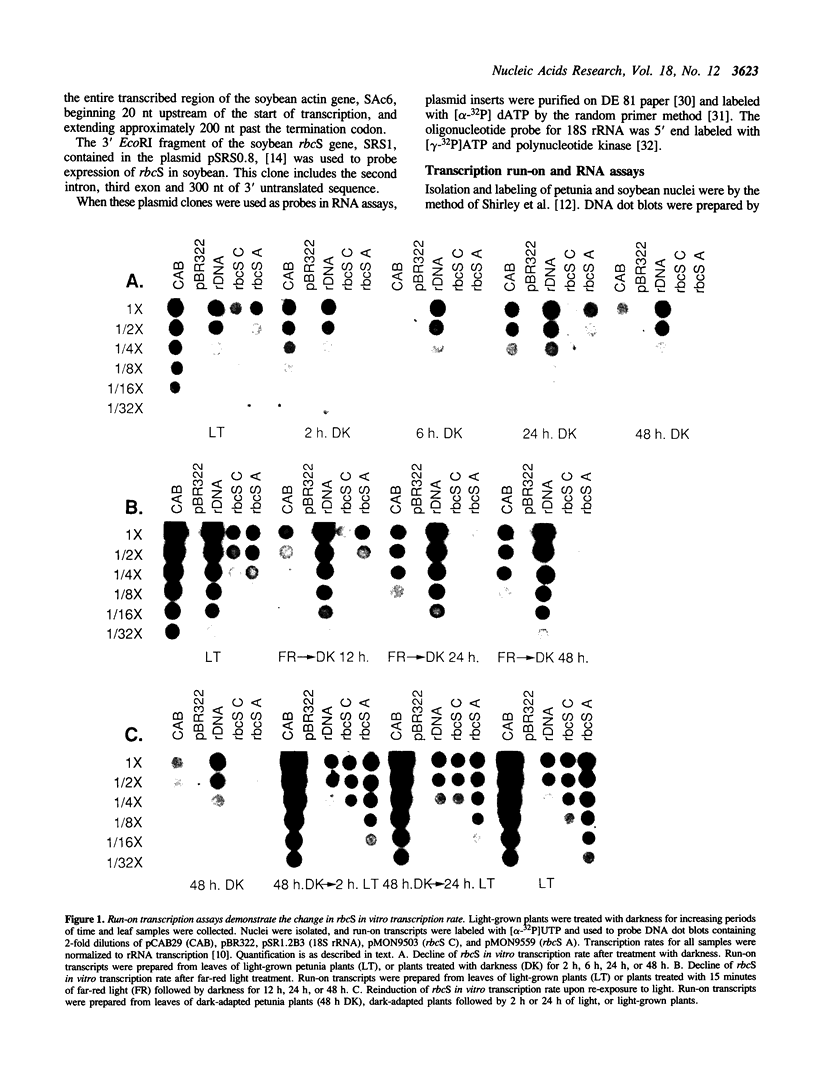
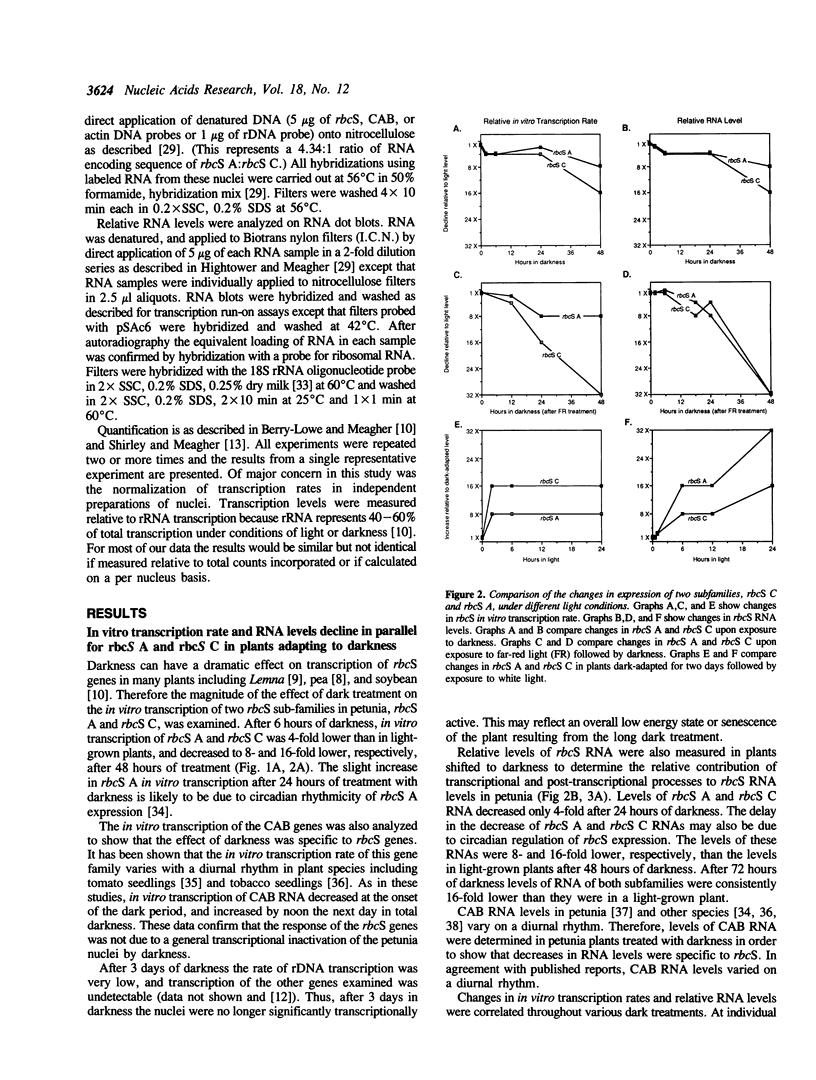
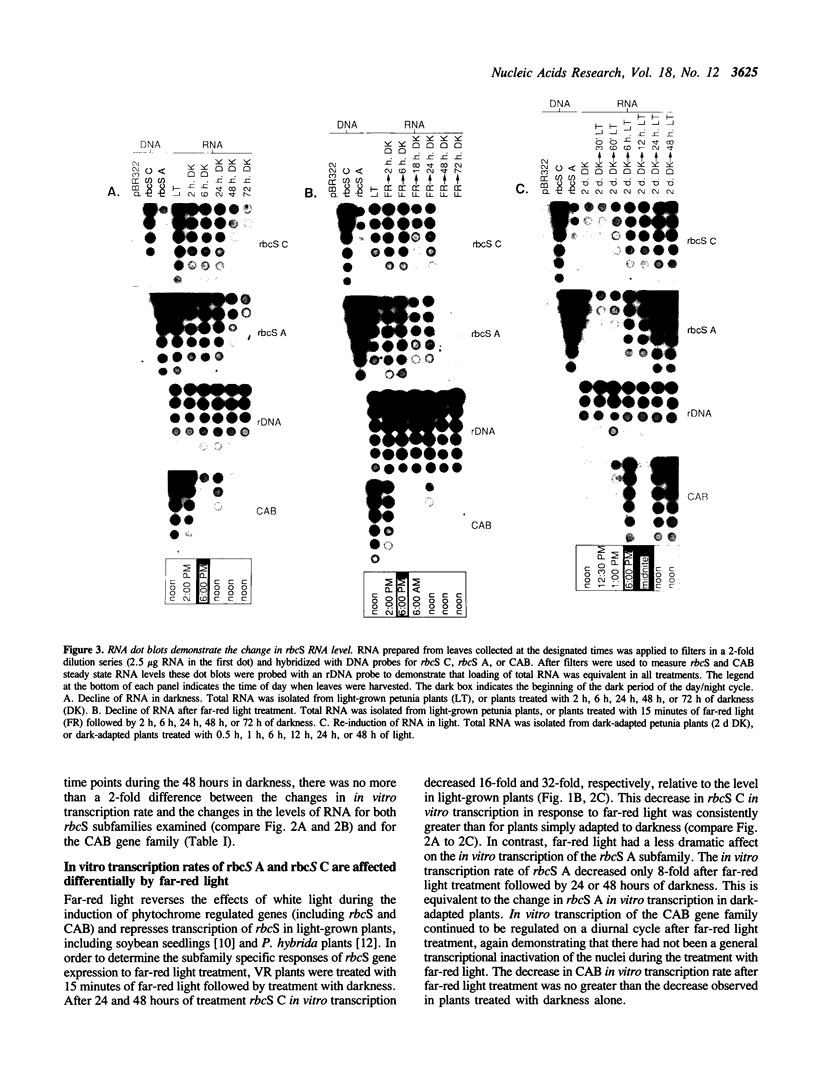
Abstract
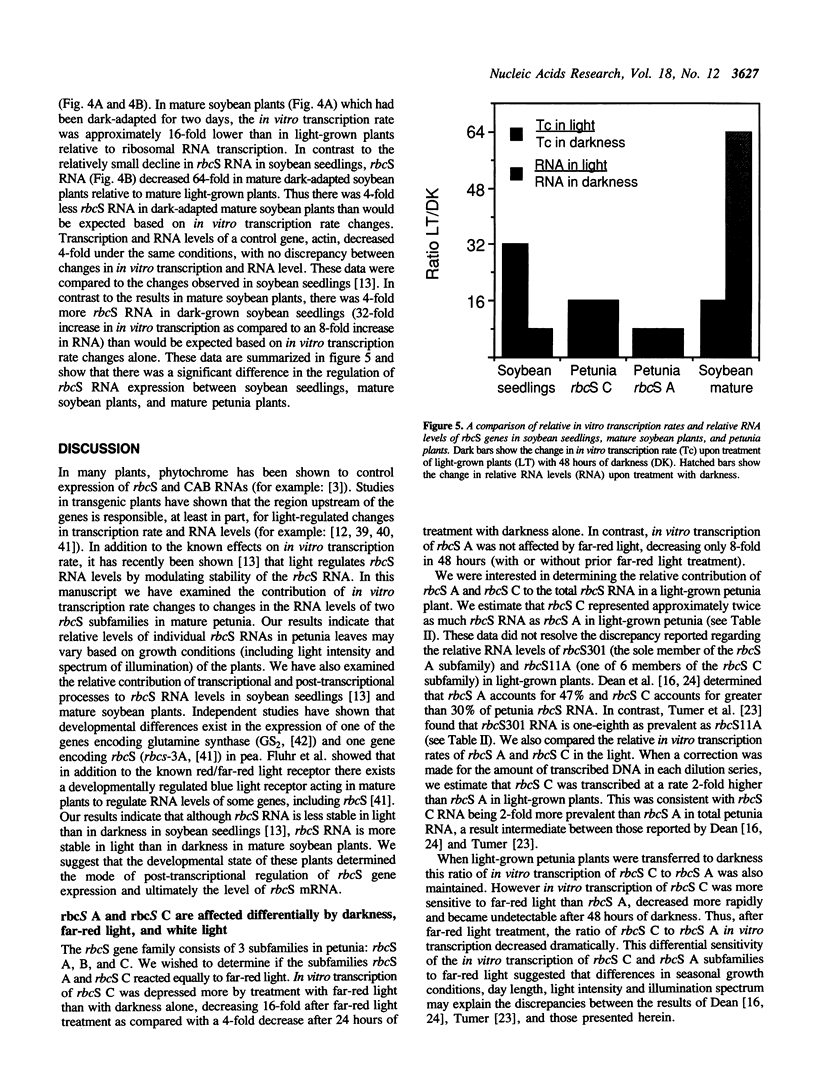
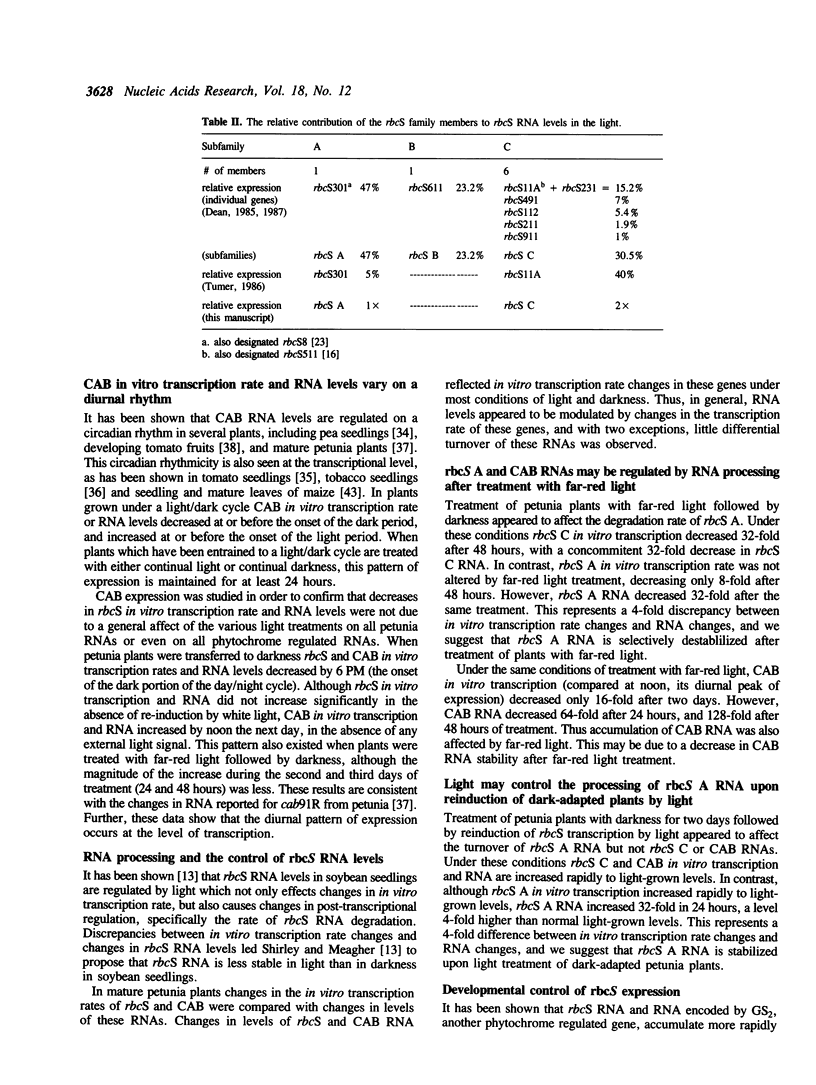
The effects of white light, far-red light and darkness on the in vitro transcription and RNA levels of the small subunit of ribulose-1,5-bisphosphate carboxylase (rbcS) were investigated in petunia and in soybean. In petunia plants treated with 48 hours of darkness the in vitro transcription rate of two of the rbcS subfamilies of petunia, rbcS A and rbcS C, declined 32- and 8-fold respectively, whereas treatment of dark-adapted plants with light caused the in vitro transcription rate of these subfamilies to return to their light-grown levels. Relative RNA levels of rbcS A and rbcS C declined in parallel with in vitro transcription rate changes upon treatment of petunia plants with darkness. However, while relative RNA levels of rbcS C changed in parallel with in vitro transcription rate under all conditions of far-red light and white light tested, there were differences between the changes in rbcS A in vitro transcription rate and RNA levels which were consistent with post-transcriptional regulation of rbcS A RNA. In addition we observed that nuclei isolated from the leaves of plants which were exposed to darkness for