Abstract
Early crystal structures of prokaryotic CLC proteins identified three Cl– binding sites: internal (Sint), central (Scen), and external (Sext). A conserved external GLU (GLUex) residue acts as a gate competing for Sext. Recently, the first crystal structure of a eukaryotic transporter, CmCLC, revealed that in this transporter GLUex competes instead for Scen. Here, we use molecular dynamics simulations to investigate Cl– transport through CmCLC. The gating and Cl–/H+ transport cycle are inferred through comparative molecular dynamics simulations with protonated and deprotonated GLUex in the presence/absence of external potentials. Adaptive biasing force calculations are employed to estimate the potential of mean force profiles associated with transport of a Cl– ion from Sext to Sint, depending on the Cl– occupancy of other sites. Our simulations demonstrate that protonation of GLUex is essential for Cl– transport from Sext to Scen. The Scen site may be occupied by two Cl– ions simultaneously due to a high energy barrier (∼8 Kcal/mol) for a single Cl– ion to translocate from Scen to Sint. Binding two Cl– ions to Scen induces a continuous water wire from Scen to the extracellular solution through the side chain of the GLUex gate. This may initiate deprotonation of GLUex, which then drives the two Cl– ions out of Scen toward the intracellular side via two putative Cl– transport paths. Finally, a conformational cycle is proposed that would account for the exchange stoichiometry.
Introduction
The CLC membrane protein family consists of nine members in mammalian species, which play critical roles in regulating the membrane action potential, muscle excitability (CLC-1), renal intravascular transport (CLC-K and CLC-5), and cell flexibility (CLC-2 and CLC-3) (1–4). The CLC superfamily includes both prokaryotic and eukaryotic members. It is composed of two subclasses: channels for selective permeation of Cl– ions and transporters for exchange of two Cl– ions in one direction with one proton in the opposite direction (5–7). Despite fundamental differences in functionality, members of the CLC family of channel/transporter protein are believed to share structural similarity (3,8,9) and its channel members are now thought to be evolutionally degraded transporters (3). The CLC protein is a dimer that adopts a double-barreled configuration in the transmembrane (TM) domain (8,10). Each subunit comprises an independent ion transport pore (11) regulated by a fast gate (3). The eukaryotic family members possess an additional cytosolic cystathionine β-synthase domain on the intracellular side (3,12).
Early x-ray crystallization of prokaryotic EcCLC and StCLC transporters (8,10) identified a narrow 15 Å selectivity filter region inside the transporters with two anion-binding sites: one near the intracellular entrance to the selectivity filter (Sint), and the other in the center of the filter (Scen). The putative gating glutamate residue (GLUex; E148 in EcCLC) occupied the third anion binding site (Sext) near the extracellular entrance to the selectivity filter. Recently, the first x-ray crystal structure of a eukaryotic CLC transporter from the thermophilic red alga Cyanidioschyzon merolae (CmCLC) revealed that the homologous GLUex gate (E210 in CmCLC) occupied the Scen site instead of Sext (12). How this conformation relates to the Cl– transport cycle remains elusive due to the lack of a complete set of intermediate conformation states (12). In the CmCLC transport cycle model provided by MacKinnon's group (12), one major assumption is the existence of a high energy barrier that prevents a rapid movement of Cl– ions from Scen to Sint.
Two (8,10,12) and three (13) anion binding sites identified in the x-ray crystal structures point to the existence of a curved Cl– ion transport route connecting the intracellular and extracellular solutions. The GLUex gate, together with a central gate formed by a conserved SER residue (S107 in EcCLC and S165 in CmCLC) and TYR residue (Y445 in EcCLC and Y515 in CmCLC), are proposed to coordinate the transport of Cl– (1,3). Whereas Cl– binding to Scen is suggested to be essential for coupled proton translocation (14,15) in EcCLC, in the newly resolved CmCLC crystal structure (12), the GLUex gate rather than Cl– occupies Scen. This finding raises the possibility that occupancy of Scen by the GLUex gate residue in CmCLC may play a similar role as found for a Cl– ion at the Scen site in EcCLC (14,15).
At present, the proton transport pathway through CLC transporters is largely unknown because crystallographic resolution is not high enough to capture proton binding. It has been proposed that a common path is shared by protons and Cl– for translocation from the extracellular solution to the GLUex gate, but that the two routes bifurcate from GLUex toward the intracellular solution for chloride versus proton transport (16). In the bacterial transporters, two glutamate residues near the extracellular (E148 in EcCLC) and intracellular solution (E203 in EcCLC) are suggested as dual gates (GLUex and GLUin) (3,16–19). Theoretical studies of the proton transport (20–22) broadly agree with experimental implications. Recent reactive molecular dynamics (MD) simulations (23) have shown that Cl– binding to both Scen and Sint sites is essential for proton transport from the GLUin to GLUex gate in the bacterial EcCLC transporter.
Several types of CLC transporters exchange chloride ions with protons in a tight 2:1 stoichiometry (2,3,17,18). Currently, there is no definitive explanation how the Cl– ion and proton motions are coupled. Transport cycle models for the CLC transporters have been proposed by Beck (22), Accardi (24), Maduke (25), Chen (26), and, recently, Miloshevsky and Jordan (27) and MacKinnon's group (12). These models have a common feature: the transport cycle of 2Cl−/H+ is determined by alternately exposing substrate binding sites through appropriate protein conformation changes (25,27). Miller and Nguitragool (6) proposed a fundamentally different model, in which the exchange stoichiometry is resolved by simultaneous binding of two Cl− and one proton at the Scen site. However, this model requires the formation of HCl and the opening of the inner gate (GLUin) twice per transport cycle (6).
