Abstract
Antibody conjugates are widely used as diagnostics and imaging reagents. However, many such conjugates suffer losses in sensitivity and specificity due to nonspecific labeling techniques. We have developed methodology to site-specifically conjugate oligonucleotides to antibodies containing a genetically encoded unnatural amino acid with orthogonal chemical reactivity. These oligobody molecules were used in immuno-PCR assays to detect Her2+ cells with greater sensitivity and specificity than nonspecifically coupled fragments, and can detect extremely rare Her2+ cells in a complex cellular environment. Such designed antibody-oligonucleotide conjugates should provide sensitive and specific reagents for diagnostics, as well as enable other unique applications based on oligobody building blocks.
The ability to detect very rare cells or low concentrations of proteins in the blood with accuracy and sensitivity is still a significant problem for molecular diagnostics. With the amplification power of PCR, the detection of single nucleic acid molecules is now routine. Hybridization of DNA to its template is highly sensitive and specific, but until recently has only been applied to detect nucleic acids. Typical protein detection methods like enzyme-linked immunosorbent assays (ELISAs) are still not sensitive enough to detect low concentrations of important biological markers such as troponin, prostate-specific antigen, or viral coat proteins. More recent DNA-linked methods for sensitive protein detection have been reported (1, 2); however, these assays are not easily applied to cellular detection, as in the case with rare circulating tumor cells. Immuno-PCR, first developed by Sano et al. (3), combines the specificity of antibodies with the amplification power of PCR allowing a 10–1,000-fold increase in sensitivity compared to traditional antigen detection methods (3, 4). Moreover, rolling circular amplification (RCA) occurs isothermally, allowing visualization of endogenous proteins on cells (Fig. 1A) (5, 6). Many immuno-PCR improvements have been made, including proximity ligation with RCA which enables detection of protein-protein interactions (7–9), real-time quantitative immuno-PCR (10–12), and amplification using T7 RNA polymerase, which can afford femtomolar sensitivity (13).
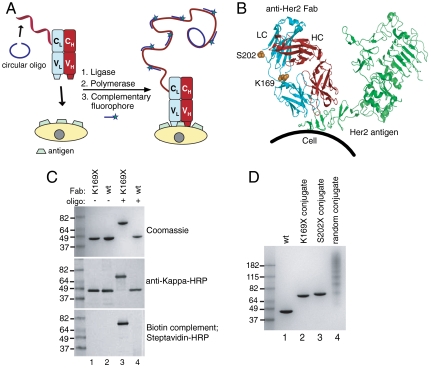
Fig. 1.
Oligobody construction for immuno-PCR. (A) Scheme depicting site-specific immuno-PCR. A complementary single-stranded oligonucleotide (68 nt) (blue) is annealed to the oligobody (red). T4 ligase is added to form a circular oligonucleotide, which is now the template for RCA. The antibody-oligonucleotide conjugate is bound to Her2 on cells and phi29 polymerase is added to isothermally amplify the DNA, creating multiple copies of the 20 nt sequence. Small complementary oligonucleotides derivatized with Alexa Fluor 488 (20 nt) are then added to obtain a fluorescent signal. (B) Residues (K169 or S202) in anti-Her2 Fab mutated to pAcF for site-specific conjugation are shown in sphere form in orange, in the light chain (LC) in blue and the heavy chain (HC) in red. The Her2 antigen (green) is distant from all mutations. (C) Oliognucleotide conjugation to Fab. Either anti-Her2 pAcF (K169X, lanes 1, 3) or wild-type Fab (lanes 2, 4) was incubated without (lanes 1, 2) or with (lanes 3, 4) 3 mM aminoxy-modified ssDNA (100 mM methoxy aniline, 37 °C, 16 h). Reactions were analyzed by SDS-PAGE (Top), or transferred to nitrocellulose and incubated with anti-kappa-HRP (Middle) or a biotinylated antisense oligonucleotide, then detected with streptavidin-HRP (Bottom). The anti-kappa-HRP and streptavidin-HRP blots were developed colorimetrically with the metal enhanced DAB kit (Pierce). (D) Purified site-specific oligonucleotide conjugates and nonspecifically labeled oligonucleotide conjugate. Lanes 2 and 3 correspond to oligonucleotide site-specifically coupled to anti-Her2 pAcF mutant Fab. Lane 4 has multiple oligonucleotides (1–6) coupled to various lysines in anti-Her2 wild-type Fab.
