Abstract
The protein kinases Akt1, Akt2, and Akt3 possess nonredundant signaling properties, few of which have been investigated. Here, we present evidence for an Akt1-dependent pathway that controls interferon (IFN)-regulated gene expression and antiviral immunity. The target of this pathway is EMSY, an oncogenic interacting partner of BRCA2 that functions as a transcriptional repressor. Overexpression of EMSY in hTERT-immortalized mammary epithelial cells, and in breast and ovarian carcinoma cell lines, represses IFN-stimulated genes (ISGs) in a BRCA2-dependent manner, whereas its knockdown has the opposite effect. EMSY binds to the promoters of ISGs, suggesting that EMSY functions as a direct transcriptional repressor. Akt1, but not Akt2, phosphorylates EMSY at Ser209, relieving EMSY-mediated ISG repression. The Akt1/EMSY/ISG pathway is activated by both viral infection and IFN, and it inhibits the replication of HSV-1 and vesicular stomatitis virus (VSV). Collectively, these data define an Akt1-dependent pathway that contributes to the full activation of ISGs by relieving their repression by EMSY and BRCA2.
Keywords: ISG15, IFITM1, RSAD2, Viperin, viral replication
The protein kinase Akt has been under intensive study for 2 decades. In the course of this work, numerous Akt substrates were identified and shown to regulate diverse cellular processes, including metabolism, cell survival, proliferation, and cell migration. However, there are three Akt isoforms (Akt1, Akt2, and Akt3), and little attention has been paid to their nonredundant functions despite strong evidence that they are functionally distinct (1–5). Thus, only a handful of isoform-specific phosphorylation substrates have been identified to date (6, 7).
Previous studies have shown that in addition to its better known functional role in cell proliferation, survival, migration, and metabolism, Akt may have a role in the control of the cellular response to IFNs. Stimulation of murine fibroblasts with IFN-α or IFN-β results in the phosphorylation and activation of Akt, which promotes the activation of mechanistic target of rapamycin (mTOR), an upstream regulator of IFN-stimulated gene (ISG) translation (8). Another study found that KO of p85α and p85β, the regulatory subunits of PI3K, compromises both IFN-induced transcription and translation (9).
EMSY was first isolated in a yeast two-hybrid screen for proteins that interact with the transactivation domain of BRCA2 (10). It binds BRCA2, but not BRCA1, through the EMSY N-terminal (ENT) domain. Although the function of EMSY has not been extensively investigated, existing evidence suggests that it may contribute to chromatin modification, DNA repair, and transcription (10, 11). EMSY was found to colocalize with BRCA2 on dsDNA breaks. Moreover, reporter assays showed that EMSY acts as a repressor to inhibit BRCA2-dependent transcription, but no target genes for EMSY were identified (10). Finally, additional yeast two-hybrid screens demonstrated that EMSY interacts with a number of proteins involved in chromatin regulation, including HP1β, BS69, and the jumonji domain histone demethylase NDY1/KDM2B (10). Changes in EMSY expression or copy number have not been linked to familial cancer. However, amplification and overexpression of EMSY are common events in sporadic breast and ovarian cancer (10, 12). Moreover, amplification and overexpression of EMSY exhibit an inverse correlation with disease-free survival in node-positive breast cancer (10).
The present study shows that EMSY functions as a transcriptional repressor of ISGs that acts in concert with BRCA2. Akt1, but not Akt2, phosphorylates EMSY at Ser209 and relieves transcriptional repression via a process that depends on this phosphorylation event. Akt1-mediated phosphorylation of EMSY is induced by treatment with IFN-α. EMSY and Akt1 act antagonistically to regulate the replication of HSV-1 and vesicular stomatitis virus (VSV). Collectively, these data suggest that Akt1 and EMSY functionally interact in a pathway that controls ISG expression and viral immunity.
Results
EMSY Is an Akt1 Substrate.
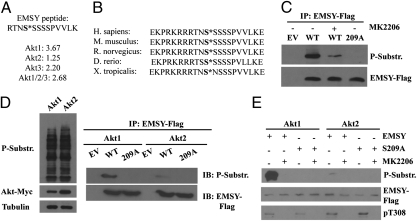
Akt1fl/fl;Akt2−/−;Akt3−/− murine lung fibroblasts, immortalized spontaneously via a 3T3 type protocol, were transduced with pBabe-puro–based retroviral constructs of Myc-Akt1, Myc-Akt2, or Myc-Akt3, or the combination of all three. Transduction of these cells with a Mig-R1-Cre retroviral construct ablated the endogenous Akt1fl/fl gene, giving rise to cells that express a single Akt isoform at a time but are otherwise identical. An antibody raised against the phosphorylated form of the Akt consensus motif (RXRXXS*/T*) was used to affinity-purify LysC-digested peptides phosphorylated at this site. Purified phosphopeptides were digested with trypsin before MS analysis, as previously described (13). Phosphopeptides from cells expressing each isoform were compared by liquid chromatography-MS with phosphopeptides from lysates of triple-Akt KO cells, devoid of Akt expression. One of the peptides phosphorylated in the Akt consensus motif maps within a highly conserved region in the N terminus of the BRCA2 interacting protein partner EMSY (C11ORF30) (Fig. 1A). The region surrounding this phosphorylation site is highly conserved across multiple species (Fig. 1B). Between residues +10 and −10, the human, mouse, and rat forms of EMSY are identical, whereas the Xenopus tropicalis and zebrafish EMSY differ by one and two amino acids, respectively. In all cases, the Akt consensus motif RXRXXS/T is conserved. The phosphorylated residue corresponds to Ser173 of the murine or Ser209 of the human protein. To validate the phosphorylation of this residue, we expressed full-length EMSY-FLAG and EMSY-FLAG S209A in 293T cells. The WT EMSY-FLAG cells were treated with the allosteric Akt1/Akt2-specific inhibitor MK2206 (14) or with the vehicle, DMSO. WT EMSY-FLAG immunoprecipitated with an anti-FLAG antibody from the DMSO-treated cells was strongly recognized by the phosphosubstrate antibody, whereas WT EMSY-FLAG immunoprecipitated from MK2206-treated cells was only faintly recognized. Finally, the EMSY S209A mutant was not recognized at all (Fig. 1C). These data suggest that EMSY is phosphorylated at the Akt phosphorylation motif at Ser209 and that phosphorylation of this site is Akt-dependent.
Fig. 1.