Extant crystal structures have revealed several stable intermediate conformations. However, a complete understanding of the CLC transport cycle will require identification of several as yet undiscovered CLC conformation states (12). To complement experimental efforts, MD simulation (20,28,29) and coarse-grained modeling (21,22,27,30) have emerged as valuable tools for elucidating dynamical processes within CLC proteins. In this study, we employ MD to investigate the Cl– transport cycle and exchange stoichiometry in CmCLC. Free energy calculations are performed to test the CmCLC transport cycle model provided by MacKinnon's group (12).
Methods
MD simulations
Three missing loops (L295 to D314, F375 to P382, and V393 to T404) in the TM domain of the CmCLC crystal structure (PDB: 3ORG) (12) were reconstructed based on the bacterial EcCLC (PDB: 1OTS) (8) using MODELER 9.0 for homology modeling (31). The missing loop between V600 and V656 in the extracellular domain was not restored, but we restrained Cα of residues (I588 to V600, and V656 to V666) near this missing loop using harmonic restraints (1.0 kcal·mol−1·Å−2) during the MD simulations. Subsequently, the TM domain of the CmCLC was inserted into the center of a preequilibrated POPE/POPG (3:1) binary lipid mixture as used in the experimental study (12). Fully equilibrated TIP3 waters and 0.3 M KCl were added to the system to form an all-atom simulation system 100 Å × 135 Å × 106 Å in extent. The complete simulation system was composed of two subunits, 199 POPE molecules, 63 POPG molecules, and ∼25,000 water molecules for a total of over 128,000 atoms. Fig. 1 illustrates the MD setup of the initial system.
Figure 1.
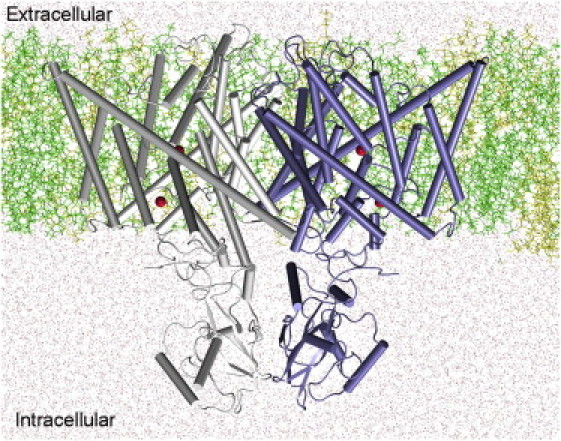
MD setup of CmCLC embedded into a POPE/POPG (3:1) binary membrane lipid bilayer and solvated by symmetric 0.3 M KCl bathing solutions (ions not shown). The two subunits in the CmCLC are colored differently. Spheres (red) depict four Cl− ions found in the crystal structure. POPE and POPG lipid molecules, represented in line format (colored by green and yellow, respectively), constitute the bilayer membrane. Water is shown as dots. The channel axis z runs from the extracellular to intracellular side. (Color online.)
Two control simulation systems were generated using different protonation states of these two GLUex gates: 1), both GLUex residues were protonated (denoted as ApBp or System 1); 2), both GLUex residues were deprotonated (termed as AdBd or System 2). To explore the transport cycle for Cl− ions, we assembled additional simulation systems (Systems 3 to 8) with different Cl− occupancies and protonation states of GLUex gates. Moreover, a Y265A mutation was performed on both subunits of System 2 to further investigate the putative proton transport path. The essential characteristics of each system are summarized in Table 1 and illustrated in Fig. S1 in the Supporting Material.
Table 1.
Summary of simulation systems
| System | Protonation state of E210 | Occupied Cl− sites | Initial protein structure | Simulation length (ns) | External potential Kcal/(mol Å e) |
|---|---|---|---|---|---|
| 1 (APBP) | Protonated A B | Sext and Sint | X-ray | 100 | After 80 ns, −0.1 |
| 2 (AdBd) | Deprotonated A B | Sext and Sint | X-ray | 100 | After 80 ns, −0.1 |
| 3 | Deprotonated A B | Scen | 84 ns of ApBp | 8 | −0.1 |
| 4 | AdBp∗ | Sext and Sint | 80 ns of ApBp | 50 | −0.1 |
| 5 | AdBp∗ | Scen and Sext | Snapshot from 4‡ | 20 | −0.1 |
| 6 | AdBp∗ | Sint Scen and Sext | Snapshot from 4‡ | 40 | −0.1 |
| 7 | Deprotonated A B | Scen | Snapshot from 5 | 40 | −0.1 |
| 8 | Deprotonated A B | Scen | Snapshot from 5 | 30 | −0.1 |
| Y265A | Deprotonated A B | Sext and Sint | X-ray | 14 ns | 0.0 |
Deprotonated E210 in subunit A and protonated E210 in subunit B.
Cl− ion at Sext was docked manually.
Simulations were carried out using NAMD (32) following standard MD procedure. The CHARMM27 force field with CMAP corrections (version 31) was used for protein, water, and lipids (33). For Systems 1 and 2, the system was first energy minimized for 20,000 conjugate gradient steps and subsequently equilibrated for 2 ns, during which the backbone constraint on CmCLC was gradually reduced from 10 kcal·mol−1·Å−2 to zero. These two control systems were subjected to unrestrained Nosé-Hoover constant pressure (P = 1 Bar) and temperature (T = 310 K) (NPT) (34,35) simulations for 80 ns, which were then continued for another 20 ns under an applied electric field (36) of −0.1 Kcal/(mol Å e) along the transmembrane direction (z axis). This applied external potential, corresponding to a −120 mV transmembrane potential (assuming the membrane thickness is ∼30 Å), drove a Cl− ion from the extracellular side to the intracellular side. Mutated CmCLC simulations were subjected to the same procedure as System 2 and were simulated for up to 14 ns. Systems 3 to 8 were energy minimized for 5,000 steps, followed by 300 ps of equilibration, during which the backbone constraint was gradually released. Subsequently, unrestrained NPT simulation was performed under the same external electric field as in the control systems. A detailed simulation protocol is provided in the Supporting Material.
