Abstract
Individuals with schizophrenia (SZ) or psychotic bipolar disorder (BPP) may share neurophysiological abnormalities as measured in auditory paired-stimuli paradigms with electroencephalography (EEG). Such investigations have been limited, however, by quantifying only event-related potential peaks and/or broad frequency bands at limited scalp locations without considering possible mediating factors (e.g., baseline differences). Results from 64-sensor EEG collected in 180 age- and gender-matched participants reveal (i) accentuated pre-stimulus gamma oscillations and (ii) reduced P2 amplitudes and theta/alpha oscillations to S1 among both SZ and BPP. Conversely (iii) N1s in SZ to S1 were reduced compared to healthy and BPP, while (iv) beta range oscillations 200–300 ms following S2 were accentuated in BPP but not SZ. Results reveal a pattern of both unique and shared neurophysiological phenotypes occurring within major psychotic diagnoses.
Auditory event-related potentials (ERPs) measured in a paired stimuli paradigm have been historically important indices of auditory processing abnormalities in SZ (Light & Braff, 1999). In the simplest version of this paradigm, identical auditory “clicks” are presented in close succession (500 ms), with stimulus pairs separated by long intervals (6–10 sec). Evoked brain responses to the first (S1) and second (S2) stimuli are measured with electroencephalography (EEG), especially the positive voltage signal at vertex sensors peaking near 50 ms after stimuli onset (the P50 or P1). There is a larger difference between S1 and S2 responses for healthy subjects than for SZ (Brockhaus-Dumke et al., 2008), caused by either larger ERPs to S2 (Sanchez-Morla et al., 2009) and/or an attenuated response to S1 (Blumenfeld & Clementz, 2001) among SZ (see Chang et al., 2011 for a review).
Informative measurements in paired-stimuli paradigms are not limited to P1. Reduced amplitudes of the negative voltage ERP at vertex sensors peaking approximately 100 ms after onset (the N100 or N1) of S1 but not S2 are reported in SZ (Blumenfeld & Clementz, 2001; Boutros et al., 2009; Brenner et al., 2009; Clementz & Blumenfeld, 2001). Frequency domain analyses also provide complementary data on neural oscillatory phenomena in relation to stimulus processing. For instance, evoked gamma band oscillations peaking early in stimulus processing reflect sensory integration (Uhlhaas et al., 2011); lower frequency oscillations (<25 Hz) peak later and may reflect widespread network involvement and novelty detection (Kopell et al, 2000). Beta-band responses to S1 also may predict subsequent P1 amplitudes to S2, suggesting a role in stimulus encoding (Hong et al, 2004). Oscillatory abnormalities have been identified among SZ in the gamma band (Clementz et al., 2007; Johannesen et al., 2005), low frequency oscillations to S1 (Brockhaus-Dumke et al., 2008; Clementz & Blumenfeld, 2001; Johannesen et al, 2005), and late (200–300 ms) beta band oscillations to S1(Brenner et al., 2009; Hong et al, 2004). Hong et al. (2008) also reported that oscillatory abnormalities in the theta-alpha band during a paired stimuli paradigm had higher heritability and were superior to ERP peak measurements for indexing SZ risk.
An important attribute of any neurophysiological liability indicator is relative specificity to a disease or clinical dimension of interest (Thaker et al, 2008). Studies characterizing distinct and shared neurophysiological features among psychotic disorders constitute critically important first steps for determining the extent of common and unique etio-pathophysiologies among disorders in this superordinate class. Abnormalities of P50 suppression occur among persons with bipolar disorder with psychosis (BPP; Olincy et al, 2005; Martin et al, 2007; Carrol et al, 2008), and among their first-degree relatives (Schulze et al, 2007). It may be that these abnormalities in SZ and BPP are associated with distinct neurophysiological mechanisms but, at present, there is insufficient information to verify or reject this claim.
If abnormalities during paired stimuli paradigms have distinct neurophysiological causes within disorders of the psychosis domain, there should be some method for identifying these differences at the level of brain functioning between SZ and BPP (see, e.g., Hong et al, 2008). One possibility is that ERP peak measurements, even for brain responses to simple stimuli, do not capture the complexity of neural responding that is evident in time-frequency representations (Hong et al, 2008; Privman et al, 2011). Few investigations have compared ERP and frequency domain measures of neuronal responding during paired stimuli paradigms among SZ (Hong et al, 2004; Hong et al, 2008) or BPP (Carrol et al, 2008). Further, most auditory suppression studies have assessed P1 amplitudes from few sensor locations (typically from the vertex sensor) using varied recording, signal processing, and quantification approaches (de Wilde et al, 2007). The present investigation of the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) sample compared ERP amplitude measurements and event related oscillations across a broad frequency range (4–55Hz) during a paired stimuli paradigm. Activations from whole head EEG recordings in response to these stimuli were compared between healthy, SZ, and BPP participants to describe the neural auditory processing abnormalities which were shared between and differentiated these groups.
Methods
Participants
Subject recruitment, interviews, and EEG data recording were completed at four B-SNIP (Bipolar and Schizophrenia Network on Intermediate Phenotypes) sites: University of Illinois in Chicago, Illinois, Yale University/IOL in Hartford, Connecticut, University of Texas Southwestern in Dallas, Texas, and University of Maryland in Baltimore, Maryland. As part of a large, multi-site project, stable participants were recruited from the community, linked community facilities and programs, from community advertisement, and local National Association on Mental Illness (NAMI) or NAMI-type family groups.
