Abstract
Over the past decade, large multicenter trials have unequivocally demonstrated that decreasing low density lipoprotein (LDL) cholesterol can reduce both primary and secondary cardiovascular events in patients at risk. However, even in the context of maximal LDL lowering, there remains considerable residual cardiovascular risk. Some of this risk can be attributed to variability in high density lipoprotein (HDL) cholesterol. As such, there is tremendous interest in defining determinants of HDL homeostasis.
Risk prediction models are being constructed based upon (1) clinical contributors, (2) known molecular determinants, and (3) the genetic architecture underlying HDL cholesterol levels. To date, however, no single resource has combined these factors within the context of a practice-based dataset. Recently, a number of academic medical centers have begun constructing DNA biobanks linked to secure encrypted versions of their respective electronic medical record. As these biobanks combine resources, the clinical community is in a position to characterize lipid-related treatment outcome on an unprecedented scale.
Lipoprotein homeostasis is a complex process [1,2]. Free fatty acids (FFA) and triglycerides (TG) absorbed from within the gastrointestinal lumen are shuttled to the liver in chylomicrons. FFA and TG are then combined with apolipoproteins, phospholipids, free cholesterol and cholesterol esters (CE), to form low density lipoproteins (LDL) and very low density lipoproteins (VLDL). These particles are inter-converted by lipoprotein lipase (LPL), and variability in LPL activity is associated with derangements in lipid homeostasis [3]. While VLDL and LDL are atherogenic, association between these large lipoproteins and cardiovascular disease (CVD) is modified by a number of additional lipid-dependent and lipid-independent risk factors [4]. Thus, all lipoproteins are not uniformly atherogenic.
High density lipoprotein (HDL) particles have direct anti-atherogenic properties in transgenic mouse models [5]. Although HDL particles may serve as a source of cholesterol esters for the more atherogenic LDL and VLDL, the HDL particles themselves may actually attenuate the development of cardiovascular disease in humans [6]. Nonetheless, the role of HDL in atherogenesis remains a matter of ongoing controversy [7]. HDL particles exhibit a wide degree of structural variability, and these particles participate in a variety of processes which can be either pro-atherogenic or anti-atherogenic. As such, there is tremendous interest in characterization and pharmacological optimization of the molecular and cellular mechanisms underlying HDL homeostasis.
Determinants of HDL Composition
Nascent HDL particles are initially composed of free cholesterol and apolipoprotein, ApoA-1. These early particles are discoidal and they occupy a pre-β position on non-denaturing electrophoretic profiles. Within peripheral tissues, lecithin cholesterol acyltransferase (LCAT) esterifies free cholesterol, and the resulting cholesterol esters are incorporated into maturing HDL particles through the activity of ATP-binding cassette protein transporters (e.g., ABCA1 and ABCG1) [1]. The result is a larger, spherical HDL particle that migrates in the α position on non-denaturing electrophoretic profiles.
Although these more mature HDL particles are rich in CE and phospholipid, the CE concentration of their lipid core varies considerably as they move throughout the circulation. These are dynamic particles. Cholesterol ester transfer proteins (CETP) also circulate in the plasma, bound to lipoproteins, and they redistribute both CE and TG between HDL and larger Apo-B containing lipoproteins (e.g., LDL). Thus, CETP is capable of moving CE back into VLDL and LDL, depending upon the ratio of LDL and HDL in the circulation. The net effect on HDL is depletion of CE and enrichment of TG [8].
Conversely, scavenger receptor Class B-1 (SRB1) moves CE forward into the liver and steroidogenic tissues (adrenals, ovaries, testes). Like CETP, the net effect is reduction of CE within the HDL particles. SRB1 multimers form within the plasma membranes of target cells (e.g., hepatocytes), possibly requiring the presence of HDL particles to facilitate their assembly [9]. Each SRB1 monomer has two membrane spanning regions. While the extracellular loop of SRB1 has moderate affinity for many apolipoproteins (i.e., functioning as a scavenger protein), SRB1 can only mobilize CE in the presence of ApoA-1. SRB1 is also capable of extracting phospholipids and TG. Thus, SRB1 generates HDL particles which also migrate in the α position on non-denaturing electrophoretic profiles [8].
Intravascular Remodeling
HDL particles undergo considerable remodeling within the vascular space. Nascent HDL particles contain 2 copies of ApoA-I and very little lipid (less than 10%) [8]. These particles acquire free and esterified cholesterol within the vascular lumen, through mechanisms introduced above. ApoA-I is present on most HDL particles, representing approximately 70% of the protein content of the total circulating pool of HDL [8]. Apo A-II is the second most abundant HDL protein. Other HDL proteins include inflammatory markers (e.g., serum Amyloid A) [8].
Further intravascular remodeling is facilitated by lipolytic enzymes, which transfer additional surface components (e.g., other apolipoproteins such Apo-E) and additional core components (e.g., TGs) to the maturing HDL particle. At least three lipolytic enzymes modify HDL composition [1,8]. They are endothelial lipase (LIPG), hepatic lipase (LIPC), and lipoprotein lipase (LPL).
LPL is synthesized by adipose tissue and skeletal muscle. Its enzymatic activity favors the lipolysis of TGs (i.e., phospholipase activity is minor). LIPC is synthesized by heptocytes, and it has both phospholipase and TG lipase activity. Further, LIPC has greater activity against HDL than VLDL or chylomicrons. LIPC thus converts larger HDL particles into smaller HDL remnants [10]. As discussed later, this process is accelerated during insulin resistance and hypertriglyceridemia [11, 12].
Conversely, LIPG is primarily a phospholipase, with relatively little TG-lipase activity. In general, LIPG hydrolyzes the phospholipids of HDL, while LIPC hydrolyzes the TG of HDL. Other phospholipases (e.g., soluble PLA2) are capable of hydrolyzing HDL phospholipids during inflammatory states. The observation that LIPG is upregulated by cytokines may provide a link between inflammation and low HDL levels.
Reverse Cholesterol Transport
As noted, circulating levels of HDL cholesterol are determined by the dynamic equilibrium of three processes: (1) lipoprotein biosynthesis, (2) particle remodeling in the vascular space, and (3) particle disassembly. At present, there is tremendous interest in pharmacological optimization of these processes, particularly with a goal toward leveraging HDL to shuttle cholesterol out of atherosclerotic plaques and back to the liver for hepatobiliary elimination (reverse cholesterol transport) [13].
The ATP-binding cassette transporter protein, ABCA1, may be able to export free cholesterol from macrophages in lipid plaques, by combining free cholesterol with nascent HDL and lipid-poor apolipoproteins [14]. Free cholesterol can also be moved out of macrophages through alternate membrane transporters (e.g., ABCG1), but these latter transporters appear to move macrophage cholesterol preferentially into mature cholesterol-laden HDL particles [14, 15]. After further remodeling of the HDL particle pool by enzymes such as CETP and LIPG, and driven by mass action, HDL particles can then shuttle cholesterol back into hepatocytes via SRB1.
Clinical variables influencing this dynamic equilibrium are reviewed below, followed by a discussion of ongoing efforts to quantify treatment efficacy within the world’s growing population-based Biobanks. This unique integration of clinical data facilitates the construction of improved risk prediction models.
Clinical Contributors to HDL Level
Lipoprotein homeostasis is strongly influenced by changes in body weight, body composition, and physical activity [11, 16–19]. The current obesity epidemic represents a major international health crisis [20], and the increased cardiovascular mortality associated with weight gain is observed in all races [21]. The metabolic complications of weight gain are often more life-threatening than obesity itself. For instance, obese individuals tend to have lower circulating levels of HDL cholesterol [22]; and each 1 mg/dl reduction in HDL cholesterol level is associated with a 6% increase cardiovascular risk [23].