Even with these developments, there are still significant challenges with the antibodies and conjugation methods that prevent immuno-PCR from becoming a broadly useful and reliable diagnostic tool. For example, most methods of DNA conjugation rely on nonspecific amide bond formation with lysine residues, resulting in heterogeneous mixtures that can alter antigen binding and lead to antibody aggregation (14, 15). The synthesis of intein-fusion proteins results in site-specific conjugation, but does not allow precise control over the site of conjugation (16, 17). Methods that rely on conjugated polyclonal secondary antibodies for detection can have higher background and altered specificity (18, 19), and variations in secondary antibody preparations can also impair consistency of a diagnostic (20–22). Thus, a homogeneous, chemically defined antibody conjugate that allows protein or cellular detection with the same sensitivity as traditional nucleic acid amplification techniques is highly desirable. Here we describe the synthesis of site-specific antibody-oligonucleotide conjugates using genetically encoded unnatural amino acids (UAAs) with unique chemical reactivity. Moreover, we demonstrate that these conjugates can detect antigens with improved sensitivity and lower nonspecific background than conventional methods based on lysine conjugation.
Results and Discussion
An Anti-Her2 Antibody-Oligonucleotide Model System.
To determine whether the orthogonal chemical reactivity of genetically encoded unnatural amino acids can lead to chemically defined antibody-oligonucleotide conjugates with improved properties, we used trastuzumab (Herceptin, Genentech/Roche) as a model system. The Her2 oncogene is overexpressed in 25–30% of breast cancers. Standard of care for metastatic Her2+ cancers includes treatment with trastuzumab; however, many tumors develop resistance and progress. Metastatic cancer spreads hematogenously, and enumeration of circulating tumor cells (CTCs) is becoming an important prognostic test (23). The biology of CTCs has not been extensively investigated; for example, it is unclear whether the phenotype of CTCs match the primary tumor. Given that Her2 is druggable by both trastuzumab, trastuzumab-drug conjugates, and small molecule kinase inhibitors, the detection and analysis of Her2+ CTCs could be an important predictive biomarker if proven out in a clinical trial. Additionally, the ability to isolate and characterize CTCs may provide information on the evolution of the disease within a patient under therapeutic pressure, and could guide treatment in relation to other phenotypic and genotypic markers. Molecular characterization of CTCs is difficult, however, because their rarity (1 in 109 blood cells) poses a challenge for conventional detection methods (24).
A reliable and accurate immuno-PCR method must fulfill a few important requirements. First, the antibody needs to have very high affinity and specificity for its antigen (25). Dissociation constants (Kd) below expected serum concentrations of the target (and in particular, slow off-rates), combined with a high melting temperature of the oligonucleotide primer are important characteristics of an amplifiable diagnostic reagent. The trastuzumab Fab fragment binds to domain 4 of Her2 with subnanomolar Kd and is thus a clinically relevant model with the requisite affinity. Second, covalent modification of the antibody with an oligonucleotide cannot adversely affect antibody binding or solubility; conjugation sites that affect hybridization or subsequent PCR amplification will also limit sensitivity. For example, nonspecific electrophilic conjugation can modify lysine residues in the antigen binding site. We have developed an approach using genetically encoded UAAs that allows conjugation to antibodies in a site-specific manner (26, 27). This approach relies on the site-specific incorporation of p-acetylphenylalanine (pAcF) or p-azidophenylalanine (pAzF) into the protein in response to an amber nonsense codon in either bacteria, yeast, or mammalian cells. The unique reactivity of the aryl ketone or azide allows selective modification with alkoxyamines or alkynes, respectively, under mild conditions. Third, the antibody conjugate should be a homogenous, chemically defined entity such that it can be reproducibly synthesized and its activity quantitatively assessed. A homogeneous product naturally results from site-specific conjugation through a single unnatural amino acid.
Synthesis of Anti-Her2 Fab-Oligonucleotide Conjugate.
We conjugated an oligonucleotide to the trastuzumab Fab fragment (anti-Her2 Fab) at a single uniquely reactive UAA distant from the antigen binding site such that it can bind to its protein target and serve as an amplification primer for immuno-PCR. We mutated sites K169 and S202 in anti-Her2 Fab (Fig. 1B) to pAcF because of their surface accessibility and previously demonstrated high coupling efficiencies to aminooxy-modified Alexa Fluor 488 dyes (26). pAcF was site-specifically introduced into the anti-Her2 Fab in response to the amber nonsense codon TAG with an orthogonal amber suppressor aminoacyl-tRNA synthetase/tRNA pair derived from Methanococcus jannaschii (28). Both mutants were either expressed in shake flasks or fermented in Escherichia coli with similar yields to wild-type Fab (> 200 mg/L with fermentation) and purified using Protein G chromatography. Mass spectrometry and SDS-PAGE gel showed > 95% purity (26).