EMSY is preferentially phosphorylated by Akt1 at Ser209. (A) Lysates of spontaneously immortalized triple-Akt KO lung fibroblasts expressing Akt1, Akt2, Akt3, or Akt1/Akt2/Akt3 were digested with LysC. Peptides phosphorylated at the Akt phosphorylation motif RXRXXS*/T* were affinity-purified with Akt phosphosubstrate antibodies. Purified peptides were digested with trypsin, and they were identified by MS. One of these phosphopeptides was mapped at the N terminus of EMSY. The numbers show the fold difference in the abundance of the phosphorylated form of the EMSY peptide in cells expressing a given Akt isoform, relative to Akt-null cells. (B) Sequence comparison of the Akt-phosphorylated EMSY peptide from the indicated five vertebrate species. (C) EMSY-Flag was immunoprecipitated with the anti-Flag antibody from lysates of HMEC-tert cells transduced with lentiviral constructs of EMSY-FLAG, EMSY-FLAG S209A, or EV. Cells were treated for 4 h before harvesting with the Ak1/Akt2 inhibitor MK2206, as indicated. WT EMSY and EMSY S209A were immunoprecipitated with an anti-Flag antibody, and the immunoprecipitates were probed with the Akt phosphosubstrate antibody. (D) (Left) Western blots of cell lysates from triple-Akt KO lung fibroblasts expressing either Akt1 or Akt2 were probed with an Akt phosphosubstrate antibody, an anti-Myc (Akt) antibody, and an antitubulin (loading control) antibody, as indicated. (Right) Lung fibroblasts were transduced with pLenti constructs expressing EMSY-FLAG, EMSY-FLAG S209A, or EV. EMSY (anti-FLAG) immunoprecipitates (IP) were probed with the Akt phosphosubstrate or the EMSY antibody, as indicated. (E) N-terminal fragment of EMSY was used as a substrate for immunoprecipitated Akt1 and Akt2. Phosphorylation was detected with an Akt phosphosubstrate antibody. P-Substr., Akt phosphosubstrate antibody. IB, immunoblot.
To determine whether Akt1 and Akt2 differ in their ability to promote the phosphorylation of EMSY, we transduced the immortalized murine lung fibroblasts expressing Akt1 or Akt2 with retroviral constructs of WT EMSY-FLAG or EMSY-FLAG S209A, or with the empty vector (EV). Probing EMSY immunoprecipitated from these cells with the Akt phosphosubstrate antibody confirmed that EMSY phosphorylation at Ser209 is Akt1- but not Akt2-dependent (Fig. 1D). Probing total lysates of Akt1- and Akt2-expressing cells with the anti-Myc (Akt) antibody or the phosphosubstrate antibody, however, revealed that Akt expression and total Akt activity in these cells were similar. Therefore, the observed differential phosphorylation is EMSY-specific.
These data suggest that EMSY is phosphorylated downstream of Akt1, but they do not address whether it is a direct Akt phosphorylation substrate. To address this question, we cloned a fragment of EMSY corresponding to the N terminus of the protein (AA8-AA374) as a GST fusion in a bacterial expression vector. Using the bacterially expressed fragment of WT EMSY and its S209A mutant as substrates in Akt1 and Akt2, in vitro kinase assays revealed that Akt1 phosphorylates the WT protein strongly, whereas Akt2 phosphorylates it weakly and that neither of the two phosphorylates the S209A mutant (Fig. 1E). We conclude that EMSY is a direct phosphorylation target of Akt1.
EMSY Is a Repressor of ISGs.
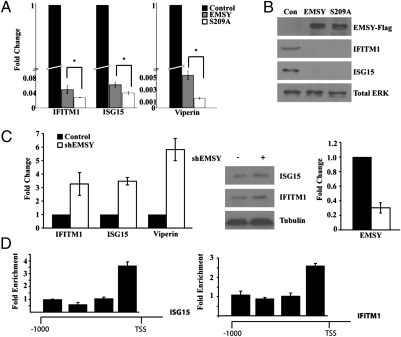
BRCA2 has been shown to regulate the expression of a small set of genes, including several ISGs, such as IFITM1, ISG15, and Viperin (15). Because EMSY interacts with BRCA2 and other proteins involved in transcriptional regulation, we hypothesized that EMSY could regulate the expression of BRCA2 target genes. To address this hypothesis, we examined the expression of BRCA2-regulated ISGs in the immortalized human mammary epithelial cell line (HMEC)-tert, before and after the transduction of these cells with WT EMSY-FLAG or EMSY-FLAG S209A. Transduction with EMSY repressed the expression of the ISGs ISG15, IFITM1, and Viperin, whereas transduction with EMSY-FLAG S209A resulted in more substantial repression (Fig. 2A). Other BRCA2 target genes, such as UBE2L6, WARS, KRT6A, and FGFR2, were also repressed by EMSY, but their repression was substantially weaker (Fig. S1A). The repression of ISG15 by EMSY was also confirmed by Western blotting (Fig. 2B). HMEC-tert cells not transduced with EMSY or EMSY S209A express low levels of IFITM1 and ISG15 in the absence of IFN stimulation. This is a result of growth in serum. The ability of serum to induce ISG expression was confirmed by Western blotting (Fig. S1C). Knockdown of EMSY in HMEC-tert cells up-regulated ISG expression (Fig. 2C). ChIP experiments in HMEC-tert cells using FLAG antibody showed binding of EMSY near the transcription start site of both ISG15 and IFITM1 (Fig. 2D). We conclude that EMSY acts directly on the promoters of certain ISGs to repress transcription.
Fig. 2.
EMSY functions as a repressor of ISGs. (A) HMEC-tert cells were transduced with retroviral constructs of WT FLAG-EMSY, FLAG-EMSY S209A, or EV. The expression of the indicated ISGs was measured by real-time RT-PCR. Changes in expression induced by EMSY or EMSY S209A are presented as fold change, relative to the expression in control cells. Error bars show the SD (*P < 0.05; n = 3). (B) Western blotting of cell lysates from the same cells as in A confirmed that EMSY and EMSY S209A repress the expression of IFITM1 and ISG15. Con, control. (C) HMEC-tert cells were transduced with a lentiviral shRNA construct of EMSY or EV. The expression of EMSY, IFITM1, ISG15, and Viperin was measured by real-time RT-PCR (n = 3). (D) ChIP of EMSY-FLAG in HMEC-tert cells demonstrated binding of EMSY near the transcription start site (TSS) of ISG15 and IFITM1. Data show real-time RT-PCR of immunoprecipitated DNA in EMSY-expressing cells relative to control (n = 3).
Akt1 Selectively Relieves the Repression of ISGs via EMSY Phosphorylation at Ser209.
