Abstract
The calcium-sensing (CaS) receptor is a G-protein-coupled receptor (GPCR) that is of fundamental importance for extracellular calcium signalling and calcium homeostasis. The CaS receptor detects changes in free, ionized extracellular calcium concentration and initiates pathways that constantly re-adjust levels of circulating calcium. In addition, the CaS receptor is involved in processes such as stem-cell homing and regulation of neuronal-process outgrowth. To perform these functions, the CaS receptor must be appropriately targeted to the plasma membrane so that its large N-terminal calcium-sensing domain is positioned in the extracellular environment to detect dynamic changes in ionic calcium concentration. Here, we provide an overview of the molecular determinants controlling CaS receptor forward traffic and highlight the roles of CaS receptor interactors such as receptor-activity-modifying proteins and subunits of other class C GPCRs in this process.
Introduction to the calcium-sensing receptor: cloning, structure and physiological roles
Calcium is a well-characterised and fundamentally important intracellular messenger [1]. In addition, calcium ions also have essential roles as extracellular messengers, mainly via the binding to surface-expressed calcium-sensing (CaS) receptors [2]. The parathyroid gland orchestrates calcium homeostasis by secreting parathyroid hormone (PTH) in response to variations in extracellular calcium concentration. Systemic calcium is normally at a concentration of ~1.2 mM and a rise or decrease in systemic calcium inhibits or stimulates PTH secretion, respectively [3]. These fluctuations in environmental calcium are detected by calcium-sensing receptors situated at the plasma membrane of parathyroid cells [4]. The CaS receptor was originally cloned by functional screening in an assay in which RNAs from a bovine parathyroid-gland library were expressed in Xenopus oocytes and scrutinised for their capacity to respond to gadolinium, an effective CaS receptor agonist [5]. This strategy led to the isolation of a single clone, named BoPCaR (bovine parathyroid calcium receptor). CaS receptors were subsequently isolated from other species and additional tissues including kidney [6] and brain [7]. For simplicity, here, we use CaS receptor to encompass all these highly related receptor variants.
The CaS receptor is a class C G-protein-coupled receptor (GPCR) closely related to metabotropic γ-aminobutyric acid (GABA)B and glutamate (mGlu) receptors. It contains an extracellular N terminal that includes a venus flytrap domain required for calcium binding [8]. The CaS receptor is a homodimer at the cell surface [9] (Figures 1 and 2), and intermolecular interactions between the monomers are important for receptor function [10]. Intriguingly, calcium is also an important extracellular signal in plants in which it regulates the degree of stomatal closure through an increase in cytosolic free calcium [11]. A calcium-sensing receptor named CAS was cloned from Arabidopsis thaliana [12]. The vegetal CAS exhibits structural features distinct from animal CaS receptors. For example, CAS contains only one transmembrane (TM) domain in contrast to seven TM domains for the CaS receptor. Nevertheless, the fact that receptors sensing extracellular calcium are present in both animal and plant kingdoms underlines the profound importance of extracellular calcium signalling and homeostasis.
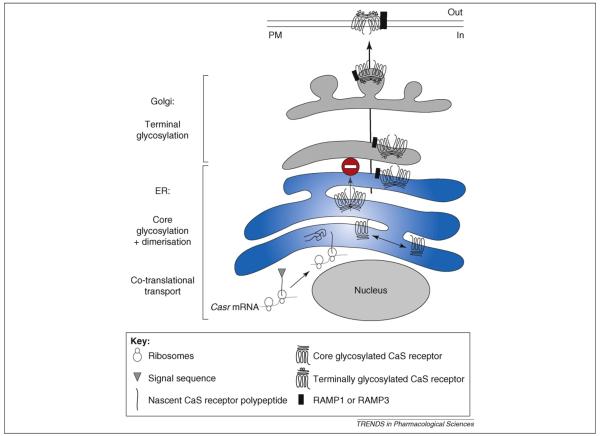
Figure 1.
