Abstract
The modification of transcriptional regulation is a well-documented evolutionary mechanism in both plants and animals, but post-transcriptional controls have received less attention. The derived hermaphrodite of C. elegans has regulated spermatogenesis in an otherwise female body. The PUF family RNA-binding proteins FBF-1 and FBF-2 limit XX spermatogenesis by repressing the male-promoting proteins FEM-3 and GLD-1. Here, we examine the function of PUF homologs from other Caenorhabditis species, with emphasis on C. briggsae, which evolved selfing convergently. C. briggsae lacks a bona fide fbf-1/2 ortholog, but two members of the related PUF-2 subfamily, Cbr-puf-2 and Cbr-puf-1.2, do have a redundant germline sex determination role. Surprisingly, this is to promote, rather than limit, hermaphrodite spermatogenesis. We provide genetic, molecular and biochemical evidence that Cbr-puf-2 and Cbr-puf-1.2 repress Cbr-gld-1 by a conserved mechanism. However, Cbr-gld-1 acts to limit, rather than promote, XX spermatogenesis. As with gld-1, no sex determination function for fbf or puf-2 orthologs is observed in gonochoristic Caenorhabditis. These results indicate that PUF family genes were co-opted for sex determination in each hermaphrodite via their long-standing association with gld-1, and that their precise sex-determining roles depend on the species-specific context in which they act. Finally, we document non-redundant roles for Cbr-puf-2 in embryonic and early larval development, the latter role being essential. Thus, recently duplicated PUF paralogs have already acquired distinct functions.
Keywords: PUF proteins, Caenorhabditis, Evolution, Translation, Germ cells, Hermaphroditism
INTRODUCTION
In convergent evolution, different lineages acquire similar phenotypes independently. However, the extent to which key modifications to development and physiology are reproduced in convergent lineages is only beginning to be addressed. In the nematode family Rhabditidae, self-fertile hermaphrodites have evolved from female ancestors at least ten times (Kiontke and Fitch, 2005). Even for the closely related Caenorhabditis elegans and Caenorhabditis briggsae, for which XX spermatogenesis is similar in extent and timing, self-fertility has evolved convergently (Cho et al., 2004; Kiontke et al., 2004). Because germline sex determination is well studied in C. elegans, comparisons between C. elegans and C. briggsae offer an experimentally tractable way to explore the molecular and genetic details of convergent evolution.
Genetic comparisons between C. elegans and C. briggsae reveal the conservation of the global sex determination pathway (Hill et al., 2006; Kelleher et al., 2008). The sex determination cascade is initiated in the early embryo by the ratio between the number of X chromosomes and sets of autosomes (X:A ratio) (Nigon, 1951), with a high ratio (2X:2A) in the hermaphrodite repressing her-1 transcription and a low ratio (1X:2A) in the male activating her-1 transcription (Dawes et al., 1999; Trent et al., 1991). In hermaphrodites, low her-1 expression permits activity of the membrane protein TRA-2, which represses the male-promoting FEM proteins (Chin-Sang and Spence, 1996; Mehra et al., 1999). The resulting lower fem activity allows accumulation of the transcription factor TRA-1, which represses genes required for male development (Chen and Ellis, 2000; Conradt and Horvitz, 1999; Mason et al., 2008; Yi et al., 2000). This global sex determination pathway is modified at the post-transcriptional level in the C. elegans XX hermaphrodite germ line to allow transient spermatogenesis. Translational repression of tra-2 by the STAR family RNA-binding protein (RBP) GLD-1 and its co-factor, the F-box protein FOG-2, is required to initiate hermaphrodite spermatogenesis (Clifford et al., 2000; Goodwin et al., 1993; Jan et al., 1999; Schedl and Kimble, 1988), and the translational repression of fem-3 by the PUF (Pumilio and FBF) (Wickens et al., 2002) family RBPs FBF-1/2 is required for the transition from spermatogenesis to oogenesis (Ahringer and Kimble, 1991; Zhang et al., 1997).
Despite overall conservation of the global pathway, species-specific germline sex determination genes and gene regulation have been described in convergent hermaphrodites. Both C. elegans and C. briggsae utilize F-box genes (fog-2 and she-1, respectively) to promote spermatogenesis, but they are both species-specific gene duplicates (Guo et al., 2009; Nayak et al., 2005). Another example is the role of the FEM genes. Although they promote male somatic fate in both species, their germline sex determination function differs. In C. elegans, fem mutations transform spermatocytes into oocytes in both males and hermaphrodites (Hodgkin, 1986), whereas XX C. briggsae Cbr-fem-2 and Cbr-fem-3 mutants are normal hermaphrodites and XO counterparts are transformed to hermaphrodites, not to females as in C. elegans (Hill et al., 2006). The different genetic architecture at the level of fem-3 regulation suggests that the C. briggsae homologs of fbf-1/2 might have different roles in germline sex determination, or none at all (Haag, 2009b).
In C. elegans, the PUF genes fbf-1, fbf-2 and puf-8 are key regulators of the sperm/oocyte switch. The nearly identical FBF paralogs bind specifically to the conserved FBF binding elements (FBEs) at the 3′ untranslated region (UTR) of fem-3, and enable the sperm/oocyte switch by repressing fem-3 translation (Zhang et al., 1997). FBF interaction with the nanos homolog NOS-3 and the bicaudal C homolog GLD-3 is also required for the sperm/oocyte switch. NOS-3 acts like an FBF activator, and together they repress fem-3 expression to promote oocyte fate (Kraemer et al., 1999). By contrast, GLD-3 antagonizes FBF function and this interaction derepresses fem-3 expression to promote sperm fate (Eckmann et al., 2002). The sex determination function of puf-8 is less clear, but it acts redundantly with fbf-1 to allow oogenesis (Bachorik and Kimble, 2005). Aside from their roles in germline sex determination, fbf-1, fbf-2 and puf-8 are also important regulators of the transition from mitosis to meiosis. FBF-1 and FBF-2 act together to promote germline cell proliferation by directly repressing translation of gld-1 mRNA (Crittenden et al., 2002). PUF-8 also promotes faithful meiotic entry in spermatocytes at elevated temperatures (Subramaniam and Seydoux, 2003).
The above observations indicate that PUF proteins pattern germline development by working with a limited set of other RBPs to form a combinatorial network of translational controls. This important role for translation is consistent with its general prominence in regulating gene expression in the C. elegans germ line (Merritt et al., 2008). The comparison of PUF functions in different species thus provides an opportunity to study regulatory evolution at the translational level. Here we present genetic and molecular analyses of PUF family genes in C. briggsae and other, gonochoristic Caenorhabditis species, focusing on their roles in germline sex determination. We find that two homologs of fbf, Cbr-puf-2 and Cbr-puf-1.2, act redundantly to promote hermaphrodite spermatogenesis, much as fbf-1/2 act to promote oogenesis in C. elegans. Cbr-PUF-2/1.2 directly repress the expression of GLD-1, which itself has opposite roles in germline sex determination in C. elegans and C. briggsae (Beadell et al., 2011). Similar to gld-1 (Beadell et al., 2011), PUF protein involvement in germline sex determination coincides phylogenetically with the origin of hermaphrodite development. Thus, C. briggsae and C. elegans PUF genes have opposite effects on germline sex determination because the role of a conserved target mRNA has diverged. Finally, we show that a C. briggsae-specific PUF paralog has already acquired additional essential functions, which might explain why such duplicate genes are so common.
MATERIALS AND METHODS
Phylogenetic analysis
Protein datasets for C. elegans, C. briggsae, C. remanei, C. brenneri and C. japonica were retrieved from the Nematode Genome Annotation Assessment Project (nGASP, http://www.sanger.ac.uk/projects/C_elegans/Wormbase/current/wormpep_download.shtml). A PUF domain hidden Markov model (HHM; PUF_ls.hmm) from Pfam (Sonnhammer et al., 1998) was used to search for PUF domain proteins using HMMER v2.3.2 (Eddy, 1998). Based on test searches for known C. elegans PUF homologs, an E-value of 1.0 was used as the cut-off threshold. Removal of likely alternative alleles in the C. remanei and C. brenneri predictions (Barriere et al., 2009) reduced family sizes to ten and nine sequences, respectively. To validate predictions with unexpected features, some sequences were reverse transcribed using FirstChoice RLM-RACE kit (Ambion) from total RNA, PCR amplified and sequenced. This revealed errors in the WS213 splicing predictions for Cbr-puf-2, Cbr-puf-1.2 and Cja-fbf-1 and confirmed the structure for Cre-puf-1.2. For Cbr-puf-2, earlier WormBase releases (e.g. WS190 and many prior releases) had the correct prediction, and Cja-fbf-1 was corrected in WormBase release WS227. The corrected coding sequence for Cbr-puf-1.2, however, has not been reported elsewhere, and has been submitted to GenBank as accession JQ655294.
Fifty-four Caenorhabditis PUF proteins were aligned with PUMILIO, the unique PUF protein in Drosophila melanogaster. Multiple sequence alignment quality was improved by first aligning sequences in three separate subgroups using MUSCLE v3.6 (Edgar, 2004) with default settings, after which the three alignments were combined using the Profile-profile alignment in MUSCLE v3.6. The combined alignment was manually curated using Se-Al v2.0 (http://tree.bio.ed.ac.uk/software/seal/), and the PUF domain with its flanking regions (335 characters) was extracted according to known PUF protein sequence features (Wickens et al., 2002). Maximum likelihood tree search was performed five times independently using GARLI 2.0 (D. J. Zwickl, PhD thesis, The University of Texas at Austin, 2006), and the tree with the best likelihood score was picked. One hundred non-parametric bootstrap runs were generated using GARLI 2.0. Trees were read in PAUP* (Swofford, 2002) for majority-rule consensus branch values, which were manually mapped onto the best tree and visualized in Dendroscope v2.6.1 (Huson et al., 2007).
