Abstract
For reasons of safety and effectiveness many forces in healthcare, especially the Affordable Care Act of 2010, are pressing for improved identification and management of substance use disorders within mainstream healthcare. Thus, standard information about patient substance use will have to be collected and utilized by providers within electronic health record systems (EHRS). While there are many important technical, legal and patient confidentiality issues that must be dealt with to achieve integration, this paper focuses upon efforts by the National Institute on Drug Abuse and other federal agencies to develop a common set of core questions to screen, diagnose and initiate treatment for substance use disorders as part of a national EHRS. This paper discusses the background and rationale for these efforts and presents the work to date to identify the questions and to promote information sharing among health care providers.
Keywords: Substance Abuse, Health Information, Electronic Health Record System, Patient Confidentiality, Patient Safety
Introduction
Information standardization, sharing and utilization issues have become critical as healthcare teams of the future will increasingly involve many types of providers coordinating their individual interventions and tracking outcomes in a compatible manner. This will require fully functioning electronic healthcare record systems (EHRS) to provide accessible, standardized information for all members of the care team at different sites and over an extended time frame. Standardized, shared information should increase the efficiency and integration of different parts of healthcare that so often hamper quality and produce unnecessary costs. One area of healthcare which has been relatively segregated from mainstream healthcare and health information sharing is substance use disorders, including tobacco, alcohol, illicit drugs and prescribed drugs used in non-medical ways. In the past, only the most severe forms of substance use disorders – abuse and dependence – have been eligible for treatment, and then almost always by specialty care providers, most of whom have not been part of general healthcare systems. Also, with the exception of tobacco use, substance use information has not been collected or stored within EHRS, due in part to different federal confidentiality regulations (42CFR part 2 vs. HIPAA). Thus substance use information has rarely been addressed in most healthcare settings.
This situation cannot continue. New federal legislation and new requirements from quality assurance and credentialing agencies will promote inclusion of substance use identification and management information into EHRS. This four-part paper discusses the clinical rationale for this change; considers some of the implications for care delivery and reimbursement in substance use; and describes the efforts of the National Institute on Drug Abuse (NIDA) thus far to develop consensus standards for patient information collection and protection in EHRS.
Part 1 – Forces for Integrating Care for Substance Use Disorders into General Healthcare
Lack of physician training, prejudice regarding patients with substance use disorders, absence of medical treatments for these disorders and poor or nonexistent reimbursement have historically prevented physicians and other healthcare workers from identifying or treating substance use problems. But four significant forces are now counteracting these historical barriers.
Improved Safety of Medical Care
Substance dependence is usually easy to identify and is an important, treatable illness in its own right. But less severe substance use disorders regularly co-occur with other medical illnesses and can impair ongoing medical care for those illnesses (Turner & McLellan, 2009). This type of substance use has been called “medically harmful use” (Saitz, 2005). While it is far more common, and is responsive to brief forms of treatment, medically harmful use is also more difficult to identify without standard information collection.
The failure to identify and account for medically harmful substance use has significant clinical and cost-of-care implications. For example, even sub-diagnostic levels of alcohol and other drug use can produce harmful and even lethal interactions with commonly prescribed medications. A 2008 study (Walley, Farrar, Cheng, Alford, & Samet, 2009) examined drug-drug interactions among 87 patients in a methadone maintenance program who were concurrently being treated by a primary care clinic within the same university medical system. However, the patients’ methadone medication status and drug use histories had not been revealed to the primary care physicians. A one-year medical records check revealed that all 87 patients (100%) had received at least one medication that could produce a potentially dangerous interaction with their opioid medication. On average these patients had five potentially dangerous drug-drug prescriptions, and 15 of the 87 (17%) had been seen in the emergency room because of an untoward interaction. Similarly, a recent study of fatal medication errors in the United States found a tenfold increase in deaths attributed to the interaction of a prescribed medication with patient alcohol and/or other drug use (Phillips, Barker, & Eguchi, 2008). Thus, including substance use information within EHRS could enable physicians and pharmacies to work more collaboratively to reduce the frequency of drug-drug interactions and overdose incidents.