An aminooxy-hexyl maleimide bifunctional linker (27), was coupled to commercially available 5′-thiol modified ssDNA primer (32 nt, IDT Technologies) (See SI Materials and Methods) (9). To synthesize the antibody-oligonucleotide conjugate, we reacted 100 μM anti-Her2 Fab (K169pAcF or S202pAcF) at 37 °C with 3 mM aminooxy-ssDNA in the presence of 100 mM methoxyaniline (29) in 100 mM acetate buffer (pH 4.5). After 16 h, the product was analyzed by SDS-PAGE (Fig. 1C, lane 3 and Fig. 1D, lanes 2 and 3). A new lower mobility band was formed that migrated at a slightly higher molecular weight than the expected molecular weight of the Fab-ssDNA complex (60 kDa). This band only formed in the presence of pAcF mutant Fabs and DNA (compare Fig. 1C, lanes 1 and 3), and not with wild-type Fab (compare Fig. 1C, lanes 2 and 4). The yield of this band was > 90% as determined by densitometry (Fig. S1). To verify that the new band contained both Fab and ssDNA, we performed a Western blotting analysis with anti-kappa-HRP (Fig. 1C, Middle, lane 3), as well as a Southwestern blotting analysis with the biotin-labeled complement of the oligonucleotide (Fig. 1C, Bottom, lane 3). Indeed, the high molecular weight band contained both Fab (Fig. 1C, Middle) and ssDNA (Fig. 1C, Bottom). The exact mass of the monoconjugated antibody-oligonucleotide molecule was confirmed by mass spectrometry (Fig. S2).
Immuno-PCR Detection of Her2+ Cells.
We performed immuno-PCR using the RCA method with the breast cancer cell lines SK-BR-3 (Her2hi) and MDA-MB-231 (Her2-) to test the oligobody specificity for Her2. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X 100 and blocked for 1 h at 37 °C with BSA and salmon sperm DNA. The circle oligonucleotide, 68 nt in length and complementary with the antibody-conjugated oligonucleotide (See Materials and Methods), was incubated with the oligobody (K169pAcF or S202pAcF) (2 μg/mL) and T4 ligase for 1 h at 37 °C before adding to cells. Phi29 polymerase was used to amplify the DNA. After 1.5 h at 37 °C, a complementary 20 nt oligonucleotide-Alexa Fluor 488 conjugate and Hoechst 33342 nuclear stain were added to cells (Fig. S3). The cells were imaged with either a fluorescence or confocal microscope. Both the anti-Her2 K169pAcF and S202pAcF Fab-oligonucleotide conjugates readily detected Her2 antigen on SK-BR-3 cells, as seen by the amplified fluorescent signal (Fig. 2 A and B). On MDA-MB-231 cells (Her2-), the signal was undetectable, even with a longer exposure time (Fig. 2 E and F). To further demonstrate the sensitivity of the site-specific conjugates, we used MCF-7 cells which express low levels (30) of Her2. With longer exposure, the Her2 antigen was detectable, whereas there was still no detectible signal for the Her2 negative cell line (Fig. S4). Furthermore, the oligobodies showed no signal on CHO cells that do not express any human Her2 antigen, but did recognize CHO cells transiently transfected with human Her2 (Fig. S5). This result shows that the Fab-oligonucleotide conjugates are specific only for cells expressing the Her2 antigen. For comparison, immuno-PCR was approximately 15-fold more sensitive in detection of Her2+ SK-BR-3 cells than an anti-Her2 Fab site-specifically conjugated with a single Alexa Fluor 488 dye (Fig. S6).
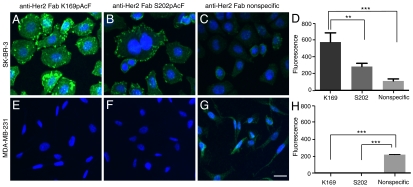
Fig. 2.
Oligobody sensitivity and specificity in detecting Her2+ cells by immuno-PCR. Immuno-PCR on (A–C) SK-BR-3 (Her2Hi) and (E–G) MDA-MB-231 (Her2-). Site-specific oligonucleotide-Fab conjugates (K169pAcF or S202pAcF) or the nonspecifically labeled oligonucleotide-Fab conjugate were added to fixed Her2 cell lines and immuno-PCR was performed as previously described. Hoechst 33342 was used as the nuclear stain (blue) and Alexa Fluor 488 complementary fluorescent probes were used to detect Her2 (green). (D and H) Fluorescence from the immuno-PCR signal was quantified with ImageJ and error bars correspond to the standard deviation from triplicate frames. One-way ANOVA test indicated p value < 0.001. Tukey’s post hoc test, p value *< 0.05, **< 0.01, ***< 0.001. Exposure times: A–C, 40 ms; E–G, 200 ms. Scale bar, 25 μm.
Because the site of conjugation can potentially affect the immuno-PCR signal through steric interactions with the antigen, oligonucleotide, or cell surface, we tested the activities of the S202pAcF versus K169pAcF oligobodies. The K169pAcF mutant provided a 2-fold greater signal compared to the S202pAcF conjugate on SK-BR-3 cells (Fig. 2 A and B, and quantified in D, Left). Importantly, neither mutant had background signal on Her2- cells (Fig. 2 E and F, and H, Right). Thus, steric factors can influence either the binding and/or amplification. Interestingly, a biotinylated S202pAcF mutant was previously shown to more efficiently form neutravidin tetramers and inhibited Her2 phosphorylation more effectively than the K169pAcF mutant in previous studies (26).
