Abstract
Background
Mixed donor-host chimerism, established through hematopoietic cell transplantation (HCT), is a highly reproducible strategy for the induction of tolerance towards solid organs. Here, we ask whether a nonmyeloablative conditioning regimen establishing mixed donor-host chimerism leads to tolerance of highly antigenic vascularized composite allografts.
Methods
Stable mixed chimerism was established in dogs given a sublethal dose (1–2 Gy) total body irradiation before and a short course of immunosuppression after dog leukocyte antigen-identical marrow transplantation. Vascularized composite allografts from marrow donors were performed after a median of 36 (range 4-54) months after HCT.
Results
All marrow recipients maintained mixed donor-host hematopoietic chimerism and accepted composite tissue grafts for periods ranging between 52 and 90 weeks; in turn, marrow donors rejected vascularized composite allografts from their respective marrow recipients within 18–29 days. Biopsies of muscle and skin of vascularized composite allografts from mixed chimeras showed few infiltrating cells compared to extensive infiltrates in biopsies of vascularized composite allografts from marrow donors. Elevated levels of CD3+ FoxP3+ T-regulatory cells were found in skin and muscle of vascularized composite allografts of mixed chimeras compared to normal tissues. In mixed chimeras, increased numbers of T-regulatory cells were found in draining compared to non-draining lymph nodes of vascularized composite allografts.
Conclusion
These data suggest that nonmyeloablative HCT may form the basis for future clinical applications of solid organ transplantation and that T-regulatory cells may function towards maintenance of the vascularized composite allograft.
Keywords: dog, nonmyeloablative conditioning regimen, mixed hematopoietic chimerism, skin grafting, hematopoietic cell transplantation, CTA transplantation, FoxP3, tolerance
INTRODUCTION
A primary goal in the field of transplantation has been the establishment of immunological tolerance in the patient towards donor-specific tissue antigens. Immunological tolerance would eliminate the need for life-long immunosuppressive therapy required to prevent rejection of the organ graft and eliminate the risks of morbidity, chronic tissue graft rejection, renal toxicity, and malignancy (1,2). Hematopoietic or mixed donor-host chimerism reasserts the immunologic repertoire by encompassing tolerance towards donor antigens and allowing transplantation of solid organs without immunosuppression (3).
Several small animal studies have shown that tolerance towards transplanted solid organs such as kidney (4), heart (5) and pancreas (6) can be achieved as a result of mixed chimerism after hematopoietic cell transplantation (HCT). Using mixed chimerism to induce tolerance to solid organs and vascularized composite allografts in larger animal models has shown signs of success; however, tolerance to the skin has been a major obstacle (7-9). The most common outcome of these experiments, even across minor genetic barriers, has been the establishment of split tolerance where tolerance to the transplanted muscle and bone was established while the skin component was rejected (10,11). While successful transplantation of all components of the VCA have been reported in large animals, they have largely been either cases of a single tolerant animal or significant prolongation observed with the addition of donor mesenchymal stem cells to the regimen (12-14). Presumably, the antigenicity of skin renders it a difficult tissue to transplant under conditions of both immunosuppression (15) and established mixed chimerism (9,16). For any tolerance protocol to be clinically applicable in the emerging field of vascularized composite allotransplantation, tolerance must be established to all components of the allograft including skin (10,11).
We previously reported consistent and sustained hematopoietic cell engraftment in dogs given a nonmyeloablative dose (1-2 Gy) of total body irradiation (TBI) before, and immunosuppression consisting of mycophenolate mofetil (MMF) and cyclosporine (CSP) for 28 and 35 days, respectively, after dog leukocyte antigen (DLA)-identical HCT (17,18). We have also demonstrated that, after establishing mixed chimerism, canine marrow recipients accepted kidney allografts long-term from their marrow donors without the need for immunosuppression (19). However, tolerance was not generally extended to marrow donor skin grafts as rejection occurred in half of the mixed chimeric dogs despite ongoing tolerance to their kidney allografts (20). The intestine, arguably a more antigenic organ to transplant than the kidney, was also successfully transplanted heterotopically into the neck region of four mixed chimeric dogs (21).
A vascularized composite allograft is composed of skin, muscle, fat, nerves, lymphatic and blood vessels. Following nonmyeloablative HCT, vascularized composite allografts allow for the evaluation of tolerance towards different tissues, which ostensibly express tissue-specific antigens. Furthermore, such grafts represent the best transplantation model for complex tissues such as the hand or any reconstructive surgery involving skin and muscle.
Here, we test the hypothesis that mixed chimerism can induce a state of tolerance towards a vascularized composite allograft in the DLA-identical canine model. We show that the well-tolerated nonmyeloablative conditioning regimen of 1–2 Gy TBI, preceding marrow transplantation and followed by a short course of postgrafting immunosuppression, is sufficient for stable engraftment towards all components of a vascularized composite allograft. Furthermore, engraftment may be influenced by the presence of infiltrating lymphocytes of the T-regulatory phenotype.
RESULTS
Stable Mixed Donor/Host Hematopoietic Chimerism
Table 1 summarizes the conditioning regimen, postgrafting immunosuppression, percentages of donor chimerism in recipient dogs, and engraftment outcome. Donor granulocyte chimerism ranged from 20–98% and lymphocytes chimerism from 40–100%. Between 5.5 months and >4 years after HCT, composite tissue grafts were transplanted from marrow donors to their respective mixed chimeric recipients. Two dogs, G244 and G969, showed an increase in chimerism for granulocytes and lymphocytes from mixed to 98% and 100% donor, respectively. The remaining three recipients showed slight reductions in donor chimerism for both of these cell populations (Table 1 and Supplemental Figure 1).
Table 1.