Furthermore, obesity (i.e., increased visceral fat mass) is associated with a reduction in mean HDL particle size, yielding a shift on density gradient ultracentrifugation toward a decreasing concentration of the larger HDL2 subclass, and an increasing concentration of the smaller HDL3 subclass [24]. In obese study subjects, low-HDL dyslipidemia is further characterized by HDL3 particles that are deficient in otherwise normal anti-atherogenic properties [25]. Thus, the shift to HDL3 as well as the development of HDL3 particle dysfunction contributes to net cardiovascular risk [26].
Atherogenic dyslipidemia is also defined by a constellation of lipoprotein abnormalities that include high serum TG levels, and a shift toward smaller more dense LDL particles [19, 27, 28]. LDL particles typically migrate in the β position on non-denaturing electrophoretic profiles, and (as noted above) mature HDL particles migrate in the α position. The distribution of each (and therefore the mean particle size) is highly inter-correlated with TG concentration [28].
While elevated TG levels and alteration in HDL cholesterol can occur in the absence of other metabolic abnormalities, both phenotypes often accompany insulin resistance [17, 24, 29]. This association appears to be driven by two mechanisms. Insulin resistance directly impacts HDL particle composition by altering the expression and activity of LIPC [12]. As introduced above, LIPC converts larger HDL particles into smaller HDL remnants [10], and this process is clearly accelerated during insulin resistance [12] The resulting remnant HDL particles have altered fluidity, as well as changes in ApoA-I conformation and higher concentrations of free fatty acids. In this context, the HDL particles generated by LIPC activity appear to be cleared faster (i.e., insulin resistance increases HDL catabolism). HDL catabolism is further altered in insulin resistant subjects who develop hypertriglyceridemia [8]. CETP-mediated heterotransfer of lipids (i.e., depletion of CE and enrichment of TG) is increased during hypertriglyceridemia, further altering the stability of HDL.
In fact, hypertriglyceridemia is the most common lipid abnormality observed within clinical syndromes of insulin resistance, and this abnormality is due in part to increased hepatic production of VLDL [8]. Due to competition for saturable removal processes [30], elevated TG levels become particularly evident during the postprandial period [31]. In the fasting state, a net increase in TG/HDL ratio is felt by some investigators to be more predictive of insulin resistance than the presence of abdominal obesity [32]. The prevalence of this phenomenon is rapidly rising.
Biological Candidate Genes
The physiologic insulin-mediated stimulation of LPL activity within skeletal muscle and adipose tissue is also altered in obese subjects with insulin-resistance [33, 34]. Rare subjects with heritable LPL deficiency are known to have low circulating levels of HDL cholesterol [35]. Other monogenic forms of low HDL dyslipidemia have also been reported (e.g., Tangier disease, due to variants in ABCA1, ApoA-I or LCAT) [2, 36].
Recently, one of the most interesting treatment targets for obesity-related dyslipidemia has been the endocannabinoid (eCB) system [37]. The eCB system consists of two cannabinoid receptors, CB1 and CB2 [38], and emerging data support the fundamental hypothesis that dyslipidemia is partly influenced by overactive signaling at the level of CB1 [37]. This claim is supported by multiple recent reports. In Europe, randomized placebo-controlled clinical trials have demonstrated that use of a CB1 receptor antagonist (rimonabant, 20 mg daily) is associated with a greater mean weight reduction than placebo after one year of therapy (−6.6 ±7.2 kg versus −1.8 ±6.4 kg; p<0.001) [39]. This finding was subsequently validated in a similar study involving North American study subjects, wherein rimonabant (20 mg daily) was also associated with a greater mean weight reduction than placebo after one year of therapy (−6.3 ±0.2 kg versus −1.6 ±0.2 kg; p<0.001) [40]. Both trials revealed a greater improvement in metabolic parameters (including HDL cholesterol) than would have been expected based upon weight loss alone [41]. In an analysis of covariance (correcting for weight loss from baseline through standard regression methods), investigators found that patients taking rimonabant developed an increase in HDL levels, and a decrease in TG levels, more than twice that anticipated based upon weight loss alone [39, 40, 41].
Since an acquired (drug-induced) change in eCB/CB1 signaling clearly modifies HDL and TG levels, it is likely that genetic variability in eCB/CB1 signaling contributes to the development of dyslipidemia [42]. We recently tested this claim in one of the most rigorously phenotyped family-based obesity study cohorts in the United States [43]. CNR1 gene variability was associated with high TG and low HDL levels. Studies in the original cohort are providing insight into mechanism; the biological link between genetic variability in eCB/CB1 signaling and dyslipidemia appears to be independent of insulin responsiveness (based upon phenotypes derived from a frequently sampled intravenous glucose tolerance test: Si, Sg, AIRg, and DI) [44]. Whether the eCB/CB1 signaling variants identified in this cohort will be causative in other populations of unrelated individuals remains unclear [45, 46].
Positional Candidate Genes
Characterization of the genetic architecture underlying clinical derangements in lipid homeostasis has not been limited to biological candidate genes; early on, some progress was made in the identification of positional candidate genes as well. Prior to 2004, linkage studies (in families) reported associations between various loci and lipoprotein levels as quantitative traits (QTs). Many of these associations were never replicated. More recently, resources have been reallocated toward whole genome scanning, with dense scanning platforms in large cohorts of unrelated individuals. While these efforts appear to be providing some insight into signaling pathways previously unrecognized as impacting lipid homeostasis, most associations represent the re-identification of previously characterized biological candidate genes.
For instance, in a recent multicenter genome scanning effort involving more than 8000 study subjects from three combined cohorts, six previously recognized loci (containing twelve biological candidate genes) were validated with respect to their association with HDL cholesterol [47]. Each of these six loci contained at least one of the biological candidate genes discussed above (e.g., ABCA1, APOA1, CETP, LIPC, LIPG, and LPL). In addition to these six previously recognized loci, the investigators also identified a novel gene associated with HDL, GALNT2 (encoding an enzyme involved in the post-translational glycosylation of proteins) [47]. Each copy of the minor allele for GALNT2 was associated with a 1.6 mg/dl decrease in HDL. Further, GALNT2 was also idenitifed as one of five new loci associated with TG levels in the same study population [47]. These results have clearly been replicated. Willer and colleagues identified nine loci that were associated with HDL in a large multicenter study of similar design [48]. Many of the lipolytic enzymes and transporters outlined above were also contained within the 9 loci identified by the latter group (e.g., ABCA1, LPL, LIPG, CETP, LCAT, and GALNT2). However, since these loci collectively explain ≤5% of the HDL trait variance in these populations, much remains to be learned about the basic biology of dyslipidemia.
Large consortia are therefore being constructed to facilitate the identification of additional loci through meta-analyses leveraging genome wide data in populations and treatment trials [49]. To date, nearly 100 variants influencing lipid homeostasis have been reported. A small subset of these loci appear to be independently associated with cardiovascular disease [50].
Therapeutic Interventions
Statins
The large majority of cholesterol within cells is derived through de novo synthesis. Free cholesterol is built from simple 2-carbon fragments through a relatively linear biosynthetic pathway [51], and the rate limiting enzyme in cholesterol biosynthesis is HMG-CoA reductase. Large, multicenter trials have demonstrated that attenuation of endogenous cholesterol biosynthesis through the use of HMG-CoA reductase inhibitors (statins) can reduce the likelihood of both primary and secondary cardiovascular events in patients at risk [52–56]. At present, there are six commercially available drugs within this class: lovastatin (approved by the FDA in 1987), simvastatin (1988), pravastatin (1991), fluvastatin (1994), atorvastatin (1997), and rosuvastatin (2003) [57]. Each of these drugs is highly efficacious (representing 30–50% risk reduction depending upon dose and upon clinical context).
While statins are known to interact with a variety of cellular processes, each drug in this class appears to derive its primary therapeutic effect by attenuating endogenous lipoprotein synthesis and up-regulating the expression of membrane bound LDL cholesterol receptors [58]. Although these agents markedly reduce circulating levels of LDL cholesterol, their effect on HDL has been modest [59]: HDL levels increase 5–10% on statins [60, 61]. Further, the shape of the dose response relationship between statins and HDL cholesterol varies from drug to drug [61]. While simvastatin appears to raise HDL consistently at all common doses, the response to atorvastatin may be biphasic (diminished at higher doses) [61]. This observation may reflect a differential effect of inhibiting cholesterol biosynthesis on the remodeling of lipoproteins.
