Abstract
Background:
There is an increasing incidence of Human immunodeficiency virus (HIV) and tuberculosis (TB) co-infection. This has led to an increasing number of atypical features on magnetic resonance imaging (MRI). We postulated that the type 4 hypersensitivity response causing granulomatous inflammation may be disrupted by the HIV resulting in less vertebral body destruction. This study compares the MRI features of spinal tuberculosis in HIV positive and negative patients.
Materials and Methods:
Fifty patients with confirmed spinal tuberculosis, HIV status and available MRI scans at a single institution from 2003-2009 were identified. HIV status was positive in 20 and negative in 30. Females were predominant (34:16). The HIV positive group was younger at 32.4 versus 46 years (P=0.008). Blood parameters (WCC, ESR, Hb, Lymphocyte count) were not significantly different between the HIV groups. MRI scans were reviewed by a radiologist who was blinded to the HIV status. Site, extent of disease, body collapse, abscess location and volume, kyphotic deformity and cord signal were reported.
Results:
There was no difference between the number of vertebral bodies affection with TB involvement, presence of cord signal or incidence of non-contiguous lesions. The HIV negative group had significantly more total vertebral collapse (P=0.036) and greater kyphosis (P=0.002). The HIV positive group had a trend to larger anterior epidural pus collection (P=0.2).
Conclusion:
HIV negative patients demonstrate greater tuberculous destruction in terms of total percentage body collapse and resultant kyphosis. There is no difference in the incidence of cord signal or presence of non-contiguous lesions. HIV positive patients show a trend to a greater epidural abscess volume. This difference may be explained by the reduced autoimmune response of the type 4 hypersensitivity reaction caused by the HIV infection.
Keywords: Human immunodeficiency virus, magnetic resonance imaging, spine, tuberculosis
INTRODUCTION
Since Percival Pott's description of spinal tuberculosis (TB) in 1779, preferred management continues to be debated.1,2 This has become more complex in recent years with the co-infection of Human Immunodeficiency virus infections (HIV) becoming more and more prevalent.3,4 Seventy-one percent of South African TB patients are estimated to be HIV positive.5 Skeletal TB occurs in 3-5% of patients with pulmonary tuberculosis,6 half of these involving the spine.3 In HIV positive patients the incidence of skeletal tuberculosis increases to 60%.7–9
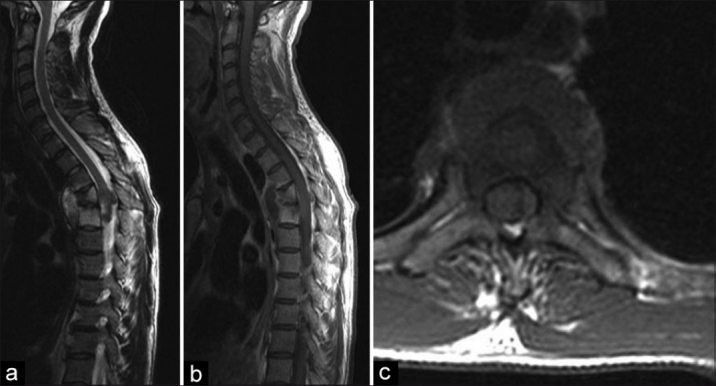
The diagnosis of spinal TB is challenging with non-specific constitutional symptoms and late presentation.2,9 Imaging plays an important role in the diagnosis and treatment decisions in these patients.3 Plain radiography remains the cornerstone of diagnosis in spinal tuberculosis; however, computerized tomography (CT) and magnetic resonance imaging (MRI) have gained popularity as special investigation methods.3,10–12 The MRI has become the gold standard for diagnosis [Figure 1a–c] and preoperative planning in spinal TB.4,7,13,14 The radiographic signs of pulmonary TB on chest radiographs have been reported to be different in HIV positive and negative patients.15,16
Figure 1.
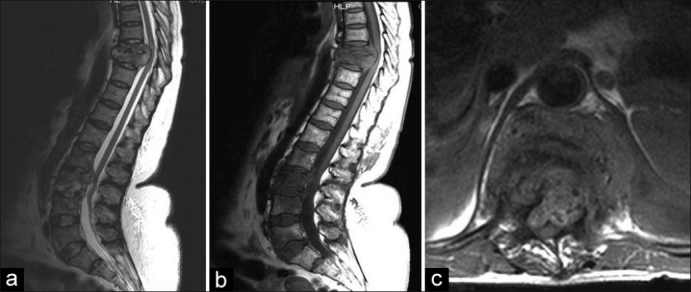
(a, b) T2W and T1W sagittal MRI of dorsolumbar spine showing destruction due to spinal TB of T8/T9 with some preservation of the disc. The importance of screening the entire spine is highlighted with the non-contiguous lesion noted at L3 and (c) T1W axial MRI of same patient showing cord compression due to an abscess
We performed a retrospective, blinded comparison of MRI's of TB spine patients to establish if there were any distinguishing features between HIV positive and negative patients.