Donor Chimerism after Marrow and Subsequent Composite Tissue Grafts from DLA-Identical Littermates
| Recipient # | Date of Marrow Graft | TBI Gy | Donor Marrow Cells × 108 / kg | Post-Marrow Grafting Immuno-suppression |
Date of Composite Tissue Allograft | % Donor Chimerism1 (weeks)2 | Post- Transplant Immuno-suppression |
Graft Rejection |
Weeks of follow-up3 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||||
| G767 | 4/26/07 | 2 | 4.3 | MMF/CSP | 10/15/07 | G62.3 (0) L37.5 (0) |
G7.4 (35) L25.8 (35) |
No | No | >90 |
| G244 | 5/16/03 | 1 | 9.2 | MMF/CSP | 10/29/07 | G62.5 (-1) L49.4 (-1) |
G100 (31) L100 (31) |
No | No | >77 |
| G500 | 12/22/04 | 2 | 4.8 | MMF/Rapa | 12/18/07 | G50.0 (-3) L66.0 (-3) |
G36.1 (26) L42.4 (26) |
No | No | >52 |
| G969 | 12/13/07 | 2 | 4.1 | MMF/CSP | 4/14/08 | G94.6 (-4) L49.5 (-4) |
G100 (53) L100 (53) |
No | No | >52 |
| G949 | 1/4/08 | 1 | 2.8 | MMF/CSP | 10/27/08 | G69.5 (0) L48.4 (0) |
G38.3 (52) L45.3 (52) |
No | No | >52 |
Data are presented as percent donor granulocytes (G) and lymphocytes (L).
Chimerism analyzed in weeks before or after composite tissue graft, week = 0.
Weeks of follow-up after composite tissue graft, week = 0.
G767 and G969 received 2 Gy TBI (day = 0) with postgrafting immunosuppression of MMF (10 mg/kg B.I.D. subq., days 0-27) and CSP (15 mg/kg B.I.D. orally , days -1-35) and received HCT on a protocol specifically for this study.
The following dogs received HCT under separate studies and were later transferred to the present study as mixed chimeras. G244 received 1 Gy TBI with postgrafting immunosuppression of MMF (10 mg/kg B.I.D subq., days 0-27) CSP (15 mg/kg B.I.D. orally, days -1-35) and methotrexate (0.4 mg/kg, I.V. days 1, 3, 6, 11).
G500 received 2 Gy TBI with postgrafting immunosuppression of MMF (10 mg/kg B.I.D subq., days 0-34) and sirolimus (0.05 mg/kg, daily, subq, day -5 to 25).
G949 received 1 Gy TBI on day = 0, recombinant canine CTLA4-Ig (4.0 mg/kg I.V. days -7 and -5) donor PBMC (1 × 106 cells/kg on days -7 and -5 injected subq and I.V.) and post grafting immunosuppression consisting of MMF (10 mg/kg B.I.D. subq., days 0-27) and CSP (15 mg/kg B.I.D. orally , days -1-35).
Vascularized Composite Allotransplantation in Marrow Donors and Stable Mixed Chimeric Recipients
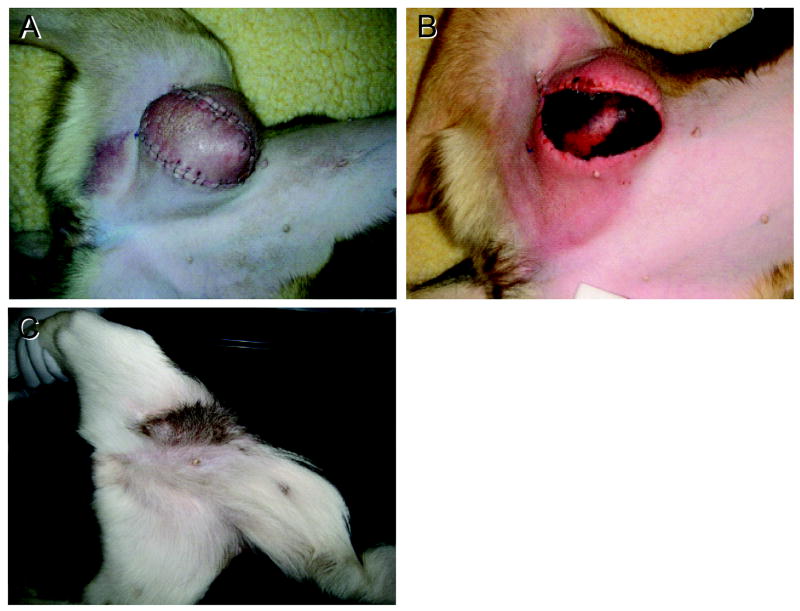
Five mixed chimeric recipients and four marrow donors received vascularized composite allografts from their respective DLA-identical littermates. All nine transplants were initially accepted and were well-healed within 2 weeks. All four non-chimeric donors rejected their composite tissue grafts within 18–29 (median 21) days. Rejection was indicated clinically by erythema and progressive edema, followed by necrosis of skin and muscle (Figure 1A and B). In contrast, the five mixed chimeras accepted the composite tissue grafts from their marrow donors with observation periods ranging from 52-90 (median 52) weeks, at which point composite tissue allografts were considered permanent (Table 1). Acceptance was indicated clinically by normal skin color and hair growth (Figure 1C). The dogs’ peripheral blood counts and blood chemistries showed no abnormalities (data not shown). Histologically, representative biopsies of skin and muscle composite tissue grafts obtained from two of the marrow donors at time of rejection revealed lymphocytic infiltrate (Banff Grade IV and Grade 3R, respectively) in both tissues (Figure 2A). Serial biopsies of skin and muscle grafts of the mixed chimeric recipients at 118–167 days after vascularized composite allograft transplantation showed minimal evidence of cellular infiltrate (Banff Grade 0 and Grade 0R, respectively) and no evidence of vascular disease, or epidermal architecture damage (Figure 2B).
Figure 1. Rejection and acceptance of vascualrized composite allografts in dogs.