Model of CaS receptor sorting traffic. During synthesis the polypeptide, together with the ribosome, is targeted to the rough ER due to the presence of the signal sequence (co-translational transport). Upon completion of synthesis, the receptor becomes anchored in the ER membrane in an orientation maintained throughout the secretory pathway. In the ER, CaS receptor monomers assemble as homodimers, which are retained (stop sign) and are core glycosylated. CaS receptor dimers in association with RAMP1 or RAMP3 bypass the ER retention and reach the Golgi apparatus where they are terminally glycosylated before being delivered to the plasma membrane (PM). For clarity, additional proteins modulating CaS receptor traffic such as GABAB1 and GABAB2 are not indicated. The stoichiometry between RAMP and the CaS receptor is not known but, for simplicity, we depict one RAMP per CaS receptor homodimer. Figure adapted and reproduced, with permission of the Company of Biologists, from Ref. [27].
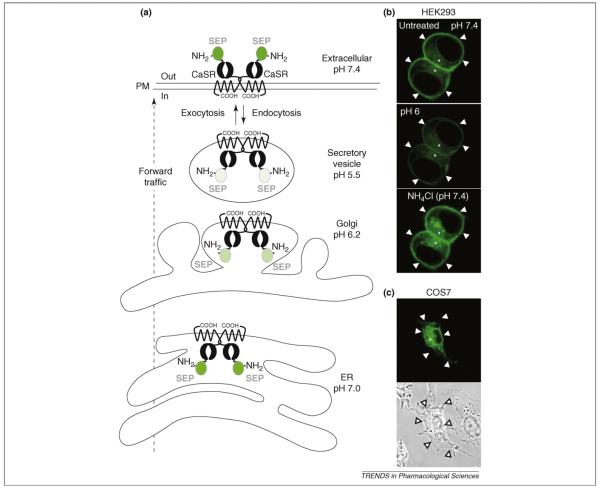
Figure 2.
Super-eclipitic pHluorin-tagged CaS receptor (SEP–CaS-receptor), a tool to track CaS receptor cell-surface expression and traffic. (a) Theoretical levels of SEP–CaS-receptor fluorescence at different pH levels in the compartments of the sorting pathway. Tagging with SEP enables real-time visualization of bulk endocytosis and exocytosis by measuring changes in fluorescence at the plasma membrane (PM). (b) Images obtained during live confocal imaging revealing the distribution of SEP–CaS-receptor fluorescence in two cultured HEK293 cells transfected with SEP–CaS-receptor. Note, the fluorescence is mainly visible at the plasma membrane (indicated by arrowheads) at an extracellular pH 7.4. At pH 6, a decrease in fluorescence is observed as surface SEP–CaS-receptor is eclipsed. By contrast, application of a solution at pH 7.4 containing NH4Cl (50 mM), which rapidly equilibrates intracellular pH levels, causes a sharp increase as all the SEP–CaS-receptor fluorescence in the cell (marked by asterisks) is revealed. (c) SEP–CaS-receptor is absent from the surface of transfected COS7 cells. A transmitted light image shows the location of the plasma membrane. Figure adapted and reproduced, with permission of the Company of Biologists, from Ref. [27]. Abbreviations: CaSR, CaS receptor.
The CaS receptor is not only involved in the control of systemic global calcium [3] but it also participates in cell-type-specific processes that involve local changes or gradients in extracellular calcium, such as stem-cells homing [13] and the outgrowth of neuronal processes in the peripheral and central nervous systems [14]. The CaS receptor can also transduce extracellular signals other than calcium ions. A diverse range of molecules has been shown to modulate CaS receptor activity. For example, the CaS receptor can be activated by magnesium, gadolinium (which was used during the cloning strategy), antibiotics (such as neomycin), several l-amino acids and by the amyloid β-peptide fragment (for reviews, see Refs [15,16]). Of these, sensing of l-amino-acids by the CaS receptor seems to be particularly physiologically relevant [17]. Thus, it seems that the CaS receptor might participate in a much broader array of sensing and effector pathways than initially appreciated.
