Abstract
The current high cure rates for children diagnosed with cancer can in part be attributed to emphasis on large cooperative group clinical trials. The significant improvement in pediatric cancer survival over the last few decades is the result of optimized chemotherapy drug dosing, timing, and intensity; however, further alterations in traditional chemotherapy agents are unlikely to produce substantially better outcomes. Furthermore, there remains a subset of patients who have a very poor prognosis due to tumor type or stage at presentation, or who have a dismal prognosis with relapse or recurrence. As such, innovative approaches to therapy and new drugs are clearly needed for introduction into the current pediatric oncology arsenal. A variety of biologically targeted therapies which have shown promise in preclinical studies and early phase adult clinical trials are now being explored in pediatric clinical trials. These novel agents hold the promise for continuing to drive forward improvements in patient survival with potentially less toxicity than exists with traditional chemotherapy drugs.
Keywords: novel therapies, targeted therapies, biologic target, pediatric oncology
INTRODUCTION
Pediatric cancer is relatively uncommon, however, it does remain the leading cause of death from disease in persons under the age of 19.[1] Despite dramatic improvements in outcome, some patients will either not achieve remission or relapse with refractory disease. Once a patient relapses, conventional chemotherapy or radiation and/or bone marrow transplant are much less effective.[2-7] Because further increases in dose intensity are neither likely tolerable nor effective, new approaches to therapy are needed, particularly for refractory or high risk patients.
Current multi-agent therapies are intensive and carry with them a significant risk for acute toxicity and late effects. It is doubtful that significant gains will be achieved by continued intensification of conventional chemotherapy regimens without paying a significant price of morbidity to patients. This makes the development of targeted therapies with greater efficacy and less toxicity of paramount importance.
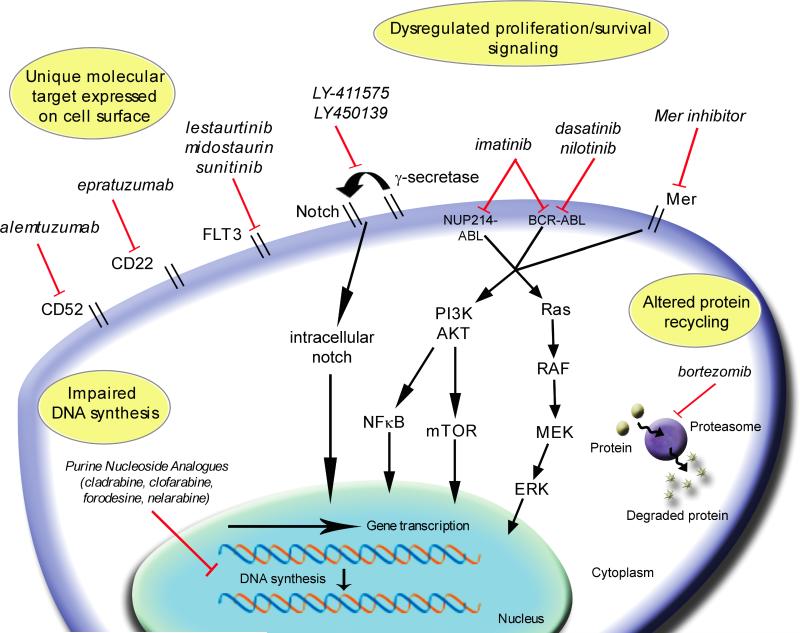
One major focus of current investigation in pediatric oncology is to differentiate patients with a high likelihood of relapse from those with a favorable prognosis. Yet even within subsets of patients with favorable prognostic factors, relapse remains problematic. New technologies have improved our understanding of cancer biology, and expanding knowledge of underlying molecular pathways and genomic aberrations is facilitating the development of new therapies for pediatric cancers.[8, 9] We present a brief review of selected new agents under investigation for pediatric malignancies, both solid tumors and hematologic malignancies (Figure 1). The majority of agents are in early phase clinical trials and some remain in pre-clinical exploration. The “novelty” of such agents comes from their more selective nature in capitalizing on a feature or features unique or more predominant in malignant cells, compared to their normal counterparts. Table 1 summarizes the drugs discussed and the current stage of clinical development. It should be noted that the current rate of drug development far outpaces the realistic ability for any review to be comprehensive in nature. However, an attempt is made to focus on a number of promising molecularly and/or mechanistically targeted agents, with the hope of characterizing pathways which may be utilized to more effectively treat patients while minimizing toxicities. Traditionally, the therapies for different pediatric tumor types have been fundamentally distinct, as our understanding of the diseases takes on a more molecular basis, analysis of the targets for therapy is increasingly likely to take on a more “cross-platform” nature.
Figure 1.
Summary of mechanisms of action of novel therapies directed against pediatric tumors and leukemia. Drugs are listed in italics demonstrating their effect on targeted proteins, selected signaling pathways, and gene transcription. RTK = receptor tyrosine kinase; VEGF = vascular endothelial growth factor; HDAC = histone deacetylase; SAHA = suberanilohydroxamic acid
Table 1.
Selected therapies under investigation in pediatric malignancies.
| Target/Class | Patient Population | Drug Name: generic (brand) | Route of Administration | Pediatric Status |
|---|---|---|---|---|
| BCR-ABL TKIs | Ph +ALL, Solid tumors | imatinib (Gleevec™) | PO | •Pediatric phase I complete •Pediatric phase III in Ph+ ALL closed, early analysis – improved early EFS (COG) •Pediatric phase II single agent trial in solid tumors – no activity |
| Ph +ALL, Solid tumors | dasatinib (Sprycel®) | PO | •Adult phase I complete; phase II and III on-going •Pediatric phase I in imatinib resistant Ph+ALL and solid tumors ongoing •Pediatric phase II in Ph+ ALL with imatinib in development |
|
| Ph +ALL, Solid tumors | nilotinib (Tasigna®) | PO | •Adult phase I complete; phase II and III on-going •Pediatric phase I in development |
|
| VEGF pathway inhibitors | Leukemias, Solid tumors | bevacizumab (Avastin®) | IV | •Pediatric phase I study in solid tumors complete •Pediatric phase I study with combination chemotherapy opening •Pediatric phase II study in combination with chemotherapy for relapsed Ewing Sarcoma ongoing |
| Solid tumors | VEGF trap | IV | • Pediatric phase I study in refractory solid tumors ongoing | |
| Solid tumors | Sunitinib (Sutent®) | PO | • Pediatric phase I study in relapsed/refractory solid tumors ongoing | |
| IGF1R inhibitors | Solid tumors | R1507 IMC A12 | IV | •Multiple adult Phase 1 complete; phase II, III ongoing •2 Pediatric Phase I studies on-going •2 Pediatric Phase II studies approved |
| Multi-target TKIs | Leukemias, Solid tumors | Sorafenib (Nexavar®) | • Adult phase I complete, phase II/ III ongoing • Pediatric phase I on-going |
|
| FLT3 inhibitors | MLL rearranged infant ALL, AML | lestaurtinib (CEP-701) | PO | •Pediatric phase I/II complete •Phase III in high risk infants with intensive chemotherapy ongoing •Pediatric pilot study in relapsed/refractory AML with cytarabine and idarubicin in development |
| Purine nucleoside analogues | Acute Leukemias | clofarabine (Clolar®) | IV | •Pediatric Phase I, II study complete •Pediatric phase I/II study in relapsed/refractory acute leukemias combined with cyclophosphamide and etoposide ongoing •Pediatric phase I/II study in relapsed/refractory leukemia with cytarabine ongoing •Pediatric phase I/II study in relapsed/refractory ALL, AML, MDS combined with cyclophosphamide ongoing |
| T-cell ALL, T-cell Lymphomas | nelarabine (Arranon®) | IV | •Pediatric phase II study complete •Pediatric phase III study in newly diagnosed T-cell ALL in combination with chemotherapy ongoing •Pediatric phase I study in relapsed/refractory T-cell ALL in combination with cyclophosphamide and etoposide in development |
|
| ALL | forodesine (Fodosine™) | IV | •Pediatric phase II study complete •Pediatric phase II and phase I/II combination study in development |
|
| Proteasome inhibitors | Leukemias, Lymphoma | bortezomib (Velcade®) | IV | •Adult Phase I study complete •Pediatric phase Ib/II study in relapsed/refractory AML with chemotherapy in development •Pediatric phase I/II combination study in ALL in development •Pediatric phase II combination in Hodgkin's Disease ongoing |
| mTOR inhibitors | Leukemias, Solid tumors | rapamycin (sirolimus, Rapamune®) | PO | •Pediatric phase III study in ALL patients post-bone marrow transplant ongoing •Phase II combination study in ALL in development •Phase I combination study with temozolamide and irinotecan in refractory sarcomas in development |
| Leukemias, Solid tumors | temsirolimus | PO | • Adult Phase I, II studies complete, analyses pending | |
| Leukemias, Solid tumors | everolimus | PO | • Pediatric phase I study in solid tumors complete | |