Improved Quality and Effectiveness of Healthcare
Systematic research reviews have shown the deleterious effects of medically harmful substance use (particularly alcohol) on common chronic illnesses such as liver diseases (Rehm et al., 2010), diabetes (Howard, Arnsten, & Gourevitch, 2004), sleep disorders (Roehrs & Roth, 2001), psychiatric disorders (Gannon, Qaseem, Snow, & Turner, 2011; Mertens, Lu, Parthasarathy, Moore, & Weisner, 2003), chronic pain (Martell et al., 2007; Mertens, et al., 2003), and various cancers (Islami et al., 2010; Ladeiras-Lopes et al., 2008; Li et al., 2010; Parsons, Daley, Begh, & Aveyard, 2010; Rehm et al., 2009; Tanaka et al., 2008). In addition, adherence to medications – a well-known cause of poor treatment outcomes and unnecessary costs – deteriorates significantly as a function of the number of drinks per day. Even three drinks per day can markedly reduce medication adherence (Golin et al., 2002; Grodensky, Golin, David, & Turner, 2011) and lead to relapse and re-treatment.
Recognizing this, clinical researchers (Babor et al., 2007; Fleming & Graham, 2001; Fleming et al., 2000; Gentilello et al., 1999; Madras et al., 2009) have developed screening and brief interventions (SBI) to identify harmful levels of substance use and to counsel and motivate patients on ways of reducing their “medically harmful” substance use. Two decades of SBI research have documented that a simple motivational conversation between a trained healthcare provider and a patient can produce significant and long lasting reductions in the patient’s substance use, with corresponding improvements in health and reductions in medical care utilization (Fleming, et al., 2000; Krupski et al., 2010). Thus, it is in the interests of generalist physicians to screen and address harmful substance use to improve the quality and efficiency of the medical care they are providing. Of course, this can only happen if patients are asked about their substance use, if they feel comfortable and safe reporting that use, and if their information is confidentially and securely stored within EHRS for healthcare providers to use.
Public Health Importance of EHRS
There are additional benefits of EHRS above and beyond improving the quality and effectiveness of patient care. Substance use data captured in EHRS may serve public health purposes, such as disease surveillance or early warning, quality improvement, as well as clinical, epidemiological and/or health services research. Such a data collection advancement has the potential to accelerate the translation of new knowledge into mainstream care and ultimately improve patient health and healthcare (Califf et al., 2002).
Changes in Healthcare Payments and Regulations
The Patient Protection and Affordable Care Act (2010), or “ACA,” could revolutionize care for substance use disorders. The following summarize key provisions of ACA(United States., United States. Congress. House. Office of the Legislative Counsel, United States. Congress. House. Committee on Ways and Means., United States. Congress. House. Committee on Energy and Commerce., & United States. Congress. House. Committee on Education and Labor., 2010). . Prevention, early intervention, office-based primary care, and specialty care comprise the full spectrum of care services for substance use disorders within ACA, and are considered “essential health benefits” of healthcare. By 2014, all health plans will be required to provide this full continuum of services for substance use disorders. In turn, and at this writing, changes are being implemented in all federal and many private insurance reimbursement codes. These changes will make available services never before covered, such as prevention, screening, early intervention, FDA-approved medications, tele-monitoring visits and most of the other standard benefits now available to patients with other chronic illnesses.
In parallel, most of the contemporary healthcare regulatory and quality assurance groups such as the National Quality Forum, the National Committee on Quality Assurance, and the Joint Commission on Accreditation of Healthcare have all indicated they will be adding substance abuse identification and quality of care indicators as part of accreditation examinations for general medical settings.
Part 2 – Federal Role in Building Electronic Health Record Systems
Access to Electronic Health Record Systems
Recognizing that less than half of all healthcare providers currently have access to any type of EHRS, the federal government instituted the Health Information Technology for Economic and Clinical Health Act (HITECH Act) (“Health Information Technology for Economic and Clinical Health Act (HITECH Act),” 2009) as part of the 2010 financial stimulus program (American Recovery and Reinvestment Act, 2009) (“Health Information Technology for Economic and Clinical Health Act (HITECH Act),” 2009). In that federal law – a separate supplement to the healthcare reform legislation – physician offices and healthcare systems would become eligible for up to $44,000 from Medicare or $63,750 from Medicaid per eligible physician (2010) to purchase necessary EHRS equipment and to implement electronic data collection and record keeping.