Rejection of a vascularized composite allograft in a marrow donor dog (G499), showing early (day 18, A) and late (day 21, B) signs of rejection, with edema, erythema, and skin necrosis. In contrast, the graft in the mixed chimeric dog (G500) shown here on day 346 with normal hair growth (brown) and no evidence of edema or erythema (C).
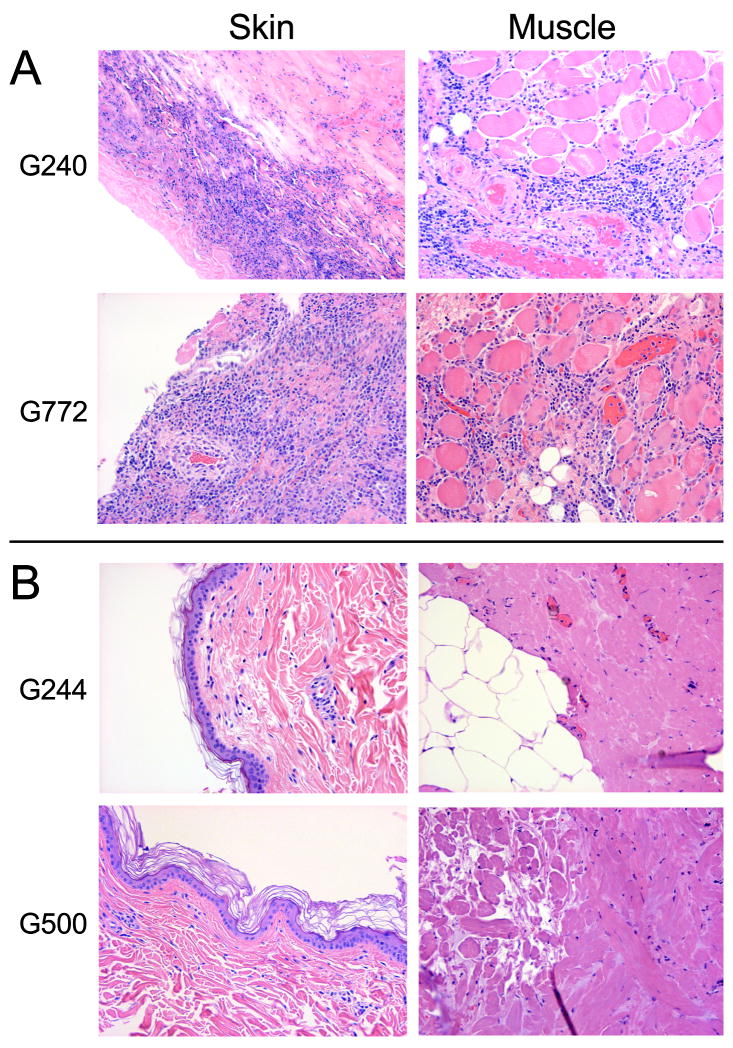
Figure 2. Histology of transplanted skin and muscle tissue from marrow donors and mixed chimeric recipients.
Recipient grafts in both marrow donors, G240 and G244 show dense lymphocytic infiltrates in skin and muscle on day 21 and 31 respectively; consistent with rejection (A). Marrow donor grafts in both mixed chimeras G244 and G500 showed normal skin and muscle histologies on days 167 and 118, respectively (B). Slides were photographed at 200X magnification.
FoxP3 and Granzyme B (Grβ) Expression in CD3+ T-cells Isolated from Tissues in Mixed Chimeric and Non-chimeric Recipients after CTA
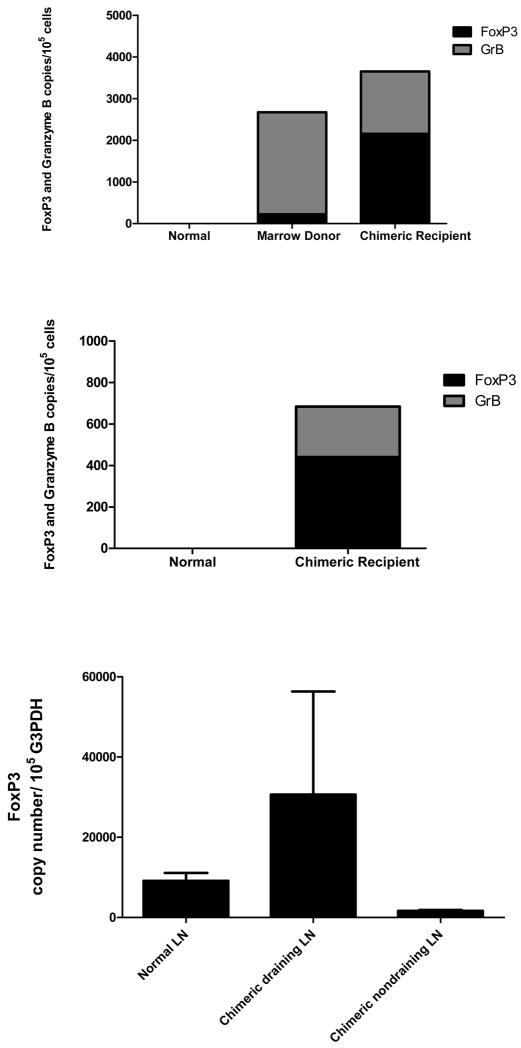
CD3+ T-cells were isolated from muscle and skin of the vascularized composite allografts and lymph nodes from mixed chimeric recipients and marrow donors. Muscle biopsies obtained from the rejected muscle in marrow donors demonstrated infiltrating T-cells with increased expression of Grβ compared to normal muscle (Figure 3A). CD3+ T-cells obtained from muscle and skin biopsies taken from vascularized composite allografts from mixed chimeric recipients had increased levels of both FoxP3 and Grβ compared to normal muscle and skin (Figures 3A, 3B). We also examined expression of FoxP3 in the CD3+ cells harvested from lymph nodes from mixed chimeras and found higher expression of FoxP3 in the draining lymph nodes (obtained from regional nodal basin of the composite tissue grafts) than the non-draining lymph nodes or the lymph nodes obtained from untreated control dogs (Figure 3C). Collectively, these data suggest that T-regulatory cells are a component of the cellular infiltrate of indefinitely accepted skin and muscle grafts possibly providing suppression of alloreactive T-cells.