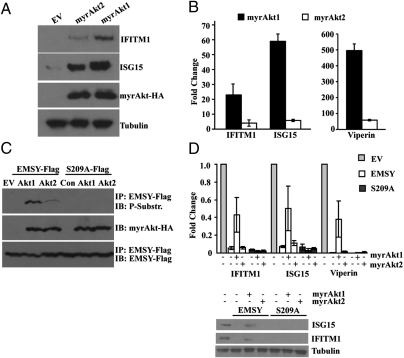
The finding that the EMSY S209A phosphorylation site mutant is a stronger transcriptional repressor of ISGs than the WT protein suggested that phosphorylation at Ser209 by Akt may relieve repression by EMSY. To address this hypothesis, we transduced HMEC-tert cells with constitutively active MyrAkt1 or MyrAkt2 and measured the expression of IFITM1 and ISG15 by real-time RT-PCR and Western blotting, and the expression of Viperin by real-time RT-PCR. The results provided support to the hypothesis by showing that both MyrAkt1 and MyrAkt2 up-regulate the expression of these genes, but MyrAkt1 is significantly more effective (Fig. 3 A and B). To confirm this conclusion, we transduced HMEC-tert cells engineered to express WT EMSY-Flag or EMSY S209A-Flag with constitutively active MyrAkt1 or MyrAkt2, or with the EV, and we examined the phosphorylation of EMSY and the expression of IFITM1, ISG15, and Viperin by both real-time RT-PCR and Western blotting. The results confirmed the phosphorylation of EMSY primarily by MyrAkt1 and showed that MyrAkt1 relieves the repression by WT EMSY but does not affect the repression by EMSY S209A. As expected, the relief of EMSY repression by MyrAkt2 was significantly weaker (Fig. 3 C and D). Consistent with this finding was the observation that MyrAkt1 and MyrAkt2 had a minor effect on the expression of BRCA2 target genes that are only weakly regulated by EMSY (Fig. S1B). The regulation of ISG expression via Akt1-mediated phosphorylation of EMSY provides a potential mechanism for earlier observations showing that the induction of ISG transcription by IFN is PI3K/Akt-dependent (8, 9).
Fig. 3.
Akt1 selectively relieves the repression of ISGs via EMSY phosphorylation at Ser209. (A) Western blots of lysates of HMEC-tert cells transduced with retroviral constructs of MyrAkt1, MyrAkt2, or EV were probed with the indicated antibodies. (B) Expression of IFITM1, ISG15, and Viperin was examined in the same cells as in A by real-time RT-PCR. Data represent fold induction of these genes in MyrAkt1- and MyrAkt2-expressing cells relative to their expression in EV cells. Error bars show the SD (n = 3). (C) Immunoprecipitates of lysates derived from HMEC-tert cells transduced with retroviral constructs of MyrAkt1, MyrAkt2, or EV and superinfected with pLenti constructs of WT EMSY or EMSY S209A were probed with the indicated antibodies. IP, immunoprecipitate; IB, immunoblot. (D) Expression of IFITM1, ISG15, and Viperin was examined in the same cells as in C by real-time RT-PCR. Data are represented as fold change relative to EV. Error bars show the SD (n = 3). Western blotting of lysates from the same cells is also shown.
Repression of ISG Expression by EMSY Is BRCA2-Dependent.
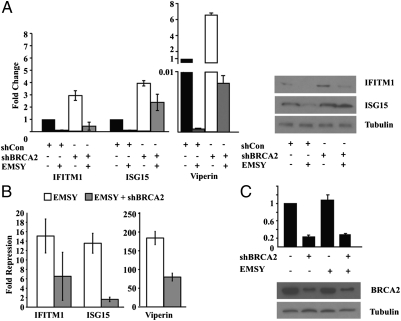
EMSY is an interacting partner of BRCA2. This raised the question of whether the binding of EMSY to BRCA2 is required for EMSY-mediated repression of ISG transcription. To determine whether BRCA2 is required for ISG repression by EMSY, we examined the ability of EMSY to repress transcription of IFITM1, ISG15, and Viperin in HMEC-tert cells, before and after the knockdown of BRCA2. The results in Fig. 4 showed that depletion of BRCA2 results in up-regulation of IFITM1, ISG15, and Viperin. Fig. 4A (Left) shows the fold change in the expression of these genes in EMSY and EMSY plus short hairpin (sh) BRCA2-transduced cells, whereas Fig. 4B shows the magnitude of the inhibition of EMSY-mediated repression by shBRCA2. In addition, the knockdown of BRCA2 in EMSY-expressing cells partially abrogated transcriptional repression. Western blotting (Fig. 4A, Right) shows the protein levels of ISG15 and IFITM1 in the same cells, and findings are in agreement with the RNA data. This result confirmed that EMSY-mediated repression of ISGs depends on BRCA2 and suggests that EMSY and BRCA2 are found in a nuclear complex that regulates transcription. The fact that the abrogation of transcriptional repression by EMSY is only partial could be because EMSY may repress the expression of ISGs by both BRCA2-dependent and independent mechanisms. We consider it more likely, however, that the partial, rather than complete, derepression is attributable to the fact that the BRCA2 shRNA lowers the expression of BRCA2 but does not completely abolish it (Fig. 4C).
Fig. 4.
Repression of ISG expression by EMSY is BRCA2-dependent. (A) Expression of IFITM1, ISG15, and Viperin was measured by real-time RT-PCR in HMEC-tert cells transduced with the indicated constructs. (Left) Data are represented as fold change in the expression of these genes in cells transduced with the indicated constructs, and error bars show the SD (n = 3). (Right) Western blot analysis of the same samples is shown. shCon, sh control. (B) Data in A were reanalyzed to show the fold repression of IFITM1, ISG15, and Viperin in EMSY and EMSY + shBRCA2-transduced HMEC-tert cells. Error bars show the SD (n = 3). (C) Analysis of BRCA2 knockdown by Western blotting and real-time RT-PCR.
The dependence of EMSY-mediated transcriptional repression on BRCA2 and the inhibition of the repression by phosphorylation at Ser209 suggest that phosphorylation may inhibit the interaction between EMSY and BRCA2. The latter may be the direct consequence of the phosphorylation, or it may be caused by changes in the subcellular localization of the protein. The mechanism of this effect would be analogous to the mechanism of regulation of FOXO family members and cell cycle regulators, which are retained in the cytoplasm by binding to 14-3-3, following phosphorylation by Akt (16–18). To determine whether EMSY phosphorylation inhibits its interaction with BRCA2, we immunoprecipitated EMSY-FLAG from nuclear and cytoplasmic extracts of HMEC-tert cells transduced with MyrAkt1 or the EVs and probed the immunoprecipitates with an anti-BRCA2 or anti-Flag antibody. Fig. S2 shows that EMSY and BRCA2 are present and interact only in the nuclear extracts and that MyrAkt1, which phosphorylates EMSY at Ser209, does not alter the interaction or the subcellular localization of EMSY. The finding that constitutively active Akt1 fails to alter the subcellular localization of the protein was confirmed by direct probing of Western blots of nuclear and cytoplasmic lysates of HMEC-tert cells. The inability of EMSY phosphorylation to alter the interaction of EMSY with BRCA2 and/or the subcellular localization of the protein suggests that it may regulate EMSY by altering its interaction with corepressors or coactivators in transcriptional complexes assembled on the promoters of BRCA2/EMSY-regulated genes.