Nematode culture and genetics
All nematode species were cultured using standard C. elegans conditions (Wood, 1988), with the use of 2.2% agar plates to discourage burrowing. All C. briggsae mutants were derived from the wild isolate AF16, and included: LGII: Cbr-puf-2(nm66), Cbr-dpy(nm4), Cbr-tra-2(nm1) and Cbr-tra-2(nm9ts); LGIII: Cbr-tra-1(nm2), Cbr-let(nm28); LGIV: Cbr-fem-3(nm63). Cbr-tra-2(nm1)/+;Cbr-fem-3(nm63) animals were the progeny of Cbr-tra-2(nm1)/+;Cbr-fem-3(nm63)/+ mothers, which came from a cross between Cbr-tra-2(nm1)/Cbr-dpy(nm4) and Cbr-fem-3(nm63)/+ males. The final genotype was confirmed by sequencing of diagnostic PCR amplicons.
RNA interference
Gene-specific templates for in vitro transcription were PCR amplified from genomic DNA (C. briggsae) or cDNA (C. sp. 9, C. remanei, C. brenneri and C. japonica) with primers flanked by the T7 promoter and sequenced to verify identity. For C. sp. 9, primers designed according to C. briggsae sequences were used. Plasmid pCR50 (gift from C. Richie, National Institutes of Health, Bethesda, MD, USA) was used to amplify green fluorescent protein (GFP) coding sequence, and pharyngeal GFP strain CP105 was used for the triple RNA interference (RNAi) efficacy test. For all experiments, double-stranded (ds) RNA was introduced by maternal microinjection (Haag et al., 2002).
Microscopy
Worms were mounted for differential interference contrast (DIC) microscopy by standard methods (Wood, 1988). For nuclear staining, worms were fixed in cold methanol, washed with M9, stained with 7.5 μM Hoechst 33258 in M9, rinsed with several changes of M9, and mounted in Vectashield (Vector Laboratories) for fluorescence microscopy. Images were captured with a Zeiss Axiocam digital camera and Open Lab software (Improvision) or an SP5 X confocal microscope (Leica). In the latter, z-stacks were collapsed for presentation.
Quantitative RT-PCR
Total RNA from staged worms was extracted in Trizol (Ambion) and purified according to the manufacturer’s instructions. For Cbr-gld-1 expression, RNA from 50 L4 Cbr-puf-2/1.2(RNAi) worms was extracted. cDNA was reverse transcribed from total mRNA using Superscript III (Invitrogen), and 2 μl was used as template for quantitative PCR using a LightCycler 480 and SYBR Green I Master (Roche) as described (Hill and Haag, 2009). Exon-exon junction primers were used for Cbr-gld-1, Cbr-puf-1.2 and Cbr-puf-2, and pan-actin was used as an internal standard. Raw data were analyzed using LinRegPCR (11.0) (Ruijter et al., 2009), which calculates the starting concentration of the sample from the mean PCR efficiency per amplicon and the Ct value per sample (Ramakers et al., 2003). For each sample, expression was normalized to actin expression.
Deletion mutant screen and transgenic rescue
A C. briggsae AF16 deletion library was produced and screened following standard C. elegans methods (Edgley et al., 2002) without the ‘poison primer’ modification. From 106 haploid genomes screened, Cbr-puf-2 deletion nm66 and Cbr-unc-119 deletion nm67 were isolated. Both alleles were outcrossed six times with the unmutagenized AF16 strain.
Production of Cbr-puf-2 transgene
Regulatory (5′), coding, and 3′ flanking sequences of Cbr-puf-2 were engineered via Gateway cloning technology (Invitrogen) into destination plasmid pCR40 (gift from C. Richie), which also contains the wild-type Cbr-unc-119 gene. This plasmid was introduced into Cbr-unc-119(nm67) mutants through biolistic bombardment (Praitis et al., 2001). Stable non-Unc lines were crossed with Cbr-puf-2(nm66)/+ mutants to test for rescue of larval arrest.
Immunoblots
Triplicate samples for quantitative Cbr-GLD-1 immunoblots comprised 50 L4 worms of Cbr-puf-2/1.2(RNAi) or AF16 controls in SDS sample buffer (Sambrook and Russell, 2001). Primary antibodies were rabbit anti-GLD-1 polyclonal (gift from T. Schedl, Washington University, St Louis, MO, USA) at 1:2000 and mouse anti-tubulin monoclonal (DM1A, Sigma) at 1:1000. Secondary antibodies were HRP-linked donkey anti-rabbit IgG (Jackson ImmunoResearch) at 1:1000 and HRP-linked sheep anti-mouse IgG (GE Healthcare) at 1:1600. ECL signal intensity was quantified using ImageJ (Abramoff et al., 2004). Cbr-GLD-1 protein expression was normalized to tubulin.
Immunohistochemistry
The immunohistochemistry protocol was slightly modified from that of T. Schedl, using a methanol/formaldehyde fix for 10 minutes at room temperature. For PH3 staining, rabbit anti-PH3 (Upstate) was used at 1:200. Fluorescently conjugated secondary antibody (goat anti-rabbit IgG, Alexa 488, Invitrogen) was used at 1:2000. All gonads were dissected and stained simultaneously and under the same conditions.
Yeast reporter constructs
DNA encoding the PUF domain and flanking regions of Cbr-puf-2 (amino acids 92-568) or Cbr-puf-1.2 (amino acids 108-554) was cloned into the GST fusion protein vector pGEX-4T-1 (GE Healthcare) using XmaI and NotI. The same fragments were cloned into pACT2-AD (Clontech) using NcoI and XmaI to allow activation domain fusion protein expression in yeast. Sense and antisense 45 bp DNA oligomers (Integrated DNA Technologies) flanking the putative FBF binding element of the Cbr-gld-1 and Cbr-fem-3 3′UTR were annealed and inserted into the pIIIA/MS2-2 vector using XmaI and SphI for hybrid RNA expression in yeast. Cbr-gld-1 and Cbr-fem-3 wild-type and ACA mutant forms were made similarly. All constructs were confirmed by direct sequencing. pIIIA/MS2-2-Ce-fem-3, pIIIA/MS2-2-Ce-gld-1, pIIIA/MS2-2-NRE and pACT2-FBF-2 (amino acids 121-632) are as previously described (Bernstein et al., 2005).
Gel mobility shift assays
GST fusion proteins were isolated from T7 Express lysY competent E. coli (New England BioLabs) and purified using the following elution buffer: 1×PBS, 0.2% Tween 20, 150 mM NaCl, 0.1% 2-mercaptoethanol, 50 mM glutathione (reduced, pH 8.0). Twenty femtomoles 32P end-labeled oligoribonucleotides (Dharmacon) were combined with GST-Cbr-PUF-2 or GST-Cbr-PUF-1.2 at various concentrations as described (Bernstein et al., 2005).
Yeast three-hybrid assay
In all experiments, RNA plasmids and activation domain fusion plasmids were co-transformed into the YBZ1 yeast strain. The three-hybrid assay was followed as described (Stumpf et al., 2008b). The strength of the interaction was measured using the beta-Glo Assay System (Promega) quantified in a luminometer (Turner 20/20n or Spectra Max M5e).
Statistics
For yeast three-hybrid assay data analysis, standard errors for the ratios of test to vector RNAs (see Fig. 4 and supplementary material Fig. S1) were estimated using the ‘delta method’, which is based on Taylor series expansions to account for multivariate nonlinear transformations of the data (Powell, 2007). Otherwise, standard two-tail t-tests were applied.
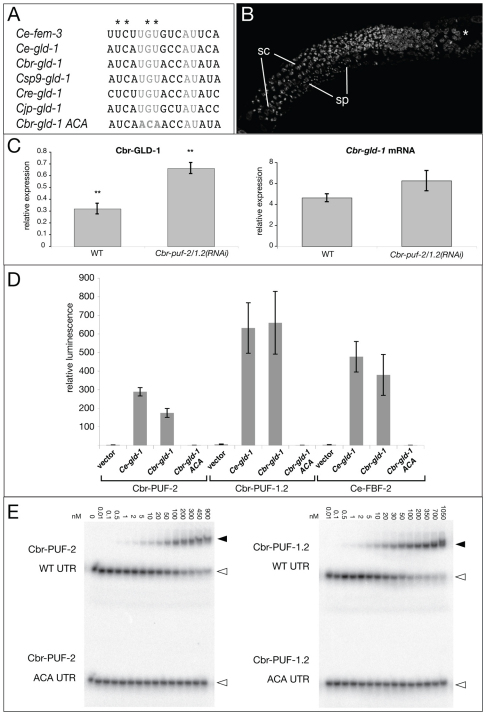
Fig. 4.
Cbr-gld-1 is a direct target of Cbr-PUF-2/1.2. (A) Alignment of FBF binding sites of C. elegans fem-3 and gld-1 with their orthologs from various Caenorhabditis species. Gray indicates the invariant core residues mutated in the ACA variant tested in D,E. Asterisks indicate fem-3(gf) (gain-of-function allele) point mutations. (B) Masculinization of germ line by Cbr-gld-1(RNAi);Cbr-puf-2/1.2(RNAi). Hoechst staining reveals spermatocytes (sc) at the gonad arm bend and highly condensed sperm (sp) nuclei at the proximal end of the gonad. Asterisk marks the distal tip of the gonad. (C) Cbr-GLD-1 level is significantly higher in Cbr-puf-2/1.2(RNAi) than in wild-type L4 worms, whereas the Cbr-gld-1 mRNA level is not. Error bars indicate s.e.; P=0.006 and P=0.168 for protein level and mRNA level, respectively (unpaired Student’s t-test). (D) Yeast three-hybrid interactions among C. elegans and C. briggsae PUF proteins and gld-1 mRNA variants. RNA plasmid pIIIa serves as the vector control. Error bars indicate s.e.m. (E) Cbr-PUF-2 and Cbr-PUF-1.2 bind to the putative Cbr-gld-1 FBE in vitro in a UGU-dependent manner. Black arrowheads indicate retarded complexes between labeled RNA oligomers and pure PUF proteins, and white arrowheads indicate free RNA oligomers.