Comparison of Site-Specific and Nonspecific Antibody-Oligonucleotide Conjugates.
Although site-specific oligobodies can easily detect Her2+ cells, it is possible that the signal afforded as a result of greater DNA loading per Fab by nonspecific lysine coupling might be higher. Therefore we directly compared our oligobodies to the corresponding nonspecifically conjugated Fab. The latter was synthesized by coupling an aldehyde modified oligonucleotide to an anti-Her2 Fab, in which the lysine residues were nonspecifically modified with hydrazine (Solulink Technologies) (31). The purified conjugate was resolved on an SDS-PAGE gel and stained with Coommassie blue (Fig. 1D). Whereas only one oligonucleotide was coupled to both the anti-Her2 K169pAcF and anti-Her2 S202pAcF Fabs, there were multiple oligonucleotides (1–6) coupled to various lysines in the nonspecifically labeled Fab (Fig. 1D, compare lanes 2 and 3 with lane 4) on the basis of mobility on a Coommassie blue-stained gel. We performed immuno-PCR as described above, and used an Alexa Fluor 488 derivatized complementary oligonucleotide for detection. Despite increased oligonucleotide loading, the signal from the SK-BR-3 cells for the nonspecifically labeled Fab was 5- and 2.5-fold lower than for the K169pAcF- and S202pAcF-labeled Fabs, respectively (Fig. 2, compare A, B, and C, signals quantified in D). More significantly, the background signal from the MDA-MB-231 cells (Her2-) with the nonspecifically labeled Fab was at least 200-fold higher than the site-specific oligobodies (Fig. 2 E, F, and G, signals quantified in H). Although derived from a triple-negative tumor, MDA-MB-231 cells might express very low basal levels of Her2. CHO cells do not express any human Her2, and serve as a completely Her2-negative cell. Remarkably, significant signal was seen for the nonspecifically labeled Fab on CHO cells, whereas the signal was undetectable for the S202pAcF or K169pAcF oligobodies (Fig. S7). Thus, the nonspecifically labeled Fab appears to bind alternative antigens on cells that do not express human Her2, leading to a higher false positive signal. In addition, this site-specific approach was compared with proximity ligation immuno-PCR (Olink Bioscience) which utilizes secondary antibodies with nonspecifically labeled oligonucleotides (Fig. S8). The procedure using secondary antibodies showed nonspecific binding to cells, whereas there was no detectable signal with the site-specifically labeled oligobodies.
Rare Cell Detection by Immuno-PCR.
To use an oligobody as a cellular detection tool in a context similar to a patient situation, we spiked SK-BR-3 or MDA-MB-231 cells into a normal white blood cell (WBC) preparation at a ratio of 1∶3,000. Immuno-PCR was performed in addition to the use of pan anti-cytokeratin (CK) and anti-CD45 primary antibodies to visualize circulating tumor cells and leukocytes, respectively. Slides were sealed with coverglass and imaged using a customized high-throughput fluorescence microscope with 10× magnification (32–34). Cells were identified using Hoechst 33342 nuclear staining and the fluorescence intensity of SK-BR-3 and MDA-MB-231 was determined. Relative intensity measurements of the cancer cell lines were then compared against WBCs (Fig. 3D). The cells were also compiled in a four-channel assay, with no emission overlap between the nuclear stain (blue), CK (red), CD45 (gray), and Her2 immuno-PCR (green) (Fig. 3 A and B and Fig. S9). There was signal from immuno-PCR only on Her2 positive cells (SK-BR-3), whereas the CK antibodies stain both the SK-BR-3 and MDA-MB-231 cells (35). We also spiked both SK-BR-3 and MDA-MB-231 cells (stained with CellTracker Red) as a mixed population into WBCs (1∶10), and were able to distinguish between them using both immuno-PCR and CK staining (See Fig. 3C and Fig. S10). This finding is especially important because the relationship between the Her2 status of CTCs and the primary tumor is not clear. In this regard, it is known that Her2+ tumors can convert to Her2- status during progression, and patients with Her2- primary tumors can have Her2+ CTCs (36–39). In the latter case, such patients could be candidates for Her2 specific therapy. This experiment also further demonstrates the specificity of our conjugate in a complex cellular context. To assess the ability of the oligobody to detect extremely rare cells reproducibly, we determined that we could easily detect 11 Her2 positive cells in 1.4 million WBCs (Fig. 3E). Remarkably, we identified 100% of the spiked cells.
Fig. 3.