Pathologies associated with CaS receptor mutations
CaS receptor mutations in humans that either inactivate or activate the receptor have been linked to hypercalcemia and hypocalcemia, respectively [3,16,18,19]. Mutations resulting in a loss of CaS receptor function are associated with familial benign hypercalcemia (FBH) and neonatal severe primary hyperparathyroidism, a lethal disease resulting in skeletal demineralization and multiple fractures [3]. To date, >70 inactivating mutations have been identified, most of them being missense mutations. For an up-to-date and exhaustive list see the CaS receptor database CASRdb (www.casrdb.mcgill.ca). Considering the central role of the receptor on the maintenance of normal calcium levels, there has been substantial effort towards the development of CaS receptor agonists and antagonists for the treatment of disease. Allosteric activators (calcimimetics) increase CaS receptor affinity for calcium and are being developed to inhibit PTH secretion [20,21]. Calcimimetics are currently in clinical use for treating secondary hyperparathyroidism in patients receiving dialysis for renal insufficiency [20,21]. Widely employed calcimimetics include the compounds NPS R-467, NPS R-568 and cinacalcet. Negative allosteric modulators of the CaS receptor (calcilytics) that indirectly stimulate PTH secretion through a decrease in CaS receptor activity are also potentially useful compounds that might provide anabolic therapies for conditions such as osteoporosis [20,21].
Of particular interest in the context of this review are the observations that certain of the loss-of-function CaS receptor mutants display altered forward trafficking [22–26] (Table 1). Consequently, these defective receptors are either not expressed or only poorly expressed at the plasma membrane and, therefore, cannot mediate systemic calcium homeostasis. Importantly, analysis of these mutants has provided increased understanding of some causes of clinical hypercalcemia. In addition, comparison of mutated and wild-type (WT) CaS receptors has shed light on the molecular mechanisms involved in the forward trafficking of the receptor. As discussed in the following sections, it turns out that key determinants of CaS receptor forward trafficking include interaction with binding partners such as receptor-activity-modifying proteins (RAMPs), GABAB receptors or mGlu receptors.
Table 1.
List of CaS receptor mutants impaired in forward trafficking
| CaS receptor mutant | Mutation type | CaS receptor plasma-membrane expression | Affected process | Refs |
|---|---|---|---|---|
| Leu11Ser | Missense | ↓ | Co-translational transport | [23] |
| Leu13Pro | Missense | ↓ | Co-translational transport | [23] |
| Arg66Cys | Missense | ↓ | ER→Golgi transition | [24,25] |
| Arg66His | Missense | ↓ | ER→Golgi transition | [24] |
| Asn583X | Insertion | ↓ | ER→Golgi transition | [24] |
| Gly549Arg | Missense | ↓ | ER→Golgi transition | [26] |
| Cys850^851 | Insertion | ↓ | ER→Golgi transition | [26] |
| Thr876insAlufs-1X911 | Alu insertion | ↓ | ER→Golgi transition | [22] |
Early sorting of the CaS receptor: biosynthesis and homomeric assembly in the endoplasmic reticulum
As shown in Figure 1, the forward trafficking of the CaS receptor is a highly regulated multi-step process. During protein synthesis the polypeptide and ribosome are targeted to the rough endoplasmic reticulum (ER) and the nascent polypeptide enters into this lumen. This co-translational transport requires the presence of the signal peptide sequence at the extreme N terminus of the nascent receptor. On completion of synthesis, the receptor inserts into the membrane of the ER in an orientation that places domains destined to be extracellular within the lumen of the ER and subsequent compartments of the secretory pathway (Figure 1). This orientation exposes the intracellular domains of the receptor for interactions with proteins in the cytoplasmic compartment. The CaS receptor escapes the ER via the formation of cis-Golgi vesicles, reaches the other face of the Golgi (trans face) and, ultimately, is delivered to the plasma membrane (Figure 1).