Ezetimibe
Under normal physiologic conditions, 20% of cholesterol stores are derived from dietary sources (i.e., exogenous uptake). Within the gastrointestinal tract, cholesterol uptake is facilitated by Niemann-Pick C1 Like 1 (NPC1L1), a protein found in the brush border membrane of enterocytes [62]. Ezetimibe lowers cholesterol levels through inhibition of cholesterol uptake in the small intestine, at the level of NPC1L1 [63]. Ezetimibe has been used clinically since 2002.
Following absorption, more than 90% of this drug undergoes enterohepatic circulation (i.e., passes through the portal circulation to the liver, then the majority of the drug re-enters the small intestine via the bile, where it blocks further cholesterol uptake) [63]. In the liver, ezetimibe undergoes glucuronidation. Primary Phase II enzymes are UGT1A1, UGT1A3, and UGT2B15 [64], and the resulting bioactive glucuronidated derivatives keep cycling and re-blocking cholesterol uptake [65, 66]. Thus, ezetimibe monotherapy can reduce LDL levels 20% [67–72]. When combined with a statin, LDL reduction is typically 25–50% [72, 73]. The effect of ezetimibe on HDL is relatively small, and its overall clinical benefit remains controversial [74]. As discussed in a later section of this review, the ARBITER trials have been constructed to clarify this controversy [75].
Torcetrapib
Both classes of drugs discussed above modify the availability of free cholesterol. As noted, however, lipoproteins contain esterified cholesterol in large amounts. Since cholesterol ester transfer protein (CETP) facilitates the movement of cholesterol esters from HDL to the more atherogenic LDL, there has recently been tremendous interest in the potential for CETP inhibitors to reduce vascular disease [7, 14, 76, 77]. While some studies indicate that CETP deficiency may be cardio-protective, other studies suggest increased cardiovascular risk [78, 79]. Clinical studies conducted with CETP inhibitors have yielded equally conflicting results. In a recent trial evaluating 15,000 patients at risk, randomized to either LDL reduction with atorvastatin alone, or LDL reduction with atorvastatin plus HDL increase with torcetrapib, subjects on combination therapy (atorvastatin plus torcetrapib) were observed to have a higher frequency of angina, revascularization, myocardial infarction, heart failure and death [7]. This finding was not anticipated.
There is growing evidence suggesting that this discrepancy (i.e., between improved lipid panels and higher frequency of adverse cardiovascular events on torcetrapib) may have been due to altered distribution of HDL particle subtypes, toward a more pro-inflammatory atherogenic form of HDL. Newer CETP inhibitors appear to increase HDL and decrease TG levels (without altering blood pressure) [80]. Through proteomics, investigators are now gaining deeper insight into the molecular heterogeneity of HDL particles [81, 82]. In a comparison between HDL particles obtained from control patients and patients with coronary artery disease, lipoproteins from patients with greater disease burden had higher levels of many acute-phase response proteins, as well as a differential distribution of apoplipoproteins [81, 83]. Thus, in addition to HDL cholesterol concentration, HDL particle composition is an important determinant of risk.
Fibric acid derivatives
The relationship between HDL cholesterol concentration and HDL particle size is strongly dependent upon TG homeostasis. As noted, fasting TG levels are an independent predictor of HDL particle size [24]. Fibric acid derivatives lower TG levels by activating peroxisome-proliferator activated receptors (PPAR) [84]. There are several molecular PPAR subtypes (α,β,δ and γ), which can be activated by endogenous lipids (fatty acids, eicosanoids, and oxidized phospholipids) [85]. Two exogenous ligands are commonly used to modulate lipid homeostasis through PPARα. In vitro, fenofibrate acts as a full agonist at PPARα, whereas gemfibrozil appears to be a partial agonist [85]. PPAR-α activation promotes the catabolism of fatty acids through beta-oxidation [85]. When bound by ligand, PPAR-α heterodimerizes with the retinoic acid receptor (RxR) and binds to PPAR response elements (PPRE) [85, 86] in genes important to lipid metabolism and glucose homeostasis [87]. When activated, PPAR-α induces acyl-Coenzyme A synthetase, a key enzyme in the esterification of fatty acids (preventing the cellular efflux of fatty acids), as well as carnitine palmitoyltransferase type 1 (CPT-1), an enzyme involved in the mitochondrial fatty acid oxidation in highly active tissues (e.g., cardiac and skeletal muscle) [84–87]. PPARα activation thus limits the amount of FFA available for hepatic synthesis of TG-rich lipoproteins like VLDL. The net effect is a reduction in TG.
Fenofibrate and gemfibrozil directly impact HDL composition, through PPARα-mediated induction of hepatic lipase (LIPC) [85]. As noted earlier, LIPC converts larger HDL particles into smaller HDL remnants [10]. PPAR-α also regulates HDL homeostasis by modulating expression of Apo-I, Apo-II, ABCA1 and SRB1 [85,86]. Ligand-activated PPAR-RXR heterodimers facilitate the trans-repression of anti-inflammatory gene products within the vascular endothelium, vascular smooth muscle, and within cells of the immune system (e.g., CRP, COX-2) [87]. It is therefore likely that fibric acids influence the HDL proteome in a manner not yet fully characterized.
Fibrates are particularly effective in improving lipid parameters in patients with dyslipidemia due to type II diabetes mellitus [88]. Fenofibrate reduces TG levels nearly 50% in such patients [89, 90]. Modest improvements in LDL and HDL are observed as well. In the FIELD Trial (nearly 10,000 diabetic subjects with and without known coronary artery disease), the use of fenofibrate was associated with a reduction in myocardial infarction (HR 0.76, 95% CI 0.62–0.94; p = 0.01), as well as a reduction in the frequency of coronary artery revascularization (HR 0.79, 95% CI 0.68–0.93; p = 0.003) [88]. Conversely, gemfibrozil appears to lower TG levels by approximately 35% [91]. In the Helsinki Heart Study, gemfibrozil was efficacious in reducing coronary events in 4000 men at moderate risk of CAD followed longitudinally (34% risk reduction after 5 years, p < 0.05) [91]. On average, LDL levels were decreased by 11% and HDL levels were increased by 11%. In sub-analyses using overweight patients with fasting TGs > 200 mg/dl (and HDL levels < 40 mg/dl), gemfibrozil reduced coronary risk 78% (p < 0.002) [92]. Gemfibrozil was also associated with a 22% reduction in adverse coronary events in patients with known CAD followed longitudinally in the VA-HIT trial [93, 94]. Nearly 25% of all patients enrolled in the VA-HIT Trial had type II diabetes mellitus.
Niacin
Niacin is efficacious at optimizing TG levels and HDL levels [95]. Niacin improves these lipid parameters through two mechanisms: (1) it increases sequestration of TGs in adipose (resulting in a reduction in the release of FFAs), and (2) it decreases synthesis of TGs in the liver (attenuating hepatic production of VLDL). In hepatocytes, esterification of diacylglycerols to TG is catalyzed by diacylglycerol acyl transferase-2 (DGAT2). Niacin has been shown to non-competitively inhibit DGAT2, decreasing TG production [96]. In adipose, activation of a G-protein-coupled receptor called HM74A (GPR109A) results in the inhibition of hormone sensitive lipase through a mechanism dependent upon cAMP [97–99]. The net effect is attenuation of the conversion of TG into FFA, keeping TG sequestered in the adipose. While niacin is not a physiological ligand for HM74A, it can activate the receptor at high concentrations [97, 98]. Since the effect of niacin is concentration-dependent, lipase activity can be inhibited almost 100% [99].