PMF calculations
The single ion potential of mean force (PMF) profiles for transporting a Cl− ion from Sext to Scen and Sint were calculated along the transport path using the adaptive biasing force (37,38) method implemented in NAMD (32). Energy barriers for Cl− transport were estimated based on the PMF profiles. Detailed adaptive biasing force calculations are presented in the Supporting Material. The GLUex gate E210 is characterized by two dihedral angles, χ1 (defined by N CA CB and CG atoms) and χ2 (defined by CA CB CG and CD atoms); cf. Fig. S2 A. A two-dimensional (2D) dihedral PMF was calculated for both the protonated (ApBp system) and deprotonated (AdBd system) E210 residues using Metadynamics as implemented in NAMD (32). The height and width of the Gaussian potentials employed in these calculations are 0.01 and , respectively. The time interval between Gaussian functions is 0.2 ps. For each system, a total of 16 ns MD simulations were carried out, during which one Cl− ion remained bound to the Sext site identified in the crystal structure (PDB:3ORG). The standard deviations for the PMF calculations were estimated from different runs on different subunits and also from MD simulations of different lengths.
Results and Discussion
Structural stability and variability
The overall stability of CmCLC structures (in the AdBd and ApBp systems) was evaluated using the root mean-square deviation (RMSD) of backbone Cα atoms away from the initial configurations. The two protein structures, with either protonated or deprotonated GLUex gate (E210), displayed a stabilized RMSD after ∼10 ns MD simulations, with the steady-state RMSD from the initial configuration being ∼2.7 ± 0.3 Å. The most significant deviation resulted from the reconstructed loops that were missing from the crystal structure: the RMSD was reduced to 2.0 ± 0.2 Å when these missing loops were omitted from the calculations.
In the initial x-ray structure, two hydrogen bonds between the two oxygen atoms from the carboxyl group of E210 were formed, with the hydroxyl groups of S165 and Y515 (12). These two hydrogen bonds assisted the binding of the GLUex gate to the Scen site. During the simulations, the two hydrogen bonds eventually broke, thereby inducing conformational changes of the Sext and Scen sites. Fig. 2 compares the MD-relaxed structures with the corresponding initial configurations under different protonation states of the GLUex gate. Protonating the GLUex gate (E210) generated a local reorganization of the side-chain orientation of E210 or the nearby Y265 residue (not shown) and no significant secondary structural changes were observed due to protonation.
Figure 2.
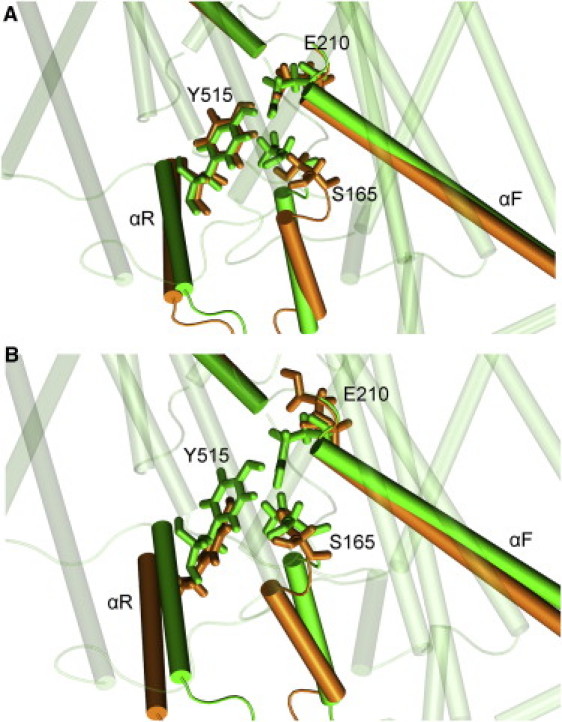
Alignment of initial configuration (colored in white) (green in the online figure) with MD equilibrated structure (colored in dark gray) (orange in the online figure) in the: (A) AdBd system and (B) ApBp system. Extracellular region is at top of plot, and intracellular region is at the bottom. (Color online.)
Protonation of the GLUex gate facilitates Cl− transport from Sext to Scen
The side-chain orientation of the GLUex gate is characterized by its dihedral angles χ1 and χ2. Only two sets of dihedral angles were observed for a deprotonated GLUex gate in our simulations of the AdBd system, one in each of the two subunits: χ1 = 50 ± 20° and χ2 = 80 ± 20°, and χ1 = 80 ± 20° and χ2 = −65 ± 20° (Fig. 3, B and A, respectively). These two dihedral orientations were also observed in Systems 3 to 8 for the deprotonated GLUex gate, as indicated by the two minima appearing in the 2D dihedral PMFs (shown in dark blue in Fig. 4 A). The first set of dihedral angles corresponds roughly to the GLUex orientation found in the x-ray structure. In this orientation, the Cl− ion initially located at the Sext site remained there during the entire simulation (Fig. S3). When the GLUex gate reoriented to the second set of dihedral angles, the binding of the Cl− ion to the Sext site became unstable and Cl− ultimately left the Sext site toward the extracellular solution without any applied potential. For the deprotonated E210, the calculated energy barrier is >30 ± 5 Kcal/mol for Cl− to transport from Sext to Scen in both subunits, with a slightly lower value for the orientation suggested by the crystal structure. Such a high energy barrier completely prevents Cl− translocation from Sext to Scen.
Figure 3.