Figure 3. FoxP3 and Granzyme B expression in biopsies of allografted and resident muscle, skin, and draining and non draining lymph nodes from marrow donors and chimeric recipients.
(A) FoxP3 (black bar) and Granzyme B (grey bar) expression of CD3+ T-cells collected from normal recipient muscle, allograft muscle from marrow donors and mixed chimeric recipients. (B) FoxP3 and Granzyme B expression of CD3+ T-cells collected from allografted skin biopsies. (C) FoxP3 expression of CD3+ cells isolated from normal dog, and draining lymph nodes or nondraining lymph nodes of the vascularized composite allograft from chimeric recipients (n=3). qRT-PCR methods were used to determine FoxP3 and GrB expression relative to G3PDH.
In situ Detection of T-regulatory Cells within Muscle and Skin Biopsies of Composite Tissue Allografts from Long-Term Mixed Chimeric Recipients
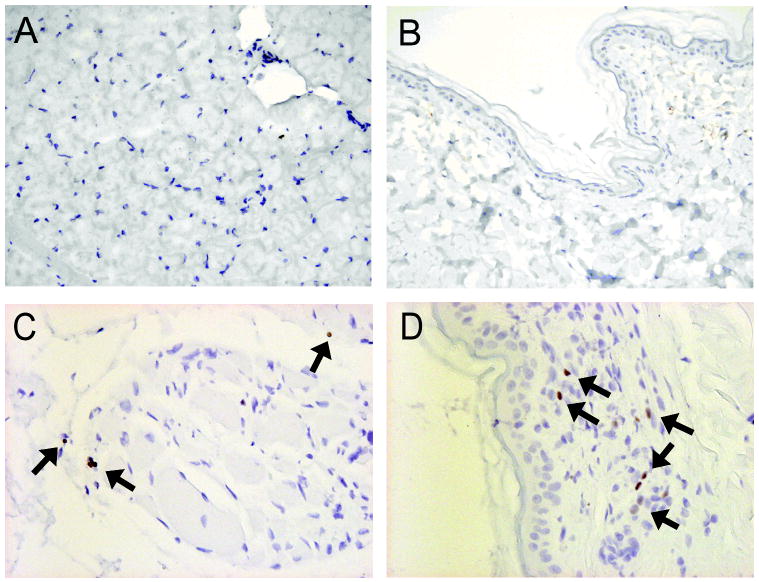
Muscle and skin biopsies from untreated control dogs and mixed chimeric recipients that demonstrated long-term acceptance of their vascularized composite allografts were stained for the presence of the intranuclear protein FoxP3. Mixed chimeric recipients maintained a consistent expression of FoxP3 over the course of the transplant (> 1 year), demonstrated both by RT-PCR and immunohistochemistry analyses. Muscle and skin biopsies from normal control dogs were negative for FoxP3 staining (Figure 4A and B). However, significant FoxP3 staining was observed in the muscle and the skin obtained from the vascularized composite allografts of mixed chimeras (Figure 4C and D). Cells staining positive for FoxP3 were localized to limited areas of higher cellularity in both muscle and skin biopsies of mixed chimeric dogs. These results further confirm the observations that T-regulatory cells are present within tissues of chimeric recipients of vascularized composite allografts (22).
Figure 4. FoxP3+ staining of muscle and skin biopsies from untreated dogs, bone marrow donor dogs and vascularized composite allografts from mixed chimeric recipients.
Representative muscle (A) and skin (B) biopsies from an untreated dog showed no areas of FoxP3+ staining (200x magnification. In contrast, representative muscle (C) and skin (D) biopsies taken from the composite tissue allograft of mixed chimeric recipients (G767 and G969) reveal increased FoxP3+ staining with limited cellular infiltrate (Arrowheads; 400x magnification).
Because lymphocytic infiltrates could be observed within some of the biopsies of transplanted skin and muscle in mixed chimeric recipients, we sought to determine whether the percentages of FoxP3+ cells present within those sections were greater than in the vascularized composite allografts taken from the control marrow donors. Analysis of images of FoxP3+ stained cells was done using ImageJ imaging software analysis. As shown in Supplemental Figure 2, the average percent of FoxP3+ cells present in the lymphocytic infiltrate of the skin of the composite tissue allograft on the marrow donor was 0.833 ± 0.508, while that of the mixed chimeric recipient was on average was significantly greater at 5.311 ± 1.926. Similarly, the average percent of FoxP3+ cells in the infiltrate of the muscle of the vascularized composite allograft on the marrow donor was 0.115 ± 0.099, while that of the mixed chimeric recipient was significantly greater at 4.023 ± 2.660.
Mixed Lymphocyte Reaction Confirms that the Immune System is Intact After Nonmyleoablative Bone Marrow Transplant
In order to determine whether mixed hematopoietic chimeras, tolerant to vascularized composite allografts, were immunocompetent, PBMC from these dogs were tested by MLR. PBMC from all mixed chimeric recipients demonstrated robust proliferative responses against PBMC obtained from DLA class II mismatched donors with a median SI of 106, (range = 10 to 126) (data not shown).