| Leukemias, Solid tumors | deforolimus | PO | •Adult phase I study complete •Pediatric phase I study in solid tumors ongoing |
|
| Anti CD22 MoABs | B-cell ALL | epratuzumab (IMMU-103) | IV | •Pediatric phase I/II study complete •Pediatric phase II combination study in relapsed CD22 positive B-cell ALL ongoing |
| B-cell ALL, Lymphoma | tositumomab (Bexxar™) | IV | • Adult Phase I studies in lymphoma complete, phase II/III ongoing | |
| B-cell ALL, Lymphoma | Ibritumomab | IV | • Pediatric phase I study in lymphoma complete, analysis pending | |
| γ-Secretase inhibitors | T-cell ALL | LY-411575 and LY450139 | PO | •Adult Phase I complete •Pediatric phase I studies in development |
| Anti CD52 MoAB | T-cell ALL | alemtuzumab (CamPath®) | IV | • Pediatric phase II in relapsed/refractory ALL with combination chemotherapy closed due to poor accrual |
| angiogenesis inhibitors | AML, Solid tumors | thalidomide, lenalidomide | PO | • Pediatric phase I studies in solid tumors completed or ongoing |
| Farnesyl transferase inhibitors | Leukemias, MDS | tipifarnib (Zarnestra™) | PO | •Pediatric phase I study in relapsed/refractory leukemia completed •Pediatric phase I trial in refractory solid tumors completed •Phase II window study in JMML with combination chemotherapy completed • Phase II study in pediatric brain tumors completed |
| MAPK pathway inhibitors | Solid tumors | AZD6244 | PO | • Adult phase I complete, phase II ongoing |
| Solid tumors | CI-1040 | PO | • Adult phase I complete, phase II ongoing | |
| HDAC inhibitors | Leukemias | valproic acid | PO | •Adult combination studies complete and on-going •Studies in pediatric solid tumors ongoing •Pediatric phase I combination study with 5-azacytadine approved |
| Leukemias, Solid tumors | Suberanilohydroxamic acid (SAHA)/vorinostat (Zolinza™) | PO | •Pediatric phase I study in solid tumors and refractory leukemias ongoing •Pediatric phase I study in relapsed/refractory leukemias and solid tumors with cyclophosphamide and topotecan opening •Pediatric phase I combination with cis-retinoic acid complete •Pediatric phase I combination with sorafenib in development |
|
| depsipeptide | IV | • Pediatric phase I study in relapsed/refractory leukemia complete | ||
| DNA methylation inhibitors | Leukemias | decitabine (Dacogen®) | IV | •Adult Phase I/II studies complete, phase III on-going •Pediatric phase I study completed, analyses pending •Pediatric phase I combination with MGCD0103 (HDAC inhibitor) in development |
| Leukemias, MDS, Solid Tumors | 5-azacytadine | IV | • Pediatric phase I trial in relapsed/refractory leukemias and solid tumors in combination with valproic acid approved | |
| Anti CD33 MoABs | AML | gemtuzumab ozogamicin (Mylotarg®) | IV | •Pediatric Phase I complete •Pediatric phase III study in newly diagnosed AML using combination therapy ongoing |
| HSP90 inhibitor | Leukemias, Solid tumors | 17-AAG | IV | • Pediatric Phase I complete |
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; PO = oral; IV = intravenous; Ph+ = Philadelphia chromosome positive; TKIs = tyrosine kinase inhibitors; VEGF = vascular endothelial growth factor; JMML = juvenile myelomonocytic leukemia; MAPK = mitogen-activated protein kinase; MoABs = monoclonal antibodies; HDAC = histone deacetylase; HSP90 = heat shock protein 90
Tyrosine Kinase Inhibitors
Tyrosine kinase receptors are implicated in a multitude of malignancies, and are currently an active approach in many targeted therapies. Therapeutics directed against selected pathways of interest are all in active development and discussed below.
BCR-ABL and Src-ABL tyrosine kinase inhibitors (TKIs)
The t(9;22)(q34;q11.2) translocation, also known as the Philadelphia chromosome, is found in fewer than 5% of pediatric ALL patients and confers a high risk of relapse and poor outcome.[10, 11] The resulting BCR-ABL fusion transcript encodes an 8.5 kB chimeric mRNA that translates to a constitutively active ABL tyrosine kinase resulting in increased cell proliferation and survival, and altered cell adhesion.[12, 13] Imatinib mesylate (STI-571, Gleevec™) is an orally available multi-targeted small molecule inhibitor that binds the ATP-binding pocket of the BCR-ABL complex, stabilizing it in the inactive form[14] and is often cited as the first molecularly targeted agent to “make it” to mass market. Its use has been applied to chronic myelogenous leukemia (CML), Philadelphia chromosome positive (Ph+) ALL, and gastrointestinal stromal tumors (GIST), with limited sustained effect as a single agent in the latter two. The most recent Children's Oncology Group clinical trial for Ph+ ALL which incorporated imatinib into a backbone of conventional chemotherapy is demonstrating substantial improvement in early EFS.[15] Despite good initial response, resistance to imatinib is an increasing concern. To circumvent this, two other orally available ABL TKIs, dasatinib (BMS-354825, Sprycel®) and nilotinib (AMN107, Tasigna®), were developed and are in advanced development. Both dasatinib and nilotinib have documented activity against many imatinib-resistant mutations, although neither has significant activity against the most refractory T315I mutation. Third generation ABL inhibitors are in early development which demonstrate activity against T315I.
Src family kinases have an important role in initiation and progression of many malignancies through regulation of cellular survival, proliferation, cellular adhesion, invasion, migration and angiogenesis.[16, 17] Dasatinib, which is much more potent than imatinib against Abl, also inhibits Src, a non receptor tyrosine kinase, with a low nM IC50, compared to the μM IC50 of imatinib for Src. The Src kinases Lyn, Hck, and Fgr are thought to be required for transformation of Ph+ALL (but not CML), therefore, given its inhibition profile, dasatinib may be an important addition to the arsenal of Ph+ALL therapy. Src overexpression has been studied in a variety of tumor types, including neuroblastoma[18], osteosarcoma[19], lymphoid [20] and myeloid leukemia,[21] making it a desirable therapeutic target for a variety of cancers. Anti-tumor activity is noted preclinically with Src kinase inhibitors against pediatric osteosarcoma [22], Ewing Sarcoma[23] and rhabdoid tumors.[24]
FLT-3 inhibitors
FLT3, a receptor tyrosine kinase (RTK) involved in hematopoiesis, is expressed in a variety of malignancies, including MLL gene rearranged infant and childhood ALL. It is the most frequently mutated gene in AML, and is aberrantly expressed in up to 90% of AML patient samples.[25-27] Dysregulation of FLT3 may occur by one of three different mechanisms: protein overexpression, internal tandem duplication (FLT3/ITD), and activating loop mutations (FLT3/ALM). Mutations leading to constitutive activation of FLT3 are seen in 20-25% of children with AML, with 10-15% having FLT3/ITD and the remainder having FLT3/ALM.[28] FLT3/ITD mutations are associated with a poorer outcome, with lower rates of remission induction and survival. Additionally, a high ratio of mutant to wild type FLT3 confers a poorer prognosis.[29] Several FLT3 TKIs, including lestaurtinib (CEP-701), the most selective of the FLT3 targeted agents, as well as midostaurin (PKC412), and sunitinib (SU11248, Sutent®), are in various stages of clinical testing for hematologic malignancies.[30-33] Lestaurtinib, an oral FLT3 TKI has selective in vitro activity against MLL-rearranged cells as well as synergistic activity with other chemotherapy agents in a sequence dependent manner.[34, 35] The addition of lestaurtinib to an intensive chemotherapy regimen is currently being evaluated in the most recent COG infant ALL trial. Several other multi-targeted kinase inhibitors, including several Aurora kinase inhibitors also demonstrate clinically achievable FLT-3 inhibition, but these agents are only now entering trials, primarily in adults, to a significant degree.