RESULTS
Caenorhabditis PUF family phylogeny reveals an ancient subfamily structure
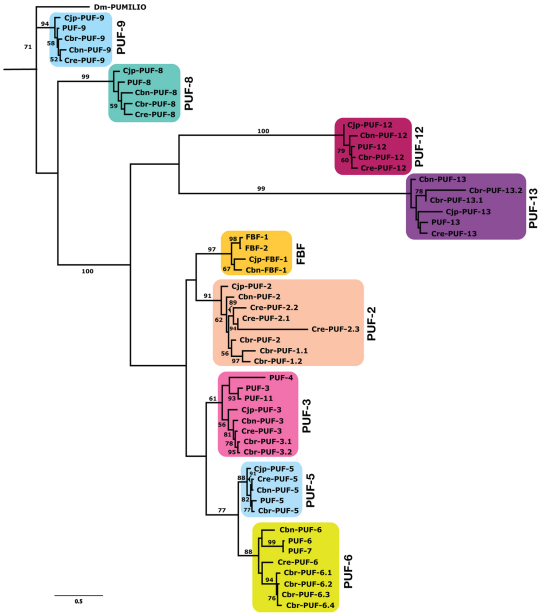
Preliminary experiments with fbf-related C. briggsae PUF homologs defined by Lamont et al. (Lamont et al., 2004) suggested they were required for XX sperm production (S. Feng, Q.L. and E.S.H., unpublished), the opposite role of C. elegans fbf-1, fbf-2 and puf-8 (Bachorik and Kimble, 2005; Zhang et al., 1997). To guide more precise experiments, we produced an expanded PUF phylogeny using all homologs from five sequenced Caenorhabditis. The most likely tree (Fig. 1) divides the PUF family into nine monophyletic subfamilies, two of which, PUF-12 and PUF-13, are newly defined here. The previously described C. elegans puf-10 is a pseudogene with stop codons throughout its former coding region and highly divergent sequence, and thus does not appear in Fig. 1. Relative to the two-species analysis of Lamont et al. (Lamont et al., 2004), one C. elegans gene and three C. briggsae genes are added. The PUF-9 subfamily is basal, with highly conserved orthologs in all sequenced species. The remaining eight subfamilies represent a more recent radiation, yet all but one has an ortholog in C. japonica, the outgroup to the other species (Cho et al., 2004; Kiontke et al., 2004). At least eight subfamilies were therefore present in the Caenorhabditis ancestor, and a more complete genome assembly for C. japonica might reveal additional PUF family genes.
Fig. 1.
PUF family phylogeny for five Caenorhabditis species. Maximum likelihood tree based on the PUF domain and conserved flanking regions. Bootstrap support is given for internal branches. See supplementary material Table S1 for WormBase gene numbers and nomenclature scheme.
Importantly for this study, C. elegans FBF proteins and C. briggsae PUF-2 proteins belong to two distinct clades. Moreover, C. elegans lacks a PUF-2 subfamily member, and C. briggsae lacks an FBF subfamily ortholog. FBF and PUF-2 subfamilies are marginally supported as sister groups. What is more certain is that both belong to a well-supported superclade of seven PUF subfamilies, two of which (PUF-5 and PUF-6/7) are closely related and share a binding preference distinct from that of FBF (and likely PUF-2) subfamilies (Stumpf et al., 2008a). Thus, the C. elegans and C. briggsae genes compared below are not orthologous but belong to subfamilies that are relatively closely related.
Opposite functions of PUF homologs in convergent hermaphrodites
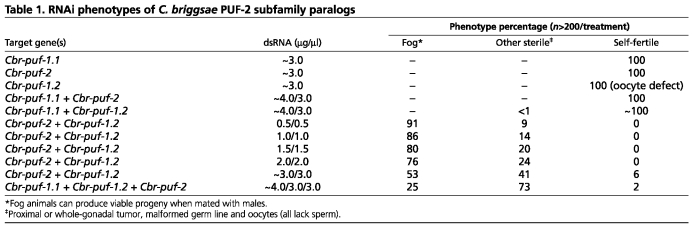
Because PUF-2 orthologs are absent from C. elegans their specific functions in C. briggsae are not readily predicted. Therefore, gene-specific knockdown of Cbr-puf-1.1, Cbr-puf-1.2 and Cbr-puf-2 was performed separately and in various combinations (Table 1). Cbr-puf-2(RNAi) alone had little effect, but simultaneous knockdown of Cbr-puf-2 and Cbr-puf-1.2 (but not other combinations) led to a strongly feminized germ line (Fig. 2B). Cbr-puf-2/1.2(RNAi) females had normal size germ lines and could mate and produce viable progeny. Cbr-puf-2/1.2(RNAi) males were overtly normal and could sire viable progeny (not shown). Thus, Cbr-puf-2 and Cbr-puf-1.2 act synthetically and specifically to promote spermatogenesis in C. briggsae hermaphrodites, but not in males. This contrasts with the role of FBF genes and puf-8 in C. elegans hermaphrodites, where they promote oogenesis (Bachorik and Kimble, 2005; Zhang et al., 1997).
Table 1.
RNAi phenotypes of C. briggsae PUF-2 subfamily paralogs
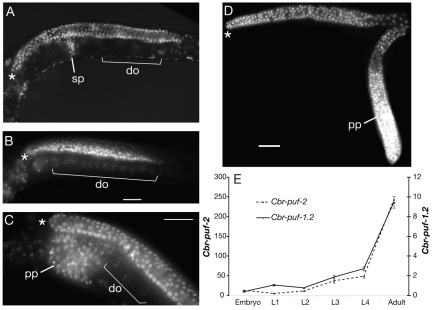
Fig. 2.
Expression and germline phenotypes of Cbr-puf-2/1.2(RNAi). (A) Wild-type C. briggsae adult hermaphrodite, stained with Hoechst 33258 to visualize DNA. Asterisk marks the distal tip of the gonad. (B) XX Cbr-puf-2/1.2(RNAi) Fog phenotype, commonly seen in low-dose RNAi, revealed by Hoechst staining. (C,D) Proximal proliferation of germline (Pro) phenotype in XX Cbr-puf-2/1.2(RNAi) animals. Tumors were observed proximal to either small populations of well-differentiated diakinesis oocytes (C) or undifferentiated germ cells (D). (E) Developmental profile of Cbr-puf-1.2 and Cbr-puf-2 mRNA levels assessed by quantitative RT-PCR. Expression levels were normalized to total actin expression and scaled (unit for Cbr-puf-2, 10–3; for Cbr-puf-1.2, 10–4). Error bars indicate s.e.m. for three biological replicates. sp, sperm, do, diakinesis oocytes; pp, proximal proliferation. Scale bars: 30 μm.
Cbr-puf-2 and Cbr-puf-1.2 also function in non-sexual aspects of germline development (Table 1). A minority of Cbr-puf-2/1.2(RNAi) worms had proximal germ cell tumors at low concentrations (0.5 μg/μl) of dsRNA (Fig. 2C,D). When the concentration of dsRNA was increased to 3.0 μg/μl, the percentage of Fog (feminization of germ line) animals decreased and more proximal tumors were observed (Table 1). In tumorous gonads, proximal overproliferated cells were followed distally by oogenic cells at various meiotic stages or abnormal pachytene cells. This oogenic region is often small and located at the bend of the gonad arm, which can be easily missed in whole-mounts. This tumor phenotype indicates that Cbr-puf-2 and Cbr-puf-1.2 are involved in the control of meiotic progression and/or the prevention of the return to mitosis. In addition, Cbr-puf-1.2(RNAi) worms produced fewer and atypically small oocytes, which indicates that Cbr-puf-1.2 is involved non-redundantly in oocyte development.
The developmental profiles of Cbr-puf-2 and Cbr-puf-1.2 mRNA levels (Fig. 2E) are qualitatively similar and are typical of germ line-expressed genes: low expression from embryo to L2 stages, slightly increasing expression at L3 and L4, and peak levels in adults. However, Cbr-puf-2 is over 100-fold more abundant than Cbr-puf-1.2, the transcripts of which are in the order of 10–5 times less abundant (body-wide) than those of total actins.
Cbr-puf-2 mutant reveals pleiotropic roles in embryogenesis and larval somatic development
To study the function of Cbr-puf-2 further, two deletion alleles were isolated. For one, a 1.9 kb genomic deletion, we failed to obtain homozygous adults and it was eventually lost. The second allele, nm66, carries a 1.7 kb genomic deletion that removes three-quarters of the coding sequence, including the entire PUF domain (Fig. 3A), and is thus a likely null allele. Again, homozygous adults could not be identified, and close inspection revealed that one-quarter of progeny from Cbr-puf-2(nm66)/+ mothers were arrested at an early larval stage (Fig. 3B), 5 days after hatching at 20°C. Genotyping of arrested larvae confirmed that they were nm66 homozygotes.