DISCUSSION
Vascularized composite allograft transplantation offers an unparalleled opportunity to reconstruct complex defects in patients. To date, approximately 50 hand transplants and 8 partial face transplants have been performed worldwide (23-28). However, it remains an experimental procedure completely dependent on immunosuppressive drug regimens similar to those used in solid organ transplantation. While immunosuppressive drugs have resulted in excellent 1-year graft survival, they have not prevented episodes of acute rejection (29). Additionally, complications from chronic immunosuppression have been widely reported in both hand and face recipients (30). Immunologic tolerance would allow for long-term survival of a vascularized composite allograft without the need for chronic immunosuppression. Establishment of hematopoietic chimerism following HCT continues to be an attractive strategy for inducing transplantation tolerance (31).
Our study was undertaken to test the hypothesis that nonmyeloablative HCT followed by a short course of postgrafting immunosuppression can establish immune tolerance of host immune cells towards donor hematopoietic cells and vascularized composite allograft from the marrow donors. While small animal models utilizing mixed chimerism to induce tolerance towards this type of transplant have been reported, translation of tolerance to vascularized composite allografts in a large animal model is limited (11,12). Additionally, the use of a canine model in studying tolerance towards vascularized composite allograft has not yet been published. Therefore, we initiated these studies with DLA-identical canines to serve as a platform from which to progress to the DLA-haploidentical and eventually unrelated settings. Because our canine mixed chimeras were DLA-antigen matched but minor antigen mismatched, successful engraftment of vascularized composite allografts was dependent on tolerance to non-MHC antigens shared between hematopoietic cells and the donor composite tissue graft. Non-MHC antigen disparities among dogs can cause potent rejections of skin (32), or kidney allografts (33,34) or cause GVHD (35). Organs that approach the skin in susceptibility to rejection are small bowel (36) and lung (37). There is limited evidence that many of these tissues can be accepted when transplanted into canine mixed hematopoietic chimeras.
Several findings in the present study were noteworthy. First, we observed 100% acceptance of all components of the vascularized composite allografts in the dog, specifically skin and muscle for all five mixed chimeric recipients for periods greater than 1 year; unlike previous large animal models where split tolerance was seen (10,11). In addition, the control transplants that received vascularized composite allografts without the benefit of HCT rejected their grafts (<21 days), confirming the requirement of HCT for inducing tolerance towards the vascularized composite allograft. This strategy of using mixed chimerism to support tolerance towards vascularized skin grafts is supported by studies in the porcine model (12). Second, vascularized composite allografts were performed well after donor marrow engraftment was established. Thus, tolerance against skin-specific antigens was established without the benefit of the tolerance-promoting conditions accompanying HCT. Third, MLR using PBMC from the stable mixed hematopoietic chimeras showed that acceptance of vascularized composite allografts was not due to lack of immunocompetence, duplicating similar studies in our laboratory (17). Fourth, by achieving tolerance against skin, a highly antigenic tissue, these results suggest that this tolerance induction regimen is likely to be successful for other commonly transplanted organs.
A second component of our study was to investigate the putative role of regulatory T-cells in the maintenance of tolerance to donor skin and muscle. Regulatory T-cells have been shown to contribute to the establishment and maintenance of tolerance of the immune system (38). The T-cell subset, CD4+/CD25high/FoxP3+ T-regulatory (T-reg) cells, have even been shown to infiltrate skin of hand transplant recipients maintained on chronic immunosuppression, but the significance of this observation is unknown (39). However, immunohistological analysis of skin biopsies from recipients of allogeneic HCT revealed that an increase in the numbers of T-reg cells correlated with less severe GVHD and was generally associated with positive clinical responses to GVHD treatment (40). Recently, we reported that markers for T-reg cell subsets were increased in CD3+ T-cells isolated from the blood and peripheral tissues after lung transplantation into canine mixed chimeric recipients (41). We have also found that canine peripheral blood CD25+ FoxP3+ cells were highly immunosuppressive in vitro (42). Additionally, lower expression of FoxP3 compared to Grβ (a cytotoxic protein effecting target cell lysis that is expressed on cytotoxic T-lymphocytes and associated with rejection) has been reported in cells collected from the urine of patients with kidney allografts following episodes of rejection (43). Thus, the long-term presence of T-reg cells after transplantation may indicate a more favorable environment for long-term allograft survival and tolerance induction.
With these studies in mind, we undertook efforts to determine to what extent T-regs (CD3+/CD25 high /FoxP3+ T-cells) were present in skin and muscle vascularized composite allografts from both marrow donors and mixed chimeric recipients. Elevated levels of FoxP3 expression were found in the transplanted muscle and skin and in the regional draining lymph node of mixed chimeras when compared to normal tissue and to the non-chimeric transplant recipients. In addition, the CD3+ cells derived from biopsies of the muscle and skin of mixed chimeric recipients maintained expression of FoxP3 for a period > 1 year. In contrast Grβ was elevated in the muscle component of the VCA harvested from the non-chimeric control transplants when compared to muscle from the tolerant chimeric VCA recipients. These findings suggest a role for T regulatory cells in the tissue and regional lymph nodes of the chimeric dogs.
In this study we have demonstrated for the first time donor specific tolerance to all components of the vascularized composite allografts in a genetically out-bred DLA-matched canine model conferred through nonmyeloablative allogeneic HCT. We saw no evidence of acute or chronic rejection in skin or muscle, GVHD, or a posttransplant lymphoproliferative disorder within follow-up periods between 52 and 90 weeks. Because of the highly antigenic nature of skin, successful methods of transplantation developed for vascularized composite allografts are likely to translate directly to other less antigenic and more commonly transplanted organs.
MATERIAL AND METHODS
Experimental Animals
Random-bred litters of beagles and mini-mongrel cross-breeds were either raised at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA, or purchased commercially. The dogs weighed from 7.0 to 13.3 (median 9.6) kg and were 7 to 27 (median 8) months old. They were observed for disease for at least 60 days before study. The Institutional Animal Care and Use Committee of the FHCRC approved the research protocols, and the American Association for Accreditation of Laboratory Animal Care certified the kennels. Five littermate donor/recipient pairs were DLA-identical on the basis of matching for highly polymorphic major histocompatibility complex (MHC) class I and class II micro-satellite markers (44). In addition, specific DLA DRB1 allelic identity was confirmed by direct sequencing (45).