Qualifying Role of “Meaningful Use”
Not all physicians and not all healthcare systems are eligible for these funds, only those who could meet criteria for “meaningful use” of these systems with certified technologies were eligible (2010) – essentially providers who were large enough and willing to commit to changing their healthcare information approaches were targeted. Thus, in addition to the push to implement EHRS from federal legislation and regulatory agencies, there is the pull of start-up funding from the HITECH Act and the potential for more efficient, effective and safe healthcare for providers. The availability of improved technology will soon make it not only necessary but smart and easy for healthcare providers to collect and integrate patient substance use information into their normal clinical and administrative activities. The next question is how this integration can take place in a sensible and secure manner.
PART 3 – Integrating Substance Use Information into Electronic Health Record Systems
Substance use, particularly use of illicit substances, has always been stigmatized and there is a long history of discrimination against substance abusers by insurers and healthcare providers. Recognizing this, special federal protections on patient information, unique to substance abuse, were instituted in the 1970’s to restrict access to this sensitive information even from healthcare providers. While all other healthcare information is regulated by the Health Insurance Portability and Accountability Act of 1996 (HIPAA) (United States. Congress (104th 2nd session : 1996), 1996), information associated with the care of patients with substance abuse disorders is regulated by provisions concerning the Confidentiality of Alcohol and Drug Abuse Patient Records (42 CFR Part 2) (1987 (1975).
Thus, while there are clear and important public health and safety benefits from integrating care for, and information about, patients’ substance use into mainstream medicine, special care is required to safeguard patients’ sensitive health information while simultaneously making that information legally accessible to healthcare providers.
Federal Efforts to Reconcile HIPAA and 42 CFR Part 2
Recognizing the growing public concerns about confidentiality and security of healthcare information, the recent federal HITECH legislation designed to foster broad utilization of EHRS by clinicians also added provisions to widen the scope of privacy and security protections under the HIPAA (Stimulus : American Recovery and Reinvestment Act of 2009 Public Law 111-5 Official Text, 2009). Specifically, the HITECH Act increased the legal liability of providers and provider organizations that were guilty of non-compliance with HIPAA, and it provided greater resources for enforcement (“HIPAA administrative simplification: enforcement. Interim final rule; request for comments,” 2009).
Nonetheless, the regulations governing information sharing in the treatment of substance abuse are still much more conservative than those found in HIPAA, due in part to the historical context under which 42 CFR, Part 2 was written. That privacy regulation, specific to substance abuse treatment providers, was enacted by Congress in 1972 and made it illegal for those treatment programs to disclose any patient identifying information without an explicit patient consent – even to another healthcare provider. This strict privacy provision was enacted under the reality that patients’ substance use information could make them legally vulnerable, and that there were no available treatments for substance use disorders in general medical settings. Until the recent ACA legislation, it was reasonable to assume that there was more risk than benefit to patients from sharing their substance use information with general healthcare providers.
However, the passing of ACA has changed the risk-benefit ratio and prompted debate on whether and how to modify HIPAA and/or 42CFR to permit greater sharing of patient information without creating greater patient vulnerability. A committee of attorneys (The Patient Protection Coalition, 2010 Febuary ) proposed an amendment to 42 CFR Part 2, which would eliminate the requirement for patient consent on the “minimum necessary” clinical information as defined by standards which are currently described under HIPAA. However, many patients’ privacy rights advocates strongly oppose modification of 42 CFR Part 2 and insist on the enhanced protections it affords (Deborah, 2011 February 25; Legal Action Center, 2010 January ; Salomon et al., 2010; Wright et al., 2010). Indeed, 42% of general healthcare consumers said that they would not feel comfortable if their doctors shared their healthcare information with other entities even if their personal identifying information were removed (Undem, 2010).
Data Segmentation and Patient Consent Technology to Protect Patient Privacy
Both the HIPAA and the 42 CFR Part 2 laws governing patient information sharing were enacted before the extraordinary advances in digital technology. In the past, “sharing patient information” literally meant transferring a patient’s medical chart (or significant parts of it) from one location to another, with any number of individuals able to gain access. Today, information management technology has advanced in sophistication and ease of use to the point where health information on EHRS can be stored safely in a single location (e.g. a server), information may be segmented into very small sections or elements (e.g. a laboratory test, a diagnostic evaluation), and access may be governed by patients by designating different levels of protection/access for different information elements. In turn, this data segmentation technology could allow specific parts of the EHRS to be controlled by different privacy policies (Melissa M. Goldstein, 2010 September).