FLT3 expression in cancer is not unique to leukemia; FLT3 and its ligand are expressed in solid tumors, including neuroblastoma.[36] FLT3 inhibition decreases cell growth and proliferation.[37] Lestaurtinib also has been evaluated in phase I study of relapsed neuroblastoma, however the target in this disease is thought to be primarily through the TrkB pathway.[38]
c-Kit inhibitors
c-Kit, a RTK important for tumor growth and progression, is normally expressed in hematopoietic progenitor cells, and is expressed in 50-80% of pediatric AML, with 11% having activating mutations.[39, 40] Three types of activating mutations are known: internal tandem duplication (ITD) of the juxtamembrane domain, activation loop mutations at D816, and exon 8 mutations. All confer a poor prognosis.[41-43] Imatinib has activity against c-Kit and platelet derived growth factor receptor. Targeting c-Kit has shown some promise pre-clinically in a spectrum of pediatric cancers including osteosarcoma, Ewing sarcoma, neuroblastoma and Wilm's tumor.[44-47] Unfortunately, a phase II trial of imatinib in pediatric solid tumors demonstrated little to no activity as a single agent.[48] However, it may have some utility in combination with chemotherapy in vitro[49] or in patients who are less heavily pretreated. Imatinib does not appear to be active against the D816 mutations seen in AML.[50] However, other more potent agents including dasatinib and midostaurin as well as other new compounds on the horizon may be effective in this setting.[51, 52]
Vascular endothelial growth factor (VEGF) inhibitors
The role of angiogenesis, particularly in solid tumors, remains a very active area of research. The effect of tumor vasculature on proliferation and migration is crucial for tumor expansion and growth. Anti-angiogenesis therapy, through vascular endothelial growth factor (VEGF) blockade has been demonstrated to be effective in many adult tumor types, including renal cell carcinoma, colorectal, breast and lung cancer.[53-57] Subsequently, targeting angiogenesis, particularly via the VEGF pathway, has become an area of increasing interest in pediatric malignancies. VEGF and its RTKs are overexpressed in acute leukemias[58, 59] and microvessel density is increased in the bone marrow of ALL, AML, and MDS patients.[60, 61] As a result, anti-angiogenic therapies (thalidomide; thalidomide analogs like lenalidomide; bevacizumab, an anti-VEGF humanized monoclonal antibody; and small molecule inhibitors such as sunitinib, vatalanib, and telatinib) are being pursued as treatment strategies in the acute leukemias, MDS, and solid tumors.[62-65]
VEGF inhibition in pediatric solid tumors, specifically neuroblastoma, glioblastoma, Wilm's tumor, hepatoblastoma, Ewing Sarcoma and rhabdomyosarcoma results in anti-tumor activity in vitro.[66-70] Pediatric preclinical testing of two small molecule tyrosine kinase inhibitors with anti-VEGF receptor activity, sunitinib and cediranib (AZD2171), showed promising anti-angiogenic effects in many of the solid tumor xenographs treated.[71, 72] Many new anti-VEGF agents are small molecule inhibitors that target more than one receptor. As a result, these “dirty” or “promiscuous” inhibitors may provide improved anti-tumor efficacy than antibodies alone through multi-target inhibition.[73] They may also have increased toxicities that are important to delineate and address.
Insulin-like Growth Factor Receptor -1 Inhibitors
Targeting of the insulin-like growth factor 1 receptor (IGF1R) pathway is a very active area of therapeutics currently. The binding of IGF1 and IGF2 to the IGF1R results in autophosphorylation and subsequent activation of multiple signaling pathways including the Ras-Raf-MAPK, PI3K/Akt pathway which both activate the mammalian target of rapamycin (mTOR) pathway, stimulating cell growth.[74] Not only is IGF1R over-expressed in many malignancies, there is also evidence to suggest that anti-cancer treatments including chemotherapy and radiation also result in increased IGF1R signaling activity.[75, 76] Many pediatric tumors have dysregulated IGF1R signaling, including rhabdomyosarcoma, Ewing sarcoma, osteosarcoma, Wilm's tumor, desmoplastic small round cell tumor, astrocytoma, and medulloblastoma.[77-82] Several in vitro studies have shown synergistic interactions with chemotherapeutic agents and IGF1R inhibitors in Ewing sarcoma.[83, 84] The development of anti-IGF1R therapies is active in adult oncology with more than a dozen antibodies and small molecule inhibitors in development.[85, 86] The role of IFG1R in leukemia is less well defined, although both ALL and AML cells have been shown to express the receptor.[87, 88]
Inhibitors of Ras Activity/ Mitogen-activated protein kinase (MAPK) pathway
The Raf-MAPK-ERK pathway including its upstream activators is often constitutively activated in tumors and is important in cell proliferation and survival; it has emerged as another attractive target for inhibition. Raf, a serine/threonine kinase is the principal effector of Ras and is required for phosphorylation of the mitogen associated/extracellular regulated kinase-1 (MEK). Inhibitors of B-Raf, sorafenib (BAY 43-9006, Nexavar®) and MEK (CI-1040/PD184352, AZD6244, PD325901) are being studied in AML and MDS, as well as a variety of solid tumors.[89, 90] Of note, sorafenib has inhibitory activity of several other protein kinases, include VEGFR2, PDGFRβ, Flt3, c-kit.[91] Specific targeting of the epidermal growth factor (EGF) signaling cascade is another means of inhibiting the downstream pathway of MAPK-ERK, although there are several other pathways, including PI3K-Akt, protein kinase C and phospholipase pathways, associated with EGF receptor signaling (reviewed in [92]). There has been success in this area, with antibodies (including cetuximab, a chimeric monoclonal IgG1 antibody) and TKIs (gefitinib and erlotinib) which are FDA approved for adult malignancies including metastatic colon carcinoma, non-small cell lung cancer, and pancreatic cancer. Cetuximab is also approved for use in combination with radiation for head and neck cancer. EGFR antibodies including cetuximab, panitumomab, and nimotuzumab are moving through pediatric cancer clinical trial development.
Ras proteins are small molecular weight GTP-binding proteins that act downstream of RTKs and upstream of the Raf-MAPK-ERK pathway. They are involved in multiple RTK signaling cascades. Activating mutations of RAS have been shown to occur in approximately 20% of pediatric AML, although the clinical significance of these mutations is unclear.[28, 39, 93] Mutations leading to RAS activation may play an important role in the pathogenesis of juvenile myelomonocytic leukemia (JMML) and MDS.[94] They have also been found in rhabdomyosarcoma.[95] The gene for neurofibromatosis encodes a protein, neurofibromin which negatively regulates Ras. Tumors in NF-1 patients, JMML and malignant peripheral nerve sheath tumors, have documented hyperactive Ras.[96]
In order to function properly, Ras must be plasma membrane bound via a series of post-translational modifications leading to the attachment of a farnesyl group to the protein. The enzyme farnesyl transferase is required for this process. The farnesyl protein transferase inhibitors (FTIs) tipifarnib (Zarnestra™, R115777) and lonafarnib (Sarasar®, SCH 66336) were developed to target Ras, although their anti-tumor effect is likely also due to inhibition of other farnesylated proteins, such as the Rho family GTPases. FTIs showed early promise in the treatment of JMML, AML, and MDS,[97-100] although their more recent development has lagged.
Purine nucleoside analogues
Nucleoside analogues, Cladribine (2-CDA, Leustatin®), fludarabine (Fludara®), and clofarabine (Clolar®) are used commonly in patients who have relapsed. Purine nucleoside analogues are similar in structure to adenosine or guanosine, but their mechanisms of action differ. Cladribine, fludarabine, and clofarabine (a second-generation purine nucleoside analog designed as a “hybrid” molecule) require intracellular phosphorylation for cytotoxicity, through the inhibition DNA polymerases and/or ribonucleotide reductase, leading to apoptosis.[101, 102]
Purine nucleoside phosphorylase (PNP) inhibitors such as nelarabine (compound 506U78, Arranon®) and forodesine (BCX-1777, Fodosine™) are directed therapies for T-lineage disease. PNP phosphorylates 2’-deoxyguanosine (dGuo) to the guanine nucleobase and 2’-deoxyribose-1-phosphate. The rare genetic deficiency of PNP results in lymphopenia and altered T-cell immunity prompting the rational development of drug-induced PNP inhibition for the treatment of T-cell malignancies.[103, 104]
Nelarabine, a 6-prodrug of ara-G, is rapidly demethylated to the active form of ara-G. Ara-G is then intracellularly phosphorylated to ara-G triphosphate, where its accumulation leads ultimately to apoptosis. [102] Nelarabine is 10-fold more soluble, thus is more attractive clinically than ara-G.[105, 106]
Forodesine, another PNP inhibitor that has shown promise in refractory ALL, blocks intracellular deoxyguanine cleavage to guanine resulting in deoxyguanosine triphosphate accumulation leading to apoptosis.[102, 103] While both agents were initially developed for T-cell disease, forodesine also has demonstrated activity against B-lineage ALL.[107, 108]
Proteasome inhibitors
The proteasome-ubiquitin pathway controls critical cell functions including transcription, apoptosis, and cell cycle progression by degrading important regulatory proteins. Malignant cells are more sensitive to proteasome blockade, a result of altering the recycling of regulatory proteins.[109] Proteasome inhibition also reduces chemoresistance and increases apoptosis by blocking chemotherapy-induced NF-κB pathway activation.[110] Bortezomib (PS-341, Velcade®) is a highly selective, reversible proteasome inhibitor with activity in B-cell leukemia.[111] It also has been shown to effect neuroblastoma cell growth both in vitro and in xenograft models.[112, 113] Ewing sarcoma and osteosarcoma cell lines have shown sensitivity to proteosome inhibitors as demonstrated by increased apoptosis.[114, 115] Preclinical evaluation of bortezomib against a panel of pediatric tumors in vitro showed uniform sensitivity. However, when applied to a selection of tumors in vivo, several ALL lines showed the greatest response.[116] It is FDA approved for use in multiple myeloma and mantle cell lymphoma, and is being investigated in combination with a variety of conventional chemotherapy and targeted agents in both adults and children presently. A phase 1 COG study of bortezomib in pediatric leukemia and solid tumors has been completed.[117, 118] Second generation proteasome inhibitors salinosporamide[110, 119, 120], CEP18770[121, 122], carfilzomib[123] are being actively developed. Salinosporamide demonstrates a synergistic interaction with bortezomib suggesting a role for different mechanisms of action.[124-126]
mTOR inhibitors
The mammalian target of rapamycin (mTOR) is important in cell proliferation and cell cycle progression.[127] Rapamycin (sirolimus, Rapamune®), a macrolide antibiotic, is commonly used as an immunosuppressant following allograft and stem cell transplantation. Rapamycin, everolimus (RAD001), and temsirolimus (CC-I779, a water soluble ester of rapamycin) also decrease cellular proliferation through inhibition of mRNA translation to proteins required for cell cycle progression from G1 to S phase, as well as decreasing angiogenesis.[128-130] Rapamycin and temsirolimus affect cellular growth in B-cell leukemia, both in vitro and in xenograft models.[131, 132] As mTOR inhibitors block activation of many signaling molecules involved in oncogenesis, they have also shown activity in solid malignancies, including rhabdomyosarcoma, neuroblastoma, osteosarcoma, and medulloblastoma.[133-136] In xenograft models of pediatric tumors, responses have been seen in ALL as well as osteosarcoma, rhabdoid tumor and rhabdomyosarcoma.[137] suggesting that these agents may have potential in the clinical setting.