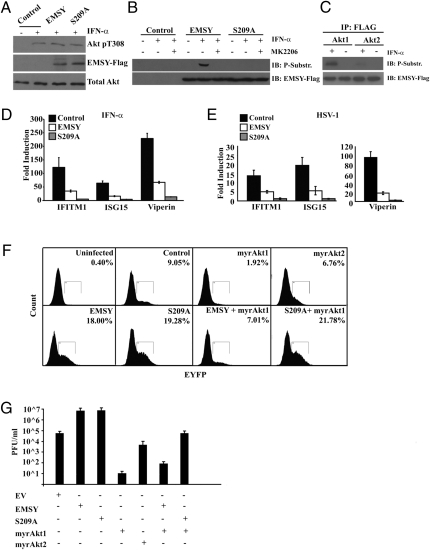
IFN-α and HSV-1 Infection Induce Robust ISG Expression in the Presence of WT EMSY and Weak ISG Expression in the Presence of EMSY S209A.
Based on the preceding data, the induction of ISGs by IFN could be mediated by the phosphorylation of EMSY by Akt1, which relieves EMSY-mediated repression of these genes. The dependence of IFN-mediated induction of ISGs on the activity of PI3K (9) is consistent with this hypothesis. To test this hypothesis, we first examined whether IFN-α promotes the phosphorylation of Akt at Thr308 in HMEC-tert cells transduced with EMSY, EMSY S209A, or the EV. The results confirmed that IFN-α activates Akt in both EMSY- and EMSY S209A-transduced and nontransduced cells (Fig. 5A). IFN-α stimulation also resulted in the phosphorylation of EMSY at Ser209, and inhibition of Akt activation by MK2206 blocked it (Fig. 5B), suggesting that EMSY phosphorylation is mediated by Akt. Stimulation of murine lung fibroblasts expressing either Akt1 or Akt2 confirmed that IFN-α–induced phosphorylation of EMSY is dependent on Akt1 but not Akt2 (Fig. 5C). Real-time RT-PCR showed that IFN-α induces robust expression of IFITM1, ISG15, and Viperin in vector-transduced cells. ISG induction was partially inhibited in cells transduced with WT EMSY. The inhibition of ISG induction was significantly more robust in cells expressing EMSY S209A (Fig. 5D and Fig. S3A). These data indicate that full ISG induction by IFN requires phosphorylation of EMSY by Akt1 at Ser209.
Fig. 5.
EMSY inhibits ISG induction and permits viral replication in a phosphorylation-dependent manner. (A) Akt is activated by IFN-α. Western blots of lysates of HMEC-tert cells transduced with constructs of WT EMSY, EMSY S209A, or EV were serum-starved overnight; stimulated with IFN-α (10 U/mL for 20 min after serum starvation); and probed with the indicated antibodies. (B) IFN-α induces EMSY phosphorylation at Ser209 in an Akt-dependent manner. EMSY-Flag was immunoprecipitated with an anti-Flag antibody from lysates of the same cells as in A, before and after treatment with MK2206. Immunoprecipitates were probed with the indicated antibodies. IB. (C) Murine lung fibroblasts expressing either Akt1 or Akt2 were stimulated with IFN-α, and EMSY phosphorylation was assessed by immunoprecipitation with FLAG antibody and blotting with Akt phosphosubstrate antibody. IP, immunoprecipitate. IB, immunblot. (D and E) Expression of IFITM1, ISG15, and Viperin was examined by real-time RT-PCR in the same cells as in A and B. RNA was collected 4 h after IFN stimulation/viral infection. The bars show the fold induction of these genes by IFN-α or HSV-1 infection in cells expressing WT EMSY or EMSY S209A, relative to their expression in EV. Error bars show the SD (n = 3). (F) HMEC-tert cells transduced with the indicated constructs were infected with HSV-1 EYFP-ICP0, and viral supernatants were collected and filtered 48 h later. The collected viral stocks were used to infect HeLa cells. The efficiency of infection was determined by measuring the percentage of EYFP-positive cells by flow cytometry 4 h later. More details are provided in the main text. This experiment was repeated twice with similar results. (G) Same cells as in E were infected with VSV tl17. Viral supernatants were collected after 24 h and titered using plaque assays in Vero cells (n = 3).
IFNs are induced in the course of viral infection and interfere with viral replication (19). To determine whether the induction of ISGs by IFNs induced in the course of viral infection also depends on the phosphorylation of EMSY at Ser209, we repeated the real-time RT-PCR assays for IFITM1, ISG15, and Viperin in HMEC-tert cells transduced with WT EMSY, EMSY S209A, or the EV and infected with HSV-1 strain 17+. The results confirmed that EMSY inhibits virus-induced ISG expression and that mutation of Ser209 enhances this effect (Fig. 5E).
EMSY Promotes Viral Replication, Whereas Activated Akt1 Inhibits It by Phosphorylating EMSY at Ser209.
IFNs inhibit the replication of a wide range of viruses. Given that their antiviral activities depend on the induction of ISGs (20, 21), we hypothesized that the efficiency of viral replication would be enhanced by EMSY and inhibited by Akt1. This hypothesis is consistent with the results of earlier studies showing that ISG15 KO mice are highly susceptible to HSV-1 infection (22). To address this hypothesis, HMEC-tert cells transduced with WT EMSY, EMSY S209A, or the EV were infected with an engineered variant of HSV-1 in which the immediate early gene ICP0 was replaced by an enhanced yellow fluorescent protein-infected cell polypeptide 0 (EYFP-ICP0) fusion (23). The viral progeny collected from these cultures at the peak of the lytic infection were used to infect HeLa cells, and the percentage of infected cells was determined by monitoring the expression of EYFP-ICP0 via flow cytometry. The percentage of infected cells in this assay is a measure of the viral titer. The results showed that EMSY-expressing cells produce more viral progeny than control cells and MyrAkt1-expressing cells produce fewer viral progeny than control cells. In addition, MyrAkt1 inhibited the enhancement of viral replication by WT EMSY but not by EMSY S209A (Fig. 5F). IFN treatment of HMEC-tert cells inhibited viral replication, as expected. Expression of WT EMSY partially blocked the inhibition of viral replication, whereas S209A almost completely abolished it (Fig. S3B).