Niacin also increases HDL levels, by blocking the catabolism of these particles. Plasma turnover studies in humans have indicated that niacin decreases the fractional catabolic rate of HDL without altering Apo A synthesis [95, 96]. Thus, raising HDL through the use of niacin may represent a valid clinical strategy for the reduction of cardiovascular risk. Data reported by the ARBITER 2 Trial have indicated that niacin provides added benefit when given in addition to statin monotherapy in the stabilization of atherosclerotic carotid artery disease [100]. At this point, it remains unclear whether the added benefit attributed to niacin was due to additional LDL lowering, versus optimization of HDL and/or lipoprotein particle composition. To address this question within the context of carotid disease, the ARBITER 6-HALTS Trial will determine if combined optimization of LDL and HDL (statins plus niacin) is clinically superior to more aggressive LDL lowering (statin plus ezetimibe) [75].
As noted earlier, a 1 mg/dl increase in HDL cholesterol can decrease the risk for cardiovascular disease as much as 6% [23]. There is also additional clinical benefit to be gained by lowering TG level (even when LDL cholesterol has already been substantially reduced). As shown in a post hoc evaluation of the PROVE IT-TIMI 22 trial [101], on-treatment plasma TG levels <150 mg/dl were independently associated with reduced risk of recurrent coronary events. In fact, after adjusting for non-HDL-cholesterol and other clinical covariates, each 10 mg/dl decrease in TG level is associated with an additional 1.4% reduction in the overall risk of developing cardiovascular disease.
Modeling Outcome
Treatment trials such as those introduced above will continue to inform our ongoing efforts to direct pharmacological intervention based on individualized risk (i.e., the presence or absence of modifiable cardiac risk factors). There is also growing interest in the characterization of genetic risk determinants. The application of such determinants will be challenging, because the genetic architecture underlying cardiac risk factors such as low HDL dyslipidemia may be quite different from the genetic architecture underlying adverse cardiovascular events [50]. Further, data indicate that the clinical response to pharmacological intervention (e.g., drug-induced change in lipid levels) is attributable to yet another set of genetic predictors [49, 102]. The identification of genetic determinants of treatment response has, however, been somewhat limited. While markers for change in LDL cholesterol are now being reported, the genetic predictors of change in HDL cholesterol remain largely unsurveyed.
Modeling treatment-induced changes for HDL cholesterol will require large population-based cohorts. As discussed, drug-induced changes in HDL cholesterol are somewhat smaller in magnitude than drug-induced changes in LDL cholesterol, and the clinical variables influencing HDL cholesterol are many. To facilitate the construction of accurate prediction models for cardiometabolic risk, the eMERGE network (electronic Medical Records and Genomics) has begun extracting lipid data from several of the world’s growing Biobank cohorts (www.gwas.net). We have begun modeling these variables in the Marshfield Clinic Personalized Medicine Research Project (PMRP), a dynamic community-based Biobank located in the Midwestern United States [103]. This population has a distribution of fasting lipid levels similar to that reported by NHANES III [104]. At present, the Marshfield Biobank contains data from nearly 20,000 adult participants; more than 10,000 individuals (54%) have impaired fasting glucose; >8000 (41%) have hypertriglyceridemia; and >9000 (48%) have reduced levels of HDL cholesterol according to criteria published by NCEP ATP-III [6, 105].
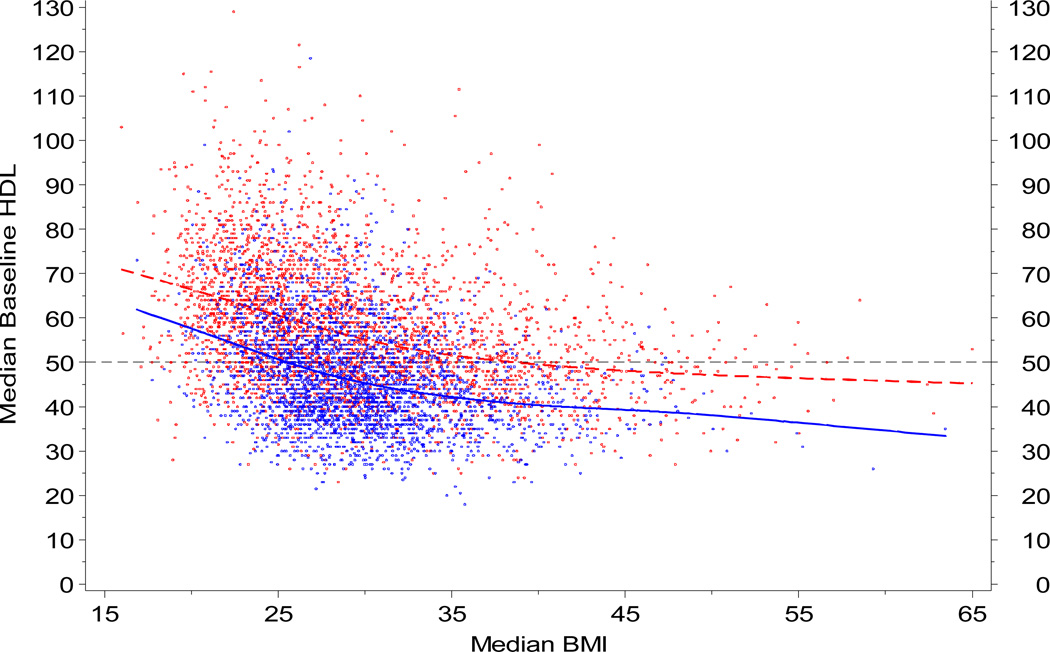
Using this cohort of 20,000 adults (age range 18 to 98 yrs, median 46 yrs), we previously extracted all lipid data, censored HDL data according to relevant co-morbidities, and applied population trends to adjust age and BMI for individual differences in the availability of HDL over time. A repeated measures model was published for males and females [103]. To illustrate the relative contribution of body composition, representative data are shown below for HDL as a function of BMI in this cohort.
If individualized pharmacologic intervention is to become a common clinical reality, risk assessment must be based upon the interaction(s) of metabolic risk determinants such as these within a variety of subpopulations. A recent comparative effectiveness review commissioned by the Agency for Healthcare Research and Quality (AHRQ) specifically addressed this need by evaluating outcome in subjects with (and without) various dyslipidemias. The findings suggest that treatment modality should be directed by co-morbidity. While the evidence supporting combination therapy (i.e., multiple lipid-modifying agents) versus high-dose statin therapy remains insufficient to guide clinical decisions in the general population (AHRQ Publication No. 09-EHC024-EF, 2009), patients with metabolic comorbidities - such as those subjects with elevated BMI in the right half of the figure shown above - are likely to benefit from combination therapy targeted toward all lipid components. Thus, biobanks in the eMERGE network (www.gwas.net) may have the capability to more accurately characterize the impact of pharmacologic intervention on outcome, for all possible permutations of common cardiometabolic risk determinants.