Histone Deacetylase (HDAC) inhibitors
Histone deacetylase inhibitors regulate transcription and protein function of genes through the control of histone acetylation. The acetylation of histones results in a relaxed chromatin structure, promoting transcriptional activation of genes (reviewed in [138]). HDAC inhibition likely leads to alteration in transcription regulation of genes important in cell cycle regulation and regulation of apoptosis, including p21WAF1/CIP1, p53, RB, bcl2, bcl6, bcl-xl, and mcl-1[138]. Several HDAC inhibitors, including valproic acid, suberanilohydroxamic acid (SAHA, vorinostat, Zolinza™), entinostat (formerly MS-275, now SNDX-275), and depsipeptide (Romidepsin), are under broad investigation in malignancies particularly hematologic, both alone and in combination[139, 140], although their use has been limited in pediatric malignancies.
DNA methyltransferase inhibitors
DNA methylation in promoter regions of genes is another control mechanism for gene transcription. When cytosine methylation occurs, promoters and gene transcription are suppressed. DNA hypermethylation in promoters regions and consequent inactivation of tumor suppressor genes, including p15INK4B, p16INK4, p14ARF, and p21WAF1/CIP1, is thought to play a role in the pathogenesis of many tumors, including AML, Ewing sarcoma, osteosarcoma, neuroblastoma and rhabdomyosarcoma.[141-144] DNA methylation inhibitors bind cytosine to prevent DNA methylation resulting in increased or restoration of normal gene transcription. Several methyltransferase inhibitors, including 5-azacytidine (Vidaza®), decitabine (5-aza-2-deoxycitadine, Dacogen®), and zebularine, are being studied in the adult acute leukemias, particularly in combination with HDAC inhibitors.[145-147]
Antibody therapies
Another strategy employed in current drug development is the targeting of proteins expressed in cancer cells but with limited expression in normal cells. Antibodies targeted to these proteins may be used alone, or can then be conjugated to radioactive or cytotoxic agents.
Gemtuzumab ozogamicin
CD33 is a transmembrane receptor expressed on the surface of myeloid and monocytic lineage cells, as well as AML cells. Gemtuzumab ozogamicin (Mylotarg®) is a humanized anti-CD33 antibody conjugated to a derivative of the cytotoxic antibiotic calicheamicin. Upon binding to CD33 on the surface of an AML cell, the antibody is internalized and the calicheamicin molecule is released through hydrolysis, leading to DNA damage and apoptosis. [148] This antibody is actively being evaluated in pediatric AML trials.
Anti-CD-22 targeted therapies
CD22 is normally expressed on the surface of mature B-cells and serves as a negative modulator of B-cell activation via B-cell antigen receptors, though its function is not fully understood. It is also expressed in more than 90% of B-precursor ALL cases[149], making it an attractive and specific target against CD22 positive malignancies. Epratuzumab (IMMU-103) and BL22 are monoclonal antibodies directed against CD22. BL22 is a monoclonal antibody fused to a portion of Pseudomonas exotoxin A.[150] Once bound, these antibodies are rapidly internalized and in vitro studies show increased cell death via antibody-dependent cellular cytotoxicity.[151, 152] Epratuzumab is currently being studied in the COG relapsed pediatric ALL trial. Because of the rapid internalization, these agents are also an attractive carrier molecule for cytotoxic drugs, such as inotuzumab ozogamicin, a humanized anti-CD-22(CMC-544) conjugated with calicheamin[153], or with radiation, such as the anti-CD20 antibodies linked to 90yittrium ibritumomab tiuxitan (Zevalin™) or I131 linked tositumomab (Bexxar™). A COG Phase I trial has been completed with ibritumomab tiuxitan.[154]
Alemtuzumab
Alemtuzumab (CamPath®) is a humanized monoclonal antibody against CD52. CD52 is normally expressed on all lymphocytes as well as lymphoblasts. As with many monoclonal antibodies, the mechanism of action of alemtuzumab is poorly understood. Apoptosis may be induced by either antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity. The degree of apoptosis is dependent on CD52 antigen density on the cell surface. Alemtuzumab is used in stem cell transplantation and chronic lymphocytic leukemia and its use is being extrapolated to acute leukemias.[155, 156]
Other Targeted Therapies
Heat Shock Protein Inhibitors
17-AAG, a geldanamycin that disrupts the chaperone function of hsp90, has shown initial promise in the treatment of both AML and ALL, particularly those leukemias with defined fusion proteins.[157] Hsp90 has an essential role in promoting cell survival by stabilizing and enhancing the activity of many signaling proteins involved in oncogenesis, including tyrosine kinases, raf and steroid hormone receptors.[158-161] It is thought that inhibition of function of heat shock protein 90 by 17-AAG, and a newer water-soluble analog, 17-DMAG, leads to depletion of many intracellular proteins, including Raf-1, AKT, and SRC kinases, as well as regulators of angiogenesis, possibly explaining sensitivity of some AML cell lines to the drug.[162, 163] 17-AAG has also shown promise preclinically against malignant glioma as well as neuroblastoma and osteosarcoma.[164-166] Phase I testing of 17-AAG has been completed in children[167, 168] and newer generation HSP90 inhibitors are being evaluated now, particularly in combination with proteasome inhibitors and a range of multi-targeted tyrosine kinase inhibitors.[169]
γ-Secretase inhibitors
The NOTCH1 gene encodes a regulatory transmembrane receptor essential for normal T-cell development. After post-translational modification of Notch, γ-secretase, a membrane bound protease, cleaves Notch at the transmembrane domain, generating intracellular Notch which enters the nucleus as a transcriptional activator that upregulates NFκB. NFκB promotes proliferation and activates the anti-apoptotic PI3 Kinase/AKT pathways.[170] Aberrant NOTCH1 activation has been implicated in tumorigenesis of many cancers, including T-ALL [170], via the PI3/AKT or c-MYC pathways. Notch has also been implicated in neural development, playing a role in the determination of fate for multipotent neural stem cells.[171] This role for Notch is thought to explain why its expression is upregulated in neuroblastoma and the more neural phenotype of Ewing sarcoma.[172, 173] Hes-1, a notch pathway gene, has been shown to be important in osteosarcoma invasion and metastasis.[174] In the hematologic malignancies, approximately 50% of T-cell ALL patients express activating mutations in NOTCH1 in the heterodimerization domain and/or the PEST domain.[175, 176] Interestingly, NOTCH1 mutations have not been observed in B-ALL patients.[176]
Targeting γ-secretase prevents intracellular Notch formation. Patients treated with γ-secretase inhibitors (LY-411575 and LY450139) for Alzheimer's disease, the drug's initial intended application, experienced altered lymphopoiesis and thymocyte development, supporting interest in use for T-cell malignancies.[177] One γ-secretase inhibitor, MK-0752 entered clinical trials for patients with refractory acute leukemias after passing initial safety and tolerability studies in normal volunteers. However, in phase I trial for patients with refractory acute leukemias, neurologic toxicities limited progression of these studies, and further work is ongoing. The mechanism for such neurotoxicity in leukemia patients has been unclear, although Notch has been implicated in neural development.[178, 179] While evidence for the role of Notch in solid tumors is increasing, trials in solid malignancies are just proceeding in adults with several newer generation γ-secretase inhibitors.
CONCLUSIONS
A wide variety of targeted agents are being studied for the treatment of a variety of malignancies in both adult and pediatric settings, and early results are promising. The molecular characterization of each patient's cancer will likely be important in the development of tailored therapy. Despite the overall cure rate in pediatric cancer, many patients cured of their cancer with current treatment protocols will suffer some late effects. The development of targeted agents may not only improve cure rates, but also help decrease the usage and thereby, the side effects of standard cytotoxic chemotherapy. A significant challenge is to determine the optimal combinations of one or several agents in conjunction with more traditional chemotherapy that will improve cure rates and decrease short- and long-term morbidity associated with treatment for these diseases. Because there are a host of new agents available, it is imperative to design clinical trials that will optimize patient utilization and resources, and increase the “signal to noise” ratio in order to develop a more comprehensive understanding of who is likely to benefit from which targeted agents. The ability to identify targeted agents with efficacy in specific patient populations will hopefully lead to continued improvements in overall patient survival in pediatric cancers and diminished toxicities from therapy.
