Abstract
Astroglial cells, due to their passive electrical properties, were long considered subservient to neurons and to merely provide the framework and metabolic support of the brain. Although astrocytes do play such structural and housekeeping roles in the brain, these glial cells also contribute to the brain's computational power and behavioural output. These more active functions are endowed by the Ca2+-based excitability displayed by astrocytes. An increase in cytosolic Ca2+ levels in astrocytes can lead to the release of signalling molecules, a process termed gliotransmission, via the process of regulated exocytosis. Dynamic components of astrocytic exocytosis include the vesicular-plasma membrane secretory machinery, as well as the vesicular traffic, which is governed not only by general cytoskeletal elements but also by astrocyte-specific IFs (intermediate filaments). Gliotransmitters released into the ECS (extracellular space) can exert their actions on neighbouring neurons, to modulate synaptic transmission and plasticity, and to affect behaviour by modulating the sleep homoeostat. Besides these novel physiological roles, astrocytic Ca2+ dynamics, Ca2+-dependent gliotransmission and astrocyte–neuron signalling have been also implicated in brain disorders, such as epilepsy. The aim of this review is to highlight the newer findings concerning Ca2+ signalling in astrocytes and exocytotic gliotransmission. For this we report on Ca2+ sources and sinks that are necessary and sufficient for regulating the exocytotic release of gliotransmitters and discuss secretory machinery, secretory vesicles and vesicle mobility regulation. Finally, we consider the exocytotic gliotransmission in the modulation of synaptic transmission and plasticity, as well as the astrocytic contribution to sleep behaviour and epilepsy.
Keywords: astrocyte, exocytosis, epilepsy, sleep, synaptic transmission, traffic
Abbreviations: ADA, adenosine deaminase; ADK, adenosine kinase; ANP, atrial natriuretic peptide; 2-APB, diphenylboric acid 2-aminoethyl ester; [Ca2+]i, cytosolic/intracellular Ca2+ levels; Cm, membrane capacitance; dnSNARE, dominant negative SNARE; ECS, extracellular space; EGFP, enhanced GFP; Emd, emerald green; ENT, equilibrative nucleoside transporter; ER, endoplasmic reticulum; GABA, γ-aminobutyric acid; GAT-1, GABA transporter-1; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; GluR, glutamate receptor; HEK-293 cells, human embryonic kidney cells; IF, intermediate filament; InsP3R, inositol 1,4,5-trisphosphate receptor; LTP, long-term potentiation; mGluR, metabotropic GluR; NCX, Na+/Ca2+ exchanger; NMDAR, N-methyl-d-aspartate receptor; Ru360, Ruthenium 360; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; SNARE, soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor; SOCE, store-operated Ca2+ entry; Sb2, synaptobrevin 2; SNAP-23, 23 kDa synaptosome-associated protein; SWA, slow wave activity; TIRFM, total internal reflection microscopy; TRP, transient receptor potential; TRPC1, TRP canonical 1; V-ATPase, vacuolar type of proton ATPase; VGCC, voltage-gated Ca2+ channels; VGLUT, vesicular glutamate transporter
INTRODUCTION
The first description of neuroglia was made by Rudolf Virchow as a 'substance… which lies between the proper nervous parts, holds them together and gives the whole its form in a greater or lesser degree' (Virchow, 1858). The original concept of glia being just a goo soon after was refined to rather receive cellular attributes, as glial cells were stained using 'reazione nera' and visualized by Camillo Golgi (Golgi, 1873). A subset of these cells was termed astrocytes by Michael von Lenhossek (Lenhossek, 1891). Interestingly, the gold chloride stain, used by Santiago Ramón y Cajal in 1913 to visualize astrocytes, targets IFs (intermediate filaments; Kimelberg, 2004), which consist mainly of GFAP (glial fibrillary acidic protein) that is today used as an astrocytic marker, while its promoter became a prime tool in molecule genetics approaches (e.g. Pascual et al., 2005; Casper et al., 2007) to study the role of astrocytes in the operation of the brain. It is at the beginning of the 20th century that glial research was pushed aside to a great extent by the victory of the neuronal doctrine with neurons being the sole determinant of the brain signalling. Of course, the fact that glial cells lack classical electrical excitability was a contributing factor. Following somewhat less than three quarters of a century of neglect, there has begun a period of about the past three decades during which extraordinary evidence has mounted to present astrocytes as an integral component of the brain operation. Owing to the development of fluorescent Ca2+ indicators, with non-disruptive intracellular loading, for optical measurements of cellular