Medical history, structured clinical interview for DSM-IV diagnosis (SCID patient or nonpatient version as appropriate; First et al, 1997), Positive and Negative Symptom Scale (PANSS; Lancon et al, 2000), Young Mania Rating Scale (Lancon et al, 2000), Montgomery Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979), and Global Assessment of Functioning scale (GAF; axis V of Diagnostic and Statistical Manual of Mental Disorders IV [DSM-IV]) were acquired by trained Masters or Doctoral-level nurses, psychologists, or psychiatrists. The presence of serious medical, neuro-opthalmological, or neurological illness (e.g., cancer, seizure disorders, coarse brain-disease), mental retardation, head trauma with >30 minutes unconscious, current substance use ascertained by history as well as urine drug screens on the day of testing, abuse in the past three months, and dependence within 6 months or extensive history of drug dependence (DSM-IV) were criteria for exclusion. Healthy persons were free of any lifetime psychotic or mood disorder and a family history of psychotic or BP disorders in their first degree relatives according to Family History Research Diagnostic Criteria (Andreasen et al., 1977). All clinical information and diagnoses for each subject were reviewed and confirmed in a best estimate diagnostic meeting including a senior psychiatrist/psychologist and the clinician who conducted the structured interviews.
Three age- and gender-matched groups were constructed blind to brain activity measurements: 60 SZ (30 female; mean age = 36.3; range 19–55); 60 BPP (30 female; mean age = 36.3; range 18–53); and 60 healthy persons (30 female; mean age = 36.2; range 18–54; recruited via random digit dialing or community advertisements. Among SZ, all but 5 were taking psychotropic medications. Among BPP, 9 were free of such medication. Detailed information regarding medication is presented in supplementary table 1.
Stimuli and Procedures
Ambient recording conditions and stimulus presentation equipment were identical across sites. Subjects were seated in with their eyes open in a sound and electrically shielded booth (ambient sound = 61 to 63dB; luminance = 0.11–0.12 foot-candles). Subjects at all sites passively listened to 150 binaural broadband auditory stimuli pairs (4 ms duration at 75dB) which were delivered by two 8 ohm speakers located 50 cm in front of the participants. Loud speakers placed in the same locations relative to the subjects were used to deliver stimuli in order to ensure maximal reliability in sound intensity and characteristics between recording sites. Stimuli pairs were separated by an average of 9.5 sec (9–10 sec inter-pair interval; rectangular distribution), with 500 ms between stimuli in a pair. Participants refrained from smoking 1 hour prior to testing.
EEG recording
EEG was collected from 64 Ag/AgCl sensors (impedance was kept below 5 K KΩ; Quik-Cap, Compumedrics Neuroscan, El Paso, TX) positioned according to the standard 10–10 EEG system (with the inclusion of mastoids and CP1/2 locations to provide for greater signal sampling below the cantho-meatal line), with a forehead ground and nose reference. EEG recordings were amplified (12,500x) and digitized (1000 Hz) using a commercially available electrophysiological recording system (Neuroscan Acquire and Synamp2; Compumedrics Neuroscan, El Paso, TX).
EEG processing
Raw EEG data were inspected for bad sensors and artifacts. Bad sensors were interpolated (no more than 5% for any subject) using a spherical spline interpolation method (as implemented in BESA 5.3; MEGIS Software, Grafelfing, Germany). Blink and cardiac artifacts were removed using Independent Components Analysis (EEGLAB 6.0; Delorme & Makeig, 2004). EEG data on each trial were then segmented into 1250 ms epochs extending from 250 ms before to 1000 ms after S1 (500 ms after S2). Trials containing activity greater than 75 μV at any sensor were eliminated from further processing. Groups did not differ on the number of trials included (see Table 1); all subjects had at least 60% of trials accepted (or 90 out of 150 total trials). Data were digitally bandpass filtered from 0.5 – 55 Hz (zero phase filter; rolloff: 6 and 48 dB/octave, respectively), and the 100 ms before S1 was used for baseline adjustment over the remaining epoch.
Table 1.
Subject demographic and clinical data.
| Healthy | Schizophrenia | Bipolar | Statistic | p-value | |
|---|---|---|---|---|---|
| Subjects | 58 | 56 | 56 | Χ2(2)=.047 | p>.976 |
| Age | 36.4 (18–54) | 36.6 (19–55) | 36.1 (19–53) | F(2,167)=.037 | p>.964 |
| Number female | 24 | 22 | 23 | Χ2(2)=.058 | p>.971 |
| UIC | 20 | 20 | 20 | ||
| YU | 16 | 12 | 15 | ||
| UTS | 14 | 12 | 12 | ||
| UM | 8 | 12 | 9 | ||
| Site effects | - | - | - | Χ2(6)=1.66 | p>.952 |
| Trials used | 141 (107–152) | 140 (95–150) | 138 (106–155) | F(2,167)=1.25 | p>.291 |
| GAF | - | 47.8 (30–90) | 59.1 (30–82) | t(112)=4.73 | p<.001 |
| PANSS-positive | - | 17.4 (8–29) | 13.6 (7–24) | t(112)=4.47 | p<.001 |
| PANSS-negative | - | 17.2 (7–32) | 11.9 (7–30) | t(112)=5.45 | p<.001 |
| PANSS-general | - | 33.3 (17–52) | 29.1 (18–56) | t(112)=2.65 | p<.010 |
| MADRS | - | 10.7 (0–30) | 11.3 (0–43) | t(112)=.327 | p>.744 |
| Young Mania Scale | - | 4.47 (0–19) | 5.15 (0–21) | t(112)=.714 | p>.477 |
UIC, University of Illinois, Chicago; YU, Yale University; UTS, University of Texas Southwestern; UM, University of Maryland; GAF, Global Assessment of Functioning; PANSS, Positive and Negative symptom; MADRS, Montgomery-Åsberg Depression Rating Scale; APS, antipsychotic medication.