The signal peptide in the nascent CaS receptor protein comprises a stretch of hydrophobic amino acids. Three mutations have been identified within the human CaS receptor signal peptide (Leu11Ser, Leu13Pro and Thr14Ala) of three FBH patients, two of whom had elevated PTH levels [23] (Table 1). In vitro transcription and translation of the mutant DNAs yielded proteins of the correct molecular weight but, when expressed in HEK293 cells, the Leu11Ser and Leu13Pro mutants generated receptors with defective signalling response to an increase in extracellular calcium and altered plasma-membrane expression [23]. This indicates that co-translational processing, the initial step of the forward-trafficking pathway, is altered in Leu11Ser and Leu13Pro mutant CaS receptors. To test this hypothesis, fragments of RNAs encoding WT, Leu11Ser or Leu13-Pro CaS receptors were in vitro translated. All the constructs generated proteins of the expected size (33 KDa). Inclusion of microsomes (small vesicles of ER obtained by cell disruption) in the cell-free assay resulted in a 16 KDa increase in size of WT protein. The size of the mutants, however, remained unchanged indicating that core glycosylation of the WT CaS receptor that occurs in the ER is prevented in the mutants. Furthermore, the WT CaS receptor was resistant to proteinase K, a proteolytic enzyme that cannot enter the ER lumen. The Leu11Ser and Leu13Pro mutant CaS receptor, by contrast, were both degraded by proteinase K, indicating that they do not enter the ER lumen [23]. This elegant work identified some of the molecular events involved in the biosynthesis and early traffic of the CaS receptor (Figure 1). In particular, the demonstration that signal-peptide mutants of the CaS receptor are incapable of entering the initial step of the forward-trafficking pathway provide a possible mechanistic explanation for the cause of some diseases characterised by deficient calcium sensing and associated signalling [23].
The ER also plays a crucial part in assembly and stability of the CaS receptor homodimer, as demonstrated recently using confocal studies combining fluorescence resonance energy transfer (FRET) and fluorescence recovery after photobleaching (FRAP) [24]. Interestingly, two loss-of-function mutants on the same arginine residue (Arg66Cys and Arg66His) failed to reach the plasma membrane (Table 1) despite the fact that they assemble as dimers in the ER [24]. This indicates that dimerization is not sufficient to drive the exit from the ER and that an additional mechanism might account for the traffic to the Golgi apparatus.
Traffic of the CaS receptor from the ER to the plasma membrane
Surface expression of rat CaS receptor at the plasma membrane has been investigated using the pH-sensitive green-fluorescent-protein variant super ecliptic pHluorin (SEP) [27] (Figure 2a). SEP is non-fluorescent at acidic pH values (≤6.0) and its brightness increases with pH values up to 8.5 [28] (Figure 2a). Vesicular compartments of the forward-trafficking pathway have an acidic lumen, with the exception of the ER [29]. The fluorescence from the CaS receptor tagged at the N terminus with SEP is eclipsed in these compartments because the receptor is anchored in sorting vesicles with the N terminus orientated within the acidic environment of the lumen. Thus, SEP–CaS-receptor is only visible at the cell surface or in the ER, which has a neutral luminal pH of ~7 [28]. Using this construct it was possible to reliably track CaS receptor surface expression in near real time in intact HEK293 cells (Figure 2b). Furthermore, this indicated that this tagging approach could be applied to other GPCRs [27]. Since this original report, similar strategies using the same SEP reporter have been applied to study the mGlu7 receptor [30] and the cannabinoid receptor CB1 [31].
SEP–CaS-receptor trafficking and surface expression differs markedly when expressed in HEK293 cells (Figure 2b) compared with COS7 cells [27] (Figure 2c). Although SEP–CaS-receptor is efficiently expressed in COS7 cells it is not able to exit the ER. Thus, SEP–CaS-receptor displays robust surface localisation in HEK293 cells but is absent from the surface of transfected COS7 cells (Figure 2b–c).
RAMPs are involved in the forward trafficking of several GPCRs [32]. RAMPs form a family of three single TM-domain proteins (RAMP1, RAMP2 and RAMP3). RAMP1 has been shown to be integral in the transport of the calcitonin-receptor-like receptor (CRLR) to the plasma membrane, generating a receptor responding to calcitonin-gene-related peptide (CGRP) [33]. Surprisingly, however, when CRLR is transported by RAMP2 or RAMP3, the receptor selectively responds to adrenomedullin [33]. Using RAMP translocation to the cell surface as a marker of RAMP–receptor interaction, six different class B GPCRs have been identified as RAMP interactors [34], indicating that RAMPs might interact with a wider variety of GPCRs than previously estimated [33].