free Ca2+ (Tsien, 1980, 1981; Grynkiewicz et al., 1985; Minta et al., 1989), in 1990, Cornell-Bell et al. (1990) made a seminal observation that activation of GluRs (glutamate receptors) in astrocytes raised their cytosolic Ca2+ levels. This finding was expanded to the observation that astrocytes with their Ca2+ responsiveness have the ability to 'sense' glutamatergic synaptic transmission (Dani et al., 1992). This was followed by the discovery that astrocytic Ca2+ dynamics can yield astrocyte–neuron signalling, with two initial studies immediately pointing to the complexity of this intercellular communication by describing two divergent underlying mechanisms: direct, perhaps using gap junctions (Nedergaard, 1994); and indirect utilizing glutamate released from astrocytes via Ca2+-dependent exocytosis (Parpura et al., 1994). The later path has led to the discovery of gliotransmission-based modulation of synaptic transmission (Araque et al., 1998a) and formulation of the concept that three, pre- and post-synaptic neuronal along with glial elements constitute a synapse (Kettenmann et al., 1996), whose structural–functional partnership was termed the tripartite synapse (Araque et al., 1999). An additional level of complexity in the role of gliotransmission was then the finding that the Ca2+-dependent release of vasoactive agents from astrocyte endfeet is at the basis of neurovascular coupling (Zonta et al., 2003) and that vesicles carrying gliotransmitters are subject to regulation (Stenovec et al., 2007). This represents the current view of synaptic physiology where astrocytes are actively involved in the brain's computational power. The aim of this review is to highlight up-to-date newer findings with respect to Ca2+ signalling in astrocytes and gliotransmission. We start with the description of Ca2+ sources and sinks that provide the astrocytic intracellular Ca2+ dynamics necessary and sufficient for regulation of exocytotic release of gliotransmitters. This is followed by a discussion on secretory machinery which underlies this release mechanism, and a description of secretory vesicles in astrocytes. It is the traffic of these organelles throughout the astrocyte which ultimately results in vesicular fusion to the plasma membrane that liberates gliotransmitter(s) into the ECS (extracellular space). It should be noted, however, that exocytosis also acts as a delivery route for insertion and retrieval of, for instance, receptors at the plasma membrane. Subsequently, we discuss exocytotic gliotransmission in modulation of synaptic transmission and plasticity, as well as astrocytic contribution to sleep behaviour. Finally, we describe the consequences of astrocytic Ca2+ excitability in epilepsy, as an example of a brain disorder with astrocytes as an aetiology share holder.
Astroglial Ca2+ excitability: Ca2+ sources and sinks
Calcium, rather than electrical, signalling is generally considered a substrate for glial excitability (Deitmer et al., 1998; Verkhratsky et al., 1998; Agulhon et al., 2008). Calcium intracellular signalling system is present in virtually all living cells, being highly evolutionarily conserved (Case et al., 2007; Verret et al., 2010). In eukaryotes, Ca2+ signalling can couple input to output, i.e. plasma membrane excitation to muscle contraction, metabolic reactions, gene expression and secretion. The excitation–secretion coupling that occurs through regulated exocytosis provides the basis for chemical transmission in the nervous system (reviewed in Parpura et al., 2010). Astrocytes, the main homoeostatic cells in the CNS (central nervous system), are no exception to this eukaryotic evolutionary trait (reviewed in Parpura and Zorec, 2010). The Ca2+ signalling system involves several sets of molecules that generate Ca2+ concentration gradients, and provide pathways for Ca2+ diffusion between various cellular compartments, which serve as sources and/or sinks for this ion. Cell stimulation triggers concerted activation of these molecular sets that produce a spatio-temporally organized transient increase in the [Ca2+]i (cytosolic/intracellular Ca2+ levels), which is sufficient and necessary for the engagement of Ca2+-sensitive effectors protein(s) of the secretory machinery. Subsequently, this leads to vesicular fusion to the plasma membrane liberating transmitters into the ECS, a process that has been referred to as gliotransmission when occurring in astrocytes. Below we summarize the role of several compartments, the ER (endoplasmic reticulum), ECS and mitochondria, for gliotransmission, in particular for Ca2+-dependent release of glutamate from astrocytes. The portrayal of this subject is largely driven by results obtained in vitro, although the final corroboration awaits further experimentation using more intact preparations.