MATERIALS AND METHODS
Spinal TB patients managed by the senior author (RD) from 2003–2009 were reviewed. Inclusion criteria for this study were spinal TB confirmed on biopsy (histology and/or culture), a documented HIV status and an available MRI scan. As a secondary review, epidemiological data including age, gender and laboratory data, including hemoglobin, white cell count (WCC) with lymphocyte count, erythrocyte sedimentation rate (ESR), albumin and cluster of differentiation 4 (CD4) count were collected. Fifty patients, 34 females and 16 males fulfilled the criteria for inclusion into the study. Twenty patients were HIV positive (15 Females, 5 Males) and thirty patients were HIV negative (19 females, 11 males). HIV positive patients where significantly younger (P=0.008) with an average age of 32.4 years (range 1.9–57.7±12.0) as opposed to HIV negative patients being 45.5 years (range 9.4-79.8±21.3).
All MRI's were reported by a radiologist, who was blinded to the HIV status of the patients. These MRI's were performed with the Siemens Symphony System (Magnetom) operated at 1.5 tesla in a supine position using spine surface coils, neck array coils and head coils as needed. Contiguous 4 mm slices were obtained in the sagittal plane in T1 sequences (TR±500-650, TE±15), and T2 sequences (TR±4200, TE±110) and in the axial plane using T2 sequences (TR±4400, TE±90-100). Contrast was not used in any of the patients. The features reported by the radiologist included anatomical site (including non-contiguous lesions), number of vertebral bodies involved, total vertebral body collapse, abscess location and volume, kyphotic deformity and cord signal.
All vertebral bodies, including non-contiguous lesions, with signs of TB infection were recorded. If a vertebral body was noted to have any signs of TB, that body was counted as one. The average was then calculated for both groups. In addition, vertebral body collapse was assessed. Each vertebral body was divided into tenths based on the first normal body above or below. The collapse was then calculated as an estimated percentage of the original vertebral body size. The percentages were then added together and an average was calculated for both groups of patients.17 Accepting the limitations of the supine position of the patient within the MRI scan, kyphosis was measured by calculating the angle of deformity along the posterior aspect of the vertebral body. The spinal cord was assessed for the presence of intensity changes on both T1 and T2 sequences.
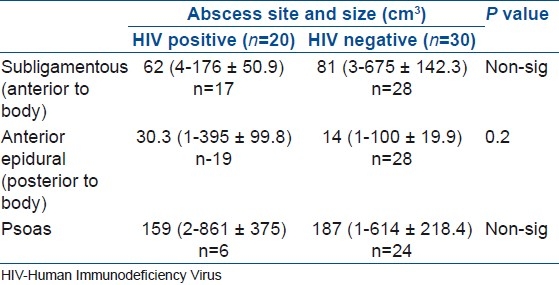
Abscesses were reviewed and classified according to three predominant locations, namely psoas, anterior epidural (posterior to vertebral body) and subligamentous (anterior to vertebral body). An abscess volume was approximated by multiplying the size on coronal, sagittal and axial views. Results were analyzed using Statistica software with Student T for continuous data and Fisher Exact Tests for categorical data. Ethical approval was obtained from the Ethics Committee.
RESULTS
There is no significant difference between HIV positive and negative patients with regards to hemoglobin, lymphocyte count, erythrocyte sedimentation rate (ESR) and albumin [Table 1]. A trend towards a lower lymphocyte count was noted in HIV positive patients (P=0.06). In the HIV negative patients, the infection was confined to the thoracic, thoracolumbar or lumbar regions in all patients. In contrast, three HIV positive patients presented with TB outside of these regions, with a case each in the cervical, cervicothoracic and lumbosacral areas. There is no statistically significant difference between the two groups with regards to the site of infection. Seven (14%) patients presented with non-contiguous lesions with no difference between HIV positive and negative patients. When assessing vertebrae for the presence of tuberculous infection, the HIV positive patients had an average of 3.5 (1-10±2.4) bodies involved as compared to 3.6 (2-10±2.0) in HIV negative patients. There is thus no statistically significant difference between the two groups with regards to the incidence of vertebral bodies involved.