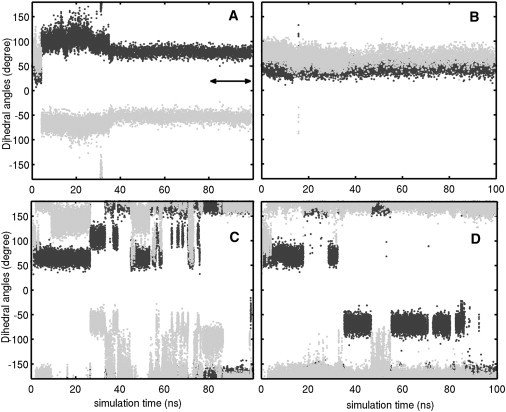
Time evolution of dihedral angles χ1 (black) and χ2 (gray) in (A) subunit A and (B) subunit B of the AdBd system; and in (C) subunit A and (D) subunit B of the ApBp system. Top and bottom panels show simulations with deprotonated and protonated E210, respectively. In both systems, an external potential was applied after 80 ns NPT simulations, as demarcated by the double-arrow line in panel A. Typical orientations of E210 in the different systems are shown in Fig S2 of the Supporting Material.
Figure 4.
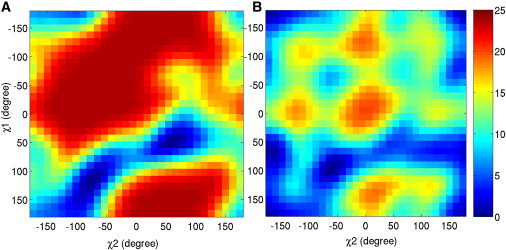
2D dihedral PMFs with (A) deprotonated E210 in the AdBd system and (B) protonated E210 in the ApBp system. PMFs are given in Kcal/mol (see scale bar at right). (Color online.)
Once the GLUex gate was protonated (ApBp system), its dihedral angles χ1 and χ2 significantly deviated from their original values in the crystal structure. The free energy increase for a protonated GLUex gate to adopt the orientation identified in the crystal structure is ∼5.6 ± 1.0 Kcal/mol, as compared to the most favorable orientations for a protonated GLUex gate (Fig. 4 B). This suggests that the GLUex gate in the x-ray structure may be deprotonated. As shown in Fig. 3, C and D, χ1 and χ2 of the protonated GLUex gate displayed a much broader range of values than those found for a deprotonated GLUex, varying between 50 ± 20° and 180° or −50 ± 20° and −180°. Such a large variation of dihedral orientation is consistent with our 2D dihedral PMFs for the protonated E210 (Fig. 4 B). Under an applied external transmembrane potential of −120 mV, within 4 ns the dihedral angles in both protonated GLUex gates quickly evolved to become χ1 = 180° (or −180°) and χ2 = 180° (or −180°) and remained at these values as Cl− transported from Sext to Scen. Simultaneously, a continuous water path was formed from the extracellular solution to the Sext site (Fig. S4). For the protonated GLUex gate, the calculated energy barrier for Cl− transport from Sext to Scen was around 2.5 ± 1.0 Kcal/mol (cf. Fig. 5), which is significantly reduced in comparison to the deprotonated GLUex. Protonation of GLUex opened the gate for Cl− transport (Fig. S5). During 80 ns of the MD simulation without any applied external potential, the side chain of the protonated GLUex gate swung away from the Scen site and gradually rotated toward the Sext site (Fig. S5), consistent with the CmCLC transport model (12) proposed by Mackinnon's group.
Figure 5.
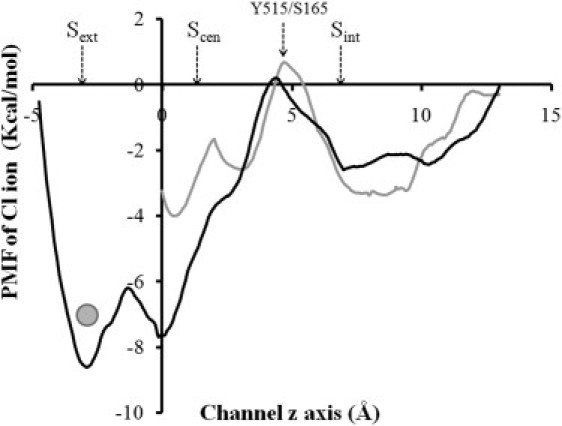
Potentials of mean force for Cl− transport from the Sext site to the intracellular solution in the protonated GLUex system (ApBp system). The single ion PMF is shown via a black line. Gray line represents the PMF for transport of one Cl− ion from Scen to intracellular solution with another Cl− bound to the Sext site. The position of the bound Cl− is indicated by a solid circle in the figure. In the PMF calculations, the bound Cl− ion is restrained using an isotropic three-dimensional harmonic restraint (with force constant of 1.0 kcal·mol−1·Å−2) at the external binding site Sext suggested by the crystal structure (PDB: 3ORG).
Putative proton transport path inferred from continuous water path
In both subunits of the ApBp system with protonated GLUex gates, the αF helix underwent a 5° clockwise rotation toward the intracellular domain and the helix αR (containing Y515) moved (or rotated) toward the intracellular domain (Fig. 2). The motion of the αR helix together with a local reorganization of residues near GLUex induced a stable continuous water wire starting from the intracellular solution and extending to the GLUex gate (Fig. S4).