PCA data reduction
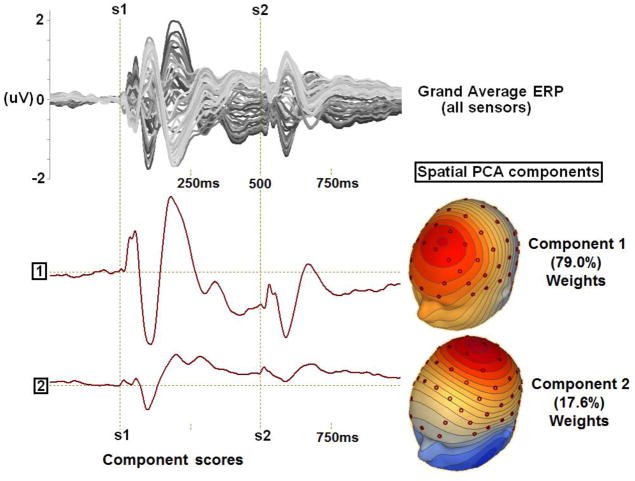
In order to use EEG data recorded from every sensor and, thus, to most accurately and comprehensively capture the spatial topography of the evoked brain responses across time, a data reduction technique using spatial principal components analysis (PCA) on grand average data (Clementz & Blumenfeld, 2001; Carrol et al, 2008) was implemented using BESA (MEGIS Software, Grafelfing, Germany) and Matlab (The Mathworks, Matick, Massachusetts, United States) to identify spatial patterns in the EEG topography. First, responses were averaged within groups (see Figure 1). Then, for each group average, a 64×64 sensor covariance matrix was calculated (using 1250 time points as observations) and a PCA was carried out with promax (oblique) vector rotation and Kaiser normalization (Dien et al., 2007). Scree tests (Cattell, 1966) for each group identified 2 components. The spatial distributions of these components and amount of variance accounted for in the grand average (always over 95%) were nearly identical across groups (see Supplementary Figure 1, for all correlations between groups greater than r=.975), so the same procedure was carried out again after averaging over all subjects in all groups. In this PCA, the first two components accounted for 79.0 and 17.6 percent of the variance, respectively. Plots of the component weights (Figure 1) show components with FCz maximum (Component 1) and CPz maximum (Component 2) activities, a result similar to previous reports using this method for ERP quantification (Clementz & Blumenfeld, 2001; Carrol et al, 2008). Each set of component weights was multiplied by each subject’s ERP data (EEG data averaged across trials), summed across sensors, and divided by the plus sum of the component weights. This reduced waveforms from 64 (one for each sensor) to 2 (one for each component) for each subject (see Figure 2, averaged within groups). This reduction can be conceptualized as the application of statistically derived spatial filters which take advantage of the high correlations among EEG channels while allowing separate neural substrates (separate PCA components) to be analyzed.
Figure 1.
Spatial PCA completed on a) grand averaged data (n=170; displayed as a butterfly plot with each sensor represented as a line) yields b) component weights displayed as waveforms and c) component scores displayed as topographies (each varying only in magnitude across the entire epoch).
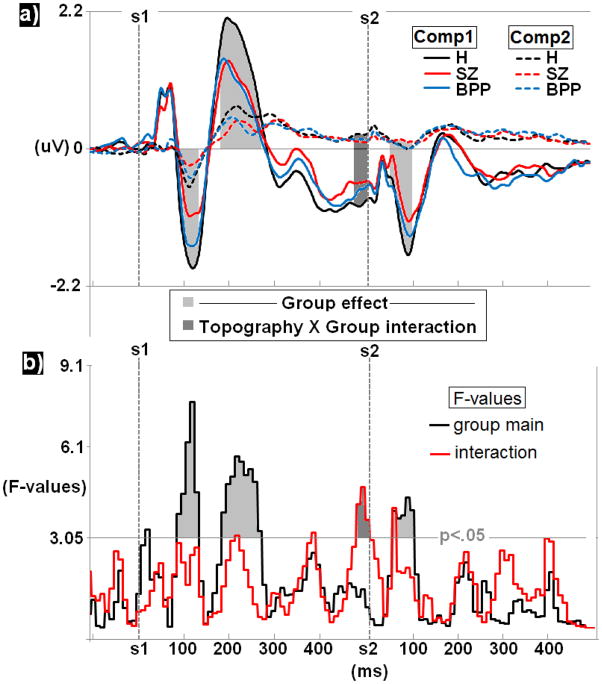
Figure 2.
Group comparisons for PCA derived ERP waveforms a) averaged within group yield significant effects in the N100-S1, P200-S1, pre-S2, and P50/N100-S2 ranges. b) F-values for these effects are also presented with highlighted significant regions extending above the p<.05 cutoff for 3 consecutive bins.