Intriguingly, it has been reported previously that RAMPs are expressed in some HEK293 cell lines but not in COS7 cells [35]. On further investigation, we determined that COS7 cells lack all RAMPs, whereas our HEK293 cells expressed only RAMP1 [27]. Gain-of-function experiments in which RAMP1, RAMP2 or RAMP3 were coexpressed with the CaS receptor in RAMP-deficient COS7 cells showed that RAMP1 and RAMP3 (but not RAMP2) restored cell-surface targeting of the GPCR (Figure 1). In the converse experiments, RNA-interference-mediated suppression of RAMP1 expression in HEK293 cells impaired forward trafficking of the CaS receptor [27].
Other CaS receptor binding partners affecting its surface expression
In addition to RAMPs, other proteins modulate CaS receptor trafficking. GABAB receptors also belong to the class C GPCRs and assemble as heterodimers of GABAB1–GABAB2 subunits to form functional receptors at the cell surface. Recently, it has been demonstrated that GABAB-receptor subunits can heterodimerize with the CaS receptor to modulate CaS receptor surface expression [36,37] (Table 2). Coexpression of GABAB2 promotes an increase of both CaS receptor surface and total expression in HEK293 cells, whereas GABAB1 exerts the opposite effect (Table 2) and also inhibits the positive-forward traffic effects of the GABAB2 subunit [36]. Consistent with this, CaS receptor surface expression is enhanced in GABAB1-gene knockout cells. Reciprocally, the CaS receptor enhances both GABAB2 and GABAB1 surface expression, possibly by masking an ER retention site on GABAB1. In solubilised brain extracts both GABAB1 and GABAB2 co-immunoprecipitate with the CaS receptor and the proteins show some immunocytochemical colocalisation in neurones [36] and in growth-plate chondrocytes [37]. Thus, heterodimerization of the CaS receptor with GABAB receptors might provide another mechanism to modulate receptor function [36,37].
Table 2.
List of interactors affecting CaS receptor traffica.
| Interactor | Interaction | CaS receptor plasma-membrane expression | Mechanism of action | Biological system | Refs |
|---|---|---|---|---|---|
| RAMP1 | Co-IP | ↑ | ER→Golgi | Heterologous | [27] |
| RAMP3 | Co-IP | ↑ | ER→Golgi | Heterologous | [27] |
| mGlu1 | Co-IP | Unaffected | Unknown | Heterologous, native | [38] |
| mGlu5 | Co-IP | ↑ | Unknown | Heterologous, native | [38] |
| GABAB1 | Co-IP | ↓ | Unknown | Heterologous, native | [36,37] |
| GABAB2 | Co-IP | ↑ | Unknown | Heterologous, native | [36,37] |
| Filamin | Co-IP, Y2H | ↑ | Stabilization | Heterologous | [44] |
| Dorfin | Co-IP, Y2H | ↓ | Degradation | Heterologous | [42] |
Abbreviations: Co-IP, co-immunoprecipitation; Y2H, yeast two-hybrid.
When co-transfected in HEK293 cells, the CaS receptor associates with the group I metabotropic glutamate receptors, mGlu1 and mGlu5, through disulfide-bond interactions (Table 2). Co-expression of mGlu5 in HEK293 cells enhances CaS receptor surface expression, whereas mGlu1 has no marked effect [38] (Table 2). Interestingly, both mGlu1 and GABAB are also sensitive to extracellular calcium concentration [39,40]. Thus, heterodimerization between these receptor types could act to modulate extracellular calcium signalling by fine-tuning CaS-receptor-dependent sensitivity and/or responses. For example, when transfected together with mGlu1, the CaS receptor then internalises in response to glutamate [38]. Although this has not yet been shown to occur in a native system, such a mechanism could enable the extracellular levels of glutamate to adjust the number of surface receptors available to sense extracellular calcium, for example in conditions of intense synaptic activity or ischaemia. Another possibility is that the CaS receptor heterodimerizes with different GPCR partners to facilitate targeting to specific subcellular locations. For example, the CaS receptor is crucial for neuronal and dendrite outgrowth [14], but the mechanisms by which the receptor is targeted to axons and dendrites remain enigmatic. It is, therefore, possible that the CaS receptor could use its association with mGlu5 to be targeted to synapses via the Homer1–mGlu5 interaction [41]. Consistent with this, Homer1c has been shown to increase the plasma-membrane localisation of the CaS receptor when co-expressed with group I mGlu receptors [38].
