Grg3 may be an important factor to suppress non-β-cell transcriptional programs and promote maturation. Our studies indicate that Grg3 is needed for the efficient delamination of endocrine cells from the trunk epithelium, suggesting that repression of the trunk/ductal transcriptional program is needed for the full differentiation of a functional β-cell. Indeed, the field of regenerative medicine has yet to reproducibly generate fully mature, glucose-responsive β-cells from ES cells in culture. Furthermore, many differentiation protocols result in double hormone-positive (insulin+/glucagon+) cells that are not functional as β-cells (Borowiak and Melton, 2009). This suggests that a general understanding of transcriptional repression during development is needed to understand better how the β-cell fate is promoted at the expense of other endocrine fates. Our finding of how a Groucho co-repressor promotes a specific step in β-cell differentiation provides further insight into this process.
Supplementary Material
Acknowledgments
We thank Angela Hines for help with animal husbandry and technical advice, and Natalie Terry for comments on the manuscript.
Footnotes
Funding
D.E.M. was supported by postdoctoral fellowships from Fox Chase Cancer Center [T32 CA-009035-32] and from the University of Pennsylvania [T32 DK007314]. The research was supported by a Beta Cell Biology Consortium grant from the National Institutes of Health [UO1 DK072503 to K.S.Z.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.072892/-/DC1
References
- Ahlgren U., Jonsson J., Jonsson L., Simu K., Edlund H. (1998). beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. R., Singer R. A., Balderes D. A., Hernandez-Lagunas L., Johnson C. W., Artinger K. B., Sussel L. (2011). The L6 domain tetraspanin Tm4sf4 regulates endocrine pancreas differentiation and directed cell migration. Development 138, 3213–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 [DOI] [PubMed] [Google Scholar]
- Arce L., Pate K. T., Waterman M. L. (2009). Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer 9, 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucher A., Martin M., Spenle C., Poulet M., Collin C., Gradwohl G. (2011). Competence of failed endocrine progenitors to give rise to acinar but not ductal cells is restricted to early pancreas development. Dev. Biol. 361, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M., Melton D. A. (2009). How to make beta cells? Curr. Opin.Cell Biol. 21, 727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantjes H., Roose J., van De Wetering M., Clevers H. (2001). All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 29, 1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Fernandez J., Mische S., Courey A. J. (1999). A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 13, 2218–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinnamon E., Paroush Z. (2008). Context-dependent regulation of Groucho/TLE-mediated repression. Curr. Opin. Genet. Dev. 18, 435–440 [DOI] [PubMed] [Google Scholar]
- Cole L., Anderson M., Antin P. B., Limesand S. W. (2009). One process for pancreatic beta-cell coalescence into islets involves an epithelial-mesenchymal transition. J. Endocrinol. 203, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. J., Loomis Z. L., Sussel L. (2007). Nkx2.2-repressor activity is sufficient to specify alpha-cells and a small number of beta-cells in the pancreatic islet. Development 134, 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., LeLay J., Vatamaniuk M. Z., Rieck S., Friedman J. R., Kaestner K. H. (2008). Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 22, 3435–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperowicz M., Otto F. (2005). Mammalian Groucho homologs: redundancy or specificity? J. Cell. Biochem. 95, 670–687 [DOI] [PubMed] [Google Scholar]
- Gouzi M., Kim Y. H., Katsumoto K., Johansson K., Grapin-Botton A. (2011). Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev. Dyn. 240, 589–604 [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Dierich A., LeMeur M., Guillemot F. (2000). neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G., Dubauskaite J., Melton D. A. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 [DOI] [PubMed] [Google Scholar]
- Hoffman B. G., Zavaglia B., Beach M., Helgason C. D. (2008). Expression of Groucho/TLE proteins during pancreas development. BMC Dev. Biol. 8, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings B. H., Ish-Horowicz D. (2008). The Groucho/TLE/Grg family of transcriptional co-repressors. Genome Biol. 9, 205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings B. H., Pickles L. M., Wainwright S. M., Roe S. M., Pearl L. H., Ish-Horowicz D. (2006). Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol. Cell 22, 645–655 [DOI] [PubMed] [Google Scholar]
- Jensen J., Pedersen E. E., Galante P., Hald J., Heller R. S., Ishibashi M., Kageyama R., Guillemot F., Serup P., Madsen O. D. (2000). Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36–44 [DOI] [PubMed] [Google Scholar]