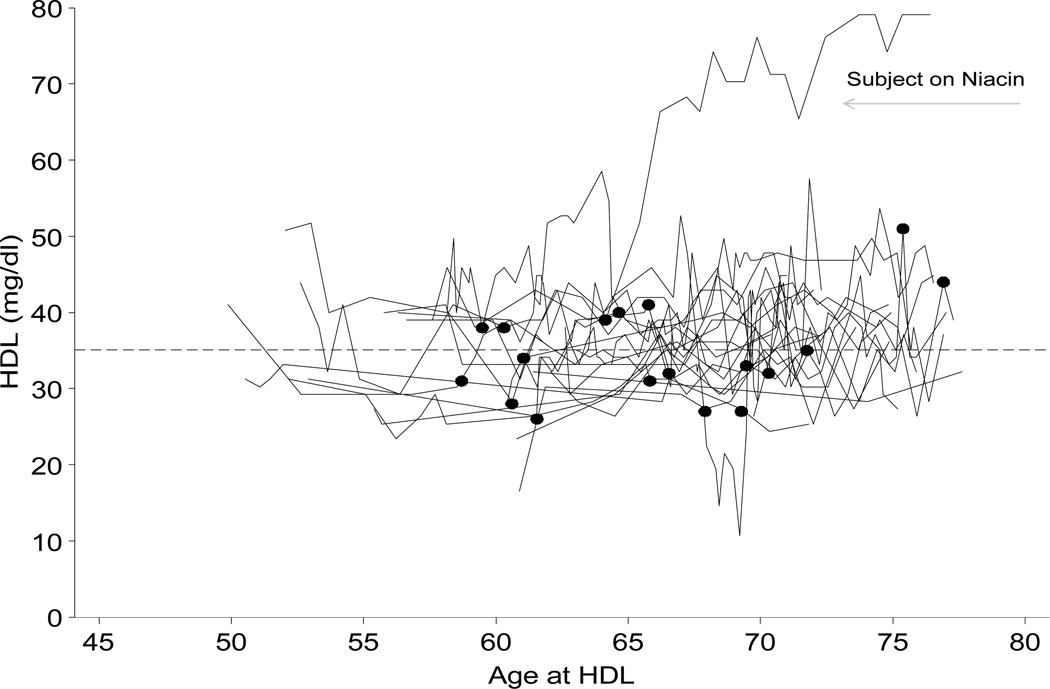
We have also previously shown that lipid data can be efficiently extracted and annotated at first exposure to a relevant lipid-altering medication using electronic health records linked to secure encrypted databases. This is illustrated below for 20 randomly sampled male subjects from eMERGE.
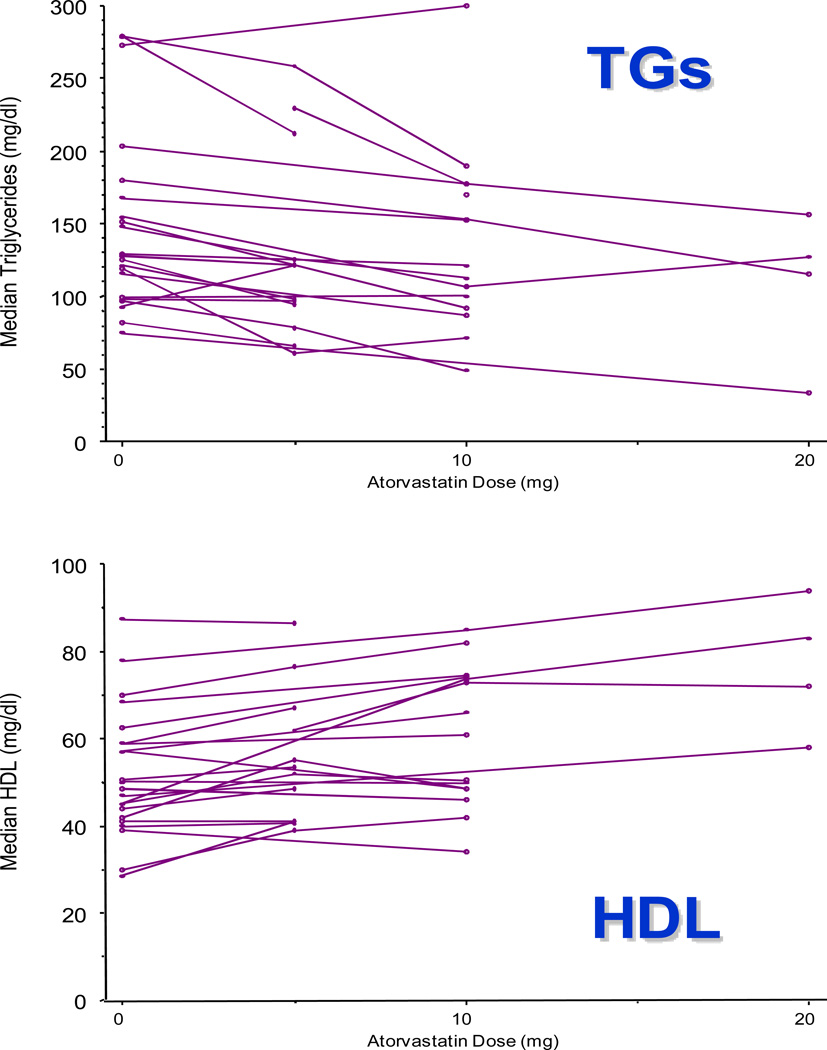
This approach can be used to describe variation in drug response in large populations. For example, we have also previously linked accurate drug exposure histories (including start date, stop date, and dose) with clinical lipid data to construct full dose-response relationships for HMG-CoA reductase inhibitors (statins) [106, 107]. An example is shown below, for HDL and TG, using a test set of 100 participants exposed to atorvastatin in the PMRP biobank. The observation that lipid levels trend in the direction anticipated clinically (i.e., HDL decreases while TGs increase)suggests that these algorithms can be applied to existing electronic data across large health care networks to characterize outcome on an unprecedented scale. The accuracy of these algorithms has been published [106].
By linking high-throughput genotyping technologies to electronic health care records within the context of these biobanks, improved risk prediction models will eventually allow investigators to distinguish the genetic architecture underlying specific cardiac risk factors (e.g., baseline HDL cholesterol level) from the genetic architecture underlying response to treatment (e.g., change in HDL cholesterol level induced by a pharmacologic intervention). However, this effort will require cohorts of extremely large sample size [108, 109]. BioVU, located at Vanderbilt University, is currently the largest population-based Biobank in the United States. At present, enrollment is approaching 80,000 unique subjects. It is anticipated that BioVU will contain DNA and securely encrypted clinical data from more than 100,000 study subjects by the end of the current academic year. To illustrate the potential of this resource to facilitate large scale studies of outcome related to the management of low HDL cholesterol, the number of participants with various metabolic traits related to body composition has been summarized below.
These phenotypes are highly inter-correlated. For example, in obese study subjects, diabetes mellitus often represents the extreme end of a disease process known to occur along a clinical continuum. Prediabetic states include impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). The former (IFG) is defined by fasting glucose levels above 100 mg/dl [reference 110]. Clinical derangements in lipid homeostasis often become evident in subjects with IFG long before they develop diabetes mellitus. As shown above, there are many different permutations of these phenotypic subsets. For example, while 8738 of the 24,299 individuals with clinical lipid data available in BioVU have IFG and high TGs, only 2076 of these individuals also have low HDL cholesterol levels. Fewer still meet all current diagnostic criteria for the constellation of clinical abnormalities commonly referred to as the Metabolic Syndrome (MTS), particularly if the definition includes a hemodynamic component (hypertension), a prothrombotic component, an anti-fibrinolytic component, and altered renal indices (e.g., proteinuria) [110].
Because all permutations of these cardiometabolic risk determinants do not carry the same risk [2, 111], clinical intervention must be guided by a more thorough understanding of their interactions within the community. It remains uncertain whether genetic markers will provide added value. Although investigators within the Pharmacogenetics and Pharmacogenomics Research Network (PGRN) have begun characterizing the genetic determinants contributing to change in lipid levels within the context of treatment trials, these markers will need to be tested for generalizability in population-based cohorts that can be stratified according to the cardiometabolic risk determinants shown above.
Table 2 represents a compilation of the genetic predictors of HDL cholesterol from 3 treatment trials, before and after exposure to atorvastatin, simvastatin, or pravastatin (adapted from Baber, et al. 2010 [reference 49]). While baseline HDL level was associated with SNPs in several well-characterized biological candidate genes (CETP, LIPC, LPL), no SNPs were associated with treatment-induced change in HDL (i.e., all frequentist p values for the difference trait were >0.05, and posterior probabilities were low for HD). Conversely, our analyses did identify an association between another well-characterized candidate gene (GCKR) and statin-induced changes in fasting triglyceride levels (data not shown) [49].
TABLE 2.