Perspectives on CaS receptor traffic
The most important currently known mechanisms involved in CaS receptor forward traffic are summarized in Figure 1. Two main checkpoints have been identified that must be passed during the sorting of the receptor. First, co-translational transport requires the integrity of the signal peptide. Second, sorting from the ER involves the binding of proteins acting as chaperones such as RAMPs or the GABAB2 receptor. There are undoubtedly additional quality checkpoints that control CaS receptor anterograde transport. For example, the CaS receptor C terminus interacts with the E3 ubiquitin ligase Dorfin [42] (Table 2). Dorfin triggers ubiquitination and degradation of immature, non-fully glycosylated CaS receptors via the ER-associated degradation pathway when the receptor and the enzyme are co-expressed in HEK293 cells [42]. This degradation response probably acts to prevent the presence of non-functional CaS receptors at the plasma membrane and might be triggered by misfolded receptors accumulating in the ER. Interestingly, this degradation process occurs in physiological conditions in cells that endogenously express the CaS receptor such as Madin-Darby canine kidney cells.
The traffic of some loss-of-function CaS receptor mutants that are ordinarily degraded and, thus, less abundant at the plasma membrane can be rescued by overnight treatment with the calcimimetic NPS-R568 [43]. NPS-R568 might produce a conformational change that rescues the misfolded receptor. This pharmacological chaperone-like property might provide a fruitful avenue of investigation for potential therapeutic interventions. The degradation process is finely balanced by protective mechanisms such as stabilization of the CaS receptor by the scaffolding molecule filamin [44] (Table 2). A similar mechanism might account for GABAB2 action on the CaS receptor total expression [36].
Once the homodimeric CaS receptor is correctly expressed at the plasma membrane, it responds to extracellular stimuli, primarily extracellular calcium. As with other GPCRs, a complex process of desensitization is set in train thereby contributing to the control of the extent and duration of the intracellular signalling response. It is established that CaS receptor desensitization involves phosphorylation by G-protein-receptor kinase and the binding of β-arrestin [45,46]. β-arrestin is also required for CaS-receptor-induced plasma-membrane ruffling [47], indicating that extracellular calcium desensitization and signalling to the cytoskeleton are intimately linked. Desensitization does not seem to involve internalisation, at least in the short term, because little CaS receptor internalisation is detected in response to agonist treatment [46]. However, the CaS receptor might redistribute along the plasma membrane to selected microdomains such as caveolin-rich domains [48] or plasma-membrane ruffles [47]. Redistribution of the CaS receptor to plasma-membrane ruffles at the leading edge of the migrating cell might provide a mechanism for sensing extracellular ionic environment to orientate the migrating cell accordingly [47].
Conclusion
Despite recent progress, important questions remain about the mechanics, activity dependence and pathways of CaS receptor trafficking. For example, it seems that RAMPs act to enable forward trafficking of SEP–CaS-receptor from the ER to the Golgi and, in that way, facilitate mature glycosylation of SEP–CaS-receptor exogenously expressed in clonal cell lines (Table 2). However, it remains to be determined if RAMPs are involved in the traffic of endogenously expressed CaS receptors, and possibly other class C GPCRs, in functionally relevant cells. The CaS receptor and RAMP1 or RAMP3 are co-expressed in the brain and kidney [33]. However, the expression of RAMPs in other cell types in which the CaS receptor has a physiological role, such as stem or parathyroid cells, remains to be determined. The roles of RAMPs in the targeting and asymmetrical localisation of the CaS receptor in polarised cells [49] also remain undefined. RAMPs might also influence the pharmacological properties of the receptors. Given that the CaS receptors respond to multiple agonists including spermine or amino acids [16], RAMPs could potentially alter the ligand potency and/or selectivity analogous to their roles in CRLR pharmacology. At a more fundamental level, the sites of interaction between RAMPs and the CaS receptor have not been established. Thus, future elucidation of the roles of RAMPs in CaS receptor signalling, pharmacology and trafficking will provide insight into the mechanisms regulating CaS receptor function and, therefore, could lead to the development of therapeutic strategies to manipulate surface functional expression of the CaS receptor in diseased cells.