It is possible that EMSY may stimulate viral production by targeting viral replication at different steps (24). ISG15 has been shown to inhibit the replication of HSV-1 as well as the release of viral progeny during infection by retroviruses (22, 25). If the enhancement of viral replication is attributable to the repression of ISG expression, it would be unlikely for the targeted step to be viral entry. We therefore addressed the question of whether EMSY promotes HSV-1 cellular entry. We infected the HMEC-tert cells transduced with the EMSY and Akt constructs we used in the preceding experiment with the EYFP-ICP0 recombinant HSV-1. All cell types were infected with the virus at the same multiplicity of infection (MOI). Four hours after infection, viral entry was assessed by measuring EYFP expression through FACS. No significant differences between the control and the EMSY- or MyrAkt1-expressing cells were found (Fig. S3C). In addition, we assessed viral entry using real-time PCR primers specific for the HSV-1 Pol I gene. The results showed that neither EMSY nor constitutively active Akt1 alters viral entry (Fig. S3D). Therefore, EMSY and Akt1 regulate HSV-1 replication by targeting steps other than viral entry or immediate early gene expression.
Titration of the tl17 thermolabile mutant of VSV, harvested from infected HMEC-tert cells transduced with EMSY, EMSY S209A, or the EV, with or without MyrAkt1, showed that the effects of EMSY and MyrAkt1 on the replication of VSV, a rhabdovirus, were similar to their effects on the replication of HSV-1 (Fig. 5G). Overall, the effects of EMSY and MyrAkt1 on viral replication mirrored the effects of these proteins on ISG transcription, suggesting that MyrAkt1 inhibits viral replication by blocking the repression of ISGs by EMSY through phosphorylation at Ser209.
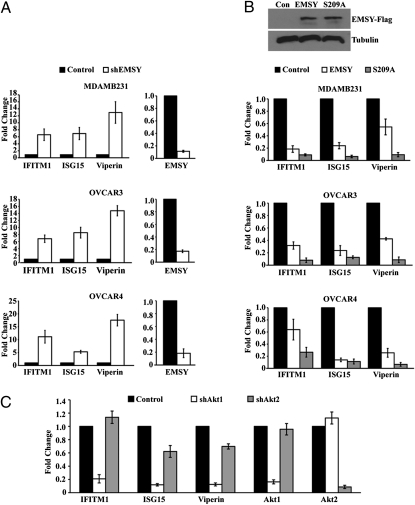
EMSY and Akt1 Antagonistically Regulate ISG Expression in Mammary and Ovarian Carcinoma Cell Lines.
EMSY is overexpressed in a significant percentage of human mammary and ovarian carcinomas. To determine whether EMSY represses ISGs in these tumors as well, we transduced the breast cancer cell line MDA MB 231 and the ovarian cancer cell lines OVCAR3 and OVCAR4 with a lentiviral shRNA construct targeting EMSY or with the EV. EMSY knockdown resulted in a significant up-regulation of IFITM1, ISG15, and Viperin (Fig. 6A). To confirm the repression of these genes by EMSY, which was suggested by the preceding experiment, and to determine whether it may be regulated by phosphorylation of EMSY at Ser209, we transduced the same tumor cell lines with WT EMSY, EMSY S209A, or the EV. The results showed that WT EMSY repressed IFITM1, ISG15, and Viperin in all the cell lines and that EMSY S209A was a more robust repressor (Fig. 6B). The data presented in Figs. 1 and 3 indicate that Ser209 of EMSY is a direct phosphorylation target of Akt1 and that phosphorylation of EMSY at this site in HMEC-tert cells relieves the EMSY-mediated repression of ISGs. To determine whether this applies to mammary carcinoma cells, we knocked down Akt1 or Akt2 in these cells and examined the effects of the knockdown on the expression of IFITM1, ISG15, and Viperin. The results confirmed that the knockdown of Akt1 repressed the expression of all three genes strongly, whereas the knockdown of Akt2 repressed their expression weakly (Fig. 6C).
Fig. 6.
EMSY represses the expression of ISGs in human mammary and ovarian carcinoma cell lines, and repression is relieved via phosphorylation at Ser209. (A) Expression of the indicated ISGs was measured by real-time RT-PCR in MDA MB 231, OVCAR3, and OVCAR4 cells transduced with a lentiviral construct of shEMSY or EV. Error bars show the SD (n = 3). (B) Expression of the indicated ISGs was measured by real-time RT-PCR in MDA MB 231, OVCAR3, and OVCAR4 cells transduced with pLenti constructs expressing WT EMSY, EMSY S209A, or EV. As in A, the bars show fold repression and the error bars show the SD (n = 3). Con, control. (C) Expression of the indicated ISGs was measured by real-time RT-PCR in MDA MB 231 cells transduced with lentiviral constructs of shAkt1, shAkt2, or EV. As in A and B, the bars show fold repression and the error bars show the SD (n = 3).
Discussion
MS analysis of murine lung fibroblasts expressing only Akt1, Akt2, or Akt3 identified a significant number of potential Akt substrates, including some that appear to be preferentially phosphorylated by a specific Akt isoform. We selected EMSY from this screen because it appeared to be phosphorylated preferentially by Akt1. Data presented in this report confirmed that EMSY is phosphorylated directly by Akt1 at Ser209, which is the only apparent Akt phosphorylation site on this protein. These data make EMSY one of only a handful of known isoform-specific Akt substrates. Inhibition of Akt activity by MK2206, a specific Akt1/Akt2 inhibitor, blocked phosphorylation at this site, and exogenously expressed EMSY was phosphorylated in cells expressing Akt1 but not Akt2. An in vitro kinase assay demonstrated that EMSY is a direct phosphorylation target of Akt1. Interestingly, the preferential phosphorylation of EMSY by Akt1 over Akt2 was maintained in vitro, suggesting that it was not caused by differences in the activation and subcellular localization of Akt1 and Akt2, or by differences between Akt1 and Akt2 in scaffold protein requirements.