ERP time course analyses
Subject component waveforms from 100 ms prior to S1 onset to 500 ms post S2 (1100 ms total) were grouped into 100 separate 10 ms bins and averaged within each bin. Group means, medians, and standard deviations for each bin were quantified. An evaluation for extreme observations resulted in 2 H, 4 SZ, and 4 BPP being eliminated from comparisons because they were clear outliers, with time-bin values exceeding 3.5 inter-quartile ranges(Brenner et al., 2009) from their respective group medians, leaving 58 H, 56 SZ, and 56 BPP (see Table 1).
Time frequency analyses
For each subject, separately for each component waveform, oscillatory power for frequencies from 4–55 Hz was calculated in 1 Hz steps using a modified Morlet wavelet transformation every 4 ms (Delorme & Makeig, 2004) on the grand averaged ERPs. The length of the wavelets increased linearly from 1 cycle at 4 Hz to 8 cycles at 55 Hz. These parameters were selected to optimize the tradeoff between temporal resolution at lower frequencies and stability at higher frequencies (Busch & VanRullen, 2010). At each time (t) and frequency (f), the result of the wavelet transform is a complex number in which A represents the amplitude of the signal and ϕ its phase:
Oscillatory power (in decibels) was defined as 10 times the log10 of A. This procedure yielded two time-frequency spectra of oscillatory power from −125 ms to 875 ms post S1 (375 ms post S2) for each subject (see Figure 3). Using the same interquartile range criterion described above in 2.4.3, no further outliers were identified.
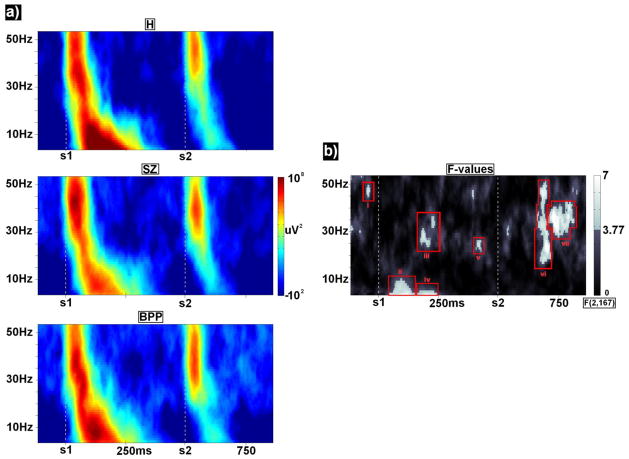
Figure 3.
a) Time-frequency spectra for each group averaged over components 1 and 2. For ease of presentation, each point in each time-frequency spectrum was rescaled with respect to its frequency’s mean over all groups and time points. b) F-values for group effects are presented with clusters satisfying alpha<.05 outlined in red.
Statistical analyses
For each 10 ms time bin (from −100 pre- to 1000 ms post-S1) and for each time-frequency point of interest (from 4- to 55-Hz, −125 ms to 875 ms post S1) groups were compared with a mixed model ANOVA with one between subject factor (diagnosis [DX]: H, SZ, BPP) and one within subject factor ([topography]: PCA-components 1 and 2). This yielded distributions of F-Values for each time bin and time-frequency point of interest and for each effect (Figures 2 and 3). To control for the possibility that large effects were the result of a small number of deviant values, this same set of ANOVAs was then run 15000 times (bootstrap procedure) with group membership randomly shuffled at each step (sampling with replacement). Probability estimates of the actual F-values were then calculated as the proportion of randomly generated F-values greater than the actual estimate. To control for increased family-wise error rate due to multiple comparisons, a clustering method (Forman et al, 1995) was used to take account of the non-independence of data from adjacent time-frequency points and time bins (for an example, see Hamm et al, 2011, and Monte Carlo simulations calculated using AlphaSim; Cox, 1996). Time bin effect “clusters” were considered significant at overall family-wise alpha<.01 if at least 3 adjacent time bins were significant p<.05; time-frequency effect “clusters” were considered significant at family-wise alpha<.01 if at least 8 adjacent time-frequency points were significant p<.025. All analyses were also conducted including data collection site as a separate factor. There were no significant interactions involving site and group on any of the variables that significantly differentiated groups (Supplementary Figure 2).
Post-hoc discriminant analyses
To efficiently summarize the EEG variables that uniquely differentiated DX groups, values from significant time bin and time-frequency clusters were averaged within adjacency clusters for each subject and submitted to a linear discriminant analysis with DX as the dependent variable (H, SZ, BPP). Variables which minimized the overall Wilks’ lambda and had individual multiple F-statistics significant at p<.05 were entered in a stepwise fashion (Mardia et al., 2008), leaving a parsimonious selection of neurophysiological measures. This additional statistical analysis was included specifically to describe the variables most important to group separations rather than as an attempt to classify observations. The latter approach will be more appropriate when we can add additional tasks and observations to our data collection efforts.