This continuous water path has been proposed as the proton transport pathway in the bacterial CLC transporters (20–22). Previously, the GLUin gate was suggested to be strictly conserved in CLC transporters but to be substituted by a hydrophobic residue VAL in all-known CLC channels (16). Neutralization of the GLUin gate was found to abolish coupled proton and Cl− transport (16) in EcCLC as well as in CLC-4 and CLC-5 (39). The CmCLC transporter does not possess a GLUin gate. Instead, a hydrophilic THR residue (T269) occupies the homologous position. This THR residue was found to line the water pore observed in our MD simulations. Feng et al. (12) proposed that this THR residue may play the role of the GLUin gate for proton transport. However, the question has been raised as to the ability of THR to act as a proton donor (21). Hence, we examined other residues in CmCLC along this putative proton transport pathway. In addition to E210, T269, and Y515 residues, whose homologous residues have previously been found to be important for proton transport in the bacterial CLC transporter (14,16), Y265 appears to be a good candidate to further test its role in the proton transport process based on our MD simulations. Aromatic Y265 is conserved in the eukaryotic CLC family (see Fig. S1 of (12)) and lines the extracellular pore entrance of this putative proton transport path (Fig. S4). During a 100-ns MD simulation of the AdBd system (Fig. S6 A), a close interaction between Y265 and Y515 (i.e., hydrogen bonding) constricted this pathway and prevented the formation of a continuous water wire leading from the intracellular solution to the GLUex gate. Motion of helix αR toward the intracellular domain (as shown in Fig. 2 B) resulted in the breakage of several hydrogen bonds (such as those between Y515 and Y265 or Y515 and E210) and contributed to the formation of such a continuous water path for a deprotonated GLUex gate. In particular, in Systems 3 to 8 (where one GLUex gate was deprotonated after a simulation of 80 ns in the ApBp system), we observed that an initially continuous water path remained intact over the entire simulation interval (over 50 ns), running from either K171 or T269 to the deprotonated GLUex gate (Fig. S6 B). Interestingly, a Y265A mutation performed on the AdBd system was found to induce this putative proton transport water path within ∼10 ns (Fig. S6 C). We thus suggest that a Y265A mutation may affect proton transfer due to its influence on the constriction of the putative proton transport path identified here.
Cl− transport from Scen to the intracellular solution
With a protonated GLUex gate (i.e., in the ApBp system), the calculated free energy barrier for a Cl− ion to transport from Scen to Sint was ∼8.0 ± 2.5 Kcal/mol (Fig. 5). Such a large energy barrier would prevent rapid Cl− translocation from Scen to Sint, thus directly supporting the major hypothesis for the CmCLC transport cycle proposed by the Mackinnon group (12). It should be noted that in motions of Cl− ions that involve simultaneous large changes in the configuration of intrapore water molecules, the Cl− coordinate itself may not represent an optimal reaction coordinate. However, because we are using these PMFs simply as a qualitative guide to possible steps in the transport mechanism of CmCLC (not for the quantitative prediction of equilibrium constants or rate constants), the treatment we have given here should suffice.
In the PMF calculations, the side chains of S165 and Y515 constricted the Cl− permeation path and impeded a partially dehydrated Cl− ion from transporting between Scen and Sint (Fig. 5 and Fig. S7). Thus, S165 and Y515 in the CmCLC may function as a second gate for Cl− permeation, as found for their bacterial homologous residues in EcCLC (1,3,6). Overcoming the energy barrier imposed by S165 and Y515 may in principle be initiated or facilitated by i), deprotonation of E210 or ii), by the arrival of another Cl− ion from the extracellular solution. We performed several sets of MD simulations (System 3 to 8) to further test these two hypotheses.
Deprotonating E210
In System 3 (Fig. S1 A), we deprotonated the GLUex gates in a protein structure taken from the ApBp system, where the Cl− ion originally at the Sext site in the x-ray structure had already transported to the Scen site in both subunits under an externally applied transmembrane potential of −120 mV. With a protonated GLUex gate, no Cl− ion was observed to transport from Scen to Sint in 20 ns of MD simulation. Upon deprotonation, the dihedral angles χ1 and χ2 of the GLUex gate evolved from identical 180° ± 20° (or −180° ± 20°) at the beginning (favorable orientation for a protonated GLUex) to become 80° ± 20° and −65° ± 15° (favorable orientation for a deprotonated GLUex), respectively (Fig. S8). As soon as the GLUex gate altered its orientation, the Cl− ion at the Scen site was observed to quickly translocate to Sint within 6 ns of MD simulation. This transport is facilitated both by repulsive electrostatic interactions and by the orientation change of the GLUex side chain.
By protonating (ApBp system) and subsequently deprotonating (System 3) the GLUex gate, Cl− ions located at Sext and Sint in the x-ray crystal structure were observed to depart from their original binding sites and eventually transport to the intracellular solution in our simulations. This appears to result in translocation of two Cl− ions in one transport cycle. However, there is one difficulty explaining the exchange stoichiometry of two Cl– ions for one H+ via this transport cycle. Namely, the motion of a Cl− ion occupying the Sint site was not strongly correlated with the transport of Cl− at the Sext (or Scen) site to the intracellular reservoir. Because the Sint site is exposed to the intracellular solution directly, it is easy for Cl− at Sint to move in and out of Sint (see Fig. S3), thus producing motion that is uncoupled from that of Cl− at Sext (or Scen), and resulting in a Cl− transport cycle that loses tight stoichiometric coupling.
Two Cl− ions competing for the Scen site
For the bacterial CLC transporters, Miller and Nguitragool (6) proposed that an exchange stoichiometry of 2Cl−/H+ requires binding of two Cl− ions to the Scen site. To test this hypothesis in the context of CmCLC, we designed several numerical simulations. In one system (System 5; see Fig S1 C), two Cl− ions were bound simultaneously to Sext and Scen in one subunit having a protonated GLUex gate. Binding of Cl− ions simultaneously to Sext and Scen has been observed in the crystal structure of E148A of the bacterial EcCLC transporter (13). Recent isothermal titration calorimetry measurement has also found that binding of two Cl− ions to the E148A mutant (EcCLC) at Sext and Scen shows 10- to 60-fold higher affinity than to the isolated sites (1,40), thus supporting our initial configuration of binding two Cl− ions to Sext and Scen (one at each site), because E148A is considered as a mimic of the protonated GLUex gate (1). The binding of Cl− to the Sext site reduced the energy barrier for a Cl− ion to transport from Scen to Sint by 4.0 ± 1.5 Kcal/mol (Fig. 5). Such a reduction is in good agreement with a similar calculation performed on the bacterial StCLC transporter (41).