References
Recently published papers of interest which elucidate the concepts and targeted pathways discussed in this review have been highlighted as:
* Of importance.
** Of major importance
- 1.Heron M. National Vital Vstatistics Reports. 5. Vol. 56. National Center for Health Statistics; Hyattsville, MD: 2007. Deaths: Leading causes for 2004. [PubMed] [Google Scholar]
- 2.Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107(12):4961–7. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynon PS, Harris RE, Altman AJ, Bostrom BC, Breneman JC, Hawks R, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol. 2006;24(19):3150–6. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 4.Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplant. 2004;34(9):799–806. doi: 10.1038/sj.bmt.1704689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker LM, Pendergrass TW, Sanders JE, Hawkins DS. Survival After Recurrence of Ewing's Sarcoma Family of Tumors. J Clin Oncol. 2005;23(19):4354–4362. doi: 10.1200/JCO.2005.05.105. [DOI] [PubMed] [Google Scholar]
- 6.Ceschel S, Casotto V, Valsecchi MG, Tamaro P, Jankovic M, Hanau G, et al. Survival after relapse in children with solid tumors: a follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer. 2006;47(5):560–6. doi: 10.1002/pbc.20726. [DOI] [PubMed] [Google Scholar]
- 7.Crompton BD, Goldsby RE, Weinberg VK, Feren R, O'Donnell RJ, Ablin AR. Survival after recurrence of osteosarcoma: a 20-year experience at a single institution. Pediatr Blood Cancer. 2006;47(3):255–9. doi: 10.1002/pbc.20580. [DOI] [PubMed] [Google Scholar]
- 8.Bhojwani D, Kang H, Moskowitz NP, Min DJ, Lee H, Potter JW, et al. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2006;108(2):711–7. doi: 10.1182/blood-2006-02-002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll WL, Bhojwani D, Min DJ, Moskowitz N, Raetz EA. Childhood acute lymphoblastic leukemia in the age of genomics. Pediatr Blood Cancer. 2006;46(5):570–8. doi: 10.1002/pbc.20722. [DOI] [PubMed] [Google Scholar]
- 10.Gaynon PS, Crotty ML, Sather HN, Bostrom BC, Nachman JB, Steinherz PG, et al. Expression of BCR-ABL, E2A-PBX1, and MLL-AF4 fusion transcripts in newly diagnosed children with acute lymphoblastic leukemia: a Children's Cancer Group initiative. Leuk Lymphoma. 1997;26(1-2):57–65. doi: 10.3109/10428199709109158. [DOI] [PubMed] [Google Scholar]
- 11.Schlieben S, Borkhardt A, Reinisch I, Ritterbach J, Janssen JW, Ratei R, et al. Incidence and clinical outcome of children with BCR/ABL-positive acute lymphoblastic leukemia (ALL). A prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM-90 and CoALL-05-92. Leukemia. 1996;10(6):957–63. [PubMed] [Google Scholar]
- 12.Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood. 1998;91(5):1700–5. [PubMed] [Google Scholar]
- 13.Cortez D, Reuther G, Pendergast AM. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene. 1997;15(19):2333–42. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- 14.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289(5486):1938–42. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 15.Schultz KR, Bowman WP, Slayton W, Aledo A, Devidas M, Sather H, et al. Improved Early Event Free Survival (EFS) in Children with Philadelphia Chromosome-Positive (Ph+) Acute Lymphoblastic Leukemia (ALL) with Intensive Imatinib in Combination with High Dose Chemotherapy: Children's Oncology Group (COG) Study AALL0031. ASH Annual Meeting Abstracts. 2007;110(11):9a. [Google Scholar]
- 16.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga T, Shirasawa H, Tanabe M, Ohnuma N, Kawamura K, Etoh T, et al. Expression of neuronal src mRNA as a favorable marker and inverse correlation to N-myc gene amplification in human neuroblastomas. Int J Cancer. 1994;58(6):793–8. doi: 10.1002/ijc.2910580607. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Montero CM, Wygant JN, McIntyre BW. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur J Cancer. 2006;42(10):1491–500. doi: 10.1016/j.ejca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Li S. Src kinase signaling in leukaemia. Int J Biochem Cell Biol. 2007;39(7-8):1483–8. doi: 10.1016/j.biocel.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozawa Y, Williams AH, Estes ML, Matsushita N, Boschelli F, Jove R, et al. Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT). Leuk Res. 2008;32(6):893–903. doi: 10.1016/j.leukres.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Spreafico A, Schenone S, Serchi T, Orlandini M, Angelucci A, Magrini D, et al. Antiproliferative and proapoptotic activities of new pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human osteosarcoma cells. Faseb J. 2008;22(5):1560–71. doi: 10.1096/fj.07-9873com. [DOI] [PubMed] [Google Scholar]
- 23.Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, et al. Dasatinib Inhibits Migration and Invasion in Diverse Human Sarcoma Cell Lines and Induces Apoptosis in Bone Sarcoma Cells Dependent on Src Kinase for Survival. Cancer Res. 2007;67(6):2800–2808. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- 24.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock RB, Tajbakhsh M, et al. Initial testing of dasatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(6):1198–206. doi: 10.1002/pbc.21368. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong SA, Mabon ME, Silverman LB, Li A, Gribben JG, Fox EA, et al. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood. 2004;103(9):3544–6. doi: 10.1182/blood-2003-07-2441. [DOI] [PubMed] [Google Scholar]
- 26.Birg F, Courcoul M, Rosnet O, Bardin F, Pebusque MJ, Marchetto S, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80(10):2584–93. [PubMed] [Google Scholar]
- 27.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 28.Meshinchi S, Stirewalt DL, Alonzo TA, Zhang Q, Sweetser DA, Woods WG, et al. Activating mutations of RTK/ras signal transduction pathway in pediatric acute myeloid leukemia. Blood. 2003;102(4):1474–9. doi: 10.1182/blood-2003-01-0137. [DOI] [PubMed] [Google Scholar]
- 29.Zwaan CM, Meshinchi S, Radich JP, Veerman AJP, Huismans DR, Munske L, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102(7):2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 30.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 31.O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 32.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 33.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–43. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 34.Brown P, Levis M, McIntyre E, Griesemer M, Small D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia. 2006;20(8):1368–76. doi: 10.1038/sj.leu.2404277. [DOI] [PubMed] [Google Scholar]
- 35.Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005;105(2):812–20. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- 36.Shimada A, Hirato J, Kuroiwa M, Kikuchi A, Hanada R, Wakai K, et al. Expression of KIT and PDGFR is associated with a good prognosis in neuroblastoma. Pediatr Blood Cancer. 2008;50(2):213–7. doi: 10.1002/pbc.21288. [DOI] [PubMed] [Google Scholar]
- 37.Timeus F, Ricotti E, Crescenzio N, Garelli E, Doria A, Spinelli M, et al. Flt-3 and its ligand are expressed in neural crest-derived tumors and promote survival and proliferation of their cell lines. Lab Invest. 2001;81(7):1025–37. doi: 10.1038/labinvest.3780314. [DOI] [PubMed] [Google Scholar]
- 38.Maris J, Minturn J, Evans A, Brophy P, Matthay K, Brown P, Hellriegel E, Brodeur G. Phase I Trial of the Orally Bioavailable TRK Tyrosine Kinase Inhibitor CEP-701 in Refractory Neuroblastoma: A New Approaches to Neuroblastoma Therapy (NANT) Study; SIOP XXXVII Annual Conference; Vancouver, Canada. 2005.2005. p. 416. [Google Scholar]
- 39.Goemans BF, Zwaan CM, Miller M, Zimmermann M, Harlow A, Meshinchi S, et al. Mutations in KIT and RAS are frequent events in pediatric core-binding factor acute myeloid leukemia. Leukemia. 2005;19(9):1536–42. doi: 10.1038/sj.leu.2403870. [DOI] [PubMed] [Google Scholar]
- 40.Uckan D, Hicsonmez G, Yetgin S, Gurgey A, Cetin M, Karaagaoglu E, et al. CD34/CD117 co-expression in childhood acute leukemia. Leuk Res. 2000;24(3):201–6. doi: 10.1016/s0145-2126(99)00183-6. [DOI] [PubMed] [Google Scholar]
- 41.Paschka P, Marcucci G, Ruppert AS, Mrozek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24(24):3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 42.Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spiekermann K, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107(5):1791–9. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 43.Shimada A, Taki T, Tabuchi K, Tawa A, Horibe K, Tsuchida M, et al. KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood. 2006;107(5):1806–9. doi: 10.1182/blood-2005-08-3408. [DOI] [PubMed] [Google Scholar]
- 44.Entz-Werle N, Gaub MP, Lavaux T, Marcellin L, Metzger N, Marec-Berard P, et al. KIT gene in pediatric osteosarcomas: could it be a new therapeutic target? Int J Cancer. 2007;120(11):2510–6. doi: 10.1002/ijc.22593. [DOI] [PubMed] [Google Scholar]