Survey for genetic predictors of HDL-cholesterol before and after treatment with three commonly used statin drugs (atorvastatin, simvastatin, pravastatin). Data were obtained in the context of clinical treatment trials.
| SNP | Posterior Probability | P-value | MAF | Chr. | Nearest Genes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H0 | HS | HD | H(S+D) | Sum | Diff | Gene Symbols (Distance from variant, kb) | |||||
| strong association* | |||||||||||
| rs247616 | <0.01 | 0.99 | <0.01 | <0.01 | 2.50E | 0.45 | 0.32 | 16 | CETP (6) | HERPUD1 (12) | SLC12A3 (42) |
| rs4775041 | <0.01 | 0.99 | <0.01 | <0.01 | 1.00E | 0.86 | 0.25 | 15 | LIPC (49) | LOC441726 (181) | AQP9 (197) |
| rs1011685 | <0.01 | 0.98 | <0.01 | 0.01 | 2.10E | 0.58 | 0.12 | 8 | LPL (6) | INTS10 (121) | SLC18A1 (172) |
| moderate association** | |||||||||||
| rs10091038 | 0.67 | 0.32 | <0.01 | 0.01 | 2.80E-06 | 0.18 | 0.43 | 8 | LOC646909(129) | DUSP4 (152) | KIF13B (240) |
| rs10123041 | 0.81 | 0.18 | <0.01 | <0.01 | 5.90E-06 | 0.96 | 0.16 | 9 | SLC28A3 (0) | RMII (303) | LOC729388 (325) |
| rs1535 | 0.83 | 0.17 | <0.01 | <0.01 | 6.80E-06 | 0.56 | 0.32 | 11 | FADS2 (0) | FADS1 (14) | FEN (33) |
| rs11641231 | 0.85 | 0.14 | <0.01 | 0.01 | 8.30E-06 | 0.07 | 0.23 | 16 | LOC401864 (87) | LOC283904 (140) | LOC729464 (148) |
| rs16839962 | 0.86 | 0.13 | <0.01 | <0.01 | 8.70E-06 | 0.48 | 0.13 | 2 | NR4A2 (256) | GPD2 (355) | LOC728038 (367) |
| rs1431315 | 0.88 | 0.11 | <0.01 | <0.01 | 1.20E-05 | 0.41 | 0.11 | 17 | ANKFN1 (80) | PCTP (297) | TMEM100 (351) |
H0: null model; HS: sum model; HD: difference model; H(S+D): sum and difference model.
Variants associated with HDL-cholesterol shown with posterior probablity less than 0.50 for association based on the null hypothesis.
Variants associated with HDL-cholesterol shown with posterior probablity between 0.50 and 0.89 for association based on the null hypothesis.
Adapted and reprinted with Permission from PLOS One 2010 (Barber, et. al. 2010) [reference 49].
Due to the heterogeneity of low HDL dyslipidemia as a clinical phenotype, the small contribution of individual genetic risk determinants, and the complexity of the genetic architecture underlying response to pharmacological intervention, the construction of accurate risk prediction models will require the coordinated sharing of data across institutions. Academic medical centers are utilizing electronic health records to merge practice-based datasets on an unprecedented scale [112]. As these biobanks become linked, the scientific community will draw nearer to personalized medicine within the context of dyslipidemia.
Summary
Clinical data available in population-based biobanks should facilitate the construction of accurate risk prediction models by integrating various combinations of cardiometabolic risk determinants with a variety of adverse cardiovascular outcomes, in the context of treatment within the community.
As the scientific community moves rapidly toward large-scale application of genetic epidemiology and pharmacogenetics, the world’s population-based biobanks represent a unique resource for assessing clinical variables in directing the care of complex phenotypes like dyslipidemia [www.gwas.net].
FIG 1.
Fasting clinical lipid data (HDL) shown as a function of body mass index (BMI) for PMRP Biobank participants. Reprinted with permission from Wilke et al. Preventive Cardiology, 2009 [104].
FIG 2.
Fasting clinical lipid data (HDL) shown as a function of patient age for 20 randomly selected PMRP Biobank participants. Reprinted with permission from Wilke et al. Preventive Cardiology, 2009 [104].
FIG 3.
Dose-response plots for the three most commonly prescribed doses of atorvastatin (i.e., 5 mg daily, 10 mg daily, and 20 mg daily), generated using clinical data from a test set of 100 PMRP Biobank participants. Upper panel: TGs by atorvastatin dose. Lower panel: HDL by atorvastatin dose.
TABLE 1.
Vanderbilt University Biobank (BioVU) - Participant Characteristics
| Characteristic | Men | Women | Total |
|---|---|---|---|
| N | 29946 (41.98) | 41390 (58.02) | 71336 (100) |
| Deceased | 1519 (5.07) | 1137 (2.75) | 2656 (3.72) |
| Mean age (yrs) | 54.3 | 50.58 | 52.14 |
| BMI class (kg/m2) | |||
| Healthy (<25) | 3345 (18.46) | 8020 (27.28) | 11365 (23.92) |
| Overweight (25–29) | 6385 (35.24) | 7958 (27.07) | 14343 (30.18) |
| Obese (30+) | 8390 (46.30) | 13421 (45.65) | 21811 (45.90) |
| Class 1 (30–34) | 4577 | 5621 | 10198 |
| Class 2 (35–39) | 2069 | 3413 | 5482 |
| Class 3 (40+) | 1744 | 4387 | 6131 |
| Metabolic Trait | Men | Women | Total |
| Impaired fasting glucose (≥100 mg/dl) |
6580/11034 | 6892/14995 | 13472/26029 |
| Elevated triglycerides (≥150 mg/dl) |
6514/11515 | 6917/16000 | 13431/27515 |
| Subthreshold HDL (<40 mg/dl in men or <50mg/dl in women) |
2854/10742 | 3552/15043 | 6406/25785 |
Acknowledgements
Dr Wilke would like to thank Erin MacKinney for assistance in the preparation of this manuscript. This work was funded by R01DK080007, U01HL069757, and U01HG004608.