For example, some vital statistics concerning health conditions and medications which may be critical to saving a patient’s life (e.g. preventing severe and dangerous drug-drug interactions) could become “core vital information” eligible to be shared among all healthcare providers without patient written consent – but with stiff penalties for disclosure outside the boundaries of healthcare delivery. In the same record, there could be important but less critical information which a patient might elect to withhold from some providers or insurers – to maintain a level of privacy and confidentiality protection. For example, records of patients in specialty addiction treatment programs are considered “sensitive health information” (National Committee on Vital and Health Statistics (NCVHS), 2010 November) which could be protected by data segmentation. The Substance Abuse and Mental Health Services Administration (SAMHSA) and the Office of the National Coordinator for Health Information Technology (ONC) are working to develop computerized consent privacy policies for data segmentation. These are intended to be used in the Nationwide Health Information Network (NHIN) (Substance Abuse and Mental Health Services Administration (SAMHSA), 2010 October). This is likely to have broad practical appeal, as a recent study found that almost all consumers surveyed thought that they should be given control of how their health care data would be shared (Schneider, Kerwin, Robins, & Dean, 2009). At the same time, healthcare providers (both specialty and general) are facing requirements to minimize unsafe practices (e.g. drug-drug interactions, etc.). These providers will likely ask patients to consent to some minimal, necessary information sharing requirements as a condition of agreeing to treat them (McCarty, McConnell, & Schmidt, 2010).
PART 4 – NIDA’s Effort to Develop Expert Consensus on Common Clinical Data Elements for EHRS
Since 2001, the National Library of Medicine (NLM) at NIH (National Institutes of Health (NIH), 1993) has been mandated by the U.S. Congress to explore the feasibility of applying Health Information Technology (HIT) in health care and research. To that end, NIH initiated “National Electronic Clinical Trials and Research” (NECTAR) projects in 2006 to test the interoperability of different HIT platforms. Concurrently, many institutes at the NIH began developing sets of standardized common data elements (CDEs) within these platforms to be used in clinical trials and potentially in clinical practice. In this NIH-wide effort, NIDA tasked its Clinical Trials Network (CTN) to lead the initiative to develop a set of standardized CDEs for substance use research that could also become part of EHRS. NIDA’s priority in developing substance abuse CDEs for EHRS is a small core set of questions to screen for patient substance use in the primary care setting.
The primary rationale for this focus is to help foster research on, and advance the practice of, screening, brief intervention and referral to treatment (SBIRT) for patients with substance use problems (Babor, et al., 2007; Fleming, et al., 2000; Madras, et al., 2009; Saitz et al., 2010). It is expected that this small set of screening items will be widely used in general medical settings. When screening indications are positive, patients will be asked additional questions to provide more detailed information for specialty treatment settings. The specific goals of this initiative are: 1) to develop standardized, expert-defined CDEs of substance use for incorporation into EHRS; 2) to ensure the data standards incorporate certified technology which allows interoperability with other systems; 3) to meet current and future “Meaningful Use” criteria; and 4) to collaborate with partner NIH institutes and other federal agencies to realize these goals.