- 45.Do I, Araujo ES, Kalil RK, Bacchini P, Bertoni F, Unni KK, et al. Protein expression of KIT and gene mutation of c-kit and PDGFRs in Ewing sarcomas. Pathol Res Pract. 2007;203(3):127–34. doi: 10.1016/j.prp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Uccini S, Mannarino O, McDowell HP, Pauser U, Vitali R, Natali PG, et al. Clinical and molecular evidence for c-kit receptor as a therapeutic target in neuroblastic tumors. Clin Cancer Res. 2005;11(1):380–9. [PubMed] [Google Scholar]
- 47.Smithey BE, Pappo AS, Hill DA. C-kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol. 2002;26(4):486–92. doi: 10.1097/00000478-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2008;50(2):254–8. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez I, Andreu EJ, Panizo A, Inoges S, Fontalba A, Fernandez-Luna JL, et al. Imatinib inhibits proliferation of Ewing tumor cells mediated by the stem cell factor/KIT receptor pathway, and sensitizes cells to vincristine and doxorubicin-induced apoptosis. Clin Cancer Res. 2004;10(2):751–61. doi: 10.1158/1078-0432.ccr-0778-03. [DOI] [PubMed] [Google Scholar]
- 50.Corbacioglu S, Kilic M, Westhoff MA, Reinhardt D, Fulda S, Debatin KM. Newly identified c-KIT receptor tyrosine kinase ITD in childhood AML induces ligand-independent growth and is responsive to a synergistic effect of imatinib and rapamycin. Blood. 2006;108(10):3504–13. doi: 10.1182/blood-2006-05-021691. [DOI] [PubMed] [Google Scholar]
- 51.Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD, et al. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood. 2005;106(2):721–4. doi: 10.1182/blood-2004-12-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66(1):473–81. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 53.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26(11):1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 54.de Gramont A, Van Cutsem E. Investigating the potential of bevacizumab in other indications: metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology. 2005;(Suppl 3):69, 46–56. doi: 10.1159/000088483. [DOI] [PubMed] [Google Scholar]
- 55.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 56.Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(11):2544–55. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 57.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A Randomized Trial of Bevacizumab, an Anti-Vascular Endothelial Growth Factor Antibody, for Metastatic Renal Cancer. N Engl J Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kharfan-Dabaja MA, Patel SA, Osunkoya AO, Kojouri K, Kamble R, Yang J, et al. Expression of the vascular endothelial growth factor receptors 1 and 2 in acute myeloid leukemia: incidence and feasibility of immunohistochemical staining. Clin Lab Haematol. 2006;28(4):254–8. doi: 10.1111/j.1365-2257.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 59.Padro T, Bieker R, Ruiz S, Steins M, Retzlaff S, Burger H, et al. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2002;16(7):1302–10. doi: 10.1038/sj.leu.2402534. [DOI] [PubMed] [Google Scholar]
- 60.Kuzu I, Beksac M, Arat M, Celebi H, Elhan AH, Erekul S. Bone marrow microvessel density (MVD) in adult acute myeloid leukemia (AML): therapy induced changes and effects on survival. Leuk Lymphoma. 2004;45(6):1185–90. doi: 10.1080/1042819032000159915. [DOI] [PubMed] [Google Scholar]
- 61.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150(3):815–21. [PMC free article] [PubMed] [Google Scholar]
- 62.Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 63.Reichert F, Barak V, Tarshis M, Prindull G, Tarshis E, Ben-Ishay Z. Anti-angiogenic effects and regression of localized murine AML produced by anti-VEGF and anti-Flk-1 antibodies. Eur J Haematol. 2005;75(1):41–6. doi: 10.1111/j.1600-0609.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 64.Roboz GJ, Giles FJ, List AF, Cortes JE, Carlin R, Kowalski M, et al. Phase 1 study of PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor, for the treatment of acute myeloid leukemia and myelodysplastic syndrome. Leukemia. 2006;20(6):952–7. doi: 10.1038/sj.leu.2404213. [DOI] [PubMed] [Google Scholar]
- 65.Thomas DA, Estey E, Giles FJ, Faderl S, Cortes J, Keating M, et al. Single agent thalidomide in patients with relapsed or refractory acute myeloid leukaemia. Br J Haematol. 2003;123(3):436–41. doi: 10.1046/j.1365-2141.2003.04639.x. [DOI] [PubMed] [Google Scholar]
- 66.Andersson MK, Aman P. Proliferation of Ewing sarcoma cell lines is suppressed by the receptor tyrosine kinase inhibitors gefitinib and vandetanib. Cancer Cell Int. 2008;8:1. doi: 10.1186/1475-2867-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim ES, Serur A, Huang J, Manley CA, McCrudden KW, Frischer JS, et al. Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma. Proc Natl Acad Sci U S A. 2002;99(17):11399–404. doi: 10.1073/pnas.172398399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai A, Filka E, McGibbon B, Nghiemphu PL, Graham C, Yong WH, et al. Phase II Pilot Study of Bevacizumab in Combination With Temozolomide and Regional Radiation Therapy for Up-Front Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Interim Analysis of Safety and Tolerability. Int J Radiat Oncol Biol Phys. 2008 doi: 10.1016/j.ijrobp.2007.11.068. Available online 20 March 2008. [DOI] [PubMed] [Google Scholar]
- 69.McCrudden KW, Hopkins B, Frischer J, Novikov A, Huang J, Kadenhe A, et al. Anti-VEGF antibody in experimental hepatoblastoma: suppression of tumor growth and altered angiogenesis. J Pediatr Surg. 2003;38(3):308–14. doi: 10.1053/jpsu.2003.50099. discussion 308-14. [DOI] [PubMed] [Google Scholar]
- 70.Rowe DH, Huang J, Kayton ML, Thompson R, Troxel A, O'Toole KM, et al. Anti-VEGF antibody suppresses primary tumor growth and metastasis in an experimental model of Wilms’ tumor. J Pediatr Surg. 2000;35(1):30–2. doi: 10.1016/s0022-3468(00)80008-1. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 71.Maris JM, Courtright J, Houghton PJ, Morton CL, Kolb EA, Lock R, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51(1):42–8. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 72.Maris JM, Courtright J, Houghton PJ, Morton CL, Gorlick R, Kolb EA, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(3):581–7. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 73.Beaudry P, Nilsson M, Rioth M, Prox D, Poon D, Xu L, et al. Potent antitumor effects of ZD6474 on neuroblastoma via dual targeting of tumor cells and tumor endothelium. Mol Cancer Ther. 2008;7(2):418–24. doi: 10.1158/1535-7163.MCT-07-0568. [DOI] [PubMed] [Google Scholar]
- 74.Wullschleger S, Loewith R, Hall MN. TOR Signaling in Growth and Metabolism. Cell. 2006;124(3):471. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 75.Allen GW, Saba C, Armstrong EA, Huang SM, Benavente S, Ludwig DL, et al. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67(3):1155–62. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- 76.Dunn SE, Hardman RA, Kari FW, Barrett JC. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res. 1997;57(13):2687–93. [PubMed] [Google Scholar]
- 77.MacEwen EG, Pastor J, Kutzke J, Tsan R, Kurzman ID, Thamm DH, et al. IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma. J Cell Biochem. 2004;92(1):77–91. doi: 10.1002/jcb.20046. [DOI] [PubMed] [Google Scholar]
- 78.Scotlandi K, Benini S, Sarti M, Serra M, Lollini P- L, Maurici D, et al. Insulin-like Growth Factor I Receptor-mediated Circuit in Ewing's Sarcoma/Peripheral Neuroectodermal Tumor: A Possible Therapeutic Target. Cancer Res. 1996;56(20):4570–4574. [PubMed] [Google Scholar]
- 79.Wang W, Kumar P, Wang W, Epstein J, Helman L, Moore JV, et al. Insulin-like Growth Factor II and PAX3-FKHR Cooperate in the Oncogenesis of Rhabdomyosarcoma. Cancer Res. 1998;58(19):4426–4433. [PubMed] [Google Scholar]
- 80.Werner H, Idelman G, Rubinstein M, Pattee P, Nagalla SR, Roberts CT., Jr. A novel EWS-WT1 gene fusion product in desmoplastic small round cell tumor is a potent transactivator of the insulin-like growth factor-I receptor (IGF-IR) gene. Cancer Lett. 2007;247(1):84–90. doi: 10.1016/j.canlet.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 81.Werner H, Shen-Orr Z, Rauscher FJ, 3rd, Morris JF, Roberts CT, Jr., LeRoith D. Inhibition of cellular proliferation by the Wilms’ tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression. Mol. Cell. Biol. 1995;15(7):3516–3522. doi: 10.1128/mcb.15.7.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zumkeller W, Westphal M. The IGF/IGFBP system in CNS malignancy. Mol Pathol. 2001;54(4):227–9. doi: 10.1136/mp.54.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benini S, Manara MC, Baldini N, Cerisano V, Massimo S, Mercuri M, et al. Inhibition of insulin-like growth factor I receptor increases the antitumor activity of doxorubicin and vincristine against Ewing's sarcoma cells. Clin Cancer Res. 2001;7(6):1790–7. [PubMed] [Google Scholar]