Table 1.
Laboratory results comparing data between HIV positive and negative patients in the study
In contrast, the total percentage collapse in HIV negative patients [Figure 2a–c] was significantly higher (P=0.036) at 107% (0-235±56.3) compared to 75.3% (0-235±64.2) in HIV positive patients [Figure 3a–c]. This shows that on an average there is more destruction of the vertebral bodies in HIV negative patients when compared with HIV positive patients. Although a CD4 count was not available in all patients, there was no correlation with regards to the CD4 count in the patients. HIV negative patients were more kyphotic. As this was assessed on the MRI in the supine position, it should be seen as a residual local kyphosis and probably less appreciated than in the standard erect X-ray. The average kyphotic deformity in HIV positive patients was 9.5° (0-42±11.8), while the average for HIV negative patients was 19.2° (12-40±10.2) (P=0.002). There was no difference in the incidence of cord signal intensity changes between two groups. Thirteen HIV positive patients (65%) had T2 increased cord intensity as opposed to fifteen HIV negative patients (50%). No patients had cord signal changes on T1 sequences.
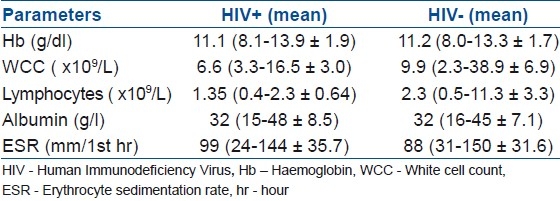
Figure 2.
(a, b) T1W and T2W sagittal MRI of dorsolumbar spine in a HIV negative patient. showing severe destruction of T11/T12 resulting in a significant kyphosis and (c) a T2W axial MRI of same patient showing an anterior subligamentous abscess
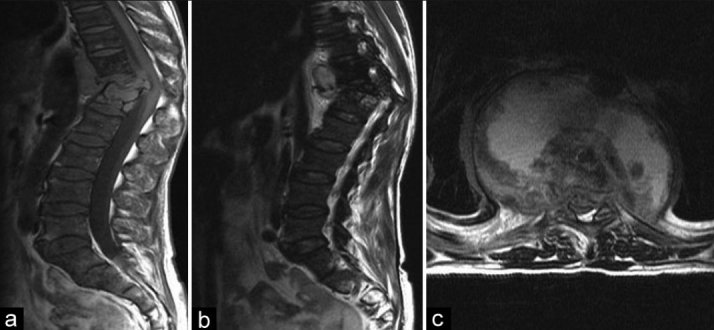
Figure 3.
(a, b) T1W and T2W sagittal MRI of spinal TB in a HIV positive patient showing minimal destruction of L3 L4 (c) T2W axial view showing spinal stenosis secondary to an abscess
The site and calculated abscess volume is demonstrated in Table 2. Overall there is no significant difference in abscess size between the two groups, although there was a trend towards increased abscess size in the epidural space in HIV positive patients.
Table 2.
Distribution and size of abscess in the human immunodeficiency virus positive and negative patients in the study
DISCUSSION
Despite the progress in modern medicine, the treatment of spinal TB remains a challenge to surgeons worldwide. Left untreated, this destructive disease has disastrous consequences with progressive deformity and eventual paralysis.13 In recent years, the increase in HIV and TB co-infection has lead to a resurgence of TB cases globally. The implications of this combination are highlighted when one considers that HIV positive patients are twelve to twenty times more susceptible to musculoskeletal TB, and therefore spinal TB, when compared with HIV negative patients.7,8,18
In 50–75% of patients exposed to TB, the bacilli are eliminated by the macrophages within the respiratory system. In the remaining 25–50%, the patients rely on the Type IV hypersensitivity (delayed type) reaction to destroy the TB bacilli following antigen presentation to the CD4+ lymphocytes by macrophages. This reaction develops within 2-4 weeks after exposure and is controlled by various the chemo- and cytokines such as Interleukin (IL)-12, Interleukin (IL)-8, interferon (IFN)-γ and Tissue necrosis factor (TNF)-α. This interaction results in the formation of a granuloma, which creates an environment in which the TB bacilli can be destroyed.19 Although the absolute number of macrophages is not decreased in HIV positive patients, their function and interaction with the CD4+ cells is negatively influenced. This coupled with the decrease in CD4+ cells leads to an impaired immune response, and thus local tissue inflammatory response to the TB bacilli in HIV positive patients.16