Nonmyeloablative Conditioning and HCT
Conditioning for HCT consisted of a single dose of either 1 or 2 Gy TBI delivered at a rate of 7 cGy/min from a high-energy linear accelerator (Varian Clinac 6, Palo Alto, CA). Two dogs (G244 and G949) received 1 Gy TBI, and three dogs (G767, G500 and G969) received 2 Gy TBI, followed by transplantation of marrow from a respective DLA-identical littermate. Marrow was infused i.v. within 4 hr of TBI at doses of 2.8 to 9.2 (median 4.4) × 108 nucleated cells/kg. The day of HCT infusion was designated as day 0 for establishing mixed chimeric dogs. Postgrafting immunosuppression consisted of MMF (10 mg/kg b.i.d., s.c., from day 0 to day 28) and cyclosporine (15 mg/kg b.i.d. orally from day -1 to 35) for 4 dogs (G767, G244, G969 and G949). One dog, (G500) received sirolimus (0.05 mg/kg s.c. from day -5 to 25) and MMF (10 mg b.i.d., s.c. from day 0 to day 34). All dogs were given standard postgrafting care (46). The dogs’ clinical statuses were assessed twice daily. White blood cell counts with differentials, platelet counts, and hematocrits, were performed daily through day 21 and twice weekly thereafter.
Assessment of Hematopoietic Cell Engraftment
Percent donor chimerism was determined as previously described (47,48). Briefly, a polymerase chain reaction (PCR)-based assay was performed using specific primers for informative microsatellite markers. Primers amplified regions throughout the genome possessing tandem repeats in which the transplant recipient and donor were non-identical. PCR products were separated by capillary electrophoresis on an ABI Prism 310 Genetic Analyzer.
Vascularized Composite Allograft Transplantation
The surgical procedures for composite tissue allotransplantation were done as previously described (49). The skin and muscle were followed via clinical examination for color of the skin and hair growth. Stable engraftment of the graft was determined by punch biopsy of the skin and muscle. Sections of tissue were fixed in 10% formalin, imbedded in paraffin, and cut sections were stained with hematoxylin-and-eosin and Jones’ stains. A pathologist (G.S.), blinded to the source of the biopsies, evaluated presence or absence of rejection.
Quantitative Reverse Transcribed-PCR of Intracellular Cytokines and FoxP3 from CD3+ T-cells and FoxP3 Immunohistochemistry
Methods and reagents for cytokine analysis and FoxP3 expression are described under Supplemental Methods.
In vitro Immune Responses
Mixed leukocyte reactions (MLR) were done as described (50). Briefly, peripheral blood mononuclear cells (PBMC) were collected from blood after Ficoll (density 1.074) gradient centrifugation. Cells were washed in phosphate buffered saline PBS and resuspended in 45% Waymouth’s Medium (GibCo, Grand Island, NY), 45% Iscove’s Modified Dulbecco’s Medium (GibCo) with 10% heat-inactivated dog serum, sodium pyruvate, nonessential amino acids and L-glutamine (all except serum from GibCo). Stimulator cells were irradiated with 22 Gy (cesium irradiator). Cells were incubated for 7 days with 3H-thymidine added on day 6.
Statistical Analysis
Comparisons of FoxP3 to Grβ ratios between accepted tissue grafts and rejected tissue grafts, and between tissue grafts and normal controls, were evaluated by Wilcoxon rank-sum tests (2-sided). Significance was defined as a P value less than or equal to 0.05.
Supplementary Material
1. Supplemental Figure 1, p.8 (Legend: Marrow donor granulocyte (left panel) and lymphocyte (right panel) chimerism in 5 recipients before and after composite tissue grafts from their marrow donors.)
2. Supplemental Figure 2, p.10 (Legend: Analysis of overall FoxP3+ staining of muscle and skin biopsies from composite tissue allografts in bone marrow donor dogs and mixed chimeric recipients. Assessing T-regulatory cells in the transplanted allograft by quantifying the overall percentage of FoxP3 staining in the muscle (left) and skin (right). In both muscle and skin, there is a greater number of FoxP3 stained cells in the composite tissue allograft of a mixed chimera dog as compared to non-mixed chimera dog (statistically significant, * designates P 0.05, two tailed Student’s T-test).)
3. Supplemental Methods, p.17
Acknowledgments
The authors thank the technicians of the Canine Shared Resources Core of the Fred Hutchinson Cancer Research Center for expert animal care. Dr. Peter Moore provided the antibody CA17.6B3. MMF was generously supplied by Roche, Nutley NJ. Dr. Michele Spector, DVM provided veterinary supervision. We are grateful to Deborah Gayle, Bonnie Larson, Helen Crawford and Sue Carbonneau for their outstanding administrative support, and Stacy Zellmer and Patrice Stroup for the DLA typing. We are grateful to Drs. Beard, Mielcarek, Parker, Thakar, Venkataraman, Wang, Rezvani, Gyurkocza, Georges, our departmental investigators who participated in weekend treatments.
Funding support information: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD, grants P01CA078902 and P30CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. In addition, David Mathes received support from the National Endowment for Plastic Surgery Grant from the Plastic Surgery Educational Foundation.
ABBREVIATIONS
- CSP
cyclosporine
- DLA
dog leukocyte antigen
- FHCRC
Fred Hutchinson Cancer Research Center
- FoxP3
Forkhead Box P3
- G3PDH
glycerol-3-phosphate dehydrogenase
- Grβ
granzyme B
- GVHD
graft versus host disease
- HCT
hematopoietic cell transplantation
- IL-10
interleukin-10
- MLR
mixed leukocyte reactions
- MMF
mycophenolate mofetil
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- RT-PCR
reverse transcribed PCR
- TBI
total body irradiation
- TGFβ
transforming growth factor beta
- T-reg cells
T-regulatory cells
Footnotes
Authors’ contributions:
D.W. Mathes: designed the study, performed the surgeries and co-wrote the manuscript.