Under an externally applied potential of −120 mV, a Cl− at Sext was able to migrate within 10 ns to the Scen site in the presence of another Cl− already occupying the central binding site. These two Cl− ions broadly exhibited two binding configurations (Fig. S9) within the Scen site. One Cl− ion bound near the backbone of the GLUex gate. The other Cl− ion either resided close to the entrance of the previously proposed proton transport path (42,43) (Fig. S9 A; termed site A) or near the Sint site (Fig. S9 B; termed site B). Occupancy by two Cl− ions of Scen induced the formation of a continuous water wire from Scen to the extracellular side through the side chain of GLUex (Fig. S9). The water path persisted as long as two Cl− ions bound simultaneously to the Scen site (life time > 10 ns). This continuous water wire may promote the dissociation of a proton from the protonated E210 residue, thus deprotonating GLUex.
To investigate how deprotonation of GLUex affects the transport of two Cl− ions, we generated two independent simulation systems (System 7 and 8) using these two snapshots (Fig. S9, A and B) as the initial configurations (shown in Fig. S1, E and F) for MD investigation. Once the GLUex gate was deprotonated, in one system (System 7) the Cl− ion at site A transported to the intracellular domain along the water path behind Y515 (black arrow in Fig. S9 A). In the other system (System 8), the Cl− ion at site B moved to the Sint site (black arrow in Fig. S9 B) along the path elucidated by x-ray crystal structures. Finally, the remaining Cl− ions took the same route (black arrow in Fig. S9 B) to Sint sites in both simulation systems within 40 ns.
Cl− transport path(s)
As just noted, we observed two potential Cl− transport paths from Scen to the intracellular side (see Fig. S9). The Cl− transport path shown in Fig. S9 A has been suggested in previous work to function as a proton transport path (22,43), but has not previously been implicated in Cl− transport. We noticed that Cl− had a higher probability to take this path when another Cl− was bound to Sext (such as state e and d of the CmCLC transport model from (12); probed in our System 6 simulations) or two Cl− ions bound simultaneously to Scen (the hypothesis employed in the Miller and Nguitragool model (6); probed in our System 7 simulations). With only one Cl− bound to the Scen site and no Cl− ion bound to the Sext site (System 3), deprotonation of the GLUex gate drove Cl− exclusively along the path shown in Fig. S9 B.
Although it is premature to confidently predict the novel Cl− transport route suggested above without explicitly considering coupled proton transport, it is worth testing in future experiments. Pore lining residues such as T269, K171, and Y265 were observed to coordinate the permeation of Cl− through this new path, and thus mutations of these residues (such as T269E, K171L, and Y265A) may assist in assessing this pathway's role in Cl− transport.
Cl− transport cycle in the CmCLC
The Cl− ions originally in Sint sites were observed under MD simulation to escape to the intracellular reservoir, and to be intermittently replaced by other Cl− ions from the intracellular reservoir (Fig. S3). This process is thus assigned a negligible role in the transport cycle proposed here. To minimize the complexity of the state model, we only consider transport of one Cl− initially at the Sext site, similar to the strategy employed by Miller and Nguitragool (6). On the basis of our MD simulations, we propose a five-state Cl− transport cycle (Fig. 6). The essential steps of the cycle are as follows:
-
A.
Deprotonated GLUex gate (E210) blocks Cl− transport from Sext to Scen.
-
B.
Protonation of GLUex opens the gate for Cl− to transport from Sext to Scen. A proton enters from the intracellular solution along a continuous water path formed behind Y515 (Fig. 6 B), which is presumably induced by the motion of helix αR toward the intracellular domain (as shown in Fig. 2 B). Upon protonation, the E210 residue moves away from the Scen site.
-
C.
One Cl− ion moves in from the Sext site via the backbone of the GLUex gate to occupy the Scen site. A significant free energy barrier prevents this Cl− ion from transporting to the intracellular reservoir.
-
D.
A second Cl− ion moves in from the extracellular reservoir to occupy the Scen site. Occupancy by two Cl− ions induces a continuous water wire from Scen to the extracellular solution through the side chain of the GLUex gate.
-
E.
The proton on E210 dissociates and exits to the extracellular solution. The resultant deprotonated E210 drives both Cl− ions that are in the Scen site to the intracellular solution. The E210 residue then swings back to the Scen site and reorients as shown in state A. Another Cl− ion moves to Sext from the extracellular solution. The cycle thus returns to its initial state.
Figure 6.