Immuno-PCR in a complex mixture derived from human blood. Cells are identified by nuclear staining (blue). The anti-CK antibodies (anti-CK19, 1∶100, Dako and anti-panCK, 1∶100, Sigma) (red) stained both the (A) SK-BR-3 cells and (B) MDA-MB-231 cells, whereas the Her2 immuno-PCR with K169pAcF oligobody (green) only stained (A) SK-BR-3 cells. Anti-CD45 (1∶125, AbD Serotec) (gray) only stain WBCs. (C) MDA-MB-231 cells were stained with CellTracker Red (Invitrogen) (orange) and both SK-BR-3 and MDA-MB-231 cells were spiked into WBCs. SK-BR-3 cells were stained with Her2 immuno-PCR (green) and CK antibodies (red), whereas MDA-MB-231 cells were only stained with CK antibodies (red). (D) Approximately 1,000 cells were spiked into approximately three million WBCs and scanned on a fluorescent microscope (1∶3,000). The Alexa Fluor 488 standard deviation of the mean (SDOM) of a representative amount of cells were graphed. (E) Eleven SK-BR-3 cells were spiked into 1.4 million WBCs, scanned and all cells were identified by immuno-PCR. Scale bar, 25 μm.
Conclusion
In summary, we site-specifically labeled a Fab fragment with an oligonucleotide to afford a homogeneous oligobody. We demonstrated that (i) site-specific conjugation generates enhanced positive signal and lower background signal compared to nonspecific conjugation, (ii) the conjugation position affects immuno-PCR signal, and (iii) Her2 antigen can be detected on cells with high selectivity and sensitivity by oligobodies both on isolated cells and in a mixed population of cancer cells and WBCs. We and others have shown that nonspecific conjugation to an antibody surface lowers binding affinity to its target (26, 40, 41). It is not surprising that specificity can also be altered. Wild-type Fab or oligobody does not bind CHO or MDA-MB-231 cells, whereas the nonspecifically labeled anti-Her2 Fab generated significant signal on these Her2-negative cell lines. Apparently, the nonspecifically labeled anti-Her2 acquires affinity to one or more unknown antigens that are expressed on these Her2- cells. This decrease in specificity may result from either the affects of nonspecific labeling at or near the antigen binding site, or from the attachment of multiple oligobodies to an antibody surface, which may result in nonspecific binding interactions. As most antibodies are tested for specificity by analyzing a few antigens by ELISA, our results show that it is important to evaluate the large number of antigens on the surface of cells to ensure that a nonspecifically labeled conjugate retains specificity when used to identify cells in a complex environment, as is the case with circulating tumor cells.
There are a number of advantages in the use of site-specifically labeled oligobodies for immuno-PCR. Antibodies conjugated with enzymes can also amplify signal, however immuno-PCR has the advantage of maintaining a covalent link with the amplified nucleic acid. Thus, fluorescent products do not wash away in solvent, allowing for spatial resolution as well as amplification in immunofluorescent applications. Additionally, proximity ligation immuno-PCR has the unique ability to detect specific protein-protein interactions. For example, the ability to detect and quantify Her2-Her2 dimers, Her2 heterodimers or other members of the ErbB family (ErbB1/EGFR, ErbB3, ErbB4) could provide insights into tumor biology, and in particular the biology of CTCs. Given the proven prognostic significance of CTCs, elucidation of the molecular details of this pathway in CTCs could be important in developing predictive tests for cancer therapy. Importantly, the ability to create a recombinantly produced, homogeneous antibody∶oligonucleotide conjugate in a 1∶1 ratio is likely to have utility beyond immuno-PCR. The Watson–Crick base pairing properties of DNA should allow unique oligobody multimeric structures to be designed and self-assembled. Thus, oligonucleotides could be generated that allow formation of bispecific antibodies or even higher order hetero- or homomultimers. Such multivalent, multispecific molecules are very difficult to produce using genetic fusion technology. The ability to couple an oligonucleotide to any surface exposed residue could also enable orientation and steric control of the oligobody’s antigen binding site for immobilization on surfaces. Beyond medical applications, oligobodies could also allow creation of unique nanostructures or even become tools used in unique self-assembly biocomputation applications (42).
Materials and Methods
Site-Specific Oligonucleotide Conjugation.
Expression and purification of anti-Her2 Fabs containing unnatural amino acids was carried out as described previously (26, 27). The anti-Her2 K169pAcF or anti-Her2 S202pAcF Fab (100 µM) was reacted with 3 mM aminooxy-modified oligonucleotide (5-ThiolC6-AAA AAA AAA ATA TGA CAG AAC TAG ACA CTC TT -3, IDT Technologies) in 100 mM acetate buffer, pH 4.5, at 37 °C. After 16 h, the reaction was buffer exchanged with PBS (pH 7.0) with Zeba desalting columns (Pierce), and the crude reaction was purified by anion exchange chromatography (Mono Q 5/50 G, Buffer A: 20 mM Tris, 10 mM NaCl, pH 7.5; Buffer B: 20 mM Tris, 2 M NaCl, pH 7.5) to remove excess DNA and unreacted protein. The conjugate was analyzed with a reducing SDS-PAGE (4–20% Tris-Glycine, Invitrogen) and stained with Coommassie blue (Fig. 1C, Top), or transferred to nitrocellulose and probed with anti-kappa-HRP (Sigma, 1∶1,000 dilution) (Fig. 1C, Middle) or the complementary biotinylated oligonucleotide (Fig. 1C, Bottom). The latter was then detected with streptavidin-HRP (Sigma, 1∶1,000 dilution). Middle and Bottom blots were developed colorimetrically using the metal enhanced DAB kit (Pierce). Benchmark Prestained Protein Ladder (Invitrogen) was used as the molecular weight marker.