Results
Voltage time bins
Average waveforms for each PCA component and group (Figure 2a) and plots of F-values for DX main effects and DX by topography interaction effects (Figure 2b) reveal four time bins which significantly differentiated groups. Three time bins differentiated DX regardless of head topography (DX main effects):
From 70–120 ms post-S1, peaking at 105 ms, F(2,167)=7.54, p<.001, SZ had significantly weaker voltage changes (less difference from baseline) than both H, t(112)=3.87, p<.001, and BPP, t(110)=2.38, p<.02;
From 170–260 ms post-S1, peaking at 205 ms, F(2,167)=5.73, p<.005, H had significantly stronger voltage than SZ, t(112)=2.71, p<.008, and BPP, t(112)=2.87, p<.005;
From 50–100 ms post-S2, peaking at 85 ms, F(2,167)=4.35, p<.015, SZ had less voltage deviations (less difference from baseline) than H, t(112)=2.81, p<.006 (BPP did not differ significantly from the other groups).
There was also a DX by PCA component (topography) interaction in the 30 ms period immediately prior to the onset of S2. This effect peaked at 15 ms pre-S2, F(2,167)=4.72, p<.010. Investigation of simple main effects indicated that this interaction was driven by a difference between H and SZ on PCA component 1, t(112)=2.61, p<.010; SZ had less extreme voltage (less difference from baseline) than H (BPP did not differ significantly from the other groups).
Time-frequency
Average time-frequency spectra for each group (Figure 3a) and plots of F-values for DX main effects (Figure 3b) revealed seven time-frequency clusters which significantly differentiated groups. No time-frequency clusters showed significant DX by topography interactions, so group averages are shown averaged across PCA components in Figure 3a. For descriptive purposes and for post-hoc analyses, clusters of time-frequency effects were grouped based on traditional frequency bands (e.g. Hong et al, 2008) and temporal proximity (red outlines in Figure 3b).
Centered at 40 ms prior to S1, and peaking at 47 Hz, F(2,167)=5.40, p<.005, H had significantly less power than SZ, t(112)=2.50, p<.015, and BPP, t(112)=2.99, p<.003;
Centered at 115 ms post-S1, and peaking at 4 Hz, F(2,167)=6.29, p<.002, SZ had significantly less power than H, t(112)=3.39, p<.001, and BPP, t(112)=2.08, p<.040.
Extending from 190 to 240 ms post-S1, but peaking at 190 ms and 28 Hz, F(2,167)=5.63, p<.004, BPP had significantly higher power than H, t(112)=2.85, p<.005 and SZ, t(112)=3.1, p<.002;
Centered at 225 ms post-S1, and peaking at 4 Hz, F(2,167)=5.79, p<.004, H had significantly more power than SZ, t(112)=3.24, p<.002, and BPP, t(112)=2.76, p<.007;
Centered at 420 ms post-S1, and peaking at 25 Hz, F(2,167)=5.42, p<.005, H had significantly less power than SZ, t(112)=2.23, p<.027, and BPP, t(112)=3.05, p<.003;
Centered at 200 ms post-S2, and peaking at 24 Hz (but extending from approximately 20–50 Hz), F(2,167)=7.57, p<.001, H had significantly less power than SZ, t(112)=2.71, p<.008, and BPP, t(112)=3.65, p<.001;
Centered at 235 ms post-S2, and peaking at 36 Hz, F(2,167)=8.23, p<.001, BPP had significantly more power than H, t(112)=4.18, p<.001, and SZ, t(112)=2.91, p<.004.
Based on the distinct frequency and time ranges of significant regions in the F-plots (Figure 3b), none of the above mentioned effects appear to be harmonic/subharmonic concomitants.
Discriminant analyses
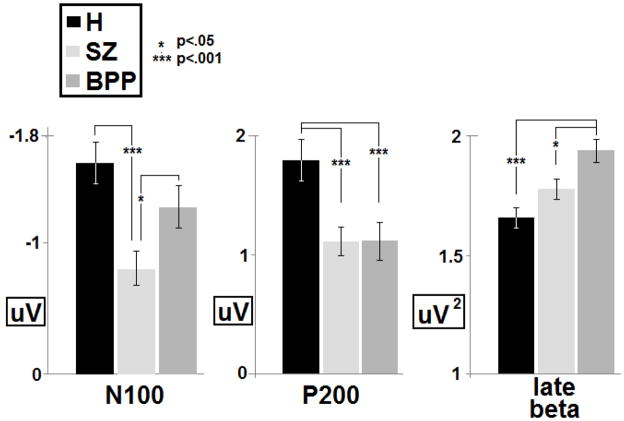
Linear discriminant analyses using the 11 variables with significant effects of DX indicated that just 3 variables uniquely differentiated groups (Table 2), thus accounting for the group variance explained by the 8 other voltage time series and time-frequency effects. First, high-beta/low-gamma range (28–42 Hz) oscillatory activity in the period from 210–305 ms post-S2 significantly differentiated BPP from both H and SZ (BPP were higher). Second, voltage data in the N1 range (70–120 ms) following S1 significantly differentiated SZ from both H and BPP (SZ were lower). Third, voltage data in the P2 range (170–260 ms) following S1 significantly differentiated both SZ and BPP from H (H were higher). Group means and standard errors are presented in figure 4.
Table 2.
Results from linear discriminant analysis.
| Measure | Wilk’s Lambda | F-value* | H vs SZ | H vs BPP | BPP vs SZ |
|---|---|---|---|---|---|
| Late beta post-S2 | .896 | 9.689 | t(112)=1.94, p<.05 | t(112)=4.28, p<.001 | t(112)=2.46, p<.05 |
| N100-S1 | .807 | 9.398 | t(112)=3.91, p<.001 | t(112)=1.49, p<.15 | t(112)=2.24, p<.05 |
| P200-S1 | .772 | 7.590 | t(112)=12.0, p<.001 | t(112)=4.70, p<.001 | t(112)=0.01, p>.99 |
all F-values are significant at p<.001
Figure 4.