B. Hwang: designed and performed the Treg cell studies and co-wrote the manuscript.
S.S. Graves: assisted in the design of the studies and co-wrote the manuscript.
J. Edwards: assisted in the surgeries.
J. Chang: assisted in the surgeries and co-wrote the manuscript.
B.E. Storer: performed the statistical analyses.
T. Miwongtum: performed chimerism analyses.
G. Sale: evaluated the histopathological samples.
R.A. Nash: designed the Treg studies.
R. Storb: designed and supervised the study and revised the manuscript.
Disclosures: This manuscript was neither prepared nor funded in any part by a commercial organization, including educational grants. The authors of this manuscript have no conflicts of interest to disclose as described by the journal Transplantation.
NOTE: Manuscript also includes Supplemental Digital Content.
References
- 1.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 3.Sykes M. Immune tolerance: mechanisms and application in clinical transplantation. J Intern Med. 2007;262:288. doi: 10.1111/j.1365-2796.2007.01855.x. Review. [DOI] [PubMed] [Google Scholar]
- 4.Guttmann RD, Santos GW, Lindquist RR. Acceptance of renal allografts in rat bone marrow chimeras. Transplantation. 1971;12:408. doi: 10.1097/00007890-197111000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Colson YL, Zadach K, Nalesnik M, Ildstad ST. Mixed allogeneic chimerism in the rat. Donor-specific transplantation tolerance without chronic rejection for primarily vascularized cardiac allografts. Transplantation. 1995;60:971. [PubMed] [Google Scholar]
- 6.Luo B, Nanji SA, Schur CD, Pawlick RL, Anderson CC, Shapiro AM. Robust tolerance to fully allogeneic islet transplants achieved by chimerism with minimal conditioning. Transplantation. 2005;80:370. doi: 10.1097/01.tp.0000167724.38038.ae. [DOI] [PubMed] [Google Scholar]
- 7.Steinmuller D. Skin allograft rejection by stable hematopoietic chimeras that accept organ allografts still is an enigma (Letter) Transplantation. 2001;72:8. doi: 10.1097/00007890-200107150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DMJ, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchimoto Y, Gleit ZL, Huang CA, et al. Skin-specific alloantigens in miniature swine. Transplantation. 2001;72:122. doi: 10.1097/00007890-200107150-00024. [DOI] [PubMed] [Google Scholar]
- 10.Mathes DW, Randolph MA, Solari MG, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75:25. doi: 10.1097/00007890-200301150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Hettiaratchy S, Melendy E, Randolph MA, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77:514. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 12.Horner BM, Randolph MA, Duran-Struuck R, et al. Induction of tolerance to an allogeneic skin flap transplant in a preclinical large animal model. Transplant Proc. 2009;41:539. doi: 10.1016/j.transproceed.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo YR, Chen CC, Shih HS, et al. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model. Plastic & Reconstructive Surgery. 2011;127:569. doi: 10.1097/PRS.0b013e318200a92c. [DOI] [PubMed] [Google Scholar]
- 14.Kuo YR, Goto S, Shih HS, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87:1769. doi: 10.1097/TP.0b013e3181a664f1. [DOI] [PubMed] [Google Scholar]
- 15.Moseley RV, Sheil AG, Mitchell RM, Murray JE. Immunologic relationships between skin and kidney homografts in dogs on immunosuppressive therapy. Transplantation. 1966;4:678. doi: 10.1097/00007890-196611000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Steinmuller D, Lofgreen JS. Differential survival of skin and heart allografts in radiation chimaeras provides further evidence for Sk histocompatibility antigen. Nature. 1974;248:796. doi: 10.1038/248796a0. [DOI] [PubMed] [Google Scholar]
- 17.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048. [PubMed] [Google Scholar]
- 18.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523. [PubMed] [Google Scholar]
- 19.Kuhr CS, Allen MD, Junghanss C, et al. Tolerance to vascularized kidney grafts in canine mixed hematopoietic chimeras. Transplantation. 2002;73:1487. doi: 10.1097/00007890-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 20.Kuhr CS, Yunusov M, Sale G, Loretz C, Storb R. Long-term tolerance to kidney allografts in a preclinical canine model. Transplantation. 2007;84:545. doi: 10.1097/01.tp.0000270325.84036.52. [DOI] [PubMed] [Google Scholar]
- 21.Yunusov MY, Kuhr C, Georges GE, et al. Survival of small bowel transplants in canine mixed hematopoietic chimeras: preliminary results. Transplant Proc. 2002;34:3366. doi: 10.1016/s0041-1345(02)03614-x. [DOI] [PubMed] [Google Scholar]
- 22.Bozulic LD, Wen Y, Xu H, Ildstad ST. Evidence that FoxP3+ regulatory T cells may play a role in promoting long-term acceptance of composite tissue allotransplants. Transplantation. 2011;91:908. doi: 10.1097/TP.0b013e31820fafb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lantieri L, Meningaud JP, Grimbert P, et al. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: a 1-year follow-up study. Lancet. 2008;372:639. doi: 10.1016/S0140-6736(08)61277-5. [DOI] [PubMed] [Google Scholar]
- 24.Lanzetta M, Petruzzo P, Margreiter R, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation. 2005;79:1210. doi: 10.1097/01.tp.0000157118.28394.fa. [DOI] [PubMed] [Google Scholar]
- 25.Siemionow M, Papay F, Alam D, et al. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet. 2009;374:203. doi: 10.1016/S0140-6736(09)61155-7. Review. [DOI] [PubMed] [Google Scholar]