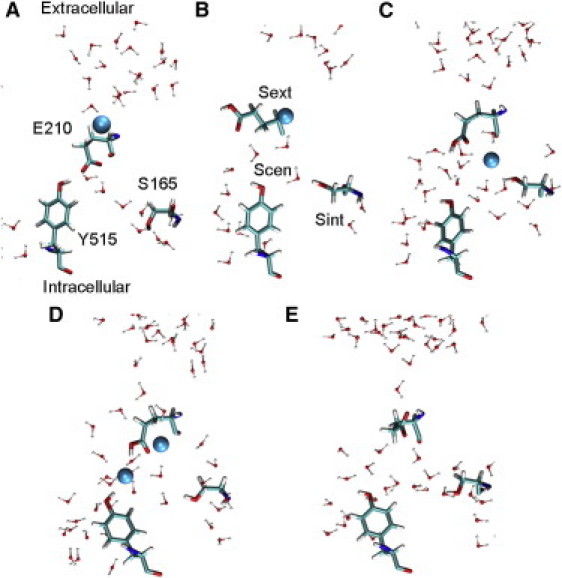
Schematic state diagram for Cl− transport cycle in the CmCLC. (A) Deprotonated E210 blocks Cl− translocation from Sext to Scen. (B) Protonation of E210 opens the GLUex gate. (C) One Cl− ion transports to and occupies the Scen site. (D) Two Cl− ions reside in the Scen site and a continuous water wire is formed from the Scen site to the extracellular solution. (E) Deprotonation of the GLUex gate drives two Cl− ions to the intracellular side. The cycle thus returns to its initial state A. Cl− ions are shown as spheres (cyan). Each picture is taken from MD snapshots of different simulation systems. For simplification, only residues E210, Y515, and S165 are shown in licorice format and waters within 12 Å of E210 are shown in CPK format. Oxygen (red), nitrogen (blue), carbon (cyan), and hydrogen (white) atoms are colored differently. States A and E have deprotonated GLUex and states B, C, and D have protonated GLUex. (Color online.)
Note that the five-state transport model proposed herein does not require the formation of HCl and the opening of the inner gate (GLUin) twice per transport cycle, as hypothesized in the EcCLC transport model by Miller and Nguitragool (6). In our model, we hypothesize that to maintain a strict 2Cl−/H+ exchange ratio, the GLUex gate will only deprotonate when two Cl− ions bind simultaneously to Scen. As we observed in Systems 5 and 6, one Cl− ion initially occupying Sext transported to Scen and resided in Scen with another Cl− ion already there. However, it should be noted that if the Cl− originally occupied Scen transports to the intracellular solution before the permeation of a second Cl− from Sext to Scen, the strict 2Cl−/H+ exchange ratio will be broken. The degree of leakage via this mechanism depends on how easy it is for Cl− at Sext to transport to Scen with a protonated GLUex gate (as explored in System 5), and how rapidly the GLUex deprotonates once a second Cl− ion arrives at Scen. Further analysis will be required to quantify the importance of this potential leakage mechanism with respect to the tight 2:1 antiport cycle process described above.
Conclusions
Using MD simulations, we have investigated the role of the GLUex gate for Cl− transport in the CmCLC transporter. For the deprotonated GLUex gate, the calculated free energy barrier is >30 ± 5 Kcal/mol for a Cl− ion to transport from Sext to Scen, which is sufficiently high to prevent Cl− transport. The opening of the GLUex gate involves the protonation of the GLUex gate and subsequent alteration of both dihedral angles to χ1 = ±180° and χ2 = ±180°, which consequently reduces the energy barrier to a value of 2.5 ± 1.0 Kcal/mol for Cl− to translocate from Sext to Scen. The Scen site may be occupied by two Cl– ions simultaneously due to a high free energy barrier (∼8 Kcal/mol) for a single Cl– ion to permeate from Scen to Sint. Binding two ions to the Scen site may be a crucial determinant of the 2Cl−/H+ exchange stoichiometry: it can induce formation of a continuous water wire from Scen to the extracellular solution. This may then initiate deprotonation of the GLUex gate, ultimately driving two Cl− ions out of Scen toward the intracellular side. Finally, we observed that Cl− had a higher probability to transport through the previously-suggested proton transport path (20–22) than through the crystal-structure elucidated Cl– conduction pathway when another Cl− was bound to Sext (such as state e and d of the CmCLC transport model from (12).), or two Cl− ions bound simultaneously to Scen (the hypothesis employed in the Miller and Nguitragool model (6)). To further evaluate this newly discovered Cl− transport path and the coupled proton/Cl− transport model proposed in this study, we suggest that experimental mutations be performed on residues Y265, T269, and K171 because these residues line a putative transport pathway for protons and/or Cl− ions.
Acknowledgments
We gratefully acknowledge financial support from the National Science Foundation (NSF) grant CHE-0750332 and computational support from the NSF through TeraGrid resources (TG-MCB100061 and TG-MCB110137). TeraGrid systems are hosted by Indiana University, Louisiana Optical Network Initiative (LONI), National Center for Atmospheric Research (NCAR), National Computational Science Alliance (NCSA), National Institute of Computational Sciences (NICS), Oak Ridge National Laboratory (ORNL), Pittsburgh Supercomputing Center (PSC), Purdue University, San Diego Supercomputer Center (SDSC), Texas Advanced Computer Center (TACC), and University of California/Argonne National Laboratory (UC/ANL).