Development of Substance Use CDEs
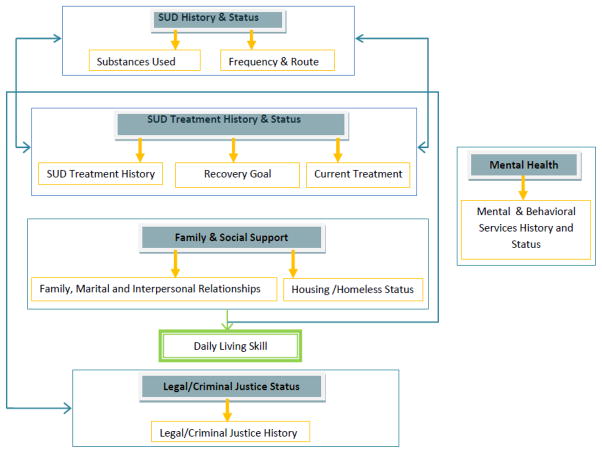
At the outset of this initiative, NIDA’s CTN formed an EHRS workgroup comprised of researchers, community treatment providers, technical consultants from the CTN’s data and statistics coordinating center (The EMMES Corporation) and NIDA staff. This workgroup solicited and arrayed 62 sets of paper-based and/or computer-based treatment records from the CTN’s community-based specialty addiction treatment programs. Additional sets of patients records were collected from large healthcare systems such as Kaiser Permanente, the Department of Veterans Affairs (VA). After considering issues such as health system interoperability, ability to permit patient data aggregation, clinical research and health services research (Gliklich & Dreyer, 2010), the substance use items (i.e. CDEs) were organized into clinically relevant domains and sub-domains. (see Figure 1).
Figure 1.
Substance Use EHRS Five Domains (gray filled-box) and Nine Sub-Domains (gold frame)
Consensus Building
The draft substance use domains and sub-domains of the recommended CDEs were further reviewed and refined through a series of consensus building meetings with interested and relevant parties. The first set included agencies charged with direct provision or regulation of prevention and services, such as the Department of Veterans Affairs, SAMHSA, the Indian Health Service (IHS), the Centers for Disease Control and Prevention (CDC), the Health Resources and Services Administration (HRSA), and the Centers for Medicare and Medicaid Services (CMS). The second set was agencies responsible for administrative regulation or oversight of substance use issues. These included the Health Information Technology Certification and Utilization section of the ONC, the Agency for Healthcare Research and Quality (AHRQ), the NLM of NIH, and the Office of National Drug Control Policy (ONDCP). Beyond the federal agencies listed above, meetings were also held with attendees from a wide variety of healthcare providers’ professional societies, particularly those that serve substance abuse patients.
Recommendations for Three Core Questions
This review process, covering a wide range of behavioral health screening, diagnosis and assessment tools, ultimately led to a recommendation to use three screening questions and (when screening is positive) individual assessment tools for tobacco, alcohol and other substance use (see Table 1) (“EHR meeting executive summary”).
Table 1.
Consensus Substance Use Screening Items in Primary Healthcare Settings
| Substance Screened | 1st Single Screening Question
|
Answer “yes”
|
2nd Screening Questions |
|---|---|---|---|
| Tobacco | Have you used tobacco, smoked cigarettes or used smokeless tobacco product in the last 30 days | yes | Would you be interested in quitting tobacco use within the next few weeks? |
| Alcohol | How many times in the past year have you had X or more drinks in a day? (X is 5 for men and 4 for women) | yes | three questions from the Alcohol Use Disorders Interview (AUDIT-C)1 -2 |
| Other drug | How many times in the past year have you used an illegal drug or used a prescription medication for non-medical reasons? 3 | yes | ten questions from Drug Abuse Screening Test (DAST10)4–5 |
Saunders, Aasland et al. 1993;
Bush, et al. 1998;
Smith, 2010;
Skinner, 1982;
Yudko, 2007.
Next Steps in Information Integration Within EHRS
As is apparent from Table 1, the three recommended screening items have different time frames (one year, past 30 days). These differences will likely produce confusion for both clinicians and patients. Thus NIDA is continuing to work toward evidence-based consensus on a common screening time frame for tobacco, alcohol and other drug use questions. An additional question is how often these core questions should be asked in general health settings: – upon first visit to a new provider? at every visit to any provider? once or twice per year? There is a need for empirical determination of action thresholds; It is likely that different thresholds will emerge for patients with different illnesses, different combinations of illnesses and at different levels of severity/acuity of the illness. For example, the research literature on the effects of alcohol on chronic sleep disorders and breast cancer is rather clear in showing that any amount of alcohol is a potential impediment to ongoing care (Arroll, Fernando, & Falloon, 2008; Kwan et al., 2010). However, it is also clear that one (for women) to two (for men) drinks of alcohol – but no more than that – are not disruptive to and can even be helpful in the management of some forms of hypertension (Sesso, Cook, Buring, Manson, & Gaziano, 2008; Thadhani et al., 2002) and diabetes (Davies et al., 2002; Koppes, Dekker, Hendriks, Bouter, & Heine, 2005; Wannamethee, Shaper, Perry, & Alberti, 2002)).