Footnotes
Conflict of Interest
None
References
- 1.Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10:109–121. doi: 10.1038/nrg2481. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120 doi: 10.1016/j.amjmed.2007.01.003. S12-S1. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, et al. Haplotypes in the lipoprotein lipase gene influence high-density lipoprotein cholesterol response to statin therapy and progression of atherosclerosis in coronary artery bypass grafts. Pharmacogenomics J. 2007;7:66–73. doi: 10.1038/sj.tpj.6500402. [DOI] [PubMed] [Google Scholar]
- 4.Krauss RM. Is the size of low-density lipoprotein particles related to the risk of coronary heart disease? JAMA. 2002;287(6):712–713. doi: 10.1001/jama.287.6.712. [DOI] [PubMed] [Google Scholar]
- 5.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National cholesterol Education Program (NCEP: Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Tall AR. CETP inhibitors to increase HDL cholesterol levels. N Engl J Med. 2007:1364–1366. doi: 10.1056/NEJMe078029. [DOI] [PubMed] [Google Scholar]
- 8.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96(12):1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 9.Sahoo D, Darlington YF, Pop D, Williams DL, Connelly MA. Scavenger receptor class B Type I (SR-BI) assembles into detergent-sensitive dimers and tetramers. Biochim Biophys Acta. 2007;1771(7):807–817. doi: 10.1016/j.bbalip.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Thuren T, Weisgraber KH, Sisson P, Waite M. Role of apolipoprotein E in hepatic lipase catalyzed hydrolysis of phospholipid in high-density lipoproteins. Biochemistry. 1992;31(8):2332–2338. doi: 10.1021/bi00123a018. [DOI] [PubMed] [Google Scholar]
- 11.Després JP, Ferland M, Moorjani S, Nadeau A, Tremblay A, Lupien PJ, Thériault G, Bouchard C. Role of hepatic-triglyceride lipase activity in the association between intra-abdominal fat and plasma HDL cholesterol in obese women. Arteriosclerosis. 1989;9(4):485–492. doi: 10.1161/01.atv.9.4.485. [DOI] [PubMed] [Google Scholar]
- 12.Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem. 2003;36(6):421–429. doi: 10.1016/s0009-9120(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 13.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol. 2003;92(4A):42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 14.Nissen SE, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 15.Soro-Paavonen A, Naukkarinen J, Lee-Rueckert M, Watanabe H, Rantala E, Soderlund S, Hiukka A, Kovanen PT, Jauhiainen M, Peltonen L, Taskinen MR. Common ABCA1 variants, HDL levels, and cellular cholesterol efflux in subjects with familial low HDL. J Lipid Res. 2007;48(6):1409–1416. doi: 10.1194/jlr.P600012-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 17.Bittner V. Perspectives on dyslipidemia and coronary heart disease in women. J Am Coll Cardiol. 2005;46(9):1628–1635. doi: 10.1016/j.jacc.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294(3):326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 19.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83(5):1025–1031. doi: 10.1093/ajcn/83.5.1025. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 22.Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci U S A. 2000;97:14478–14483. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashen MD, Blumenthal RS. Clinical practice - low HDL cholesterol levels. N Engl J Med. 2005;353:1252–1260. doi: 10.1056/NEJMcp044370. [DOI] [PubMed] [Google Scholar]
- 24.Pascot A, Lemieux I, Prud'homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Després JP. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res. 2001;42(12):2007–2014. [PubMed] [Google Scholar]
- 25.Navab M, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41(9):1495–1508. [PubMed] [Google Scholar]
- 26.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58(3):342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 27.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–1379. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 29.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9(11):819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunzell JD, Hazzard WR, Porte D, Jr, et al. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen H, Breedveld B, Schoonderwoerd K. Role of lipoprotein lipases in postprandial lipid metabolism. Atherosclerosis. 1998;14 Suppl 1:S31–S34. doi: 10.1016/s0021-9150(98)00214-7. [DOI] [PubMed] [Google Scholar]
- 32.Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–288. doi: 10.1161/01.cir.0000019884.36724.d9. [DOI] [PubMed] [Google Scholar]
- 33.Glaser DS, Yost TJ, Eckel RH. Preheparin lipoprotein lipolytic activities: relationship to plasma lipoproteins and postheparin lipolytic activities. J Lipid Res. 1992;33(2):209–214. [PubMed] [Google Scholar]
- 34.Eckel RH, Yost TJ, Jensen DR. Sustained weight reduction in moderately obese women results in decreased activity of skeletal muscle lipoprotein lipase. Eur J Clin Invest. 1995;25(6):396–402. doi: 10.1111/j.1365-2362.1995.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 35.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res. 2002;43(12):1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 36.Brooks-Wilson A, Marcil M, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22(4):336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 37.Scheen AJ, Paquot N. Use of cannabinoid CB1 receptor antagonists for the treatment of metabolic disorders. Best Pract Res Clin Endocrinol Metab. 2009;23:103–116. doi: 10.1016/j.beem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 39.van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365(9468):1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 40.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295(7):761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 41.van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31 Suppl 2:S229–S240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- 42.Wangensteen T, Akselsen H, Holmen J, Undlien D, Retterstøl L. A Common haplotype in NAPEPLD Is associated with severe obesity in a Norwegian population-based cohort (the HUNT Study) Obesity. 2010 September 30; doi: 10.1038/oby.2010.219. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Baye TM, Zhang Y, Smith E, et al. Genetic variation in cannabinoid receptor 1 (CNR1) is associated with derangements in lipid homeostasis, independent of body mass index. Pharmacogenomics. 2008;9:1647–1656. doi: 10.2217/14622416.9.11.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Sonnenberg G, Baye TM, Littrell J, Gunnell J, DeLaForest A, MacKinney E, Hillard CJ, Kissebah AH, Olivier M, Wilke RA. Genetic variation in fatty acid amide hydrolase (FAAH) is associated with obesity-related dyslipidemia, independent of insulin responsiveness, in multigenerational families of Northern European descent. Pharmacogenomics. 2009;10:1929–1939. doi: 10.2217/pgs.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360(17):1696–1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- 46.Li B, Leal SM. Discovery of rare variants via sequencing: implications for the design of complex trait association studies. PLoS Genet. 2009;5(5):e1000481. doi: 10.1371/journal.pgen.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kathiresan S, Melander O, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willer CJ, Sanna S, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barber MJ, Mangravite LM, Hyde CL, Chasman D, Smith J, McCarty CA, Wilke RA, Rieder M, Williams P, Ridker PM, Chatterjee A, Rotter JI, Nickerson DA, Stephens M, Krauss RM. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS One. 2010;5(3):e9763. doi: 10.1371/journal.pone.0009763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teslovich TM, Musunuru K, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilke RA, Mareedu RK, Moore JH. The pathway less traveled – moving from candidate genes to candidate pathways in the analysis of genome-wide data from large scale pharmacogenetic association studies. Current Pharmacogenomics and Personalized Medicine. 2008;6:150–159. doi: 10.2174/1875692110806030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trial results: I. reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 53.Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary artery disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 54.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. New Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 55.Yee HS, Fong NT. Atorvastatin in the treatment of primary hypercholesterolemia and mixed dyslipidemias. Ann Pharmacother. 1998;32:1030–1043. doi: 10.1345/aph.17231. [DOI] [PubMed] [Google Scholar]
- 56.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 57.Tobert JA. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nature Reviews Drug Discovery. 2003;2:517–526. doi: 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- 58.Kinley S. What do we know about plieotropic effects? Lipid letter. 2005;4(3):1. [Google Scholar]
- 59.Asztalos BF, Horvath KV, McNamara JR, Roheim PS, Rubinstein JJ, Schaefer EJ. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis. 2002;164(2):361–369. doi: 10.1016/s0021-9150(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 60.Schaefer EJ, Asztalos BF. The effects of statins on high-density lipoproteins. Curr Atheroscler Rep. 2006;8(1):41–49. doi: 10.1007/s11883-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 61.Wierzbicki AS, Mikhailidis DP. Dose-response effect of atorvastatin and simvastatin on high-density lipoprotein cholesterol in hypercholesterolaemic patients: a review of five comparative studies. International Journal of Cardiology. 2002;84:53–57. doi: 10.1016/s0167-5273(02)00118-3. [DOI] [PubMed] [Google Scholar]
- 62.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 63.van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR., Jr In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283(1):157–163. [PubMed] [Google Scholar]