- 26.Breidenbach WC, Gonzales NR, Kaufman CL, Klapheke M, Tobin GR, Gorantla VS. Outcomes of the first 2 American hand transplants at 8 and 6 years posttransplant. Journal of Hand Surgery - American Volume. 2008;33:1039. doi: 10.1016/j.jhsa.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Guo S, Han Y, Zhang X, et al. Human facial allotransplantation: a 2-year follow-up study. Lancet. 2008;372:631. doi: 10.1016/S0140-6736(08)61276-3. [DOI] [PubMed] [Google Scholar]
- 28.Brandacher G, Ninkovic M, Piza-Katzer H, et al. The Innsbruck hand transplant program: update at 8 years after the first transplant. Transplant Proc. 2009;41:491. doi: 10.1016/j.transproceed.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Whitaker IS, Duggan EM, Alloway RR, et al. Composite tissue allotransplantation: a review of relevant immunological issues for plastic surgeons. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2008;61:481. doi: 10.1016/j.bjps.2007.11.019. Review. [DOI] [PubMed] [Google Scholar]
- 30.Hettiaratchy S, Randolph MA, Petit F, Lee WP, Butler PE. Composite tissue allotransplantation--a new era in plastic surgery? British Journal of Plastic Surgery. 2004;57:381. doi: 10.1016/j.bjps.2004.02.012. Review. [DOI] [PubMed] [Google Scholar]
- 31.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353. doi: 10.1146/annurev.med.52.1.353. Review. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder ML, Storb R, Graham TC, Weiden PL. Canine radiation chimeras: An attempt to demonstrate serum blocking factors by an in vivo approach. J Immunol. 1975;114:540. [PubMed] [Google Scholar]
- 33.Junghanss C, Takatu A, Little M-T, et al. Adoptive immunotherapy against kidney targets in dog-leukocyte antigen-identical mixed hematopoietic canine chimeras. Transplantation. 2003;75:268. doi: 10.1097/01.TP.0000045224.52516.FC. [DOI] [PubMed] [Google Scholar]
- 34.Graves SS, Hogan WJ, Kuhr C, et al. Adoptive immunotherapy against allogeneic kidney grafts in dogs with stable hematopoietic trichimerism. Biol Blood Marrow Transplant. 2008;14:1201. doi: 10.1016/j.bbmt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storb R, Rudolph RH, Thomas ED. Marrow grafts between canine siblings matched by serotyping and mixed leukocyte culture. J Clin Invest. 1971;50:1272. doi: 10.1172/JCI106605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Zhu L, Quan D, et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 1996;62:1267. doi: 10.1097/00007890-199611150-00016. [DOI] [PubMed] [Google Scholar]
- 37.Prop J, Nieuwenhuis P, Wildevuur CR. Lung allograft rejection in the rat. I. Accelerated rejection caused by graft lymphocytes. Transplantation. 1985;40:25. doi: 10.1097/00007890-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Mahnke K, Ring S, Bedke T, Karakhanova S, Enk AH. Interaction of regulatory T cells with antigen-presenting cells in health and disease. Chemical Immunology & Allergy. 2008;94:29. doi: 10.1159/000154854. Review. [DOI] [PubMed] [Google Scholar]
- 39.Eljaafari A, Badet L, Kanitakis J, et al. Isolation of regulatory T cells in the skin of a human hand-allograft, up to six years posttransplantation. Transplantation. 2006;82:1764. doi: 10.1097/01.tp.0000250937.46187.ca. [DOI] [PubMed] [Google Scholar]
- 40.Fondi C, Nozzoli C, Benemei S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15:938. doi: 10.1016/j.bbmt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Nash RA, Yunusov M, Abrams K, et al. Immunomodulatory effects of mixed hematopoietic chimerism: immune tolerance in canine model of lung transplantation. Am J Transplant. 2009;9:1037. doi: 10.1111/j.1600-6143.2009.02619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graves SS, Stone DM, Loretz C, et al. Antagonistic and agonistic anti-canine CD28 monoclonal antibodies: tools for allogeneic transplantation. Transplantation. 2011;91:833. doi: 10.1097/TP.0b013e31820f07ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 44.Wagner JL, Burnett RC, Storb R. Molecular analysis of the DLA DR region. Tissue Antigens. 1996;48:549. doi: 10.1111/j.1399-0039.1996.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 45.Wagner JL, Hayes-Lattin B, Works JD, Storb R. Molecular analysis and polymorphism of the DLA-DQB genes. Tissue Antigens. 1998;52:242. doi: 10.1111/j.1399-0039.1998.tb03039.x. [DOI] [PubMed] [Google Scholar]
- 46.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11. [PubMed] [Google Scholar]
- 47.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701. [PubMed] [Google Scholar]
- 48.Graves SS, Hogan W, Kuhr CS, et al. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathes DW, Noland M, Graves S, Schlenker R, Miwongtum T, Storb R. A preclinical canine model for composite tissue transplantation. Journal of Reconstructive Microsurgery. 2010;26:201. doi: 10.1055/s-0030-1247717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Supplemental Figure 1, p.8 (Legend: Marrow donor granulocyte (left panel) and lymphocyte (right panel) chimerism in 5 recipients before and after composite tissue grafts from their marrow donors.)
2. Supplemental Figure 2, p.10 (Legend: Analysis of overall FoxP3+ staining of muscle and skin biopsies from composite tissue allografts in bone marrow donor dogs and mixed chimeric recipients. Assessing T-regulatory cells in the transplanted allograft by quantifying the overall percentage of FoxP3 staining in the muscle (left) and skin (right). In both muscle and skin, there is a greater number of FoxP3 stained cells in the composite tissue allograft of a mixed chimera dog as compared to non-mixed chimera dog (statistically significant, * designates P 0.05, two tailed Student’s T-test).)
3. Supplemental Methods, p.17