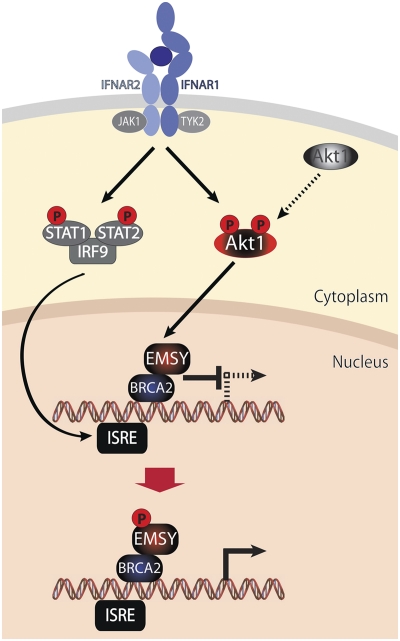
The goal of the work in this study was to determine the biological significance of the phosphorylation of EMSY by Akt1. Our data showed that unphosphorylated EMSY functions in concert with BRCA2 to repress the expression of a set of ISGs and that phosphorylation of EMSY by Akt1 at Ser209 relieves the repression. These findings raised the question of whether the Akt1/EMSY/BRCA2 pathway contributes to the IFN response. IFNs are a family of secreted proteins that function in an autocrine or paracrine fashion to regulate viral infection and replication, as well as innate and adaptive immunity (19). There are three different types of interferons: types I, II, and III, with types I and III represented by multiple members. Type I IFNs include IFN-α (13 members) and IFN-β (1 member), and type II IFNs are represented by IFN-γ (1 member), which is expressed by natural killer and activated T cells. Type I IFNs engage IFN alpha receptors 1 and 2 (IFNAR1 and IFNAR2) type II IFN-γ engages IFN gamma receptors 1 and 2 (IFNGR1 and IFGNR2), and type III IFNs engage IFN lambda receptor 1 (IFNLR1) and IL-10 receptor 2 (20). Engagement of these receptors activates members of the JAK kinase family, which, in turn, phosphorylate and activate members of the STAT family of transcription factors. The latter dimerize and translocate into the nucleus, where they associate with their respective DNA binding sites in the promoters of IFN-inducible genes and stimulate transcription. The specificity in the pathways activated by different types of IFNs stems, at least in part, from the unique combinations of JAK and STAT molecules that are activated specifically by each type of IFN. For example, type I IFNs require the tyrosine kinases JAK1 and TYK2, which phosphorylate STAT1 and STAT2. The latter form a trimeric complex with IRF9, which binds to palindromic sequences in ISG promoters called IFN-stimulated response elements (26, 27). The work presented in this paper provides evidence for an additional component of the IFN response pathway that depends on BRCA2, EMSY, and Akt1. Our data show that ISGs are normally repressed via the concerted action of EMSY and BRCA2. IFNs activate Akt1, which phosphorylates EMSY at Ser209 and relieves the repression, allowing the full activation of ISGs (model in Fig. 7). These findings support and explain earlier observations suggesting that the full induction of ISGs depends on the activation of the PI3K/Akt pathway (9).
Fig. 7.
Model for the functional role of Akt1, EMSY, and BRCA2 in the regulation of ISGs. EMSY is an Akt substrate that shows a strong preference for phosphorylation by Akt1 over Akt2. Akt1 is activated by IFN-generated signals. Activated Akt1 phosphorylates EMSY at Ser209 and relieves EMSY-mediated repression. The phosphorylation of EMSY by Akt1 is required for the full activation of ISGs by IFN. The diagram depicts the pathway activated by type I IFNs. ISRE, interferon-stimulated response element.
IFNs were discovered more than 50 y ago, by virtue of the fact that they inhibit infection by many different types of viruses. Today, we know that IFNs are induced by both viral and bacterial products through the activation of different Toll-like receptor signaling pathways or through the activation of the RIG1/MDA5 pathway (28). Moreover, we know that IFNs induced in the course of viral infection stimulate the expression of ISGs, which inhibit viral infection and replication through multiple mechanisms (24). Because the Akt1/EMSY/BRCA2 pathway regulates the induction of ISGs, we hypothesized that it would also regulate the efficiency of viral infection and replication. This was confirmed with experiments showing that overexpression of both WT EMSY and EMSY S209A enhances the efficiency of viral infection and that MyrAkt1 inhibits the effect of WT but not mutant EMSY. It is also noteworthy that the Akt1/EMSY/BRCA2 pathway similarly affected the replication of HSV-1 (a DNA herpesvirus) and VSV (a dsRNA rhabdovirus).
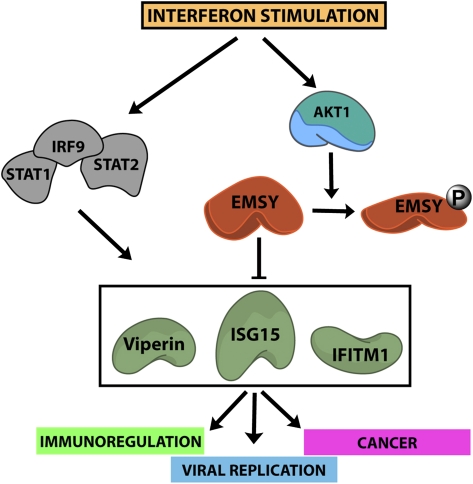
IFNs also play an important role in cancer. They promote tumor infiltration with natural killer cells and activated T cells, as well as antigen processing and presentation by dendritic cells (20). In addition, they inhibit angiogenesis and promote tumor cell apoptosis by a variety of mechanisms (29). Many of these effects are mediated by ISGs. Of the ISGs that appear to be regulated by the Akt1/EMSY/BRCA2 pathway, IFITM1 and ISG15 have already been linked to human cancer. IFITM1 is overexpressed in squamous cell carcinomas, and its overexpression has been linked to tumor cell invasiveness (30), perhaps attributable to regulation of the expression of metalloproteinases. Knockdown of IFITM1 inhibits the invasiveness of squamous cell carcinomas. It also inhibits the proliferation and migration of glioma cells in culture (31). An ISG expression signature is also associated with chemo- and radioresistance in breast cancer cells (32). Along these lines, knockdown of ISG15 or IFITM1 sensitizes breast cancer cells to chemotherapy (33). The data presented in this report suggest that the functional role of IFITM1 and ISG15 in cancer may be regulated by signals initiated by IFNs and transduced via the Akt1/EMSY/BRCA2 pathway. Additionally, they identify a new and so far unsuspected role for EMSY in the biology of cancer cells and their response to IFN stimulation.
The data presented in this report may also have implications in cancer treatment with oncolytic viruses. A variety of such viruses have been tried to date. Among them are variants of HSV-1 and VSV (34, 35), both of which appear to be regulated by the Akt1/EMSY/BRCA2 pathway. Our data show that virus infection and replication are enhanced in cells expressing the ISG transcriptional repressor EMSY and that activated Akt1, which relieves the EMSY-mediated repression, inhibits virus replication. These data suggest that oncolytic viruses may synergize with Akt inhibitors to kill cancer cells that express both EMSY and activated Akt.
In conclusion, the data presented in this report show that EMSY, in concert with its binding partner BRCA2, represses the expression of a set of IFN-inducible genes and that the full activation of these genes by IFN depends on the relief of the repression via phosphorylation of EMSY by Akt1. The Akt1/EMSY/BRCA2 pathway described in this report assigns additional functional activities to BRCA2, EMSY, and Akt1, and plays an important role in the control of viral replication. The same pathway may also play an important role in the biology of human cancer.
Materials and Methods
Constructs and Cell Culture.