- 84.Martins AS, Mackintosh C, Martin DH, Campos M, Hernandez T, Ordonez JL, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res. 2006;(11 Pt 1):12, 3532–40. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 85.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13(19):5834–40. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 86.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13(18 Pt 2):5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 87.Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21(9):1921–30. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- 88.Vorwerk P, Wex H, Hohmann B, Mohnike K, Schmidt U, Mittler U. Expression of components of the IGF signalling system in childhood acute lymphoblastic leukaemia. Mol Pathol. 2002;55(1):40–5. doi: 10.1136/mp.55.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JT, McCubrey JA. BAY-43-9006 Bayer/Onyx. Curr Opin Investig Drugs. 2003;4(6):757–63. [PubMed] [Google Scholar]
- 90.Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108(6):851–9. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 92.Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, et al. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6(3):243–57. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- 93.Boissel N, Leroy H, Brethon B, Philippe N, de Botton S, Auvrignon A, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia. 2006;20(6):965–70. doi: 10.1038/sj.leu.2404188. [DOI] [PubMed] [Google Scholar]
- 94.Lu D, Nounou R, Beran M, Estey E, Manshouri T, Kantarjian H, et al. The prognostic significance of bone marrow levels of neurofibromatosis-1 protein and ras oncogene mutations in patients with acute myeloid leukemia and myelodysplastic syndrome. Cancer. 2003;97(2):441–9. doi: 10.1002/cncr.11036. [DOI] [PubMed] [Google Scholar]
- 95.Chen Y, Takita J, Hiwatari M, Igarashi T, Hanada R, Kikuchi A, et al. Mutations of the PTPN11 and RAS genes in rhabdomyosarcoma and pediatric hematological malignancies. Genes Chromosomes Cancer. 2006;45(6):583–91. doi: 10.1002/gcc.20322. [DOI] [PubMed] [Google Scholar]
- 96.Weiss B, Bollag G, Shannon K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am J Med Genet. 1999;89(1):14–22. [PubMed] [Google Scholar]
- 97.Emanuel PD, Snyder RC, Wiley T, Gopurala B, Castleberry RP. Inhibition of juvenile myelomonocytic leukemia cell growth in vitro by farnesyltransferase inhibitors. Blood. 2000;95(2):639–45. [PubMed] [Google Scholar]
- 98.Fenaux P, Raza A, Mufti GJ, Aul C, Germing U, Kantarjian H, et al. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007;109(10):4158–63. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 99.Harousseau JL, Lancet JE, Reiffers J, Lowenberg B, Thomas X, Huguet F, et al. A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood. 2007;109(12):5151–6. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]
- 100.Karp JE, Lancet JE, Kaufmann SH, End DW, Wright JJ, Bol K, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97(11):3361–9. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 101.Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103(3):784–9. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 102.Robak T, Lech-Maranda E, Korycka A, Robak E. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity. Curr Med Chem. 2006;13(26):3165–89. doi: 10.2174/092986706778742918. [DOI] [PubMed] [Google Scholar]
- 103.Bantia S, Ananth SL, Parker CD, Horn LL, Upshaw R. Mechanism of inhibition of T-acute lymphoblastic leukemia cells by PNP inhibitor--BCX-1777. Int Immunopharmacol. 2003;3(6):879–87. doi: 10.1016/S1567-5769(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 104.Gandhi V, Kilpatrick JM, Plunkett W, Ayres M, Harman L, Du M, et al. A proof-of-principle pharmacokinetic, pharmacodynamic, and clinical study with purine nucleoside phosphorylase inhibitor immucillin-H (BCX-1777, forodesine). Blood. 2005;106(13):4253–60. doi: 10.1182/blood-2005-03-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen MH, Johnson JR, Massie T, Sridhara R, McGuinn WD, Jr., Abraham S, et al. Approval summary: nelarabine for the treatment of T-cell lymphoblastic leukemia/lymphoma. Clin Cancer Res. 2006;12(18):5329–35. doi: 10.1158/1078-0432.CCR-06-0606. [DOI] [PubMed] [Google Scholar]
- 106.Kurtzberg J, Ernst TJ, Keating MJ, Gandhi V, Hodge JP, Kisor DF, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23(15):3396–403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 107.Furman RR, Gore L, Ravandi F, Hoelzer D. Forodesine IV (Bcx-1777) Is Clinically Active in Relapsed/Refractory T-Cell Leukemia: Results of a Phase II Study (Interim Report). ASH Annual Meeting Abstracts. 2006;(11):108, 1851. [Google Scholar]
- 108.Ritchie E, Gore L, Roboz G, Feldman E, Ravandi F, Furman R. Phase II study of forodesine, a PNP inhibitor, in patients with relapsed or refractory B-lineage acute lymphoblastic leukemia; 48th Annual Meeting of the American Society of Hematology; Orlando, Fl. 2006.2006. [Google Scholar]
- 109.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Invest. 2004;22(2):304–11. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 110.Cusack JC, Jr., Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61(9):3535–40. [PubMed] [Google Scholar]
- 111.Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10(10):3371–6. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 112.Brignole C, Marimpietri D, Pastorino F, Nico B, Di Paolo D, Cioni M, et al. Effect of bortezomib on human neuroblastoma cell growth, apoptosis, and angiogenesis. J Natl Cancer Inst. 2006;98(16):1142–57. doi: 10.1093/jnci/djj309. [DOI] [PubMed] [Google Scholar]
- 113.Khan T, Stauffer JK, Williams R, Hixon JA, Salcedo R, Lincoln E, et al. Proteasome inhibition to maximize the apoptotic potential of cytokine therapy for murine neuroblastoma tumors. J Immunol. 2006;176(10):6302–12. doi: 10.4049/jimmunol.176.10.6302. [DOI] [PubMed] [Google Scholar]
- 114.Soldatenkov VA, Dritschilo A. Apoptosis of Ewing's sarcoma cells is accompanied by accumulation of ubiquitinated proteins. Cancer Res. 1997;57(18):3881–5. [PubMed] [Google Scholar]
- 115.Yan XB, Yang DS, Gao X, Feng J, Shi ZL, Ye Z. Caspase-8 dependent osteosarcoma cell apoptosis induced by proteasome inhibitor MG132. Cell Biol Int. 2007;31(10):1136–43. doi: 10.1016/j.cellbi.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 116.Houghton PJ, Morton CL, Kolb EA, Lock R, Carol H, Reynolds CP, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(1):37–45. doi: 10.1002/pbc.21214. [DOI] [PubMed] [Google Scholar]
- 117.Blaney SM, Bernstein M, Neville K, Ginsberg J, Kitchen B, Horton T, et al. Phase I study of the proteasome inhibitor bortezomib in pediatric patients with refractory solid tumors: a Children's Oncology Group study (ADVL0015). J Clin Oncol. 2004;22(23):4804–9. doi: 10.1200/JCO.2004.12.185. [DOI] [PubMed] [Google Scholar]
- 118.Horton TM, Pati D, Plon SE, Thompson PA, Bomgaars LR, Adamson PC, et al. A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a Children's Oncology Group study. Clin Cancer Res. 2007;13(5):1516–22. doi: 10.1158/1078-0432.CCR-06-2173. [DOI] [PubMed] [Google Scholar]
- 119.Ahn KS, Sethi G, Chao TH, Neuteboom ST, Chaturvedi MM, Palladino MA, et al. Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products. Blood. 2007;110(7):2286–95. doi: 10.1182/blood-2007-04-084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller CP, Ban K, Dujka ME, McConkey DJ, Munsell M, Palladino M, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110(1):267–77. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dorsey BD, Iqbal M, Chatterjee S, Menta E, Bernardini R, Bernareggi A, et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem. 2008;51(4):1068–72. doi: 10.1021/jm7010589. [DOI] [PubMed] [Google Scholar]
- 122.Piva R, Ruggeri B, Williams M, Costa G, Tamagno I, Ferrero D, et al. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood. 2008;111(5):2765–75. doi: 10.1182/blood-2007-07-100651. [DOI] [PubMed] [Google Scholar]
- 123.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110(9):3281–90. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8(5):407–19. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 125.Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111(3):1654–64. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruiz S, Krupnik Y, Keating M, Chandra J, Palladino M, McConkey D. The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia. Mol Cancer Ther. 2006;5(7):1836–43. doi: 10.1158/1535-7163.MCT-06-0066. [DOI] [PubMed] [Google Scholar]
- 127.Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Curr Mol Med. 2005;5(7):653–61. doi: 10.2174/156652405774641034. [DOI] [PubMed] [Google Scholar]
- 128.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19(56):6680–6. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 129.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 130.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 131.Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, et al. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci U S A. 2003;100(25):15113–8. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107(3):1149–55. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61(4):1527–32. [PubMed] [Google Scholar]
- 134.Johnsen JI, Segerstrom L, Orrego A, Elfman L, Henriksson M, Kagedal B, et al. Inhibitors of mammalian target of rapamycin downregulate MYCN protein expression and inhibit neuroblastoma growth in vitro and in vivo. Oncogene. 2008;27(20):2910–22. doi: 10.1038/sj.onc.1210938. [DOI] [PubMed] [Google Scholar]