The ER represents a dynamic network of tubules and cisternae distributed throughout the cell body, forming the nuclear envelope and extending towards most distal processes (Baumann and Walz, 2001; Verkhratsky, 2005). One of the ER functions is to act as a dynamic Ca2+ store that generates and shapes cytosolic Ca2+ signals. The ER has its own Ca2+ homoeostatic machinery represented by: (i) Ca2+ pumps of SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) type that transport Ca2+ into the ER lumen against the concentration gradient; (ii) opposing leakage pathway from the ER (Camello et al., 2002; Beck et al., 2004); and (iii) Ca2+ release channels. The latter are archetypal ligand-gated channels, InsP3Rs (inositol 1,4,5-trisphosphate receptors) and RYRs (ryanodine/caffeine receptors), activated by the second messengers InsP3 and Ca2+ ions respectively (Bezprozvanny, 2005; Galione and Ruas, 2005; Hamilton, 2005), which when open allow Ca2+ release down its concentration gradient resulting in transient cytosolic Ca2+ signals. The host of metabotropic receptors expressed at the astrocytic plasma membrane activate the phospholipase C/InsP3 signalling cascade that results in the opening of InsP3Rs (Berridge, 1993; Kostyuk and Verkhratsky, 1994; Petersen et al., 1994), which has been demonstrated in all types of glial cells in vitro and in situ (see e.g. McCarthy and Salm, 1991; Kastritsis et al., 1992; Finkbeiner 1993; Kirischuk et al., 1995, 1996; Peakman and Hill, 1995; Verkhratsky et al., 1998; Agulhon et al., 2008). Although the role for RyRs in shaping Ca2+ release in astrocytes in vivo remains debatable (Parpura et al., 2011), there are some indices of their contribution to the physiology of astrocytes, for instance, in ventrobasal thalamus (Parri and Crunelli, 2003).
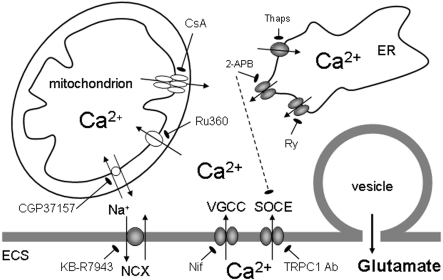
The contribution of the ER Ca2+ release to gliotransmission was deduced from experiments on cultured astrocytes, which showed that inhibition of ER Ca2+ accumulation by thapsigargin, a specific SERCA blocker, largely reduced Ca2+-dependent glutamate release (Innocenti et al., 2000; Jeremic et al., 2001; Hua et al., 2004) (Figure 1). Both InsP3Rs and RyRs were conduits for Ca2+ release from the ER to the cytosol (Hua et al., 2004). This was evident by the reduction of cytosolic Ca2+ loads and glutamate release when astrocytes were mechanically stimulated while being exposed to a variety of pharmacological agents including: (i) 2-APB (diphenylboric acid 2-aminoethyl ester), a cell-permeable InsP3R antagonist (Maruyama et al., 1997), which can also block the SOCE (store-operated Ca2+ entry) (Kukkonen et al., 2001; Bootman et al., 2002); (ii) ryanodine at concentration which can block RyRs (Rousseau and Meissner, 1989; Henzi and MacDermott, 1992; Golovina and Blaustein, 1997); (iii) caffeine, which depletes the ER through persistent activation of RyRs, but can also block InsP3Rs (Osipchuk et al., 1990; Wakui et al., 1990; Ehrlich et al., 1994); and (iv) combination of 2-APB with ryanodine or caffeine, without additive effect when compared with treatments with one agent only (Figure 1).