Full-length EMSY was cloned into the Destination (DEST) vectors pLenti CMV Puro DEST and pLenti CMV Neo DEST using LR recombinase (Addgene). pLenti CMV Puro/Neo expressing GFP was used as a control. An N-terminal fragment of EMSY (both WT and S209A) corresponding to amino acids 8–374 was cloned as a GST fusion into the pDEST 565 bacterial expression vector (no. 11520; Addgene). shRNAs cloned into the pLKO.1 Puro lentiviral vector were purchased from Open Biosystems. MyrAkt1 and MyrAkt2 were cloned into the pBabe-Puro retroviral vector.
HMEC-tert cells were cultured in MEGM (Cambrex) or DMEM/F12 media supplemented with FBS (5% vol/vol), EGF (10 ng/mL), insulin (10 μg/mL), hydrocortisone (0.5 mg/mL), cholera toxin (100 ng/mL), and antibiotics. MDA MB 231 and 3T3 cells were cultured in DMEM supplemented with 10% (vol/vol) FBS and antibiotics, and OVCAR3 and OVCAR4 cells were cultured using RPMI supplemented with 10% (vol/vol) FBS and antibiotics.
Real-Time RT-PCR.
Real-time RT-PCR reactions were performed using an MJ Research Opticon 2 instrument. DNA synthesis was monitored by SYBR Green (SA Biosciences). Cellular RNA was isolated from cultured cells using QIAshredder and RNeasy Plus kits (Qiagen). cDNA was synthesized using the RETROscript kit (Ambion). To quantify the RNA levels for EMSY, BRCA2, Akt1, Akt2, and the ISGs discussed in this paper, we used the following primers: Viperin [forward (F): CAAGAC CGGGGAGAATACCTG, reverse (R): GCGAGAATGTCCAAATACTCACC], ISG15 (F: TCCTGGTGAGGAATAACAAGGG, R: GTCAGCCAGAACAGGTCGTC), IFITM1 (F: TCA TCCTGTCACTGGTATTCGGCTC, R: GTGGGTATAAACTGCTGTATCTAGGG), WARS (F: CTCGTAAGGTCCCTCAAAGCG, R: GCCATGATTACTGGTAGGTGCT), UBE2L6 (F: CATCAGCAGTGAGAACTGGAAG, R: GGCATTCTTTCTGAACAGCTCC), KRT6A (F: AGTGGATTTGGTTTCGGTGG, R: GACTCTGGTTGACGGTGAC), FGF receptor 2 (F: GAGGAT ACCACATTAGAGCCAG, R: CACCCCATCCTTAGTCCAAC), EMSY (F: ATAGCAACG GTTAAGTCTCCAAG, R: CAGGCACAGTGATCGTCTTTG), HSV-1 DNA Polymerase I (F: AGCCTGTACCCCAGCATCAT, R: TGGGCCTTCACGAAGAACA), and nonspecific genomic DNA (F: ATGGTTGCCACTGGGGATCT, R: TGCCAAAGCCTAGGGGAAGA). All primers produced a single peak when the PCR reactions were analyzed by melt curve analysis.
Antibodies, Immunoprecipitation, and Western Blotting.
Antibodies used for immunoprecipitation and Western blotting throughout this paper include the following: Phospho-Ser/Thr Akt Substrate antibody (no. 9611; Cell Signaling), FLAG (no. 2368; Cell Signaling), HA (no. 2367; Cell Signaling), MYC (no. 2276; Cell Signaling), ISG15 (no. 2758; Cell Signaling), IFITM1 (no. 66827; Santa Cruz), BRCA2 (no. 9012; Cell Signaling), and Akt pT308 (no. 4056; Cell Signaling). Antibodies were diluted according to manufacturers’ instructions. Cells were lysed with the recommended buffer from Cell Signaling [20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100] supplemented with protease and phosphatase inhibitors (Roche). Lysates were sonicated, and insoluble proteins were removed by centrifugation at 18,000 × g for 10 min. In some of the experiments, cells were fractionated with a cell fractionation kit from Thermo Scientific, and nuclear and cytoplasmic lysates were analyzed in parallel. In immunoprecipitation experiments, cell lysates obtained using Cell Signaling lysis buffer were first incubated with the primary antibody and the resulting immunoprecipitates were pulled down with Protein G agarose beads (Sigma) for 1 h at 4 °C. MK2206 (Merck) was used at a concentration of 2.5 μM.
In Vitro Kinase Assay and Kinase Inhibitors.
Residues 8–374 of EMSY and EMSY S209A were cloned as GST fusions into the pDest 565 vector, and the fusions were expressed in the BL21 strain of Escherichia coli. EMSY was induced with isopropyl-β-d-thiogalactopyranoside, and it was affinity-purified using Glutathione Sepharose beads (Amersham) and glutathione elution. Myc-tagged Akt1 and Akt2 were immunoprecipitated with anti–Myc-conjugated agarose beads from triple-KO murine fibroblasts expressing either Akt1 or Akt2. Purified EMSY or EMSY S209A (500 ng) was incubated with the Akt1 or Akt2 immunoprecipitate for 30 min at 30 °C in kinase buffer (Cell Signaling) containing 25 mM Tris-HCl (pH 7.5), 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, and 10 mM MgCl2. After boiling in 2× sample buffer, the products of the kinase reaction were immunoblotted and probed with an Akt phosphosubstrate antibody or the anti-FLAG antibody. MK2206 was used at the concentration of 2.5 μM.
IFN Stimulation and Activation of the IFN Response via HSV-1 Infection.
HMEC-tert cells grown to 50% confluence were stimulated with IFN-α. The expression of ISGs was measured in cell lysates harvested 6 h after the start of IFN-α treatment. ISG expression in HSV-1–infected HMEC-tert cells (MOI of 1) was examined in cell lysates harvested 36 h after infection.
HSV-1 Titration.
HSV-1 strain 17+ expressing EYFP-ICP0 was grown and titrated in BHK cells propagated in Glasgow modified Eagle's medium supplemented with 10% newborn calf serum, 10% tryptose phosphate broth and penicillin (100 U/mL), and streptomycin (100 μg/mL) (23). HMEC-tert cells transduced with the indicated EMSY and Akt constructs and control HMEC-tert cells transduced with the EVs were grown to confluence and then infected with HSV-1 by exposing them to the virus for 3 h at an MOI of 1. The cells were then washed twice with PBS, and the virus was allowed to grow for 48 h. Virus harvested from these cultures was filtered and then used to infect confluent HeLa cells by incubating it with the cells for 3 h (200 μL per confluent 6-cm plate). The HeLa cells were then washed, collected by trypsinization, and fixed in 0.5% formaldehyde for 10 min at room temperature. The percentage of cells expressing EYFP-ICP0 was measured by FACS.
To measure viral entry and immediate early gene expression, HMEC-tert cells were incubated with the EYFP-ICP0 recombinant HSV-1 for 4 h and total cellular DNA was extracted. Viral entry was measured by real-time RT-PCR for HSV-1 DNA Polymerase I, and primers for a nonspecific genomic region were used as a loading control. In parallel experiments, cells were also harvested after 4 h and the percentage of EYFP-positive cells was determined by flow cytometry.