Immuno-PCR on Cancer Cell Lines.
SK-BR-3, MDA-MB-231, and MCF-7 cells [American Type Culture Collection (ATCC)] were grown in DMEM (Invitrogen) supplemented with 10% FBS in a 5% CO2 humidified incubator. Cells were trypsinized and plated on poly-d-lysine coated coverslips and grown for 1–2 d. Cells were washed with 1× PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton-X 100 for 10 min. Cells were washed with 1× PBS and blocked with 1 mg/mL BSA, 50 μg/mL RNase A, 300 ng/mL salmon sperm DNA (Sigma), 5 mM EDTA, and 0.05% Tween 20 in 1× PBS for 1 h at 37 °C (9). During the blocking step, the antibody-oligonucleotide conjugate (2 μg/mL) was added to a separate solution of 1 mg/mL BSA, 2.5 mM l-cysteine, 300 ng/mL salmon sperm DNA, 1 mM ATP, 0.05% Tween 20, 250 mM NaCl, 0.05 U/μL T4 ligase (New England Biolabs), and 125 nM circle oligo (5-  -GTT CTG TCA TAT TTC AGT GAA TGC GAG TCC GTC TAA GAG AGT AGT ACA GCA GCC GTC AAG AGT GTC TA -3) in 1× ligation buffer (10 mM Tris-acetate, 10 mM magnesium acetate, 50 mM potassium acetate, pH 7.5) for 1 h at 37 °C. Nucleotides (indicated in bold) anneal to the oligobody to form a circular oligonucleotide, which is the template for RCA. Cells were washed two times with PBST (0.05% Tween 20) and then the antibody-oligonucleotide solution was added to the cells for 40–60 min at 37 °C. The cells were washed three times with PBST and the polymerase solution containing 1 mg/mL BSA, 250 μM dNTPs, 0.05% Tween 20, 0.125 U/μL phi29 polymerase (New England Biolabs) in 1× polymerase buffer [50 mM Tris·HCl, 10 mM MgCl2, 10 mM (NH4)2SO4, pH 7.5] was added for 1.5 h at 37 °C. The cells were washed three times with PBST and the detection solution containing 1 mg/mL BSA, 300 ng/mL salmon sperm DNA, 1× SSC, 0.05% Tween 20, 1 μg/mL Hoechst, and 62.5 nM of the detection oligonucleotide (5- Alexa488-CAG TGA ATG CGA GTC CGT CT -3) was added for 40–60 min at 37 °C. Cells were washed three times with PBST and once with 1× PBS. Glass coverslips were mounted on glass slides and cells were imaged with a confocal microscope (Olympus FluoView 1000) using Velocity software (Perkin Elmer), or with a fluorescent microscope (Nikon Eclipse Ti) using NIS-Elements software. Quantification of fluorescence was by ImageJ [National Institutes of Health (NIH)]. Background signal was subtracted for SK-BR-3 and MDA-MB-231 cells. Error bars represent the standard deviation between the average signal of triplicate frames.
-GTT CTG TCA TAT TTC AGT GAA TGC GAG TCC GTC TAA GAG AGT AGT ACA GCA GCC GTC AAG AGT GTC TA -3) in 1× ligation buffer (10 mM Tris-acetate, 10 mM magnesium acetate, 50 mM potassium acetate, pH 7.5) for 1 h at 37 °C. Nucleotides (indicated in bold) anneal to the oligobody to form a circular oligonucleotide, which is the template for RCA. Cells were washed two times with PBST (0.05% Tween 20) and then the antibody-oligonucleotide solution was added to the cells for 40–60 min at 37 °C. The cells were washed three times with PBST and the polymerase solution containing 1 mg/mL BSA, 250 μM dNTPs, 0.05% Tween 20, 0.125 U/μL phi29 polymerase (New England Biolabs) in 1× polymerase buffer [50 mM Tris·HCl, 10 mM MgCl2, 10 mM (NH4)2SO4, pH 7.5] was added for 1.5 h at 37 °C. The cells were washed three times with PBST and the detection solution containing 1 mg/mL BSA, 300 ng/mL salmon sperm DNA, 1× SSC, 0.05% Tween 20, 1 μg/mL Hoechst, and 62.5 nM of the detection oligonucleotide (5- Alexa488-CAG TGA ATG CGA GTC CGT CT -3) was added for 40–60 min at 37 °C. Cells were washed three times with PBST and once with 1× PBS. Glass coverslips were mounted on glass slides and cells were imaged with a confocal microscope (Olympus FluoView 1000) using Velocity software (Perkin Elmer), or with a fluorescent microscope (Nikon Eclipse Ti) using NIS-Elements software. Quantification of fluorescence was by ImageJ [National Institutes of Health (NIH)]. Background signal was subtracted for SK-BR-3 and MDA-MB-231 cells. Error bars represent the standard deviation between the average signal of triplicate frames.