Figure 1. Sources of Ca2+ in Ca2+-dependent glutamate release from astrocytes.
The accumulation of Ca2+ in cytosol could be caused by the entry of Ca2+ from the ECS through: (i) L-type VGCC [blocked by nifedipine (Nif)]; (ii) SOCE via TRPC1 containing channels [blocked by antibodies against the channel pore (Ab)]; and (iii) the plasma membrane NCX (the reverse mode blocked by KB-R7943). The predominant source of Ca2+ is available from the ER internal store that possess InsP3R (blocked by 2-APB, which can also block SOCE) and RyR [blocked by ryanodine (Ry)] channels acting as conduits for Ca2+ delivery to the cytosol. The ER store is (re)filled by SERCA [blocked by thapsigargin (Thaps)]. Cytosolic Ca2+ levels are modulated by mitochondria that can take-up Ca2+ via the Ca2+ uniporter (blocked by Ru360) during the cytosolic Ca2+ increase. As cytosolic Ca2+ declines due to extruding mechanisms, Ca2+ is slowly released by mitochondria into cytosol via the mitochondrial NCX (blocked by CGP37157) as well as by the formation of the mitochondrial permeability transition pore [its opening blocked by cyclosporin A (CsA)]. The increase in cytosolic Ca2+ levels is sufficient and necessary to cause the fusion of glutamatergic vesicles to the plasma membrane and exocytotic release of glutamate. Drawing is not to scale.
An additional source of Ca2+ for gliotransmission comes from the ECS, since exposure of astrocytes to Cd2+, a broad spectrum blocker of plasmalemmal Ca2+ membrane channels, reduced both mechanically induced cytosolic Ca2+ loads and glutamate release (Hua et al., 2004), this finding being in agreement with the dual inhibitory action of 2-APB on the InsP3R and the SOCE. Indeed, SOCE, activated following the depletion of the ER Ca2+ store, is generally present in all types of glial cells (see e.g. Tuschick et al., 1997; Toescu et al., 1998; Pivneva et al., 2008; Li et al., 2009). Some of the possible SOCE molecular entities, the products of TRP (transient receptor potential) genes, have been identified in astrocytes and play a role in the regulation of astrocytic Ca2+ homoeostasis and glutamate release (Pizzo et al., 2001; Grimaldi et al., 2003; Golovina, 2005; Malarkey et al., 2008). Hence, an acute immunological treatment using blocking antibodies against the TRPC1 (TRP canonical 1) protein channel pore significantly reduced mechanically induced cytosolic Ca2+ elevations and the consequential glutamate release from cultured astrocytes (Malarkey et al., 2008) (Figure 1).
In addition to SOCE, Ca2+ entry from the ECS to the cytosol can be mediated via plasma membrane voltage- and ligand-gated Ca2+ channels. Astrocytes in acute slices from ventrobasal thalamus showed intrinsic cytosolic Ca2+ oscillations which lead to glutamate release (Parri et al., 2001). These oscillations were thapsigargin sensitive, thus requiring Ca2+ release from the ER. However, they also required Ca2+ entry from the ECS since they were additionally sensitive to nifedipine, a blocker of L-type VGCC (voltage-gated Ca2+ channels) (Parri et al., 2001) (Figure 1). Conversely, the L-type Ca2+ channel positive modulator BayK8644 increased the number of astrocytes exhibiting cytosolic Ca2+ oscillations (Parri and Crunelli, 2003). Although astrocytes in vitro and in situ express several types of plasma membrane ligand-gated Ca2+ channels/ionotropic receptors (for detailed overview, see Steinhauser and Gallo, 1996; Gallo and Ghiani, 2000; Verkhratsky and Steinhauser, 2000; Lalo et al., 2011), their role in exocytotic glutamate release from astrocytes remains speculative at the moment.