Similarly, the observations that the CaS receptor can heterodimerize with other GPCR subunits (Table 2) raise many questions about the potential interchange of subunits from different GPCRs that would enable subtle modulation of the expression and function of the heteromeric receptors. If widespread, this ‘pick-and-mix’ mechanism would provide a potentially huge number of receptor combinations, especially when subunit splice isoforms are included. It is conceivable that subtle but functionally important variations in receptor composition dictate with very high specificity the precise cellular localisation and pharmacological properties of receptor subpopulations. This would enable individual cells or cell compartments to contain unique receptors that are determined by the environment and are exquisitely fine-tuned for their specific role. Furthermore, the exact nature of the receptors might change in response to changed intracellular or extracellular conditions.
Acknowledgements
We thank the Wellcome Trust (www.wellcome.ac.uk), Biotechnology and Biological Sciences Research Council (www.bbsrc.ac.uk) and the Medical Research Council (www.mrc.ac.uk) for financial support.
References
- 1.Berridge MJ, et al. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Hofer AM. Another dimension to calcium signaling: a look at extracellular calcium. J. Cell Sci. 2005;118:855–862. doi: 10.1242/jcs.01705. [DOI] [PubMed] [Google Scholar]
- 3.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth EF, Scarpa A. Rapid mobilization of cellular Ca2+ in bovine parathyroid cells evoked by extracellular divalent cations. Evidence for a cell surface calcium receptor. J. Biol. Chem. 1987;262:5188–5196. [PubMed] [Google Scholar]
- 5.Brown EM, et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 6.Riccardi D, et al. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc. Natl. Acad. Sci. U. S. A. 1995;92:131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruat M, et al. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brauner-Osborne H, et al. The agonist-binding domain of the calcium-sensing receptor is located at the amino-terminal domain. J. Biol. Chem. 1999;274:18382–18386. doi: 10.1074/jbc.274.26.18382. [DOI] [PubMed] [Google Scholar]
- 9.Bai M, et al. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 10.Bai M, et al. Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2834–2839. doi: 10.1073/pnas.96.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAinsh MR, et al. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han S, et al. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature. 2003;425:196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- 13.Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 14.Vizard TN, et al. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat. Neurosci. 2008;11:285–291. doi: 10.1038/nn2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouschet T, Henley JM. Calcium as an extracellular signalling molecule: perspectives on the Calcium Sensing Receptor in the brain. C. R. Biol. 2005;328:691–700. doi: 10.1016/j.crvi.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 17.Conigrave AD, et al. Physiological significance of l-amino acid sensing by extracellular Ca2+-sensing receptors. Biochem. Soc. Trans. 2007;35:1195–1198. doi: 10.1042/BST0351195. [DOI] [PubMed] [Google Scholar]
- 18.Pollak MR, et al. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 19.Pollak MR, et al. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat. Genet. 1994;8:303–307. doi: 10.1038/ng1194-303. [DOI] [PubMed] [Google Scholar]
- 20.Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell. Biochem. 2007;45:139–167. doi: 10.1007/978-1-4020-6191-2_6. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth EF. Calcimimetic and calcilytic drugs: just for parathyroid cells? Cell Calcium. 2004;35:283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Bai M, et al. Markedly reduced activity of mutant calcium-sensing receptor with an inserted Alu element from a kindred with familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. J. Clin. Invest. 1997;99:1917–1925. doi: 10.1172/JCI119359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pidasheva S, et al. Impaired cotranslational processing of the calcium-sensing receptor due to signal peptide missense mutations in familial hypocalciuric hypercalcemia. Hum. Mol. Genet. 2005;14:1679–1690. doi: 10.1093/hmg/ddi176. [DOI] [PubMed] [Google Scholar]