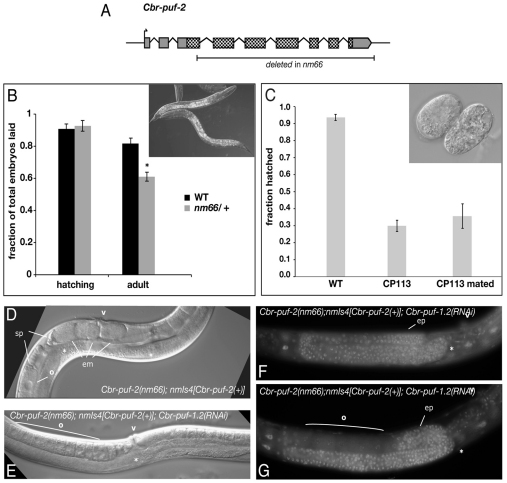
Fig. 3.
Pleiotropic functions of Cbr-puf-2 in embryogenesis and larval growth. (A) Structure of Cbr-puf-2 and extent of deletion in allele nm66. Rectangles represent exons, with coding sequence for the conserved PUF domain and flanking regions stippled, and other coding sequences in gray. *, P=0.003 (two-tailed t-test), for the fraction of embryos from wild-type versus nm66/+ mothers that reach adulthood. (B) Cbr-puf-2(nm66) embryos hatch normally but arrest as larvae. Progeny that reach adulthood were significantly fewer in number from nm66/+ mothers than from wild-type (WT) AF16 mothers (P=0.003); nm66 adults were never observed. Inset shows arrested nm66 larvae. (C) Maternally deposited Cbr-puf-2 promotes embryonic development. CP113 animals hatched at lower rates than AF16 (P<0.0001), and mating with AF16 males failed to rescue lethality (P=0.0002). Inset shows representative dead embryos. Error bars indicate s.e.m. (D) Cbr-puf-2(nm66) animals harboring a Cbr-puf-2(+) transgene (strain CP113) grow into fertile adults. (E-G) Low concentrations of Cbr-puf-1.2 dsRNA produce Fog animals (E), whereas higher doses produce tumors (F,G). Asterisk indicates the distal tip of the gonad arm. sp, sperm; v, vulva; em, embryos; o, oocytes; ep, ectopically proliferative germline tumor.
To confirm that loss of Cbr-puf-2 function causes the larval arrest phenotype in nm66, we introduced a wild-type Cbr-puf-2 transgene into nm66 mutants. This was sufficient to allow nm66 homozygotes to develop into fertile adults (Fig. 3D). The rescued strain, CP113, nevertheless had undetectably low Cbr-puf-2 mRNA levels as measured by RT-PCR. Since germline transgene silencing is a known phenomenon in C. elegans (Seydoux and Schedl, 2001), we hypothesized that CP113 was a somatic-rescued but germline-null Cbr-puf-2 mutant.
In a wild-type genetic background, both Cbr-puf-2 and Cbr-puf-1.2 must be knocked down to feminize the germ line and produce tumors (Fig. 2B). In CP113, however, Cbr-puf-1.2(RNAi) alone produced the Fog phenotype (Fig. 3E), and at high doses of Cbr-puf-1.2 dsRNA germline tumors became common (Fig. 3F,G). These results are consistent with germline silencing of the Cbr-puf-2 transgene in the CP113 strain. This also suggests that very low levels of Cbr-puf-2 expression are sufficient in somatic tissues to allow progression from larval stages to adulthood. This could also explain the observation that Cbr-puf-2(RNAi) animals did not undergo larval arrest.
XX CP113 animals also had subtle germline defects. Although they were overtly normal and fertile, they had delayed gamete maturation. Newly molted adult CP113 animals had very few yolky oocytes, with spermatocytes just beginning to differentiate. AF16 animals at this stage generally have fully differentiated sperm, and oocytes fill the proximal gonad arms. Also, ∼70% of CP113 eggs died at various embryonic stages (Fig. 3G), and this embryonic lethal phenotype could not be rescued by a paternal copy of Cbr-puf-2(+). We interpret this to be a maternal effect of nm66 caused by lack of Cbr-puf-2 activity in the maternal germ line.
Cbr-PUF-2 and Cbr-PUF-1.2 directly repress Cbr-gld-1 mRNA to promote spermatogenesis
In C. elegans, FBF-1 and FBF-2 directly regulate gld-1 and fem-3 mRNA translation via FBF binding elements (FBEs) in their 3′UTRs (Crittenden et al., 2002; Suh et al., 2009; Zhang et al., 1997). The binding elements contain a ‘core’ central region (CGUGUAUUAUA, invariable nucleotides underlined) and flanking sequences, and the core is distinct from that of other PUF proteins (Bernstein et al., 2005). The 3′UTR of Cbr-gld-1 bears a 15 nt stretch that is nearly identical to the C. elegans gld-1 FBE (Fig. 4A). Moreover, loss of Cbr-gld-1 function masculinizes the germ line (Beadell et al., 2011; Nayak et al., 2005), suggesting that its normal function is to promote oogenesis. We hypothesized that Cbr-gld-1 might be hyperactive in Cbr-puf-2/1.2(RNAi) animals and would thus completely repress hermaphrodite spermatogenesis.
We investigated the epistatic relationship of Cbr-gld-1 and Cbr-puf-2/1.2 through triple RNAi knockdown. A preliminary experiment was conducted to demonstrate the efficacy of Cbr-puf-2/1.2(RNAi) in a triple knockdown. A myo-2::gfp transgenic strain injected with a mixture of Cbr-puf-2/1.2 and gfp dsRNA had a feminized germ line with compromised pharyngeal GFP expression (data not shown). XX Cbr-gld-1(RNAi);Cbr-puf-2/1.2(RNAi) adults had masculinized germ lines (Fig. 4B), indicating that sperm production (to excess) in Cbr-puf-2/1.2 is restored when Cbr-gld-1 function is reduced. Also consistent with repression of Cbr-gld-1 by Cbr-PUF-2/1.2, Cbr-GLD-1 protein levels at the late L4 stage (when wild-type worms are at their peak of sperm production) in Cbr-puf-2/1.2(RNAi) worms were approximately double those in wild type (Fig. 4C), a statistically significant result (P=0.006, unpaired Student’s t-test). By contrast, there was no significant difference in Cbr-gld-1 transcript levels in the two treatments (Fig. 4C; P=0.168) at this stage. These results are consistent with Cbr-PUF-2/1.2 acting at the level of translation to promote spermatogenesis via direct repression of Cbr-GLD-1 expression.
Binding of Cbr-PUF-2/1.2 to the candidate FBE in the Cbr-gld-1 3′UTR was first measured using the yeast three-hybrid assay, in which interaction of an RBP activation domain fusion protein with a ‘bait’ RNA leads to activation of a reporter (Bernstein et al., 2002). Reporter activity was much higher with wild-type than with mutated versions of Cbr-gld-1 FBE bait RNA (Fig. 4D). C. elegans FBF-2 also interacted strongly and in an FBE-dependent manner with the Cbr-gld-1 bait RNA. To verify that the interactions between the Cbr-gld-1 FBE and the Cbr-PUF-2 and Cbr-PUF-1.2 proteins were direct, we used synthetic oligoribonucleotides encoding the candidate FBE and purified proteins in gel mobility shift assays. Both Cbr-PUF-2 and Cbr-PUF-1.2 bound with high affinity to the Cbr-gld-1 FBE (Fig. 4E), and this interaction required the UGU motif that is essential for FBE binding by FBF in C. elegans (Bernstein et al., 2005).
The above assays indicate that Cbr-PUF-2 and Cbr-PUF-1.2 interact with the Cbr-gld-1 FBE directly and with properties similar to those of the FBF subfamily. The C. briggsae fem-3 3′UTR also possesses a well-conserved FBF-like binding site termed the point mutation element (PME) (Haag et al., 2002). In yeast three-hybrid assays, Cbr-PUF-1.2 and Cbr-PUF-2 interacted specifically with PME-containing fragments from C. elegans and C. briggsae fem-3, and C. elegans FBF interacted with C. briggsae and C. elegans fem-3 PME fragments to a similar extent (supplementary material Fig. S1). This suggests that both PUF-2 and FBF PUF subfamilies can recognize a similar RNA motif conserved in fem-3 orthologs, but the biological significance of a Cbr-PUF–Cbr-fem-3 mRNA interaction was initially unclear (see below).
Nonlinear interactions between Cbr-puf-2/1.2 and the core sex determination pathway
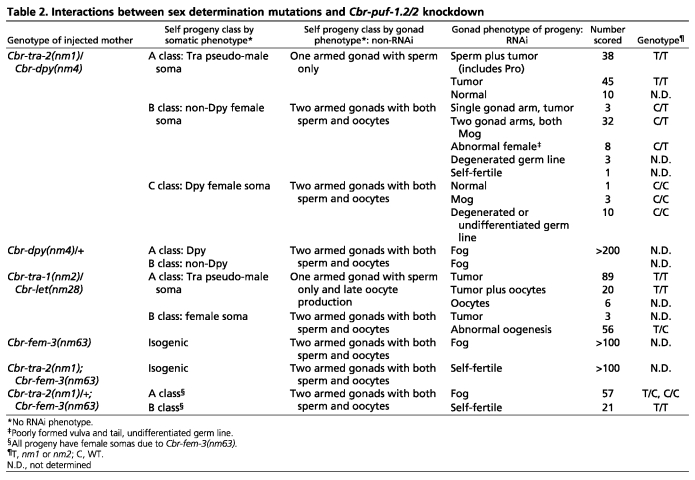
In an effort to place Cbr-puf-2/1.2 activity in the sex determination pathway, we performed approximations of epistasis tests by combining Cbr-puf-2/1.2(RNAi) with tra (masculinizing) mutants (Table 2). XX Cbr-tra-2(nm1) homozygotes develop imperfect male bodies and produce only sperm (Kelleher et al., 2008), whereas heterozygotes are normal hermaphrodites. All XX Cbr-tra-2(nm1);Cbr-puf-2/1.2(RNAi) animals developed male somas, but roughly half of these had tumorous germ lines lacking differentiated gametes and half produced sperm proximal to a tumor (Fig. 5C). None had obvious oocytes. Using the Cbr-dpy(nm4) marker closely linked to Cbr-tra-2 in trans, Cbr-tra-2(nm1)/+ and Cbr-tra-2(+/+) could also be scored reliably. Surprisingly, most Cbr-tra-2(nm1)/+;Cbr-puf-2/1.2(RNAi) animals (Table 3) had two gonads full of sperm with no sign of oogenesis (Fig. 5D). Genotyping confirmed that these female soma/Mog (masculinization of germ line) animals were indeed Cbr-tra-2(nm1)/+. Since Cbr-tra-2 germline masculinization is normally recessive and Cbr-puf-2/1.2(RNAi) has a feminizing effect, the masculinization of this combination is unexpected.