An alternative pathway for trans-plasmalemmal Ca2+ movements is controlled by NCXs (Na+/Ca2+ exchangers), abundantly expressed in astrocytes (Goldman et al., 1994; Takuma et al., 1994; Matsuda et al., 1996; Kirischuk et al., 1997). When operating in the reverse mode, the NCX can deliver Ca2+ to the cytosol (Kirischuk et al., 1997; Rojas et al., 2008). Hence, mild depolarization of astrocytes cultured from adult rats caused NCXs to operate in the reverse mode to generate cytosolic Ca2+ increases leading to glutamate release that were sensitive to 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiurea (KB-R7943) (Paluzzi et al., 2007) (Figure 1). A similar role for NCX was observed when neonatal astrocytes were mechanically stimulated (Reyes and Parpura, 2009).
Cytosolic Ca2+ levels are also modulated by mitochondria (Duchen et al., 2008). In mechanically stimulated astrocytes, mitochondria rapidly sequester high cytosolic Ca2+ via the Ca2+ uniporter. The inhibition of mitochondrial Ca2+ uniporters with Ru360 (Ruthenium 360) increased mechanically induced cytosolic Ca2+ loads and potentiated the release of glutamate (Reyes and Parpura, 2008) (Figure 1). Conversely, as free cytosolic Ca2+ is removed by pumps, such as the SERCA and/or plasma membrane Ca2+ ATPase, mitochondria slowly release Ca2+ into cytosol via the mitochondrial NCX, as well as by the formation of the mitochondrial permeability transition pore. The inhibition of mitochondrial Ca2+ release, either by blocking the mitochondrial NCX with 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157) or by applying cyclosporin A which inhibits opening of the mitochondrial permeability transition pore (Basso et al., 2005), reduced both the cytosolic Ca2+ loads and consequential glutamate release (Reyes et al., 2012) (Figure 1).
Taken together multiple Ca2+ delivery/retrieval pathways are involved in regulating cytosolic Ca2+ levels, which are sufficient and necessary for triggering exocytotic release of glutamate, and/or other gliotransmitters, from astrocytes (reviewed in Parpura and Zorec, 2010). It should be noted, however, that the sensitivity of secretory machinery to cytosolic Ca2+ can be modulated (Capogna et al., 1995; Wu and Wu, 2001), which has been recently demonstrated in cultured astrocytes (Reyes et al., 2011).
Regulated exocytosis and gliotransmission
The criteria for a chemical released from glia/astrocyte to be classified as a 'gliotransmitter' have been defined (Do et al., 1997; Volterra and Meldolesi, 2005; Martin et al., 2007; Parpura and Zorec, 2010) by a set of criteria: (i) synthesis by and/or storage in glia; (ii) regulated release triggered by physiological and/or pathological stimuli; (iii) activation of rapid (milliseconds to seconds) responses in neighbouring cells (paracrine), or to itself (autocrine); and (iv) a role in (patho)physiological processes. Astrocytes are capable of releasing a variety of gliotransmitters using three general mechanisms/conduits: (i) plasma membrane channels (Pasantes Morales and Schousboe, 1988; Cotrina et al., 1998; Duan et al., 2003; Iglesias et al., 2009); (ii) plasma membrane transporters (Szatkowski et al., 1990; Rosenberg et al., 1994; Warr et al., 1999); and (iii) Ca2+-dependent exocytosis (Parpura et al., 1994; Calegari et al., 1999; Krzan et al., 2003; Pangrsic et al., 2007); also reviewed in Parpura and Zorec (2010). Owing to the ongoing debate on the role of regulated exocytosis in gliotransmission (Smith, 2010), we here report on the exocytotic release of three classes of gliotransmitters: (i) amino acids, such as glutamate; (ii) nucleotides, such as ATP; and (iii) peptides, such as ANP (atrial natriuretic peptide).