TB also has an effect on the HIV state by increasing activation of the immune system. This results in an increased secretion of pro-inflammatory mediators which in turn increase HIV replication with CD4 cell loss and have a negative effect on the mono cytes and lymphocytes with accelerated HIV-related disease process.8,20 Early diagnosis and prompt initiation of anti tuberculous medication remains the key to successful treatment of spinal TB.7,13,18 TB is commonly referred to as the “great mimicker” highlighting the fact that TB may have an atypical presentation. Imaging plays an important role in the diagnosis of spinal TB. Plain film radiography remains the first line of investigation, however relying solely on plain film radiography to establish the diagnosis has its dangers.11,14 TB only becomes apparent on plain film radiography when 50% of the trabecular bone within the vertebral body is destroyed,10,11,14 a process which may take up to four to six months. CT scan has been used with success in the diagnosis of spinal TB; however, due to the relative lack of soft tissue definition, evaluation of the spinal cord and epidural space is not commonly done.10
MRI scan has a reported sensitivity and specificity of 100% and 88.2% respectively for the diagnosis of spinal TB.9 The major advantages of MRI are the earlier detection of spinal TB as suggested by an increased intensity of the bone marrow and allowing for overview of the whole vertebral column to diagnose non-contiguous lesions.21 Three important findings of spinal TB on MRI are endplate disruption, paravertebral soft tissue abscess and the presence of increased signal intensity of intervertebral disc on T2W.4,9,13 In addition, MRI will identify other abscesses including extension into the psoas muscle and epidural space [Figure 4a–c], posterior element involvement and spinal cord compression. Gadolinium enhanced MRI was not used in this cohort. Due to the associated cost and time, it is not part of the routine protocol at our institution. From a clinical perspective, Gadolinium did not add value in diagnosis as the standard sequences delineated the pathology adequately and diagnosis was confirmed with biopsy. Bono highlighted that the findings of spinal TB appeared more benign on MRI in HIV positive patients.22 Despite this being such a common co-infection, there however remains limited information in the literature regarding the MRI features of spinal TB in HIV positive and negative patients.
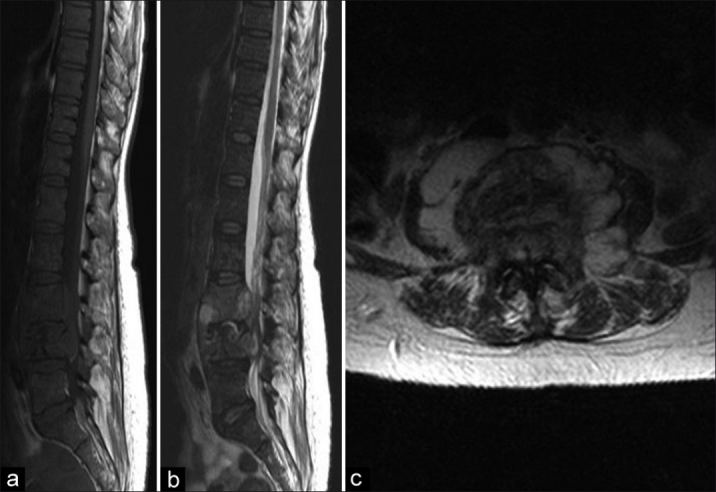
Figure 4.
(a, b) T2W and T1W sagittal MRI of a HIV positive patient showing the lack of significant kyphosis and destruction. (c) a T1 axial show demonstrating an abscess with extension into epidural space left and displacement of cord to right
In our series we found that patients with spinal TB who are HIV positive had significantly less vertebral body collapse than HIV negative patients, despite the number of bodies involved being the same. At first this may seem counter-intuitive, but this can be explained by the altered local inflammatory response, i.e. ameliorated type 4 hypersensitivity reaction. The typical granulomatous destruction of TB is less likely to occur and thus the mechanical consequences of destruction and kyphosis are less evident in HIV positive patients.
Despite less bony destruction in the HIV positive group, the abscess formation is no different. The total abscess volumes and sites were the same in both groups with only a trend towards greater epidural pus formation in the positive group. This has an influence on surgical management where frequently simple decompressive surgery, i.e. costotransversectomy, is an effective option in the HIV positive group as opposed to complex reconstructive procedures often employed when deformity is present.