Table 2.
Interactions between sex determination mutations and Cbr-puf-1.2/2 knockdown
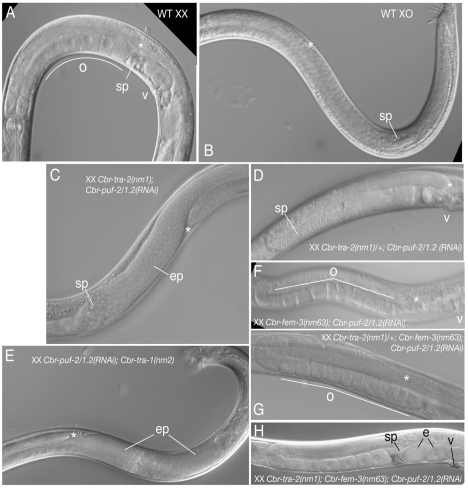
Fig. 5.
Interaction between Cbr-puf-2/1.2 knockdown and masculinizing tra mutations. (A) Wild-type XX C. briggsae hermaphrodite gonad arm (anterior), showing mature oocytes (o), sperm (sp) in the spermatheca, and vulva (v). The distal tip of the reflexed arm is marked with an asterisk. (B) Wild-type C. briggsae XO male testis (labeled as in A). (C) Cbr-tra-2(nm1);Cbr-puf-2/1.2(RNAi) XX animals develop male soma, half of which have sperm proximal to an ectopically proliferative (ep) germline tumor. (D) Cbr-tra-2(nm1)/+;Cbr-puf-2/1.2(RNAi) animals have two gonads full of sperm with no sign of oogenesis. (E) Cbr-puf-2/1.2(RNAi);Cbr-tra-1(nm2) XX animals have male soma. Among them, 77% developed a tumorous germ line without apparent gametogenesis. (F) Cbr-puf-2/1.2(RNAi);Cbr-fem-3(nm63) animals are Fog. (G) Cbr-tra-2(nm1)/+;Cbr-puf-2/1.2(RNAi);Cbr-fem-3(nm63) are Fog. (H) Cbr-tra-2(nm1);Cbr-puf-2/1.2(RNAi);Cbr-fem-3(nm63) animals are self-fertile hermaphrodites that produce embryos (e).
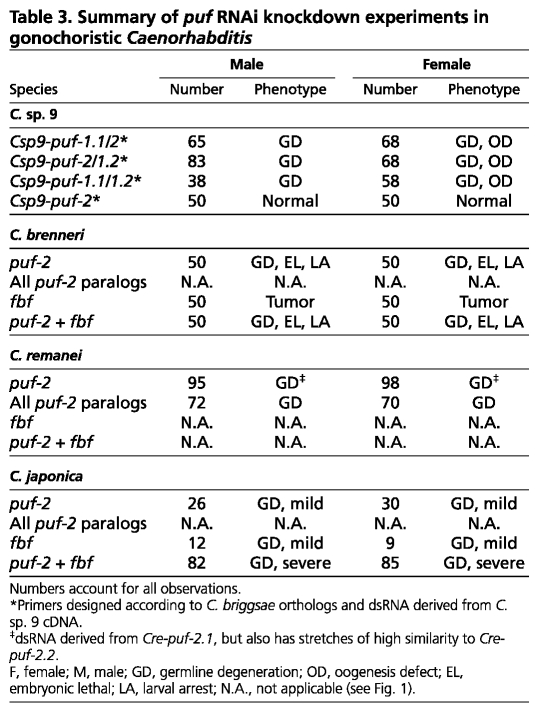
Table 3.
Summary of puf RNAi knockdown experiments in gonochoristic Caenorhabditis
Also unexpected was the lack of differentiated gametes seen in the Dpy progeny with two wild-type zygotic copies of Cbr-tra-2. To control for possible effects of the Cbr-dpy(nm4) marker, Cbr-dpy(nm4)/+ mothers lacking any Cbr-tra-2 mutation were injected with Cbr-puf-2/1.2 dsRNA. Here, all selfed progeny, including Dpy homozygotes, were Fog. Therefore, the Mog phenotype of Cbr-tra-2(nm1)/+;Cbr-puf-2/1.2(RNAi) animals requires a maternal nm1 allele, and the poorly differentiated germ line of their Cbr-dpy(nm4);Cbr-puf-2/1.2(RNAi) siblings is a dominant maternal effect of the Cbr-tra-2(nm1) mutation. Another Cbr-tra-2 allele, nm9ts (Kelleher et al., 2008), produced the same result, suggesting that the interaction between Cbr-tra-2 and Cbr-puf-2/1.2 is general.
The strong loss-of-function mutation Cbr-tra-1(nm2) causes XX animals to develop a male body and a mixture of sperm and endomitotic oocytes (Hill and Haag, 2009; Kelleher et al., 2008). Similar to Cbr-tra-2(nm1);Cbr-puf-2/1.2(RNAi), all Cbr-tra-1(nm2);Cbr-puf-2/1.2(RNAi) XX animals had a fully male soma, consistent with Cbr-puf-2/1.2 acting to determine sex exclusively in germ cells. Seventy-seven percent developed germline tumors without apparent gametogenesis (Fig. 5E), 17% had differentiated oocytes distal to tumorous germ cells, and the remainder had only oocytes with an otherwise normal germ line (Table 2). Suppression of the abundant sperm development characteristic of Cbr-tra-1(nm2) by Cbr-puf-2/1.2(RNAi) is surprising because wild-type XO males show no such defect.
We also examined interactions between Cbr-puf-2/1.2(RNAi) and the likely null Cbr-fem-3 mutant nm63, which on its own has no effect on XX hermaphrodites but sex-reverses XO animals (Hill et al., 2006). XX Cbr-puf-2/1.2(RNAi);Cbr-fem-3(nm63) animals are Fog (Fig. 5F), suggesting that Cbr-puf-2/1.2 and Cbr-fem-3 do not have obvious genetic interaction. To further test a simple linear model, we reduced Cbr-tra-2 levels via the nm1 mutation with the expectation that, in the absence of Cbr-fem-3, loss of all or part of Cbr-tra-2 activity would have no effect. However, whereas all Cbr-tra-2(nm1)/+;Cbr-puf-2/1.2(RNAi);Cbr-fem-3(nm63) animals were Fog (Fig. 5G, Table 2), homozygosity for Cbr-tra-2(nm1) restored self-fertility to the otherwise Fog Cbr-puf-2/1.2(RNAi);Cbr-fem-3(nm63) animals (Fig. 5H). Thus, the germline sex determination activity of Cbr-puf-2/1.2 is sensitive to Cbr-tra-2 dose even in the absence of Cbr-fem-3, which is inconsistent with a linear epistasis model for gene activity.
Functions of puf-2 and fbf orthologs in gonochoristic Caenorhabditis
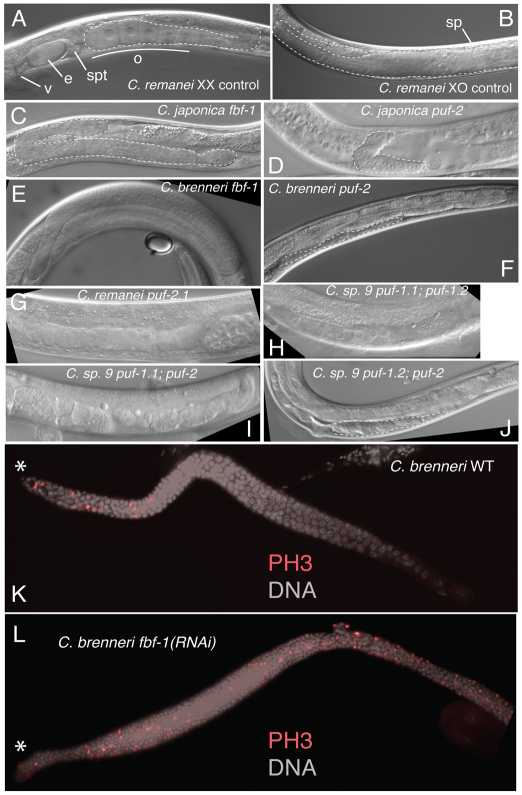
But for the production of sperm, females of gonochoristic Caenorhabditis are very similar to C. elegans and C. briggsae hermaphrodites, and males are anatomically identical. We therefore sought to clarify the evolutionary history of FBF and PUF-2 subfamily gene function in germline sex determination. RNAi knockdown by direct injection of dsRNA into the germ line is efficient in a range of Caenorhabditis species (Winston et al., 2007), so we applied this to the gonochoristic C. brenneri, C. remanei, C. japonica and C. sp. 9 (Table 3). In nearly every case, puf RNAi caused pronounced germline underproliferation, ranging from fewer germ cells than usual to complete loss (Fig. 6C-J). A notable exception, however, was knockdown of C. brenneri fbf-1. In this case, germ cells appeared to exit meiosis and re-enter the mitotic cell cycle, producing a germ cell tumor (Fig. 6E,L). The phenotype is reminiscent of loss of gld-1 function in both C. elegans (Francis et al., 1995a) and C. briggsae (Beadell et al., 2011; Nayak et al., 2005). However, straightforward germline sex determination phenotypes were not observed in either XX or XO animals.