The immediate next steps for NIDA are to place the small core set of CDEs into EHRS platforms to permit validation in real world settings and for the purposes they were suggested, such as SBIRT in general medical settings. In these tests, it will be critical to assess the feasibility, interoperability and general utility of these items and the “new” clinical procedures which they are expected to foster. These evaluations may also serve as an important platform for health care process performance and effectiveness studies, as well as implementation, health services and clinical research. One such activity is NIDA’s current collaboration with the NLM of the NIH and SAMHSA to develop Systematized Nomenclature of Medicine Clinical Terms codes (SNOMED CT) and Logical Observations Identifiers, Names, Codes (LONIC) for these CDEs to make them electronically interoperable within the SAMHSA’s SBIRT grant programs. In addition, NIDA is taking the lead in developing the Healthcare Information Technology Standards Panel (HITSP)’s “e-measure” specifications for quality performance reporting. In all these activities, NIDA will continue to consult with all principal stakeholders in the creation of additional CDEs to be recommended for health information collection and sharing in EHRS.
CONCLUSION
There are now overwhelming arguments favoring the inclusion of patient substance use information into EHRS, and favoring the integration of care for identified “medically harmful substance use” into general healthcare and health insurance. Done properly this type of integration should produce much-needed improvements in patient safety and health outcomes, as well as important reductions in healthcare costs and threats to public health and patients’ safety. Important additional effects of properly implemented integrated substance abuse care are reductions in stigma and with it the engagement of more affected individuals into treatment. The real questions facing the healthcare field are how to perform this much-needed integration and with what patient privacy protections in the era when health information technology is widely adopted and implemented.
It is impossible to definitively predict the resolution to all the legitimate information collection, storage and sharing issues associated with the integration of substance use information into general healthcare through EHRS. Debates continue at this writing, and various conceptual models of data segmentation, control and access have emerged (Melissa M. Goldstein, 2010 September). Ultimately, components of a good data segmentation model will have to offer acceptable balance across the concerns of patients, providers, Federal and State governments, institutions and organizations such as health information organizations. Yet as the healthcare field increases the accessibility of patient information to achieve greater safety, quality and efficiency, all patients – not just those with substance use disorders – are raising concerns over access to and control of that accessibility. To whom does a patient’s healthcare record belong? Who has the right to access a patient’s healthcare record – and who decides? Should there be limits on patients’ control of their own healthcare records? For example, does a patient with a serious communicable disease have the right to keep that information from involved healthcare providers, or from public health departments? What are a physician’s rights and obligations to a patient who would not permit information sharing? For example, can a primary care physician safely prescribe an opioid for pain symptoms if s/he cannot have access to the patient’s history of substance abuse-related information, or if the patient refuses to allow the prescription information to be part of his/her records? These are serious questions and they must be answered sensibly and practically if the promise of fully integrated healthcare and healthcare information is to be realized. In this regard, it is important to acknowledge that any information sharing and confidentiality answers derived from these discussions will be temporary at best, because the science and technology in this field is advancing at a remarkable rate. Many of the information problems we currently face will be resolved with new technologies in the near future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Recovery and Reinvestment Act of 2009 H.R.1(2009).
- Arroll B, Fernando A, 3rd, Falloon K. Sleep disorder (insomnia) BMJ. 2008;337:a1245. doi: 10.1136/bmj.a1245. [DOI] [PubMed] [Google Scholar]
- Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- Califf RM, Peterson ED, Gibbons RJ, Garson A, Jr, Brindis RG, Beller GA, et al. Integrating quality into the cycle of therapeutic development. Journal of the American College of Cardiology. 2002;40(11):1895–1901. doi: 10.1016/s0735-1097(02)02537-8. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services (CMS) Medicare and Medicaid programs; electronic health record incentive program. Final rule. Federal Register. 2010;75(144):44313–44588. [PubMed] [Google Scholar]
- Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287(19):2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- Deborah CP. Re: HIT policy committee meaningful use workgroup request for comments regarding meaningful use stage 2. 2011 Feb 25; Retrieved 2011 April 27, 2011, from http://patientprivacyrights.org/wp-content/uploads/2011/03/PPR-MU-Stage-2-Comments.pdf.