VSV tl17 Plaque Assay.
A thermolabile vesicular stomatitis virus mutant (VSV tl17) was propagated in National Institutes of Health 3T3 cells at 32 °C. Virus was harvested at 48 h from the start of the infection, when cell lysis was complete. HMEC-tert cells transduced with the indicated EMSY and Akt constructs and control HMEC-tert cells transduced with the EVs were infected with a 1:2,000 dilution of the filtered viral stock, and supernatants were collected and filtered 24 h later. The harvested viral stocks were titrated on Vero cells plated to confluence in six-well plates. Following a 2-h incubation of the viral dilutions with the Vero cells, the inoculum was removed from the plates and the cells were overlaid with 1% noble agar in conditioned media. Plaques were visualized by staining the infected cells with Giemsa 30–36 h postinfection.
ChIP.
HMEC-tert cells transduced with EV or EMSY-FLAG were cross-linked, and protein was immunoprecipitated with FLAG M2 (Sigma) using a Millipore kit (no. 17-295). SDS and sodium deoxycholate were removed from buffers to prevent inhibition of FLAG binding, and Triton X-100 was substituted.
Supplementary Material
Acknowledgments
We thank Dr. T. Kouzarides (University of Cambridge, Cambridge, UK) for his generous gift of a full-length EMSY construct and Dr. E. Scolnick (Stanley Center for Psychiatric Research, Cambridge, MA) for his generous gift of MK2206. We thank S. Kampranis, F. Kottakis, and C. Mao for helpful discussions and A. Degterev, O. Gjoerup, P. Hinds, and B. Schaffhausen for their input on the manuscript. This work was supported by National Institutes of Health Grant R01 CA057436 (to P.N.T.). S.A.E. was supported by funds from the Sackler School of Graduate Biomedical Sciences.
Footnotes
Conflict of interest statement: A.G. and M.J.C. are employees of Cell Signaling Technology.
This article is a PNAS Direct Submission.
See Author Summary on page 3614 (volume 109, number 10).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115029109/-/DCSupplemental.
References
- 1.Chen WS, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 3.Dummler B, et al. Life with a single isoform of Akt: Mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliopoulos D, et al. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polytarchou C, et al. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011;71:4720–4731. doi: 10.1158/0008-5472.CAN-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao D, et al. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur S, et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci USA. 2008;105:4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur S, et al. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol. 2008;181:7316–7323. doi: 10.4049/jimmunol.181.10.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes-Davies L, et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523–535. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- 11.Cousineau I, Belmaaza A. EMSY overexpression disrupts the BRCA2/RAD51 pathway in the DNA-damage response: Implications for chromosomal instability/recombination syndromes as checkpoint diseases. Mol Genet Genomics. 2011;285:325–340. doi: 10.1007/s00438-011-0612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez C, et al. Amplification of the BRCA2 pathway gene EMSY in sporadic breast cancer is related to negative outcome. Clin Cancer Res. 2004;10:5785–5791. doi: 10.1158/1078-0432.CCR-03-0410. [DOI] [PubMed] [Google Scholar]
- 13.Moritz A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3 doi: 10.1126/scisignal.2000998. ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap TA, et al. First-in-Man Clinical Trial of the Oral Pan-AKT Inhibitor MK-2206 in Patients With Advanced Solid Tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi MK, Chaudhuri G. Down-regulation of UCRP and UBE2L6 in BRCA2 knocked-down human breast cells. Biochem Biophys Res Commun. 2005;328(1):43–48. doi: 10.1016/j.bbrc.2004.12.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhou BP, et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 18.Liang J, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 19.Borden EC, et al. Interferons at age 50: Past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuang Z, Seo EJ, Leis J. Mechanism of inhibition of retrovirus release from cells by interferon-induced gene ISG15. J Virol. 2011;85:7153–7161. doi: 10.1128/JVI.02610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenschow DJ, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci USA. 2007;104:1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett RD, Sourvinos G, Orr A. Recruitment of herpes simplex virus type 1 transcriptional regulatory protein ICP4 into foci juxtaposed to ND10 in live, infected cells. J Virol. 2003;77:3680–3689. doi: 10.1128/JVI.77.6.3680-3689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borden EC, Williams BR. Interferon-stimulated genes and their protein products: What and how? J Interferon Cytokine Res. 2011;31(1):1–4. doi: 10.1089/jir.2010.0129. [DOI] [PubMed] [Google Scholar]
- 25.Pincetic A, Kuang Z, Seo EJ, Leis J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol. 2010;84:4725–4736. doi: 10.1128/JVI.02478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi SA, Salditt-Georgieff M, Darnell JE., Jr Tyrosine-phosphorylated Stat1 and Stat2 plus a 48-kDa protein all contact DNA in forming interferon-stimulated-gene factor 3. Proc Natl Acad Sci USA. 1995;92:3829–3833. doi: 10.1073/pnas.92.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung S, Qureshi SA, Kerr IM, Darnell JE, Jr, Stark GR. Role of STAT2 in the alpha interferon signaling pathway. Mol Cell Biol. 1995;15:1312–1317. doi: 10.1128/mcb.15.3.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 29.Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: Effects on tumor- and lymphocyte-induced vascular responses. Cancer Res. 1987;47:5155–5161. [PubMed] [Google Scholar]
- 30.Hatano H, et al. IFN-induced transmembrane protein 1 promotes invasion at early stage of head and neck cancer progression. Clin Cancer Res. 2008;14:6097–6105. doi: 10.1158/1078-0432.CCR-07-4761. [DOI] [PubMed] [Google Scholar]
- 31.Yu F, et al. Knockdown of interferon-induced transmembrane protein 1 (IFITM1) inhibits proliferation, migration, and invasion of glioma cells. J Neurooncol. 2011;103(2):187–195. doi: 10.1007/s11060-010-0377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci USA. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai SD, et al. ISG15 as a novel tumor biomarker for drug sensitivity. Mol Cancer Ther. 2008;7:1430–1439. doi: 10.1158/1535-7163.MCT-07-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longo SL, Griffith C, Glass A, Shillitoe EJ, Post DE. Development of an oncolytic herpes simplex virus using a tumor-specific HIF-responsive promoter. Cancer Gene Ther. 2011;18(2):123–134. doi: 10.1038/cgt.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moussavi M, et al. Oncolysis of prostate cancers induced by vesicular stomatitis virus in PTEN knockout mice. Cancer Res. 2010;70:1367–1376. doi: 10.1158/0008-5472.CAN-09-2377. [DOI] [PubMed] [Google Scholar]