Glutamate is synthesized within astrocytes via the tricarboxylic acid cycle (Westergaard et al., 1996). Owing to the expression of the enzyme pyruvate carboxylase, astrocytes can synthesize glutamate de novo (Hertz et al., 1999). ATP is produced in cells by glycolysis and oxidative phosphorylation. Extracellularly released ATP can directly act on purinergic receptors, or indirectly, upon its hydrolysis by membrane-bound ectonucleotidases to ADP and adenosine, can activate various plasma membrane receptors (reviewed in Fields and Burnstock, 2006). Both glutamate and ATP get loaded into vesicular lumen by vesicular transporters (see below), unlike ANP which enters vesicles via the synthetic secretory pathway, i.e., pro-ANP is made in the ER, and after sorting into secretory vesicles it gets processed to its final form, ANP, before being released (Dannies, 1999).
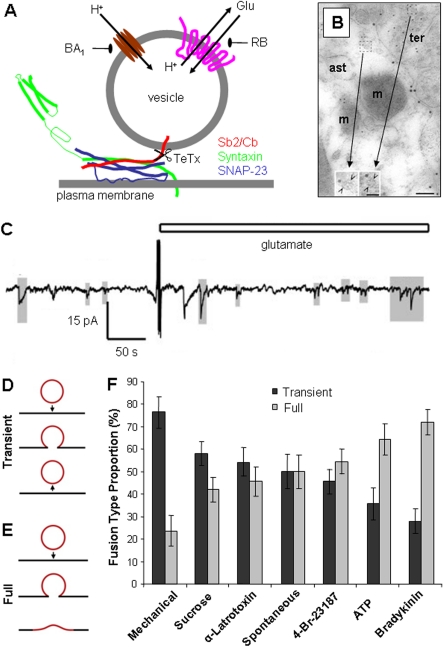
Ca2+-dependent exocytotic release of glutamate and ATP depends on the presence of two sets of proteins: (i) the SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) complex, responsible for mediating vesicular and plasma membranes merger, containing: Sb2 (synaptobrevin 2), or its homologue cellubrevin, syntaxin 1, SNAP-23 (23 kDa synaptosome-associated protein); and (ii) proteins responsible for sequestering glutamate and ATP into vesicles: the V-ATPase (vacuolar type of proton ATPase), which drives protons into the vesicular lumen creating the proton concentration gradient necessary for glutamate/ATP transport into vesicles via VGLUTs (vesicular glutamate transporters) 1, 2 and 3 and VNUT (vesicular nucleotide transporter) respectively (reviewed in Montana et al., 2006; Parpura and Zorec, 2010) (Figure 2A).
Figure 2. Release of gliotransmitters from astrocytes.
(A) Ca2+-dependent glutamate release from astrocytes utilizes a vesicular exocytotic pathway, which can be inhibited by: (i) a holoprotein of tetanus toxin (TeTx) that cleaves vesicular SNAREs Sb2 and cellubrevin (Cb, red); (ii) bafilomycin A1 (BA1) a specific inhibitor of V-ATPase (brown) which pumps protons into the vesicular lumen; and (ii) Rose Bengal (RB), an allosteric site modulator of VGLUTs (pink), transporters that utilize proton gradient to deliver glutamate (Glu) into the vesicle. The plasma membrane SNAREs syntaxin 1 (green) and SNAP-23 (blue) form an acceptor complex for the vesicular SNARE (Sb2/Cb red). Scissors and arrows indicate sites of actions for agents affecting vesicular proteins. Drawing is not to scale. (B) Glutamatergic vesicles at the putative tripartite synapse. Immunoelectron micrograph of VGLUT1 (small gold particles) in astrocytic process (ast) is identified by the plasma membrane glutamate transporters labelling (large gold particles). VGLUT1 positive vesicles (insets, open arrowheads) in an astrocyte and in the adjacent neuronal terminal (ter) have similar appearances; m, mitochondria. Scale bars, 100 nm; and 50 nm in insets. Reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience, Paola Bezzi, Vidar Gundersen, José Luis Galbete, Gerald Seifert, Christian Steinhäuser et al., 2004, Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate, Nature Neuroscience, 7(6), pp. 613−620, copyright (2004). (C) Exocytotic release of ATP from astrocytes is quantal. Small transient inward currents (shaded in grey), representing quantal release, can be recorded from ATP-sensing HEK-293 cells cultured on astrocytes at rest (spontaneous release) and after these glial cells were stimulated with glutamate (open bar); stimulation significantly decreases the mean interval between detected currents. Reproduced from Tina Pangršič, Maja Potokar, Matjaž Stenovec, Marko Kreft, Elsa Fabbretti, Andrea Nistri, Evgeny Pryazhnikov, Leonard Khiroug, Rashid Giniatullin, Robert Zorec, Exocytotic Release of ATP from Cultured Astrocytes, Journal of Biological Chemistry, 282(39), pp 28749–28758. Copyright © 2007, by the American Society for Biochemistry and Molecular Biology. (D–F) Preferred vesicular fusion type in astrocytes is stimulus dependent. (D, E) Drawings depict two vesicle fusion types described in astrocytes: (D) transient fusion, where a vesicle remains intact and opens a transient fusion pore (arrow pointing down, fusion; arrow pointing up, vesicle retrieval from the plasma membrane); and (E) full fusion where a vesicle fully collapses into the plasma membrane upon fusion (arrow). (F) Fusion events, categorized as either transient or full fusions were plotted as a percentage of the total number of fusions that occurred, either spontaneously or when cultured astrocytes were exposed to various stimuli. Reproduced from Erik B. Malarkey and Vladimir Parpura, Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at singlevesicle resolution, Journal of Physiology, John Wiley and Sons © 2011 The Authors. Journal compilation © 2011 The Physiological Society.
Secretory vesicles are compulsory morphological elements for exocytosis; a large variety of their sizes has been reported in astrocytes (reviewed in Montana et al., 2006; Parpura et al., 2010; Parpura and Zorec, 2010). In astrocytes, Sb2 can be associated with vesicular structures ranging from 30 to 700 nm, the majority of which are electron-lucent (clear), while ∼2% are dense core (Maienschein et al., 1999; Crippa et al., 2006). Dense, and not so dense, core vesicles in cultured astrocytes with diameters of ∼115 nm, contained the secretory peptide secretogranin II (Calegari et al., 1999) and ATP (Coco et al., 2003); subcellular fractions containing secretogranin II were mainly distinct from fractions containing Sb2 (Calegari et al., 1999). Consistent with this finding ANP fluorescent derivative [Emd (emerald green) appended] puncta poorly co-localized with VGLUT1 signal (Kreft et al., 2004), but they had ∼35% co-localization with ATP (Potokar et al., 2008). However, ∼85% of Sb2 containing vesicles in astrocytes co-localize with VGLUTs (Montana et al., 2004; Bowser and Khakh, 2007); a subset of these Sb2 vesicles likely contains other transmitters such as d-serine (Martineau et al., 2008). Astrocytes in situ showed association of cellubrevin and VGLUT 1 or 2 with small clear vesicles with a mean diameter of ∼30 nm (Bezzi et al., 2004) (Figure 2B). However, photoconverted recycling dye FM1–43 labelled glutamatergic vesicles with a measured diameter of 310 nm which was comparable with their sizes in live cultured astrocytes (340 nm) obtained using differential interference contrast microscopy (Chen et al., 2005). Visualization of vesicles expressing the Sb2 fluorescent derivatives [GFP (green fluorescent protein) or pHluorin appended] in astrocytes using TIRFM (total internal reflection microscopy) gave an estimated diameter of under ∼100 nm (Bowser and Khakh, 2007) and of ∼312 nm (range 161–422 nm) (Malarkey and Parpura, 2011). Similarly, diameters of VGLUT2 or VGLUT1-pHluorin expressing vesicles in astrocytes using TIRFM were estimated to be ∼40 nm (Cali et al., 2008; Marchaland et al., 2008). Glutamatergic vesicles labelled by capturing an extracellular antibody against VGLUT1 in a Ca2+-dependent manner while recycling with the plasma membrane are electron-lucent and have diameters of ∼50 nm (Stenovec et al., 2007). Similarly, recycling peptidergic vesicles, capturing an extracellular antibody against ANP, have diameters of ∼50 nm (Potokar et al., 2008). Vesicles found within gliosomes, subcellular components of astrocytic processes isolated from brain by fractionation (Nakamura et al., 1993), express Sb2 and VGLUT1 and measure ∼30 nm in diameter (Stigliani et al., 2006). Taken together, it appears that there are three classes of vesicles devoted to packaging of amino acids, nucleotides and peptides with some overlap in co-packaging (see summary in Table 1 of Parpura et al., 2010).