The differences were found on group averages; however, within the HIV positive group extensive destruction was noted in a few cases. Thus, significant group difference does not mean that all behave the same. HIV patients are clearly at different stages of immune compromise and thus behave differently. An attempt to assess immune function by CD4 showed no relationship, but our numbers with CD4 results were small. In addition, TB on its own suppresses CD4, and thus it not a good indicator of immune function in this setting. There was no difference in the incidence of cord signal intensity changes between two groups. Although not studied in this paper, we have previously published on the relationship between cord signal and ambulatory status in TB patients. Non-ambulatory patients had a 100% incidence of T2W cord signal, with a much lower incidence in Frankel D and E's.23
CONCLUSION
HIV negative patients demonstrate greater tuberculous destruction of their spine in terms of total percentage body collapse and resultant kyphosis. There is no difference in the incidence or size of abscess formation, incidence of cord signal intensity changes or presence of non-contiguous lesions. This difference may be explained by the reduced autoimmune response of the type 4 hypersensitivity reaction caused by the HIV infection.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Tuli SM. Tuberculosis of the spine: A historical review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 2.Moon MS. Tuberculosis of spine-contemporary thoughts on current issues and perspective views. Curr Orthop. 2007;21:364–79. [Google Scholar]
- 3.Jain AK, Dhammi IK. Tuberculosis of the spine: A review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318065b7c3. [DOI] [PubMed] [Google Scholar]
- 4.Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87-B:1454–8. doi: 10.1302/0301-620X.87B11.16294. [DOI] [PubMed] [Google Scholar]
- 5.No authors listed. Global tuberculosis control: A short update to the 2009 report. [last accessed on 2011 Apr 01]. Available from: http://www.who.int/tb/publications/global_report/2009/update/en/
- 6.Moon MS. Tuberculosis of the spine: Controversies and a new challenge. Spine (Phila Pa 1976) 1997;22:1791–7. doi: 10.1097/00007632-199708010-00022. [DOI] [PubMed] [Google Scholar]
- 7.Dunn R. The medical management of spinal tuberculosis. SAOJAutumn. 2010;9:37–41. [Google Scholar]
- 8.Govender S, Parbhoo AH, Kumar KP, Annamalai K. Anterior spinal decompression in HIV-positive patients with tuberculosis A prospective study. J Bone Joint Surg Br. 2001;83-B:864–7. doi: 10.1302/0301-620x.83b6.11995. [DOI] [PubMed] [Google Scholar]
- 9.Danchaivijitr N, Temram S, Thepmongkhol K, Chiewvit P. Diagnostic accuracy of MR imaging in tuberculous spondylitis. J Med Assoc Thai. 2007;90:1581–9. [PubMed] [Google Scholar]
- 10.De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, De Schepper AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–19. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]
- 11.Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am. 2001;39:329–42. doi: 10.1016/s0033-8389(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 12.Sharif HS, Morgan JL, Al Shahed MS, Thagafi MY. Role of CT and MR imaging in the management of tuberculous spondylitis. Radiol Clin North Am. 1995;33:787–804. [PubMed] [Google Scholar]
- 13.Jain AK. Tuberculosis of the spine: A fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–13. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 14.Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br. 1994;76:863–9. [PubMed] [Google Scholar]
- 15.Keiper M, Beumont M, Elshami A, Langlotz C, Miller W. CD4 T lymphocyte count and the radiographic presentation of pulmonary tuberculosis: A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest. 1995;107:74–80. doi: 10.1378/chest.107.1.74. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Evans AJ, Sedgwick PM, Acheampong JW. Pulmonary tuberculosis: Radiological differences in West Africans coinfected with HIV. Br J Radiol. 1999;71:339–44. doi: 10.1259/bjr.72.856.10474493. [DOI] [PubMed] [Google Scholar]
- 17.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am. 1987;69:503–9. [PubMed] [Google Scholar]
- 18.Moon MS. Development in the management of tuberculosis of the spine. Curr Orthop. 2006;20:132–40. [Google Scholar]
- 19.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: The impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–46. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- 20.Martin DJ. Epidemiology, pathology and clinical presentation of HIV/TB. Med Chron. 2009 Feb;:79. [Google Scholar]
- 21.Polley P, Dunn R. Noncontiguous spinal tuberculosis: Incidence and management. Eur Spine J. 2009;18:1096–101. doi: 10.1007/s00586-009-0966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bono CM. Spectrum of spine infections in patients with HIV: A case report and review of the literature. Clin Orthop Relat Res. 2006;444:83–91. doi: 10.1097/01.blo.0000203450.28899.ef. [DOI] [PubMed] [Google Scholar]
- 23.Dunn R, Zondagh I, Candy S. Spinal tuberculosis magnetic resonance imaging and neurological impairment. Spine (Phila Pa 1976) 2011;36:469–73. doi: 10.1097/brs.0b013e3181d265c0. [DOI] [PubMed] [Google Scholar]