- EHR meeting executive summary. Identifying Core Behavioral and Psychosocial Data Elements for the Electronic Health Record. 2011 May 2–3; Retrieved July 8 2011, from http://conferences.thehillgroup.com/OBSSR/EHR2011/resources.html.
- Fleming MF, Graham AW. Screening and brief interventions for alcohol use disorders in managed care settings. Recent Developments in Alcoholism. 2001;15:393–416. doi: 10.1007/978-0-306-47193-3_22. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Mundt MP, French MT, Manwell LB, Stauffacher EA, Barry KL. Benefit-cost analysis of brief physician advice with problem drinkers in primary care settings. Medical Care. 2000;38(1):7–18. doi: 10.1097/00005650-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Gannon MA, Qaseem A, Snow V, Turner B. Raising achievement: educating physicians to address effects of at-risk drinking on common diseases. Qual Prim Care. 2011;19(1):43–47. [PubMed] [Google Scholar]
- Gentilello LM, Rivara FP, Donovan DM, Jurkovich GJ, Daranciang E, Dunn CW, et al. Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Annals of Surgery. 1999;230(4):473–480. doi: 10.1097/00000658-199910000-00003. discussion 480–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliklich R, Dreyer N. Registries for evaluating patient outcomes: a user’s guide. 2010. [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, et al. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine. 2002;17(10):756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodensky C, Golin C, David R, Turner B. Unpublished Systematic Review. Rober Wood Johnson Foundation; 2011. Systematic Review: Effect of Alcohol Intake on Adherence to Outpatient Medication Regimens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIPAA administrative simplification: enforcement. Interim final rule; request for comments. Federal Register. 2009;74(209):56123–56131. [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Annals of Internal Medicine. 2004;140(3):211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- Islami F, Tramacere I, Rota M, Bagnardi V, Fedirko V, Scotti L, et al. Alcohol drinking and laryngeal cancer: overall and dose-risk relation--a systematic review and meta-analysis. Oral Oncology. 2010;46(11):802–810. doi: 10.1016/j.oraloncology.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28(3):719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- Krupski A, Sears JM, Joesch JM, Estee S, He L, Dunn C, et al. Impact of brief interventions and brief treatment on admissions to chemical dependency treatment. Drug and Alcohol Dependence. 2010;110(1–2):126–136. doi: 10.1016/j.drugalcdep.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. Journal of Clinical Oncology. 2010;28(29):4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes and Control. 2008;19(7):689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- Legal Action Center. Confidentiality of alcohol and drug records in the 21st century. 2010 Jan; Retrieved from http://www.lac.org/doc_library/lac/publications/Confidentiality_of_Alcohol_and_Drug_Records_in_the_21st_Century-1-20-10.pdf.
- Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women’s health initiative observational study. Journal of the National Cancer Institute. 2010;102(18):1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug and Alcohol Dependence. 2009;99(1–3):280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Annals of Internal Medicine. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- McCarty D, McConnell KJ, Schmidt LA. Priorities for policy research on treatments for alcohol and drug use disorders. Journal of Substance Abuse Treatment. 2010;39(2):87–95. doi: 10.1016/j.jsat.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Melissa M, Goldstein ALR, Heesters MSMelissa M. Data Segmentation In Electronic Health Information Exchange. 2010 Sep; Retrieved from http://healthit.hhs.gov/portal/server.pt?open=512&objID=1147&parentname=CommunityPage&parentid=10&mode=2&in_hi_userid=11113&cached=true&in_hi_userid=11673.
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Archives of Internal Medicine. 2003;163(20):2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- National Committee on Vital and Health Statistics (NCVHS) Recommendations Regarding Sensitive Health Information. 2010 Nov; Retrieved from http://www.ncvhs.hhs.gov/101110lt.pdf.
- Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP, Barker GE, Eguchi MM. A steep increase in domestic fatal medication errors with use of alcohol and/or street drugs. Archives of Internal Medicine. 2008;168(14):1561–1566. doi: 10.1001/archinte.168.14.1561. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29(4):437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5(4):287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Saitz R, Alford DP, Bernstein J, Cheng DM, Samet J, Palfai T. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4(3):123–130. doi: 10.1097/ADM.0b013e3181db6b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RM, Blackford JU, Rosenbloom ST, Seidel S, Clayton EW, Dilts DM, et al. Openness of patients’ reporting with use of electronic records: psychiatric clinicians’ views. Journal of the American Medical Informatics Association. 2010;17(1):54–60. doi: 10.1197/jamia.M3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SJ, Kerwin J, Robins C, Dean D. Consumer Engagement in Developing Electronic Health Information Systems Final Report. 2009. [Google Scholar]
- Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008;51(4):1080–1087. doi: 10.1161/HYPERTENSIONAHA.107.104968. [DOI] [PubMed] [Google Scholar]
- Stimulus : American Recovery and Reinvestment Act of 2009 Public Law 111-5 Official Text. Lanham, MD: Government Institutes/Bernan Press; 2009. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Leading Change: A Plan for SAMHSA’s Roles and Actions 2011–2014. 2010 Oct; Retrieved from http://store.samhsa.gov/shin/content//SMA11-4629/01-FullDocument.pdf.
- Tanaka K, Tsuji I, Wakai K, Nagata C, Mizoue T, Inoue M, et al. Alcohol drinking and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Japanese Journal of Clinical Oncology. 2008;38(12):816–838. doi: 10.1093/jjco/hyn108. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Camargo CA, Jr, Stampfer MJ, Curhan GC, Willett WC, Rimm EB. Prospective study of moderate alcohol consumption and risk of hypertension in young women. Archives of Internal Medicine. 2002;162(5):569–574. doi: 10.1001/archinte.162.5.569. [DOI] [PubMed] [Google Scholar]
- The Patient Protection Coalition. A Proposal to Promote Coordination of Care and to Strengthening Patient Protections under the Federal Alcohol and Drug Abuse Confidentiality Law. 2010 Febuary; Retrieved 2011 April 27, 2011, from www.law.virginia.edu/pdf/faculty/bonnie_patientprotection.pdf.
- Turner BJ, McLellan AT. Methodological challenges and limitations of research on alcohol consumption and effect on common clinical conditions: evidence from six systematic reviews. Journal of General Internal Medicine. 2009;24(10):1156–1160. doi: 10.1007/s11606-009-1072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Confidentiality of alcohol and drug abuse patient records--PHS. Final rule. Federal Register. 1987;52(110):21796–21814. [PubMed] [Google Scholar]
- U.S. Department of Health Education and Welfare. Confidentiality of alcohol and drug abuse patient records. Federal Register. 1975;40(91):20522–20542. [PubMed] [Google Scholar]
- Undem T. Consumers and health information technology: a national survey. 2010 Retrieved 2011 July 27, 2011, from http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ConsumersHealthInfoTechnologyNationalSurvey.pdf.
- United States., United States. Congress. House. Office of the Legislative Counsel, United States. Congress. House. Committee on Ways and Means., United States. Congress. House. Committee on Energy and Commerce., & United States. Congress. House. Committee on Education and Labor. Compilation of Patient Protection and Affordable Care Act : as amended through November 1, 2010 including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. Washington: U.S. Government Printing Office; 2010. [Google Scholar]
- United States. Congress (104th 2nd session : 1996) Health Insurance Portability and Accountability Act of 1996 : conference report (to accompany H.R. 3103) Washington, D.C: U.S. G.P.O; 1996. [Google Scholar]
- Walley AY, Farrar D, Cheng DM, Alford DP, Samet JH. Are opioid dependence and methadone maintenance treatment (MMT) documented in the medical record? A patient safety issue. Journal of General Internal Medicine. 2009;24(9):1007–1011. doi: 10.1007/s11606-009-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Perry IJ, Alberti KG. Alcohol consumption and the incidence of type II diabetes. Journal of Epidemiology and Community Health. 2002;56(7):542–548. doi: 10.1136/jech.56.7.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Soran C, Jenter CA, Volk LA, Bates DW, Simon SR. Physician attitudes toward health information exchange: results of a statewide survey. Journal of the American Medical Informatics Association. 2010;17(1):66–70. doi: 10.1197/jamia.M3241. [DOI] [PMC free article] [PubMed] [Google Scholar]