Supporting Material
References
- 1.Accardi A., Picollo A. CLC channels and transporters: proteins with borderline personalities. Biochim. Biophys. Acta. 2010;1798:1457–1464. doi: 10.1016/j.bbamem.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jentsch T.J. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 3.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 4.Chen T.Y. Structure and function of clc channels. Annu. Rev. Physiol. 2005;67:809–839. doi: 10.1146/annurev.physiol.67.032003.153012. [DOI] [PubMed] [Google Scholar]
- 5.Picollo A., Malvezzi M., Accardi A. Proton block of the CLC-5 Cl−/H+ exchanger. J. Gen. Physiol. 2010;135:653–659. doi: 10.1085/jgp.201010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller C., Nguitragool W. A provisional transport mechanism for a chloride channel-type Cl−/H+ exchanger. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:175–180. doi: 10.1098/rstb.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zifarelli G., Pusch M. CLC chloride channels and transporters: a biophysical and physiological perspective. Rev. Physiol. Biochem. Pharmacol. 2007;158:23–76. doi: 10.1007/112_2006_0605. [DOI] [PubMed] [Google Scholar]
- 8.Dutzler R., Campbell E.B., MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- 9.Dutzler R. A structural perspective on ClC channel and transporter function. FEBS Lett. 2007;581:2839–2844. doi: 10.1016/j.febslet.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Dutzler R., Campbell E.B., MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 11.Robertson J.L., Kolmakova-Partensky L., Miller C. Design, function and structure of a monomeric ClC transporter. Nature. 2010;468:844–847. doi: 10.1038/nature09556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L., Campbell E.B., MacKinnon R. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 2010;330:635–641. doi: 10.1126/science.1195230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobet S., Dutzler R. Ion-binding properties of the ClC chloride selectivity filter. EMBO J. 2006;25:24–33. doi: 10.1038/sj.emboj.7600909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Accardi A., Lobet S., Dutzler R. Synergism between halide binding and proton transport in a CLC-type exchanger. J. Mol. Biol. 2006;362:691–699. doi: 10.1016/j.jmb.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 15.Nguitragool W., Miller C. Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J. Mol. Biol. 2006;362:682–690. doi: 10.1016/j.jmb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Accardi A., Walden M., Miller C. Separate ion pathways in a Cl−/H+ exchanger. J. Gen. Physiol. 2005;126:563–570. doi: 10.1085/jgp.200509417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accardi A., Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 18.Picollo A., Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 19.Scheel O., Zdebik A.A., Jentsch T.J. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 20.Wang D., Voth G.A. Proton transport pathway in the ClC Cl−/H+ antiporter. Biophys. J. 2009;97:121–131. doi: 10.1016/j.bpj.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieseritzky G., Knapp E.W. Charge transport in the ClC-type chloride-proton anti-porter from Escherichia coli. J. Biol. Chem. 2011;286:2976–2986. doi: 10.1074/jbc.M110.163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuang Z.F., Mahankali U., Beck T.L. Proton pathways and H+/Cl− stoichiometry in bacterial chloride transporters. Proteins. 2007;68:26–33. doi: 10.1002/prot.21441. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Voth G.A. The coupled proton transport in the ClC-ec1 Cl(−)/H(+) antiporter. Biophys. J. 2011;101:L47–L49. doi: 10.1016/j.bpj.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accardi A. Structure and function of CLC chloride channels and transporters. In: Pusch M., editor. Chloride Movements Across Cellular Membranes. Elsevier; New York: 2007. pp. 59–82. [Google Scholar]
- 25.Matulef K., Maduke M. The CLC ‘chloride channel’ family: revelations from prokaryotes. Mol. Membr. Biol. 2007;24:342–350. doi: 10.1080/09687680701413874. [DOI] [PubMed] [Google Scholar]
- 26.Chen T.Y., Hwang T.C. CLC-0 and CFTR: chloride channels evolved from transporters. Physiol. Rev. 2008;88:351–387. doi: 10.1152/physrev.00058.2006. [DOI] [PubMed] [Google Scholar]
- 27.Miloshevsky G.V., Hassanein A., Jordan P.C. Antiport mechanism for Cl(−)/H(+) in ClC-ec1 from normal-mode analysis. Biophys. J. 2010;98:999–1008. doi: 10.1016/j.bpj.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostick D.L., Berkowitz M.L. Exterior site occupancy infers chloride-induced proton gating in a prokaryotic homolog of the ClC chloride channel. Biophys. J. 2004;87:1686–1696. doi: 10.1529/biophysj.104.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J., Schulten K. Mechanism of anionic conduction across ClC. Biophys. J. 2004;86:836–845. doi: 10.1016/S0006-3495(04)74159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faraldo-Gómez J.D., Roux B. Electrostatics of ion stabilization in a ClC chloride channel homologue from Escherichia coli. J. Mol. Biol. 2004;339:981–1000. doi: 10.1016/j.jmb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Šali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 32.Phillips J.C., Braun R., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKerell A.D., Bashford D., Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 34.Nosé S. A unified formulation of the constant-temperature molecular-dynamics methods. J. Chem. Phys. 1984;81:511–519. [Google Scholar]
- 35.Hoover W.G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 36.Aksimentiev A., Schulten K. Imaging alpha-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map. Biophys. J. 2005;88:3745–3761. doi: 10.1529/biophysj.104.058727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darve E., Rodríguez-Gómez D., Pohorille A. Adaptive biasing force method for scalar and vector free energy calculations. J. Chem. Phys. 2008;128:144120. doi: 10.1063/1.2829861. [DOI] [PubMed] [Google Scholar]
- 38.Chipot C., Hénin J. Exploring the free-energy landscape of a short peptide using an average force. J. Chem. Phys. 2005;123:244906. doi: 10.1063/1.2138694. [DOI] [PubMed] [Google Scholar]
- 39.Zdebik A.A., Zifarelli G., Pusch M. Determinants of anion-proton coupling in mammalian endosomal CLC proteins. J. Biol. Chem. 2008;283:4219–4227. doi: 10.1074/jbc.M708368200. [DOI] [PubMed] [Google Scholar]
- 40.Picollo A., Malvezzi M., Accardi A. Basis of substrate binding and conservation of selectivity in the CLC family of channels and transporters. Nat. Struct. Mol. Biol. 2009;16:1294–1301. doi: 10.1038/nsmb.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gervasio F.L., Parrinello M., Klein M.L. Exploring the gating mechanism in the ClC chloride channel via metadynamics. J. Mol. Biol. 2006;361:390–398. doi: 10.1016/j.jmb.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Yin J., Kuang Z., Beck T.L. Ion transit pathways and gating in ClC chloride channels. Proteins. 2004;57:414–421. doi: 10.1002/prot.20208. [DOI] [PubMed] [Google Scholar]
- 43.Wang X.Q., Yu T., Zou X. A three-state multi-ion kinetic model for conduction properties of ClC-0 chloride channel. Biophys. J. 2010;99:464–471. doi: 10.1016/j.bpj.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darden T., York D., Pedersen L. Particle mesh Ewald - an N.Log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