- Jimenez G., Paroush Z., Ish-Horowicz D. (1997). Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed. Genes Dev. 11, 3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K. A., Dursun U., Jordan N., Gu G., Beermann F., Gradwohl G., Grapin-Botton A. (2007). Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 12, 457–465 [DOI] [PubMed] [Google Scholar]
- Lee C. S., Perreault N., Brestelli J. E., Kaestner K. H. (2002). Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 16, 1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Sund N. J., Behr R., Herrera P. L., Kaestner K. H. (2005). Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev. Biol. 278, 484–495 [DOI] [PubMed] [Google Scholar]
- Lee J. C., Smith S. B., Watada H., Lin J., Scheel D., Wang J., Mirmira R. G., German M. S. (2001). Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes 50, 928–936 [DOI] [PubMed] [Google Scholar]
- Magenheim J., Klein A. M., Stanger B. Z., Ashery-Padan R., Sosa-Pineda B., Gu G., Dor Y. (2011). Ngn3(+) endocrine progenitor cells control the fate and morphogenesis of pancreatic ductal epithelium. Dev. Biol. 359, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatsuka T., Li Z., German M. S. (2009). Chronology of islet differentiation revealed by temporal cell labeling. Diabetes 58, 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel A. C., Krejci A., Tenin G., Bravo-Patino A., Bray S., Maier D., Preiss A. (2005). Hairless-mediated repression of notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol. Cell. Biol. 25, 10433–10441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papizan J. B., Singer R. A., Tschen S. I., Dhawan S., Friel J. M., Hipkens S. B., Magnuson M. A., Bhushan A., Sussel L. (2011). Nkx2.2 repressor complex regulates islet beta-cell specification and prevents beta-to-alpha-cell reprogramming. Genes Dev. 25, 2291–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierreux C. E., Poll A. V., Kemp C. R., Clotman F., Maestro M. A., Cordi S., Ferrer J., Leyns L., Rousseau G. G., Lemaigre F. P. (2006). The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 130, 532–541 [DOI] [PubMed] [Google Scholar]
- Prado C. L., Pugh-Bernard A. E., Elghazi L., Sosa-Pineda B., Sussel L. (2004). Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. USA 101, 2924–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis J. M., Habener J. F. (2007). Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr. Patterns 7, 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Sussel L., Conners J., Scheel D., Kalamaras J., Dela Cruz F., Schwitzgebel V., Hayes-Jordan A., German M. (2000). Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 127, 5533–5540 [DOI] [PubMed] [Google Scholar]
- Santisteban P., Recacha P., Metzger D. E., Zaret K. S. (2010). Dynamic expression of groucho-related genes Grg1 and Grg3 in foregut endoderm and antagonism of differentiation. Dev. Dyn. 239, 980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Zaret K. S. (2007). Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo. Mol. Cell 28, 291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar M., Cardalda C., Houbracken I., Martin M., Maestro M. A., De Medts N., Xu X., Grau V., Heimberg H., Bouwens L., et al. (2009). Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 [DOI] [PubMed] [Google Scholar]
- Soyer J., Flasse L., Raffelsberger W., Beucher A., Orvain C., Peers B., Ravassard P., Vermot J., Voz M. L., Mellitzer G., et al. (2010). Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development 137, 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. R., Wells J. M. (2007). Translational embryology: using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev. Dyn. 236, 3218–3227 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L., Kalamaras J., Hartigan-O’Connor D. J., Meneses J. J., Pedersen R. A., Rubenstein J. L., German M. S. (1998). Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 125, 2213–2221 [DOI] [PubMed] [Google Scholar]
- Villasenor A., Chong D. C., Henkemeyer M., Cleaver O. (2010). Epithelial dynamics of pancreatic branching morphogenesis. Development 137, 4295–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yan J., Anderson D. A., Xu Y., Kanal M. C., Cao Z., Wright C. V., Gu G. (2010). Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev. Biol. 339, 26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P., May C. L., Lamounier R. N., Brestelli J. E., Kaestner K. H. (2008). Defining pancreatic endocrine precursors and their descendants. Diabetes 57, 654–668 [DOI] [PubMed] [Google Scholar]
- Zaret K. S. (2008). Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat. Rev. Genet. 9, 329–340 [DOI] [PubMed] [Google Scholar]
- Zhang H., Ables E. T., Pope C. F., Washington M. K., Hipkens S., Means A. L., Path G., Seufert J., Costa R. H., Leiter A. B., et al. (2009). Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech. Dev. 126, 958–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Law A. C., Rajagopal J., Anderson W. J., Gray P. A., Melton D. A. (2007). A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell 13, 103–114 [DOI] [PubMed] [Google Scholar]
- Zufferey R., Nagy D., Mandel R. J., Naldini L., Trono D. (1997). Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15, 871–875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.