Group averages and standard errors for three main group discriminators determined in the linear discriminant analysis: N100 to S1, P200 to S1, and beta oscillatory power 250 ms after the onset of S2. P-values are based on two-tailed independent samples t-tests.
Correlations with Clinical Variables
Pearson correlations of all EEG variables which significantly differentiated groups and all available clinical measures (Table 1) were computed within SZ and BPP groups. There were no correlations significant at p<.05 (uncorrected) for any EEG-clinical variable pair (including medication status).
Discussion
This study investigated neural manifestations of auditory processing deviations during a paired stimuli paradigm among persons with psychosis, including two different categorical diagnoses: schizophrenia or bipolar disorder. The psychoses groups showed higher gamma range activity prior to presentation of S1, and deviations from normal in voltage (especially SZ) and high beta range activity during the 80 ms preceding S2 (a time range typically considered ‘baseline period’ for the S2 response). Consistent with multiple previous investigations, only SZ psychosis had lower magnitude responses to S1 in the N100 time range, an effect that was manifest in both voltage and frequency analyses. This effect uniquely differentiated SZ from BPP and healthy persons. Thus, this feature appears specific to SZ rather than general to the class of psychotic disorders. The psychosis volunteers, both SZ and BPP, shared lower magnitude responses than healthy persons to S1 in the P200 time range. Finally, BPP showed a unique accentuation of beta/gamma activity following the P200 response window in relation to both S1 and S2, with the S2 response, specifically, differentiating BPP from the SZ and H. The implications of these findings for understanding what is unique and shared among SZ and BPP are discussed below.
High levels of intrinsic neural activity characterize SZ (Clementz & Blumenfeld, 2001; Rolls et al., 2008; Clementz et al., 2008), with abnormality of pre-stimulus gamma also observed among SZ during other auditory processing paradigms (Reinhart et al., 2011). Excess intrinsic neural activity yields lower signal-to-noise ratio responses to salient stimulus events (e.g., ERPs) even if those responses are of normal magnitude. Neither SZ nor BPP, however, showed normal magnitude ERPs in relation to especially S1. The combination of higher intrinsic neural activity and lower magnitude responses to salient stimuli found among the psychotic individuals may compromise processing fidelity and the ability to discern relevance in the sensory environment.
There are multiple intriguing aspects to excess intrinsic neural activity among psychotic patients. Privman et al (2011) showed that, in a visual paired-stimuli paradigm, pre-stimulus gamma power is negatively correlated with and determines ERP magnitude. Such differences in baseline EEG gamma activity may result from disinhibition of pyramidal neurons due to decreased NMDA receptor mediated GABA-ergic modulation (Belforte et al., 2010), cellular deviations perhaps most evident in N100-generating cortical layers (Radhakrishnan et al., 2011; Lewis & Hashimoto, 2007). The frequency range within which high intrinsic neural activity manifests in SZ, however, varies across studies. For instance, Clementz et al. (2008) observed this effect in the theta/low alpha range (the stimulus salience range in that study) while in the present report this effect occurred in the high beta/gamma range (the primary range of initial stimulus registration here; see Figure 3 at −40 ms). Pre-stimulus neural bias signals are related to expected stimulus properties (Luck et al., 1997), and tend to vary with task-related cognitive control requirements (Rolls et al., 2008; Ress et al, 2000). Excess neural activity in psychosis, therefore, may signify a maladaptive effort to enhance processing of salient stimuli, a possibility that might be usefully evaluated in future investigations.
Reduction of N100 amplitude in relation to S1 was specific to SZ. This effect is frequently reported among SZ (Blumenfeld & Clementz, 2001; Boutros et al., 2009; Brenner et al., 2009; Clementz & Blumenfeld, 2001), and is consistent with reduction in low frequency oscillations in this same evoked response time window (Brockhaus-Dumke et al., 2008; Brenner et al., 2009; Clementz & Blumenfeld, 2001; Johannesen et al, 2005). Interestingly, Boutros et al (1997) also showed that N100, but not P200, differentiated SZ from BPP during auditory processing. P200 amplitudes, as in the current study, were found to be reduced in both psychosis groups. Relatively little is known about the specific functions and neural correlates of the P200 (Tremblay et al., 2009), but this reduction may indicate the psychoses share difficulties with auditory stimulus identification, classification, and/or registration processes (Naatanen & Picton, 1987). Reduced N100 magnitudes, however, as a response generated largely in supra- and infra-granualar layers in the planum temporale (Naatanen & Picton, 1987), (Barth & Di, 1991), may reflect disruptions of temporal summation in intralaminar auditory cortical circuitry among SZ (Hamm et al, 2011; Gilmore et al., 2004) perhaps associated with sparse connectivity between primary and secondary auditory cortex (Sweet et al., 2007). Assessing whether BPP have spared temporal summation effects on N100 amplitudes would be extremely important for verifying a specific pathophysiological difference between these psychotic disorders.