periods of 72 hours or longer were transcriptionally inactive. Similar experiments on the in vitro transcription and relative levels of the rbcS RNA in soybean seedlings have lead to the hypothesis that rbcS RNA is less stable in light than in darkness. In contrast, small decreases in rbcS in vitro transcription rate in mature soybean plants treated with darkness were accompanied by large decreases in rbcS RNA, suggesting that rbcS RNA was degraded more rapidly in darkness than in light in these plants. We have shown that differences in the modulation of rbcS RNA levels by post-transcriptional mechanisms exist between plants which belong to different orders, and between different developmental states of the same plant species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Berry-Lowe S. L., Meagher R. B. Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol Cell Biol. 1985 Aug;5(8):1910–1917. doi: 10.1128/mcb.5.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Favreau M., Dunsmuir P., Bedbrook J. Confirmation of the relative expression levels of the Petunia (Mitchell) rbcS genes. Nucleic Acids Res. 1987 Jun 11;15(11):4655–4668. doi: 10.1093/nar/15.11.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., van den Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Linkage and homology analysis divides the eight genes for the small subunit of petunia ribulose 1,5-bisphosphate carboxylase into three gene families. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4964–4968. doi: 10.1073/pnas.82.15.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Dunsmuir P., Smith S. M., Bedbrook J. The major chlorophyll a/b binding protein of petunia is composed of several polypeptides encoded by a number of distinct nuclear genes. J Mol Appl Genet. 1983;2(3):285–300. [PubMed] [Google Scholar]
- Eckenrode V. K., Arnold J., Meagher R. B. Comparison of the nucleotide sequence of soybean 18S rRNA with the sequences of other small-subunit rRNAs. J Mol Evol. 1984;21(3):259–269. doi: 10.1007/BF02102358. [DOI] [PubMed] [Google Scholar]
- Edwards J. W., Coruzzi G. M. Photorespiration and light act in concert to regulate the expression of the nuclear gene for chloroplast glutamine synthetase. Plant Cell. 1989 Feb;1(2):241–248. doi: 10.1105/tpc.1.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Fluhr Robert, Moses Phyllis, Morelli Giorgio, Coruzzi Gloria, Chua Nam-Hai. Expression dynamics of the pea rbcS multigene family and organ distribution of the transcripts. EMBO J. 1986 Sep;5(9):2063–2071. doi: 10.1002/j.1460-2075.1986.tb04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Hoffman N. E., Ko K., Scolnik P. A., Cashmore A. R. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988 Dec 1;7(12):3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Berry-Lowe S., Rice K. Molecular evolution of the small subunit of ribulose bisphosphate carboxylase: nucleotide substitution and gene conversion. Genetics. 1989 Dec;123(4):845–863. doi: 10.1093/genetics/123.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Nagy F., Morelli G., Fraley R. T., Rogers S. G., Chua N. H. Photoregulated expression of a pea rbcS gene in leaves of transgenic plants. EMBO J. 1985 Dec 1;4(12):3063–3068. doi: 10.1002/j.1460-2075.1985.tb04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen H., Bogorad L. Diurnal and Circadian Rhythms in the Accumulation and Synthesis of mRNA for the Light-Harvesting Chlorophyll a/b-Binding Protein in Tobacco. Plant Physiol. 1988 Dec;88(4):1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Cashmore A. R. Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3880–3884. doi: 10.1073/pnas.83.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B., Gruissem W. Diurnal mRNA fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J. 1987 Dec 1;6(12):3593–3599. doi: 10.1002/j.1460-2075.1987.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley B. W., Berry-Lowe S. L., Rogers S. G., Flick J. S., Horsch R., Fraley R. T., Meagher R. B. 5' proximal sequences of a soybean ribulose-1,5-bisphosphate carboxylase small subunit gene direct light and phytochrome controlled transcription. Nucleic Acids Res. 1987 Aug 25;15(16):6501–6514. doi: 10.1093/nar/15.16.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Wessler S., Fedoroff N. Molecular identification and isolation of the Waxy locus in maize. Cell. 1983 Nov;35(1):225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayton M. M., Brosio P., Dunsmuir P. Photosynthetic Genes of Petunia (Mitchell) Are Differentially Expressed during the Diurnal Cycle. Plant Physiol. 1989 Mar;89(3):776–782. doi: 10.1104/pp.89.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Silverthorne J., Tobin E. M. Phytochrome Control of the Expression of Two Nuclear Genes Encoding Chloroplast Proteins in Lemna gibba L. G-3. Plant Physiol. 1983 Jul;72(3):717–724. doi: 10.1104/pp.72.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Manzara T., Pichersky E., Cashmore A., Gruissem W. Genomic organization, sequence analysis and expression of all five genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from tomato. Mol Gen Genet. 1987 Sep;209(2):247–256. doi: 10.1007/BF00329650. [DOI] [PubMed] [Google Scholar]
- Taylor W. C. Transcriptional regulation by a circadian rhythm. Plant Cell. 1989 Feb;1(2):259–264. doi: 10.1105/tpc.1.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]