- 24.Pidasheva S, et al. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum. Mol. Genet. 2006;15:2200–2209. doi: 10.1093/hmg/ddl145. [DOI] [PubMed] [Google Scholar]
- 25.Bai M, et al. Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J. Biol. Chem. 1996;271:19537–19545. doi: 10.1074/jbc.271.32.19537. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza-Li L, et al. Identification and functional characterization of novel calcium-sensing receptor mutations in familial hypocalciuric hypercalcemia and autosomal dominant hypocalcemia. J. Clin. Endocrinol. Metab. 2002;87:1309–1318. doi: 10.1210/jcem.87.3.8280. [DOI] [PubMed] [Google Scholar]
- 27.Bouschet T, et al. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J. Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashby MC, et al. It’s green outside: tracking cell surface proteins with pH-sensitive GFP. Trends Neurosci. 2004;27:257–261. doi: 10.1016/j.tins.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Mellman I, et al. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 30.Pelkey KA, et al. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology. 2007;52:108–117. doi: 10.1016/j.neuropharm.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 31.McDonald NA, et al. Generation and functional characterization of fluorescent, N-terminally tagged CB1 receptor chimeras for live-cell imaging. Mol. Cell. Neurosci. 2007;35:237–248. doi: 10.1016/j.mcn.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Hay DL, et al. GPCR modulation by RAMPs. Pharmacol. Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 33.McLatchie LM, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 34.Christopoulos A, et al. Novel receptor partners and function of receptor activity-modifying proteins. J. Biol. Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- 35.Buhlmann N, et al. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- 36.Chang W, et al. Complex formation with the Type B γ-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J. Biol. Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Z, et al. Type B γ-aminobutyric acid receptors modulate the function of the extracellular Ca2+-sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148:4984–4992. doi: 10.1210/en.2007-0653. [DOI] [PubMed] [Google Scholar]
- 38.Gama L, et al. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J. Biol. Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 39.Kubo Y, et al. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- 40.Wise A, et al. Calcium sensing properties of the GABAB receptor. Neuropharmacology. 1999;38:1647–1656. doi: 10.1016/s0028-3908(99)00119-7. [DOI] [PubMed] [Google Scholar]
- 41.Ango F, et al. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J. Neurosci. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, et al. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J. Biol. Chem. 2006;281:11610–11617. doi: 10.1074/jbc.M513552200. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J. Biol. Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Breitwieser GE. High affinity interaction with filamin A protects against calcium-sensing receptor degradation. J. Biol. Chem. 2005;280:11140–11146. doi: 10.1074/jbc.M412242200. [DOI] [PubMed] [Google Scholar]
- 45.Pi M, et al. β-arrestin- and G protein receptor kinase-mediated calcium-sensingreceptordesensitization. Mol.Endocrinol. 2005;19:1078–1087. doi: 10.1210/me.2004-0450. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz S, et al. Functional desensitization of the extracellular calcium-sensing receptor is regulated via distinct mechanisms: role of G protein-coupled receptor kinases, protein kinase C and β-arrestins. Endocrinology. 2007;148:2398–2404. doi: 10.1210/en.2006-1035. [DOI] [PubMed] [Google Scholar]
- 47.Bouschet T, et al. The calcium-sensing receptor changes cell shape via a β-arrestin-1 ARNO ARF6 ELMO protein network. J. Cell Sci. 2007;120:2489–2497. doi: 10.1242/jcs.03469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kifor O, et al. m-Calpain colocalizes with the calcium-sensing receptor (CaR) in caveolae in parathyroid cells and participates in degradation of the CaR. J. Biol. Chem. 2003;278:31167–31176. doi: 10.1074/jbc.M303377200. [DOI] [PubMed] [Google Scholar]
- 49.Caroppo R, et al. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia. EMBO J. 2001;20:6316–6326. doi: 10.1093/emboj/20.22.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]