Immuno-PCR Detection in Blood Samples.
Blood was collected from The Scripps Research Institute Normal Blood Donor Service under Institutional Review Board approved protocols. WBC count was determined with a HemoCue (HemoCue). Red blood cells (RBCs) were lysed (150 mM NH4Cl, 10 mM KHCO3, 130 nM EDTA), pelleted, and the RBCs in the supernatant were aspirated. The remaining WBCs were resuspended in 1× PBS. For cell line spiking experiments, either SK-BR-3, MDA-MB-231, or a mixture of the two, were added to normal blood donor. For mixture experiments, MDA-MB-231 cells were prelabeled with CellTracker Red (Invitrogen) according to the manufacturer’s instructions before spiking into WBCs. Spiked WBCs were then prepared as previously reported (32). In addition to automated identification, WBCs spiked with both SK-BR-3 and MDA-MB-231 (labeled with CellTracker) cells were also imaged with a confocal microscope (Zeiss 710).
Supplementary Material
Acknowledgments.
The authors thank Benjamin Hutchins and Philip Dawson for helpful discussions, Danling Wang for help acquiring microscope images, and Bradley Charette for help in image analysis. This work was supported by National Institutes of Health Grant 1RC1EBO10745; American Cancer Society Grant RSG-09-1601 (to V.V.S.), R01GM062159 (to P.G.S.), and U54CA143906 (to P.K.); and American Chemical Society Medicinal Chemistry Pre-doctoral Fellowship (S.A.K.). This manuscript is number 21379 of The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120682109/-/DCSupplemental.
References
- 1.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 2.Thaxton CS, et al. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc Natl Acad Sci USA. 2009;106:18437–18442. doi: 10.1073/pnas.0904719106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano T, Smith CL, Cantor CR. Immuno-PCR: Very sensitive antigen detection by means of specific antibody-DNA conjugates. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]
- 4.Niemeyer CM, Adler M, Wacker R. Detecting antigens by quantitative immuno-PCR. Nat Protoc. 2007;2:1918–1930. doi: 10.1038/nprot.2007.267. [DOI] [PubMed] [Google Scholar]
- 5.Schweitzer B, et al. Immunoassays with rolling circle DNA amplification: A versatile platform for ultrasensitive antigen detection. Proc Natl Acad Sci USA. 2000;97:10113–10119. doi: 10.1073/pnas.170237197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konry T, Smolina I, Yarmush JM, Irimia D, Yarmush ML. Ultrasensitive detection of low-abundance surface-marker protein using isothermal rolling circle amplification in a microfluidic nanoliter platform. Small. 2011;7:395–400. doi: 10.1002/smll.201001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksson S, et al. Multiplexed protein detection by proximity ligation for cancer biomarker validation. Nat Methods. 2007;4:327–329. doi: 10.1038/nmeth1020. [DOI] [PubMed] [Google Scholar]
- 8.Jarvius M, et al. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol Cell Proteomics. 2007;6:1500–1509. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Soderberg O, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 10.Adler M, Wacker R, Niemeyer CM. A real-time immuno-PCR assay for routine ultrasensitive quantification of proteins. Biochem Biophys Res Commun. 2003;308:240–250. doi: 10.1016/s0006-291x(03)01364-0. [DOI] [PubMed] [Google Scholar]
- 11.Lind K, Kubista M. Development and evaluation of three real-time immuno-PCR assemblages for quantification of PSA. J Immunol Methods. 2005;304:107–116. doi: 10.1016/j.jim.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Niemeyer CM, Adler M, Wacker R. Immuno-PCR: High sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol. 2005;23:208–216. doi: 10.1016/j.tibtech.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Cheng X, Richter M, Greene MI. A sensitive and high-throughput assay to detect low-abundance proteins in serum. Nat Med. 2006;12:473–477. doi: 10.1038/nm1378. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Amphlett G, Blattler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci. 2005;14:2436–2446. doi: 10.1110/ps.051478705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu AM, Senter PD. Arming antibodies: Prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 16.Burbulis I, Yamaguchi K, Gordon A, Carlson R, Brent R. Using protein-DNA chimeras to detect and count small numbers of molecules. Nat Methods. 2005;2:31–37. doi: 10.1038/nmeth729. [DOI] [PubMed] [Google Scholar]