Fig. 6.
PUF family knockdown in gonochoristic Caenorhabditis. (A,B) Untreated adult C. remanei female and male, with germ lines outlined. (C-J) RNAi directed against the C. japonica, C. brenneri, C. remanei and C. sp. 9 genes indicated. RNAi of PUF homologs generally produced a germline underproliferation (C,D,F,J) or abnormal germline degeneration (G-I) phenotype. By contrast, C. brenneri fbf-1(RNAi) produced a germ cell tumor (E). (K,L) Merged fluorescent images of DNA (gray) and phospho-histone 3 (PH3, red) staining of extruded XX C. brenneri gonads from untreated (K) or Cbn-fbf-1(RNAi) (L) animals. Mitotic nuclei are localized to the distal stem cell niche (asterisk) in wild-type females (K), but distributed throughout the gonad in Cbn-fbf-1(RNAi) (L). e, embryo; o, oocytes, spt, spermatheca; v, vulva.
DISCUSSION
Additional taxa clarify the size and evolutionary history of the Caenorhabditis PUF family
Since Lamont et al. (Lamont et al., 2004) produced the first phylogeny for C. elegans and C. briggsae PUF gene family members, the genomes of three gonochoristic Caenorhabditis (C. remanei, C. brenneri and C. japonica) have been sequenced and annotated (and others now have preliminary assemblies). Searches of all five genomes revealed two PUF protein families not present in this earlier analysis: PUF-12 and PUF-13. The functions of these two newly added PUF subfamilies are unknown. Phylogenetic reconstruction unambiguously groups PUF proteins into nine distinct subfamilies and shows that C. elegans FBF and Cbr-PUF-1.1/1.2/2 are members of different subfamilies that existed prior to the divergence of C. japonica from the Elegans group species. Nevertheless, the FBF and PUF-2 subfamilies retain common RNA-binding site preferences and roles in regulating germline proliferation. Their most striking difference, which is in hermaphrodite germline sexual patterning, evolved as the C. elegans and C. briggsae lineages adopted self-fertility (Fig. 7A).
Fig. 7.
Models of FBF and PUF-2 subfamily evolution. (A) Cladogram of Elegans group Caenorhabditis [based on published data (Kiontke et al., 2004; Woodruff et al., 2010)] and summaries of knockdown phenotypes for FBF and PUF-2/1.2 subfamilies from this study: GD, germline degeneration; Mog, masculinization of germ line; Fog, feminization of germ line. Because of lineage-specific subfamily loss, some species-subfamily combinations have no data. (B) Genetic model for regulatory interactions between fbf, puf-2 and other sex determination factors in the hermaphrodite germ line of C. elegans and C. briggsae. The weight of the repression bars downstream of fbf and Cbr-puf-2/1.2 is indicative of the relative significance of the interaction for sex determination. Note that C. elegans gld-1 promotes spermatogenesis by directly regulating tra-2 (Jan et al., 1999), but this is not shown here.
The sex determination function of Cbr-puf-2/1.2 is mediated by a conserved PUF–gld-1 interaction
The PUF and GLD-1 RBPs are pleiotropic regulators with complex interactions with other factors. In C. elegans, FBF-1/2 regulate germ cell sexual fate (Zhang et al., 1997) and the entry into meiosis (Crittenden et al., 2002; Lamont et al., 2004) through repression of hundreds of target mRNAs (Kershner and Kimble, 2010). In addition, in the soma FBF-1 can act as a positive regulator of target gene expression (Kaye et al., 2009). GLD-1 is also a translational repressor (Jan et al., 1999) with many target mRNAs (Wright et al., 2010) and roles in both sex determination and meiotic progression (Francis et al., 1995b). gld-1 is itself both positively and negatively regulated at the mRNA (Crittenden et al., 2002; Suh et al., 2009; Suh et al., 2006) and protein (Clifford et al., 2000; Jeong et al., 2010) levels. Further, in a sensitized background C. elegans gld-1 mutations can have an unexpected strong masculinizing effect (Kim et al., 2009), and FBF associates with molecular complexes that have both repressive and stimulatory effects on gld-1 expression (Suh et al., 2009). These complexities suggest a number of ways that a PUF–gld-1 regulatory linkage could be modified such that homologous PUF mutants have opposite sexual phenotypes. However, in this study we tested a simple hypothesis based on three initial observations: (1) gld-1 is repressed by FBF in C. elegans (Crittenden et al., 2002; Suh et al., 2009); (2) the FBF and PUF-2 subfamilies are related (Fig. 1); and (3) the sexual transformations of both gld-1 orthologs and fbf/puf-2 PUF genes (Fig. 2) are opposite in C. elegans (Clifford et al., 2000; Francis et al., 1995a; Francis et al., 1995b; Goodwin et al., 1993; Jan et al., 1999) and C. briggsae (Beadell et al., 2011; Nayak et al., 2005).
We hypothesized that loss of FBF and PUF-2 family members in C. elegans and C. briggsae, respectively, have opposite effects on germline sex primarily because a conserved, negatively regulated target mRNA, gld-1, has itself adopted opposite sexual roles.
We have presented several lines of evidence indicating that Cbr-GLD-1 expression is indeed repressed directly by Cbr-PUF-2/1.2. First, the conserved Cbr-gld-1 FBE can be specifically bound in vitro by Cbr-PUF-2, Cbr-PUF-1.2 and C. elegans FBF-2. In yeast, fem-3 FBEs also interact with FBF-2, Cbr-PUF-2 and Cbr-PUF-1.2 (supplementary material Fig. S1). Thus, the FBF and PUF-2 subfamilies have similar RNA binding properties. Secondly, reduced Cbr-puf-2/1.2 function elevates Cbr-GLD-1 levels at the stage when spermatogenesis normally occurs. Although it is possible that this effect is indirect, the simplest interpretation is that Cbr-gld-1 translation is increased. Finally, Cbr-gld-1(RNAi) suppression of Cbr-puf-2/1.2(RNAi) feminization is consistent with GLD-1 overexpression being the chief mechanism by which Cbr-puf-2/1.2(RNAi) feminizes the hermaphrodite germ line.
Independent recruitment of a PUF–gld-1 regulatory module during evolution of hermaphroditism
PUF-2 and FBF subfamily gene knockdowns (Fig. 6) revealed defects in proliferation control, but not in sex determination, in gonochoristic Caenorhabditis, whereas both C. elegans and C. briggsae show strong masculinization or feminization, respectively. This could suggest the independent co-option of PUF proteins into C. elegans and C. briggsae hermaphroditic germline patterning. However, we have recently described complementary changes in gld-1 function in the same species (Beadell et al., 2011). Specifically, the C. elegans tra-2 3′UTR evolved to support an unusually strong in vivo association with GLD-1 that is required for XX spermatogenesis. By contrast, C. briggsae gld-1 evolved to limit XX sperm production through regulation of Cbr-puf-8. These changes in the targets of gld-1, when combined with the existence of a conserved PUF–gld-1 module described here, are largely sufficient to explain the differences in C. elegans fbf and C. briggsae puf-2 phenotypes (Fig. 7).
The repeated recruitment of PUF and gld-1 (Beadell et al., 2011) homologs into hermaphroditic germline sex determination might reflect the general reliance of germline development on post-transcriptional gene regulation (Leatherman and Jongens, 2003), especially via mRNA 3′ UTRs (Merritt et al., 2008). PUF proteins are pleiotropic germline mRNA-binding proteins (Ariz et al., 2009; Lublin and Evans, 2007; Subramaniam and Seydoux, 2003; Wickens et al., 2002), and are thus a priori on a short list of candidates for mediating germline sex determination. Also, germline sex determination has spatial and temporal overlap with events regulating germline meiotic entry and gamete differentiation, which pre-date the origins of self-fertility. This overlap might increase the probability of recruiting genes regulating these events into hermaphrodite patterning. Consistent with this, the 3′ UTR motif that allows C. elegans FBF repression of gld-1 mRNA to promote germ cell proliferation (Crittenden et al., 2002) is conserved among all sequenced Caenorhabditis species, hermaphroditic or otherwise (Fig. 4A).
Taken together, it is likely that the last common ancestor of the FBF and PUF-2 subfamilies repressed gld-1 translation in the service of regulating germline proliferation. Extant Caenorhabditis species have then modified this situation by losing one or other subfamily entirely (but never both) and duplicating genes within a given subfamily. Layered upon this is the co-option of the entire PUF–gld-1 module into hermaphrodite development. Although this occurred in both characterized selfing species (and might be true of others), the exact role of the module is variable and dependent upon the overall context in which it occurs.
Computer simulations of evolving, unconstrained genetic networks show that participation of genes in multiple traits leads to modular regulation, and that pre-existing modules have a tendency to be utilized as raw materials for subsequent evolutionary innovation (Espinosa-Soto and Wagner, 2011). The multiple developmental functions of PUF family genes and gld-1 (Ariz et al., 2009; Crittenden et al., 2002; Francis et al., 1995a; Jeong et al., 2010; Lublin and Evans, 2007; Subramaniam and Seydoux, 2003; Wickens et al., 2002) might therefore promote their continued regulatory linkage in the face of altered germline phenotypes.