An accentuation of beta-range activity following the P200 time window, especially following S2, was peculiar to BPP. Although similar accentuations have been reported previously among bipolar patients (Lee et al., 2010; Ozerdem et al., 2008), this effect appears to require stimulus processing given that Venables et al (2009) did not observe this SZ-BPP effect in resting EEG. Synchronized beta-range activity is required for distant cortico-cortical communication (Kopell et al, 2004), and is supported by GABAergic-NMDA networks that purportedly is hypofunctional among SZ in some cortical regions (Vierling-Claassen et al., 2009). Given this effect, it appears that BPP showed enhanced sustained attention/vigilance to the stimuli (Braboszcz & Delorme, 2011), indicating an approach to stimulus processing of which SZ are not capable given their ostensible pathophysiology. It would be worthwhile to investigate beta-range accentuation in non-psychotic bipolar patients to determine whether this alteration is specific to psychotic bipolar patients or a trait-marker of bipolar disorder in general.
While there are numerous similarities between the present and previous findings, there are noteworthy differences. Some prior studies found early gamma-related reductions (in the P50 time window) to S1 in SZ (Clementz & Blumenfeld, 2001; Johannesen et al, 2005), although this is not a ubiquitous finding (Brockhaus-Dumke et al., 2008; Brenner et al., 2009). The present study also indicates a difference in response to S2 among SZ on voltage in the P50 to N100 time window. Inspection of Figure 2 indicates that SZ have more positive voltages in this time range, at least consistent with Chang et al’s (2011) conclusion that the P50 response to S2 primary accounts for SZ-healthy differences in previous paired-stimuli studies. A complication for this interpretation, however, is that SZ also have more positive voltages in the typical pre-S2 baseline period (see Figure 2); if S2 responses were adjusted for this activity, SZ would no longer differ from healthy people on S2 response magnitudes in the current study. Whether this pre-S2 difference accounts for previously described P50 effects is unclear given variation across studies in i) electrode site of analysis and ii) the use of peak-to-trough versus baseline-to-peak measurements of ERP amplitudes. Nonetheless, this group difference, along with the fact that ERPS/evoked oscillations in the pre-S2 period do not fully return to pre-S1 levels (Figures 1–3), suggests that direct comparison between S1 and S2 measures may be problematic given that they are superimposed on different brain states.
Nicotine ingestion may transiently “correct” SZ abnormalities in traditional P50 gating measures (Leonard et al, 1996), so subjects in the current study were required to refrain from smoking 1 hour prior to EEG data collection. Although chronic nicotine use in H has been associated with reductions on traditional P50 measures (Brinkmeyer et al, 2011), Hong et al (2008) recently reported that traditional P50 and evoked oscillatory measures do not differ between smoking and non-smoking SZ. How chronic nicotine use may affect the electrophysiological abnormalities identified in the current paper is uncertain given their marked differences from traditional quantification schemes. Because such a high percentage of SZ use nicotine (80%; Winterer, 2010), a proper and balanced analysis involving nicotine as a mediator of neurophysiological effects would be difficult in samples of SZ, BPP, and H not selectively balanced on this measure (as in the current study). This is an issue that might be usefully investigated in subsequent within-subject and/or family/genetic designs.
These results are not directly comparable to those in the archival literature for a number of methodological reasons. Even though we used a typical paired-stimuli paradigm, our analysis method differed in multiple ways. First, we imposed minimal filtering and data processing adjustments (e.g. only using baseline correction prior to trial onset) in an effort to impose the fewest restrictions possible on data analyses. Second, rather than relying on a single sensor (or few sensors) we integrated information across a large number (64) of EEG sensors. This allowed for maximal use of available data that should optimize quantification of neural responses to stimulus events. Third, rather than arbitrarily selecting sensors for analyses, we used spatial PCA to empirically derive a multi-sensor neural response that could be quantified with enhanced signal-to-noise ratio and improved reliability of measurement (Maurer et al., 1990). This approach also clarified that the vertex sensor (Cz), typically used for quantifying brain responses in such studies, is suboptimal for quantifying neural activity given the spatial maxima of the auditory cortex dipolar response (see Figure 1). Fourth, rather than quantifying only peaks, we evaluated voltage differences over the entire time range to allow for maximal use of available information. Finally, we used both voltage and time-frequency analyses to comprehensively investigate group differences, with both approaches yielding interesting and complementary information. Despite, or perhaps because of, these differences, the present results indicate that multiple neural responses during a paired stimuli paradigm yield valuable information for understanding the pathophysiology of psychotic disorders. It appears the mechanisms supporting neural responses during this paradigm may be more complex and intriguing than had been previously supposed.
Supplementary Material
Acknowledgments
This work was supported by Grants from the National institutes of Health (R01s MH077945, MH077862, MH077851, MH078113, and MH085485).