- 17.Takeda S, Tsukiji S, Nagamune T. Site-specific conjugation of oligonucleotides to the C terminus of recombinant protein by expressed protein ligation. Bioorg Med Chem Lett. 2004;14:2407–2410. doi: 10.1016/j.bmcl.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Agaton C, et al. Selective enrichment of monospecific polyclonal antibodies for antibody-based proteomics efforts. J Chromatogr A. 2004;1043:33–40. doi: 10.1016/j.chroma.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lasne F. Double-blotting: A solution to the problem of non-specific binding of secondary antibodies in immunoblotting procedures. J Immunol Methods. 2001;253:125–131. doi: 10.1016/s0022-1759(01)00355-6. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostou VK, et al. Analytic variability in immunohistochemistry biomarker studies. Cancer Epidemiol Biomarkers Prev. 2010;19:982–991. doi: 10.1158/1055-9965.EPI-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordeaux J, et al. Antibody validation. BioTechniques. 2010;48:197–209. doi: 10.2144/000113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozner-Moulis S, Cregger M, Camp RL, Rimm DL. Antibody validation by quantitative analysis of protein expression using expression of Met in breast cancer as a model. Lab Invest. 2007;87:251–260. doi: 10.1038/labinvest.3700515. [DOI] [PubMed] [Google Scholar]
- 23.Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 24.Stott SL, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gullberg M, et al. Cytokine detection by antibody-based proximity ligation. Proc Natl Acad Sci USA. 2004;101:8420–8424. doi: 10.1073/pnas.0400552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchins BM, et al. Site-specific coupling and sterically controlled formation of multimeric antibody fab fragments with unnatural amino acids. J Mol Biol. 2011;406:595–603. doi: 10.1016/j.jmb.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchins BM, et al. Selective formation of covalent protein heterodimers with an unnatural amino acid. Chem Biol. 2011;18:299–303. doi: 10.1016/j.chembiol.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Zhang Z, Brock A, Schultz PG. Addition of the keto functional group to the genetic code of Escherichia coli. Proc Natl Acad Sci USA. 2003;100:56–61. doi: 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirksen A, Hackeng TM, Dawson PE. Nucleophilic catalysis of oxime ligation. Angew Chem Int Ed Engl. 2006;45:7581–7584. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- 30.Yuste L, Montero JC, Esparis-Ogando A, Pandiella A. Activation of ErbB2 by overexpression or by transmembrane neuregulin results in differential signaling and sensitivity to herceptin. Cancer Res. 2005;65:6801–6810. doi: 10.1158/0008-5472.CAN-04-4023. [DOI] [PubMed] [Google Scholar]
- 31.Bailey RC, Kwong GA, Radu CG, Witte ON, Heath JR. DNA-encoded antibody libraries: A unified platform for multiplexed cell sorting and detection of genes and proteins. J Am Chem Soc. 2007;129:1959–1967. doi: 10.1021/ja065930i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krivacic RT, et al. A rare-cell detector for cancer. Proc Natl Acad Sci USA. 2004;101:10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marrinucci D, et al. Case study of the morphologic variation of circulating tumor cells. Hum Pathol. 2007;38:514–519. doi: 10.1016/j.humpath.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Marrinucci D, et al. Cytomorphology of circulating colorectal tumor cells: A small case series. J Oncol. 2010:861341. doi: 10.1155/2010/861341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sommers CL, et al. Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines and in oncogene-transformed mammary epithelial cells. Cancer Res. 1989;49:4258–4263. [PubMed] [Google Scholar]
- 36.Finn RS, et al. Prognostic and predictive value of HER2 extracellular domain in metastatic breast cancer treated with lapatinib and paclitaxel in a randomized phase III study. J Clin Oncol. 2009;27:5552–5558. doi: 10.1200/JCO.2008.21.1763. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi N, et al. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol. 2011 doi: 10.1007/s10147-011-0260-0. 10.1007/s10147-011-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen JD, Knoop A, Ewertz M, Laenkholm AV. ER, HER2, and TOP2A expression in primary tumor, synchronous axillary nodes, and asynchronous metastases in breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1610-3. 10.1007/s10549-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 39.Xiao C, Gong Y, Han EY, Gonzalez-Angulo AM, Sneige N. Stability of HER2-positive status in breast carcinoma: A comparison between primary and paired metastatic tumors with regard to the possible impact of intervening trastuzumab treatment. Ann Oncol. 2011;22:1547–1553. doi: 10.1093/annonc/mdq623. [DOI] [PubMed] [Google Scholar]
- 40.Debbia M, Lambin P. Measurement of anti-D intrinsic affinity with unlabeled antibodies. Transfusion. 2004;44:399–406. doi: 10.1111/j.1537-2995.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 41.Husain M, Bieniarz C. Fc site-specific labeling of immunoglobulins with calf intestinal alkaline phosphatase. Bioconjugate Chem. 1994;5:482–490. doi: 10.1021/bc00029a017. [DOI] [PubMed] [Google Scholar]
- 42.Yin P, Choi HM, Calvert CR, Pierce NA. Programming biomolecular self-assembly pathways. Nature. 2008;451:318–322. doi: 10.1038/nature06451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.