Evolution of genetic interactions between PUF targets
C. elegans fbf-1/2 hypomorphs or mutants are Mog (Zhang et al., 1997) because of fem-3 hyperactivity (Ahringer and Kimble, 1991; Zhang et al., 1997). C. elegans GLD-1 is also hyperactive when fbf-1/2 activity is reduced (Crittenden et al., 2002; Jones et al., 1996), which might synergize with excess FEM-3 to reinforce male fate. In C. briggsae, conservation of the fem-3 PME (Haag and Kimble, 2000) and its interaction with Cbr-PUF-2/1.2 (supplementary material Fig. S1) suggest simultaneous upregulation of Cbr-GLD-1 and Cbr-FEM-3 might also occur when Cbr-puf-2/1.2 activity is reduced. If so, why would the GLD-1 side dominate phenotypically in C. briggsae? fem-3 plays a different germline role in the two species (Hill et al., 2006), so regulation of Cbr-fem-3 by Cbr-puf-2/1.2 could be inconsequential with respect to hermaphrodite sex determination. However, the genetic interactions between Cbr-puf-2/1.2 and Cbr-tra-2 suggest an alternative explanation: excess Cbr-FEM-3 is masculinizing on its own, but the simultaneous hyperactivity of Cbr-GLD-1 that occurs in the Cbr-puf-2/1.2 knockdown suppresses it via a parallel pathway (Fig. 7B). Consistent with this, loss of a single copy of Cbr-tra-2, which has no effect on its own (Kelleher et al., 2008), completely masculinizes the germ line of Cbr-puf-2/1.2(RNAi) animals (Fig. 5D). We propose that reduced function of both Cbr-tra-2 and Cbr-puf-2/1.2 synergize to activate Cbr-fem-3 to the point where this dominates over the Cbr-gld-1-mediated feminizing effect of Cbr-puf-2/1.2 alone (Fig. 7B). This is an interesting example of the inherently bi-stable nature of germline sex determination, in which subtle differences in dosage cause complete sex reversal.
Pleiotropy and redundancy in the PUF family
The nine PUF subfamilies, although generally stable, show some recent duplications and loss in particular lineages. That germline feminization requires simultaneous loss of both Cbr-puf-2 and Cbr-puf-1.2 function initially suggested that these genes would be wholly redundant. However, the nm66 mutation reveals that Cbr-puf-2 is required in the maternal germ line for reliable embryogenesis, and in the larval soma it is absolutely essential for progression beyond the L2 stage. These roles were not apparent in RNAi knockdown experiments, and similar essential roles have not been reported for any C. elegans PUF family member. Whether this somatic function was ancestral but lost in C. elegans, perhaps associated with loss of the PUF-2 subfamily, or represents a gain in C. briggsae, is unclear. What is clear, however, is that not all functions of recently duplicated PUF proteins are redundant, and this might explain their evolutionary persistence (Force et al., 1999).
Dynamic functions of puf-2 and fbf orthologs in regulation of germ cell proliferation
Cbr-puf-1.2/2 also promote germ cell meiotic progression. This effect is independent of sexual fate, as it is not fully suppressed in the XO male germ line and is never suppressed in Cbr-tra-1 and Cbr-tra-2 pseudo-males. In this respect, the role of Cbr-puf-2/1.2 is distinct from that of C. elegans fbf-1/2, which promote proliferation and repress meiotic entry (Crittenden et al., 2002). With the exception of C. brenneri fbf-1, RNAi knockdown of PUF-2 and FBF subfamily genes in gonochoristic species led to germline degeneration (Fig. 6). This suggests that the ancestral function of both the PUF-2 and FBF subfamilies is the maintenance of germline proliferation and/or integrity. If so, then Cbr-puf-2/1.2 acquired a distinct tumor-suppressing role in the C. briggsae lineage, perhaps as it acquired a role in hermaphrodite sex determination. Whether these two changes were functionally linked is unclear. In addition, in C. brenneri FBF and PUF-2 subfamilies have taken on opposite roles in regulating proliferation, with the former limiting it and the latter promoting it. If they also have similar RNA binding properties, then understanding what mediates their apparently antagonistic functions will help clarify the overall logic of PUF regulation.
Evolution of gene regulation at the translational level
Cis-regulatory DNA has emerged as a common locus of genetic variation underlying novel phenotypes, presumably because this avoids deleterious pleiotropic effects (Carroll, 2008; Stern, 2000; Stern and Orgogozo, 2008). Translational control and its evolutionary dynamics are presumably important for adaptation in tissues such as the germ line, yet it has been little explored (Haag, 2009a). The in vitro PUF–gld-1 cross-species interaction described here suggests that, at the protein sequence level, Cbr-PUF-2/1.2 and FBF are interchangeable. We recently reported similar results for GLD-1 (Beadell et al., 2011). These studies provide evidence that conserved RBP-mRNA interactions might take on altered significance due to changes in the role of the target mRNA (as appears to be the case with PUF–gld-1) or to variation in RBP protein co-factors that qualitatively or quantitatively modify conserved RBP-mRNA interactions, such as FOG-2, a GLD-1 co-factor in C. elegans (Clifford et al., 2000; Nayak et al., 2005). FBF co-factors have also been reported (Kraemer et al., 1999; Suh et al., 2009). Clarification of the precise biochemical roles(s) of such co-factors is an important subject of future research.
Supplementary Material
Acknowledgments
We thank A. Beadell, G. Woodruff and M. A. Félix for sharing results and reagents prior to publication; J. Ross for strain CP105; L. Pick and A. Bely for reagents; N. Andrews for use of a luminometer; M. Cummings and C. Delwiche for phylogenetics advice; and S. Feng for assistance with preliminary experiments that motivated this work.
Footnotes
Funding
This work was supported by a research fellowship from the University of Maryland Graduate School [to Q.L.] and National Institutes of Health (NIH) grants [GM79414 to E.S.H. and GM50942 to M.W.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.070128/-/DC1
References
- Abramoff M., Magelhaes P. J., Ram S. J. (2004). Image processing with ImageJ. Biophotonics International 11, 36–42 [Google Scholar]
- Ahringer J., Kimble J. (1991). Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3′ untranslated region. Nature 349, 346–348 [DOI] [PubMed] [Google Scholar]
- Ariz M., Mainpal R., Subramaniam K. (2009). C. elegans RNA-binding proteins PUF-8 and MEX-3 function redundantly to promote germline stem cell mitosis. Dev. Biol. 326, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachorik J. L., Kimble J. (2005). Redundant control of the Caenorhabditis elegans sperm/oocyte switch by PUF-8 and FBF-1, two distinct PUF RNA-binding proteins. Proc. Natl. Acad. Sci. USA 102, 10893–10897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere A., Yang S. P., Pekarek E., Thomas C. G., Haag E. S., Ruvinsky I. (2009). Detecting heterozygosity in shotgun genome assemblies: Lessons from obligately outcrossing nematodes. Genome Res. 19, 470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadell A. V., Liu Q., Johnson D. M., Haag E. S. (2011). Independent recruitments of a translational regulator in the evolution of self-fertile nematodes. Proc. Natl. Acad. Sci. USA 108, 19672–19677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D., Hook B., Hajarnavis A., Opperman L., Wickens M. (2005). Binding specificity and mRNA targets of a C. elegans PUF protein, FBF-1. RNA 11, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D. S., Buter N., Stumpf C., Wickens M. (2002). Analyzing mRNA-protein complexes using a yeast three-hybrid system. Methods 26, 123–141 [DOI] [PubMed] [Google Scholar]
- Carroll S. B. (2008). Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36 [DOI] [PubMed] [Google Scholar]
- Chen P., Ellis R. E. (2000). TRA-1A regulates transcription of fog-3, which controls germ cell fate in C. elegans. Development 127, 3119–3129 [DOI] [PubMed] [Google Scholar]
- Chin-Sang I. D., Spence A. M. (1996). Caenorhabditis elegans sex-determining protein FEM-2 is a protein phosphatase that promotes male development and interacts directly with FEM-3. Genes Dev. 10, 2314–2325 [DOI] [PubMed] [Google Scholar]
- Cho S., Jin S. W., Cohen A., Ellis R. E. (2004). A phylogeny of Caenorhabditis reveals frequent loss of introns during nematode evolution. Genome Res. 14, 1207–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford R., Lee M., Nayak S., Ohmachi M., Giorgini F., Schedl T. (2000). FOG-2, a novel F-box-containing protein, associates with the GLD-1 RNA-binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development 127, 5265–5276 [DOI] [PubMed] [Google Scholar]
- Conradt B., Horvitz H. R. (1999). The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell 98, 317–327 [DOI] [PubMed] [Google Scholar]
- Crittenden S. L., Bernstein D. S., Bachorik J. L., Thompson B. E., Gallegos M., Petcherski A. G., Moulder G., Barstead R., Wickens M., Kimble J. (2002). A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417, 660–663 [DOI] [PubMed] [Google Scholar]
- Dawes H. E., Berlin D. S., Lapidus D. M., Nusbaum C., Davis T. L., Meyer B. J. (1999). Dosage compensation proteins targeted to X chromosomes by a determinant of hermaphrodite fate. Science 284, 1800–1804 [DOI] [PubMed] [Google Scholar]
- Eckmann C. R., Kraemer B., Wickens M., Kimble J. (2002). GLD-3, a bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev. Cell 3, 697–710 [DOI] [PubMed] [Google Scholar]
- Eddy S. R. (1998). Profile hidden Markov models. Bioinformatics 14, 755–763 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley M., D’Souza A., Moulder G., McKay S., Shen B., Gilchrist E., Moerman D., Barstead R. (2002). Improved detection of small deletions in complex pools of DNA. Nucleic Acids Res. 30, e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Soto C., Wagner A. (2011). Specialization can drive the evolution of modularity. PLoS Comput. Biol. 6, e1000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A., Lynch M., Pickett F., Amores A., Yan Y., Postlethwait J. (1999). Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151, 1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Barton M. K., Kimble J., Schedl T. (1995a). gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Maine E., Schedl T. (1995b). Analysis of the multiple roles of gld-1 in germline development: interactions with the sex determination cascade and the glp-1 signaling pathway. Genetics 139, 607–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin E. B., Okkema P. G., Evans T. C., Kimble J. (1993). Translational regulation of tra-2 by its 3′ untranslated region controls sexual identity in C. elegans. Cell 75, 329–339 [DOI] [PubMed] [Google Scholar]