- 64.Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, Zbaida S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia) Drug Metab Dispos. 2004;32(3):314–320. doi: 10.1124/dmd.32.3.314. [DOI] [PubMed] [Google Scholar]
- 65.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe-A Review of its Metabolism, Pharmacokinetics and Drug Interactions. Clin Pharmacokinet. 2005;44(5):467–494. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 66.Patrick JE, Kosoglou T, Stauber KL, Alton KB, Maxwell SE, Zhu Y, Statkevich P, Iannucci R, Chowdhury S, Affrime M, Cayen MN. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos. 2002;30(4):430–437. doi: 10.1124/dmd.30.4.430. [DOI] [PubMed] [Google Scholar]
- 67.Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP Ezetimibe Study Group. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90(10):1092–1097. doi: 10.1016/s0002-9149(02)02798-4. [DOI] [PubMed] [Google Scholar]
- 68.Dujovne CA, Suresh R, McCrary SC, Maccubbin D, Strony J, Veltri E. Safety and efficacy of ezetimibe monotherapy in 1624 primary hypercholesterolaemic patients for up to 2 years. Int J Clin Pract. 2008;62(9):1332–1336. doi: 10.1111/j.1742-1241.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- 69.Pandor A, Ara RM, Tumur I, Wilkinson AJ, Paisley S, Duenas A, Durrington PN, Chilcott J. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568–580. doi: 10.1111/j.1365-2796.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 70.Venero CV, Venero JV, Seip RL, Thompson PD. Effectiveness of thrice weekly ezetimibe. Am J Cardiol. 2008;102(9):1205–1206. doi: 10.1016/j.amjcard.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 71.Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP Ezetimibe Study Group. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24(8):729–741. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 72.Sudhop T, Reber M, Tribble D, Sapre A, Taggart W, Gibbons P, Musliner T, von Bergmann K, Lütjohann D. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J Lipid Res. 2009;50:2117–2123. doi: 10.1194/jlr.P900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 74.Califf RM, Robert AH, Michael AB. Premature Release of Data from Clinical Trials of Ezetimibe. N Engl J Med. 2009;361:7. doi: 10.1056/NEJMsr0900910. [DOI] [PubMed] [Google Scholar]
- 75.Devine PJ, Turco MA, Taylor AJ. Design and rationale of the ARBITER 6 trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol)-6-HDL and LDL Treatment Strategies in Atherosclerosis (HALTS) Cardiovasc Drugs Ther. 2007;21(3):221–225. doi: 10.1007/s10557-007-6020-8. [DOI] [PubMed] [Google Scholar]
- 76.Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, Revkin JH, Grobbee DE, Riley WA, Shear CL, Duggan WT, Bots ML RADIANCE 1 Investigators. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356(16):1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 77.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118:2506–2514. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 78.Nagano M, et al. Molecular mechanisms of cholesteryl ester transfer protein deficiency in Japanese. J Atheroscler Thromb. 2004:110–121. doi: 10.5551/jat.11.110. [DOI] [PubMed] [Google Scholar]
- 79.Rhyne J, Ryan MJ, White C, Chimonas T, Miller M. The two novel CETP mutations Gln87X and Gln165X in a compound heterozygous state are associated with marked hyperalphalipoproteinemia and absence of significant coronary artery disease. J Mol Med. 2006:647–650. doi: 10.1007/s00109-006-0070-4. [DOI] [PubMed] [Google Scholar]
- 80.Wolk R, Chen D, Clark RW, Mancuso J, Barclay PL. Pharmacokinetic, pharmacodynamic, and safety profile of a new cholesteryl ester transfer protein inhibitor in healthy human subjects. Clin Pharmacol Ther. 2009;86(4):430–437. doi: 10.1038/clpt.2009.120. [DOI] [PubMed] [Google Scholar]
- 81.Hampton T. New clues to HDL's benefits revealed. JAMA. 2007;297(14):1537. doi: 10.1001/jama.297.14.1537. [DOI] [PubMed] [Google Scholar]
- 82.Reilly MP, Tall AR. HDL proteomics: pot of gold or Pandora's box? J Clin Invest. 2007;117:595–598. doi: 10.1172/JCI31608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaisar T, Pennathur S, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117(3):746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 85.Fruchart JC. Peroxisome proliferator-activated receptor-alpha (PPARalpha): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205(1):1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999;354(9173):141–148. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- 87.Shulman AI, Mangelsdorf DJ. Retinoid × receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353(6):604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 88.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 89.Brown WV, Dujovne CA, Farquhar JW, Feldman EB, Grundy SM, Knopp RH, Lasser NL, Mellies MJ, Palmer RH, Samuel P, et al. Effects of fenofibrate on plasma lipids. Double-blind, multicenter study in patients with type IIA or IIB hyperlipidemia. Arteriosclerosis. 1986;6(6):670–678. doi: 10.1161/01.atv.6.6.670. [DOI] [PubMed] [Google Scholar]
- 90.Genest J, Jr, Nguyen NH, Theroux P, Davignon J, Cohn JS. Effect of micronized fenofibrate on plasma lipoprotein levels and hemostatic parameters of hypertriglyceridemic patients with low levels of high-density lipoprotein cholesterol in the fed and fasted state. J Cardiovasc Pharmacol. 2000;35(1):164–172. doi: 10.1097/00005344-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 91.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 92.Tenkanen L, Mänttäri M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and treatment with gemfibrozil. Experience from the Helsinki Heart Study. Circulation. 1995;92(7):1779–1785. doi: 10.1161/01.cir.92.7.1779. [DOI] [PubMed] [Google Scholar]
- 93.Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High–Density Lipoprotein Intervention Trial. Am J Cardiol. 2000;86:19L–22L. doi: 10.1016/s0002-9149(00)01464-8. [DOI] [PubMed] [Google Scholar]
- 94.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341(6):410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 95.Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Curr Opin Lipidol. 2004;15(6):659–665. doi: 10.1097/00041433-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101(8A):20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 97.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9(3):352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 98.Plaisance EP, Lukasova M, Offermanns S, Zhang Y, Cao G, Judd RL. Niacin stimulates adiponectin secretion through the GPR109A receptor. Am J Physiol Endocrinol Metab. 2009;296(3):E549–E548. doi: 10.1152/ajpendo.91004.2008. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Schmidt RJ, Foxworthy P, Emkey R, Oler JK, Large TH, Wang H, Su EW, Mosior MK, Eacho PI, Cao G. Niacin mediates lipolysis in adipose tissue through its G-protein coupled receptor HM74A. Biochem Biophys Res Commun. 2005;334(2):729–732. doi: 10.1016/j.bbrc.2005.06.141. [DOI] [PubMed] [Google Scholar]
- 100.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 101.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E PROVE IT-TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 102.Lamon-Fava S, Asztalos BF, Howard TD, Reboussin DM, Horvath KV, Schaefer EJ, Herrington DM. Association of polymorphisms in genes involved in lipoprotein metabolism with plasma concentrations of remnant lipoproteins and HDL subpopulations before and after hormone therapy in postmenopausal women. Clin Endocrinol (Oxf) 2009 May 29; doi: 10.1111/j.1365-2265.2009.03644.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilke RA, Berg RL, Linneman JG, Peissig P, Starren J, Ritchie MD, McCarty CA. Quantification of the clinical modifiers impacting high density lipoprotein (HDL) cholesterol in the community – Personalized Medicine Research Project (PMRP) Preventive Cardiology. 2010;13(2):63–68. doi: 10.1111/j.1751-7141.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, Grundy SM, Johnson CL. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294(14):1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 105.McCarty CA, Wilke RA. Biobanking and pharmacogenetics. Pharmacogenomics. 2010;11(5):637–641. doi: 10.2217/pgs.10.13. [DOI] [PubMed] [Google Scholar]
- 106.Peissig P, Sirohi E, Berg RL, Brown-Switzer C, Ghebranious N, McCarty CA, Wilke RA. Construction of atorvastatin dose-response relationships using data from a large population-based DNA Biobank. Basic Clin Pharmacol Toxicol. 2007;100:286–288. doi: 10.1111/j.1742-7843.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 107.Wilke RA, Berg RL, Linneman JG, Zhao C-F, McCarty CA, Krauss RM. Characterization of LDL-cholesterol lowering efficacy for atorvastatin in a population-based DNA Biorepository. Basic Clin Pharmacol Toxicol. 2008;103:354–359. doi: 10.1111/j.1742-7843.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 108.Wilke RA, Reif DM, Moore JH. Combinatorial pharmacogenetics. Nat Rev Drug Discov. 2005;4(11):911–918. doi: 10.1038/nrd1874. [DOI] [PubMed] [Google Scholar]
- 109.Wilke RA, Lin D, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions – current progress and challenges. Nature Reviews Drug Discovery. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson LW, Weinstock RS. The metabolic syndrome: concepts and controversy. Mayo Clin Proc. 2006;81(12):1615–1620. doi: 10.4065/81.12.1615. [DOI] [PubMed] [Google Scholar]
- 111.Krauss RM. Insulin resistance syndrome and dyslipidemia. Endocr Pract. 2003;9 Suppl 2:67–72. doi: 10.4158/EP.9.S2.67. [DOI] [PubMed] [Google Scholar]
- 112.Wilke RA, Xu H, Denny JC, Roden DM, Krauss RM, McCarty CA, Davis RL, Skaar T, Lamba J, Savova G. The emerging role of electronic medical records in pharmacogenomics. Clinical Pharmacology and Therapeutics. 2010 doi: 10.1038/clpt.2010.260. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]