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–35. doi: 10.1097/00006199-197803000-00059. [DOI] [PubMed] [Google Scholar]
- Barth DS, Di S. The functional anatomy of middle latency auditory evoked potentials. Brain Research. 1991;565:109–15. doi: 10.1016/0006-8993(91)91741-I. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clin Neurophysiol. 2001;112(9):1650–9. doi: 10.1016/S1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Brockhaus-Dumke A, Gjini K, Vedeniapin A, Elfakhani M, Burroughs S, Keshavan M. Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophr Res. 2009;113(2–3):339–46. doi: 10.1016/j.schres.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Nasrallah H, Leighty R, Torello M, Tueting P, Olson S. Auditory evoked potentials, clinical vs. research applications. Psychiatry Res. 1997;69(2–3):183–95. doi: 10.1016/S0165-1781(96)02919-8. [DOI] [PubMed] [Google Scholar]
- Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage. 2011;54(4):3040–7. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O’Donnell BF, Hetrick WP. Event related potential abnormalities in schizophrenia: a failure to “gate in” salient information? Schizophr Res. 2009;113:332–338. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeyer J, Mobascher A, Musso F, Schmitz M, Wagner M, Frommann I, et al. P50 sensory gating and smoking in the general population. Addict Biol. 2011;16(3):485–98. doi: 10.1111/j.1369-1600.2010.00302.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biological Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci U S A. 2010;107(37):16048–53. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, Kieffaber PD, Vohs JL, O’Donnell BF, Shekhar A, Hetrick WP. Contributions of spectral frequency analyses to the study of P50 ERP amplitude and suppression in bipolar disorder with or without a history of psychosis. Bipolar Disord. 2008;10(7):776–87. doi: 10.1111/j.1399-5618.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The screen test for the number of factors. Multivariate Behav Res. 1966;1:245–76. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Chang WP, Arfken CL, Sangal MP, Boutros NN. Probing the relative contribution of the first and second responses to sensory gating indices: A meta-analysis. Psychophysiology. 2011;48(7):180–92. doi: 10.1111/j.1469-8986.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139(4):377–90. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8(18):3889–93. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci. 2008;28:13411–13418. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA. Reassessing benzene risks using internal doses and Monte-carlo uncertainty analysis. Environ Health Perspect. 1996;104:1413–1429. doi: 10.2307/3433198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophr Res. 2007;97(1–3):137–51. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Hum Brain Mapp. 2007;28(8):742–63. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Clementz BA, Buckley PF. Rate of stimulation affects schizophrenia-normal differences on the N1 auditory-evoked potential. Neuroreport. 2004;15(18):2713–7. Retrieved from http://journals.lww.com/neuroreport/ [PubMed]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV—clinical version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1997. Retrieved from http://www.appi.org. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NA, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol Psychiatry. 2011;69(10):989–96. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004;15:155–159. doi: 10.1097/00001756-200401190-00030. Retrieved from http://journals.lww.com/neuroreport/ [DOI] [PubMed]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008;65(9):1008–16. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97(4):1867–72. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lançon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS) Schizophr Res. 2000;42(3):231–9. doi: 10.1016/S0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- Lee PS, Chen YS, Hsieh JC, Su TP, Chen LF. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: a magnetoencephalographic study. J Affect Disord. 2010;123(1–3):270–5. doi: 10.1016/j.jad.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons. Int Rev Neurobiol. 2007;78:109–31. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1(1):31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. Retrieved from http://jn.physiology.org/ [DOI] [PubMed]
- Mardia KV, Kent JT, Bibby JM. Multivariate Analysis. Academic Press; 1979. Retrieved from http://www.elsevierdirect.com/imprint.jsp?iid=5. [Google Scholar]
- Martin LF, Hall MH, Ross RG, Zerbe G, Freedman R, Olincy A. Physiology of schizophrenia, bipolar disorder, and schizoaffective disorder. Am J Psychiatry. 2007;164(12):1900–6. doi: 10.1176/appi.ajp.2007.06010017. [DOI] [PubMed] [Google Scholar]
- Maurer K, Dierks T, Strik WK, Frolich L. P3 topography in psychiatry and psychopharmacology. Brain Topogr. 1990;3:79–84. doi: 10.1007/BF01128864. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134(4):382–89. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar subjects with a history of psychosis. Am J Psychiatry. 2005;162:43–49. 9. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Tunca Z, Başar E. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain Res. 2008;1235:98–108. doi: 10.1016/j.brainres.2008.06.101. [DOI] [PubMed] [Google Scholar]
- Privman E, Fisch L, Neufeld MY, et al. Antagonistic relationship between gamma power and visual evoked potentials revealed in human visual cortex. Cereb Cortex. 2011;21(3):616–24. doi: 10.1093/cercor/bhq128. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan H, Wu W, Boas D, Franceschini MA. Study of neurovascular coupling by modulating neuronal activity with GABA. Brain Res. 2011;1372:1–12. doi: 10.1016/j.brainres.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RM, Mathalon DH, Roach BJ, Ford JM. Relationships between pre-stimulus gamma power and subsequent P300 and reaction time breakdown in schizophrenia. Int J Psychophysiol. 2011;79(1):16–24. doi: 10.1016/j.ijpsycho.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3(9):940–5. doi: 10.1038/78856. Retrieved from http://www.nature.com/neuro/ [DOI] [PubMed]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nature Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Sánchez-Morla EM, Santos JL, Aparicio A, Garca-Jimenez MA, Villanueva C, Martnez-Vizcano V, Arango C. Antipsychotic effects on auditory sensory gating in schizophrenia patients. European Neuropsychopharmacology. 2009;19:905–909. doi: 10.1016/j.euroneuro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall MH, McDonald C, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol Psychiatry. 2007;62(2):121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61(7):854–64. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–73. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Shahin AJ, Picton T, Ross B. Auditory training alters the physiological detection of stimulus-specific cues in humans. Clin Neurophysiol. 2009;120(1):128–35. doi: 10.1016/j.clinph.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High- (>60 Hz) γ-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol. 2011;105(1–2):14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. 2009;35(4):826–39. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23(2):112–9. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.