- Guo Y., Lang S., Ellis R. E. (2009). Independent recruitment of F box genes to regulate hermaphrodite development during nematode evolution. Curr. Biol. 19, 1853–1860 [DOI] [PubMed] [Google Scholar]
- Haag E. S. (2009a). Chapter 3. Caenorhabditis nematodes as a model for the adaptive evolution of germ cells. Curr. Top. Dev. Biol. 86, 43–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag E. S. (2009b). Convergent evolution: regulatory lightning strikes twice. Curr. Biol. 19, R977–R979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag E. S., Kimble J. (2000). Regulatory elements required for development of Caenorhabditis elegans hermaphrodites are conserved in the tra-2 homologue of C. remanei, a male/female sister species. Genetics 155, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag E. S., Wang S., Kimble J. (2002). Rapid coevolution of the nematode sex-determining genes fem-3 and tra-2. Curr. Biol. 12, 2035–2041 [DOI] [PubMed] [Google Scholar]
- Hill R., Haag E. (2009). A sensitized genetic background reveals evolution near the terminus of the Caenorhabditis germline sex determination pathway. Evol. Dev. 4, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. C., de Carvalho C. E., Salogiannis J., Schlager B., Pilgrim D., Haag E. S. (2006). Genetic flexibility in the convergent evolution of hermaphroditism in Caenorhabditis nematodes. Dev. Cell 10, 531–538 [DOI] [PubMed] [Google Scholar]
- Hodgkin J. (1986). Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics 114, 15–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D. H., Richter D. C., Rausch C., Dezulian T., Franz M., Rupp R. (2007). Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8, 460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan E., Motzny C. K., Graves L. E., Goodwin E. B. (1999). The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 18, 258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Verheyden J. M., Kimble J. (2010). Cyclin E and Cdk2 Control GLD-1, the mitosis/meiosis decision, and germline stem cells in Caenorhabditis elegans. PLoS Genet. 7, e1001348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. R., Francis R., Schedl T. (1996). GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev. Biol. 180, 165–183 [DOI] [PubMed] [Google Scholar]
- Kaye J. A., Rose N. C., Goldsworthy B., Goga A., L’Etoile N. D. (2009). A 3′UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron 61, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher D. F., de Carvalho C. E., Doty A. V., Layton M., Cheng A. T., Mathies L. D., Pilgrim D., Haag E. S. (2008). Comparative genetics of sex determination: masculinizing mutations in Caenorhabditis briggsae. Genetics 178, 1415–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner A. M., Kimble J. (2010). Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc. Natl. Acad. Sci. USA 107, 3936–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. W., Nykamp K., Suh N., Bachorik J. L., Wang L., Kimble J. (2009). Antagonism between GLD-2 binding partners controls gamete sex. Dev. Cell 16, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K., Fitch D. (2005). The phylogenetic relationships of Caenorhabditis and other rhabditids. In WormBook (ed. the C. elegans Research Community), www.wormbook.org [DOI] [PMC free article] [PubMed]
- Kiontke K., Gavin N. P., Raynes Y., Roehrig C., Piano F., Fitch D. H. (2004). Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc. Natl. Acad. Sci. USA 101, 9003–9008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer B., Crittenden S., Gallegos M., Moulder G., Barstead R., Kimble J., Wickens M. (1999). NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr. Biol. 9, 1009–1018 [DOI] [PubMed] [Google Scholar]
- Lamont L. B., Crittenden S. L., Bernstein D., Wickens M., Kimble J. (2004). FBF-1 and FBF-2 regulate the size of the mitotic region in the C. elegans germline. Dev. Cell 7, 697–707 [DOI] [PubMed] [Google Scholar]
- Leatherman J. L., Jongens T. A. (2003). Transcriptional silencing and translational control: key features of early germline development. BioEssays 25, 326–335 [DOI] [PubMed] [Google Scholar]
- Lublin A. L., Evans T. C. (2007). The RNA-binding proteins PUF-5, PUF-6, and PUF-7 reveal multiple systems for maternal mRNA regulation during C. elegans oogenesis. Dev. Biol. 303, 635–649 [DOI] [PubMed] [Google Scholar]
- Mason D. A., Rabinowitz J. S., Portman D. S. (2008). dmd-3, a doublesex-related gene regulated by tra-1, governs sex-specific morphogenesis in C. elegans. Development 135, 2373–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra A., Gaudet J., Heck L., Kuwabara P. E., Spence A. M. (1999). Negative regulation of male development in Caenorhabditis elegans by a protein-protein interaction between TRA-2A and FEM-3. Genes Dev. 13, 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C., Rasoloson D., Ko D., Seydoux G. (2008). 3′ UTRs are the primary regulators of gene expression in the C. elegans germline. Curr. Biol. 18, 1476–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S., Goree J., Schedl T. (2005). fog-2 and the evolution of self-fertile hermaphroditism in Caenorhabditis. PLoS Biol. 3, e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigon V. (1951). Polyploidie experimentale chez un nematode libre, Rhabditis elegans Maupas. Bull. Biol. Fr. Belg. 85, 187–255 [Google Scholar]
- Powell L. A. (2007). Approximating variance of demographic parameters using the delta method: a reference for avian biologists. The Condor 109, 949–954 [Google Scholar]
- Praitis V., Casey E., Collar D., Austin J. (2001). Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C., Ruijter J. M., Deprez R. H., Moorman A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66 [DOI] [PubMed] [Google Scholar]
- Ruijter J. M., Ramakers C., Hoogaars W. M., Karlen Y., Bakker O., van den Hoff M. J., Moorman A. F. (2009). Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37, e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. (2001). Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Schedl T., Kimble J. (1988). fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119, 43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G., Schedl T. (2001). The germline in C. elegans: origins, proliferation, and silencing. Int. Rev. Cytol. 203, 139–185 [DOI] [PubMed] [Google Scholar]
- Sonnhammer E. L., Eddy S. R., Birney E., Bateman A., Durbin R. (1998). Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 26, 320–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. L. (2000). Evolutionary developmental biology and the problem of variation. Evolution 54, 1079–1091 [DOI] [PubMed] [Google Scholar]
- Stern D. L., Orgogozo V. (2008). The loci of evolution: how predictable is genetic evolution? Evolution 62, 2155–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf C. R., Kimble J., Wickens M. (2008a). A Caenorhabditis elegans PUF protein family with distinct RNA binding specificity. RNA 14, 1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf C. R., Opperman L., Wickens M. (2008b). Analysis of RNA-protein interactions using a yeast three-hybrid system. Methods Enzymol. 449, 295–315 [DOI] [PubMed] [Google Scholar]
- Subramaniam K., Seydoux G. (2003). Dedifferentiation of primary spermatocytes into germ cell tumors in C. elegans lacking the pumilio-like protein PUF-8. Curr. Biol. 13, 134–139 [DOI] [PubMed] [Google Scholar]
- Suh N., Jedamzik B., Eckmann C. R., Wickens M., Kimble J. (2006). The GLD-2 poly(A) polymerase activates gld-1 mRNA in the Caenorhabditis elegans germ line. Proc. Natl. Acad. Sci. USA 103, 15108–15112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N., Crittenden S. L., Goldstrohm A., Hook B., Thompson B., Wickens M., Kimble J. (2009). FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 181, 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D. (2002). PAUP*: Phylogenetic Analysis Using Parsimony. Sunderland, MA: Sinauer; [Google Scholar]
- Trent C., Purnell B., Gavinski S., Hageman J., Chamblin C., Wood W. B. (1991). Sex-specific transcriptional regulation of the C. elegans sex-determining gene her-1. Mech. Dev. 34, 43–55 [DOI] [PubMed] [Google Scholar]
- Wickens M., Bernstein D. S., Kimble J., Parker R. (2002). A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 18, 150–157 [DOI] [PubMed] [Google Scholar]
- Winston W. M., Sutherlin M., Wright A. J., Feinberg E. H., Hunter C. P. (2007). Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc. Natl. Acad. Sci. USA 104, 10565–10570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. (1988). Determination of pattern and fate in early embryos of Caenorhabditis elegans. Dev. Biol. 5, 57–78 [DOI] [PubMed] [Google Scholar]
- Woodruff G. C., Eke O., Baird S. E., Felix M. A., Haag E. S. (2010). Insights into species divergence and the evolution of hermaphroditism from fertile interspecies hybrids of Caenorhabditis nematodes. Genetics 186, 997–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. E., Gaidatzis D., Senften M., Farley B. M., Westhof E., Ryder S. P., Ciosk R. (2010). A quantitative RNA code for mRNA target selection by the germline fate determinant GLD-1. EMBO J. 30, 533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W., Ross J. M., Zarkower D. (2000). mab-3 is a direct tra-1 target gene regulating diverse aspects of C. elegans male sexual development and behavior. Development 127, 4469–4480 [DOI] [PubMed] [Google Scholar]
- Zhang B., Gallegos M., Puoti A., Durkin E., Fields S., Kimble J., Wickens M. P. (1997). A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390, 477–484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.