- 135.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65(6):2406–11. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 136.Wan X, Shen N, Mendoza A, Khanna C, Helman LJ. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 2006;8(5):394–401. doi: 10.1593/neo.05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Houghton PJ, Morton CL, Kolb EA, Gorlick R, Lock R, Carol H, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(4):799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 138.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 139.Kuendgen A, Schmid M, Schlenk R, Knipp S, Hildebrandt B, Steidl C, et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all trans retinoic acid in patients with acute myeloid leukemia. Cancer. 2006;106(1):112–9. doi: 10.1002/cncr.21552. [DOI] [PubMed] [Google Scholar]
- 140.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63(13):3637–45. [PubMed] [Google Scholar]
- 141.Banelli B, Di Vinci A, Gelvi I, Casciano I, Allemanni G, Bonassi S, et al. DNA methylation in neuroblastic tumors. Cancer Lett. 2005;228(1-2):37–41. doi: 10.1016/j.canlet.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 142.Obana K, Yang HW, Piao HY, Taki T, Hashizume K, Hanada R, et al. Aberrations of p16INK4A, p14ARF and p15INK4B genes in pediatric solid tumors. Int J Oncol. 2003;23(4):1151–7. [PubMed] [Google Scholar]
- 143.Park YB, Park MJ, Kimura K, Shimizu K, Lee SH, Yokota J. Alterations in the INK4a/ARF locus and their effects on the growth of human osteosarcoma cell lines. Cancer Genet Cytogenet. 2002;133(2):105–11. doi: 10.1016/s0165-4608(01)00575-1. [DOI] [PubMed] [Google Scholar]
- 144.Aggerholm A, Holm MS, Guldberg P, Olesen LH, Hokland P. Promoter hypermethylation of p15INK4B, HIC1, CDH1, and ER is frequent in myelodysplastic syndrome and predicts poor prognosis in early-stage patients. Eur J Haematol. 2006;76(1):23–32. doi: 10.1111/j.1600-0609.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 145.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108(10):3271–9. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scott SA, Lakshimikuttysamma A, Sheridan DP, Sanche SE, Geyer CR, DeCoteau JF. Zebularine inhibits human acute myeloid leukemia cell growth in vitro in association with p15INK4B demethylation and reexpression. Exp Hematol. 2007;35(2):263–73. doi: 10.1016/j.exphem.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 147.Shaker S, Bernstein M, Momparler LF, Momparler RL. Preclinical evaluation of antineoplastic activity of inhibitors of DNA methylation (5-aza-2'-deoxycytidine) and histone deacetylation (trichostatin A, depsipeptide) in combination against myeloid leukemic cells. Leuk Res. 2003;27(5):437–44. doi: 10.1016/s0145-2126(02)00222-9. [DOI] [PubMed] [Google Scholar]
- 148.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13(1):47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 149.Gudowius S, Recker K, Laws HJ, Dirksen U, Troger A, Wieczorek U, et al. Identification of candidate target antigens for antibody-based immunotherapy in childhood B-cell precursor ALL. Klin Padiatr. 2006;218(6):327–33. doi: 10.1055/s-2006-942273. [DOI] [PubMed] [Google Scholar]
- 150.Mansfield E, Amlot P, Pastan I, FitzGerald DJ. Recombinant RFB4 Immunotoxins Exhibit Potent Cytotoxic Activity for CD22-Bearing Cells and Tumors. Blood. 1997;90(5):2020–2026. [PubMed] [Google Scholar]
- 151.Carnahan J, Stein R, Qu Z, Hess K, Cesano A, Hansen HJ, et al. Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol Immunol. 2007;44(6):1331–41. doi: 10.1016/j.molimm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 152.Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;(10 Pt 2):9, 3982S–90S. [PubMed] [Google Scholar]
- 153.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5. doi: 10.1038/sj.leu.2404866. [DOI] [PubMed] [Google Scholar]
- 154.Cooney-Qualter E, Krailo M, Angiolillo A, Fawwaz RA, Wiseman G, Harrison L, et al. A Phase I Study of 90Yttrium-Ibritumomab-Tiuxetan in Children and Adolescents with Relapsed/Refractory CD20-Positive Non Hodgkin's Lymphoma: A Children's Oncology Group Study. Clin Cancer Res. 2007;13(18):5652s–5660. doi: 10.1158/1078-0432.CCR-07-1060. [DOI] [PubMed] [Google Scholar]
- 155.Giralt S. The role of alemtuzumab in nonmyeloablative hematopoietic transplantation. Semin Oncol. 2006;33(2 Suppl 5):S36–43. doi: 10.1053/j.seminoncol.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 156.McCune SL, Gockerman JP, Moore JO, Decastro CM, Bass AJ, Chao NJ, et al. Alemtuzumab in relapsed or refractory chronic lymphocytic leukemia and prolymphocytic leukemia. Leuk Lymphoma. 2002;43(5):1007–11. doi: 10.1080/10428190290021597. [DOI] [PubMed] [Google Scholar]
- 157.Yao Q, Weigel B, Kersey J. Synergism between etoposide and 17-AAG in leukemia cells: critical roles for Hsp90, FLT3, topoisomerase II, Chk1, and Rad51. Clin Cancer Res. 2007;13(5):1591–600. doi: 10.1158/1078-0432.CCR-06-1750. [DOI] [PubMed] [Google Scholar]
- 158.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, et al. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J Med Chem. 1995;38(19):3806–12. doi: 10.1021/jm00019a010. [DOI] [PubMed] [Google Scholar]
- 159.Schulte TW, Blagosklonny MV, Romanova L, Mushinski JF, Monia BP, Johnston JF, et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16(10):5839–45. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10(6):705–12. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 161.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91(18):8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.George P, Bali P, Cohen P, Tao J, Guo F, Sigua C, et al. Cotreatment with 17-allylamino demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 2004;64(10):3645–52. doi: 10.1158/0008-5472.CAN-04-0006. [DOI] [PubMed] [Google Scholar]
- 163.Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SJ, Dai NT, et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106(1):318–27. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplatin. Int J Cancer. 2005;113(2):179–88. doi: 10.1002/ijc.20611. [DOI] [PubMed] [Google Scholar]
- 165.Garcia-Morales P, Carrasco-Garcia E, Ruiz-Rico P, Martinez-Mira R, Menendez-Gutierrez MP, Ferragut JA, et al. Inhibition of Hsp90 function by ansamycins causes downregulation of cdc2 and cdc25c and G(2)/M arrest in glioblastoma cell lines. Oncogene. 2007;26(51):7185–93. doi: 10.1038/sj.onc.1210534. [DOI] [PubMed] [Google Scholar]
- 166.Kang J, Kamal A, Burrows FJ, Evers BM, Chung DH. Inhibition of neuroblastoma xenograft growth by Hsp90 inhibitors. Anticancer Res. 2006;26(3A):1903–8. [PMC free article] [PubMed] [Google Scholar]
- 167.Bagatell R, Gore L, Egorin MJ, Ho R, Heller G, Boucher N, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17- demethoxygeldanamycin in pediatric patients with recurrent or refractory solid tumors: a pediatric oncology experimental therapeutics investigators consortium study. Clin Cancer Res. 2007;13(6):1783–8. doi: 10.1158/1078-0432.CCR-06-1892. [DOI] [PubMed] [Google Scholar]
- 168.Weigel BJ, Blaney SM, Reid JM, Safgren SL, Bagatell R, Kersey J, et al. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children's Oncology Group study. Clin Cancer Res. 2007;13(6):1789–93. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- 169.Smith MA, Morton CL, Phelps DA, Kolb EA, Lock R, Carol H, et al. Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51(1):34–41. doi: 10.1002/pbc.21508. [DOI] [PubMed] [Google Scholar]
- 170.Grabher C, von Boehmer H, Look AT. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6(5):347–59. doi: 10.1038/nrc1880. [DOI] [PubMed] [Google Scholar]
- 171.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 172.Pahlman S, Stockhausen MT, Fredlund E, Axelson H. Notch signaling in neuroblastoma. Semin Cancer Biol. 2004;14(5):365–73. doi: 10.1016/j.semcancer.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 173.Baliko F, Bright T, Poon R, Cohen B, Egan SE, Alman BA. Inhibition of notch signaling induces neural differentiation in Ewing sarcoma. Am J Pathol. 2007;170(5):1686–94. doi: 10.2353/ajpath.2007.060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang P, Yang Y, Zweidler-McKay PA, Hughes DP. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin Cancer Res. 2008;14(10):2962–9. doi: 10.1158/1078-0432.CCR-07-1992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 175.Breit S, Stanulla M, Flohr T, Schrappe M, Ludwig WD, Tolle G, et al. Activating NOTCH1 mutations predict favorable early treatment response and long-term outcome in childhood precursor T-cell lymphoblastic leukemia. Blood. 2006;108(4):1151–7. doi: 10.1182/blood-2005-12-4956. [DOI] [PubMed] [Google Scholar]
- 176.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–71. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 177.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279(13):12876–82. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 178.Machold RP, Kittell DJ, Fishell GJ. Antagonism between Notch and bone morphogenetic protein receptor signaling regulates neurogenesis in the cerebellar rhombic lip. Neural Develop. 2007;2:5. doi: 10.1186/1749-8104-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(51):20558–63. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]