The process of exocytosis is characterized by quantal release of transmitter, which has been demonstrated in astrocytes using 'sniffer' cells (Pasti et al., 2001; Pangrsic et al., 2007) and fluorescent microscopy (Bezzi et al., 2004; Pangrsic et al., 2007) for ATP and glutamate, while glutamatergic vesicles containing a 'surrogate' transmitter were also interrogated using amperometry (Chen et al., 2005). Pasti et al. (2001) transfected HEK-293 cells (human embryonic kidney cells), which endogenously do not express GluR, with NMDAR (N-methyl-d-aspartate receptor) subunits 1 and 2A. These cells, termed NR cells, were co-cultured with astrocytes and used as glutamate biosensors/reporters in whole-cell patch clamp recordings. Selective glutamatergic stimulation of astrocytes caused oscillatory [Ca2+]i elevations in astrocytes as well as inward currents in adjacent NR cells consistent with quantal release of glutamate from astrocytes. Similarly, glutamate stimulation of astrocytes showed quantal release of ATP as recorded by ATP biosensor (Pangrsic et al., 2007), HEK-293 cells expressing a mutated P2X3 receptor with reduced desensitization (Fabbretti et al., 2004) (Figure 2C). Further evidence for vesicular exocytosis from astrocytes came from the use of TIRFM (Bezzi et al., 2004; Pangrsic et al., 2007). Pangrsic et al. (2007) incubated astrocytes with quinacrine, a compound that fluorescently labels ATP containing structures and shows punctate appearance. The rapid loss of quinacrine puncta was evident upon receptor stimulation using glutamate or ATP and the Ca2+ ionophore, ionomycin (Pryazhnikov and Khiroug, 2008). Similar data were obtained for glutamatergic vesicles when astrocytes in culture were transfected to express either VGLUT 1-EGFP (enhanced GFP) or VGLUT 2-EGFP chimaeric proteins loaded with Acridine Orange and stimulated using a mGluR (metabotropic GluR) agonist or ionomycin (Bezzi et al., 2004). Additional evidence for quantal release from astrocytes was obtained by amperometric measurements from cells pre-incubated with the oxidizable transmitter dopamine, which was stored in glutamatergic vesicles (Chen et al., 2005). Exocytotic release of ANP, visualized as a disappearance of fluorescent chimaeric protein ANP-Emd puncta (quanta), was observed upon stimulation of astrocytes by ionomycin (Krzan et al., 2003).
The delivery of secretory vesicles to the plasma membrane exocytotic sites can be observed as an increase in the Cm (membrane capacitance) due to a net addition of vesicular membrane to the plasma membrane (Kreft et al., 2004). Hence, stimulation to increase [Ca2+]i caused an increase in astrocyte Cm (Kreft et al., 2004; Zhang et al., 2004). Interestingly, in astrocytes the maximal rate of Cm increase (proportional to the rate of exocytosis) was at least two orders of magnitude slower when compared with that in neurons, measured by a similar method (Kreft et al., 2003). In a subset of experiments where simultaneous measurements of glutamate release and Cm were made, evoked glutamate release from astrocytes was accompanied by an increase in Cm in all cells (Zhang et al., 2004).
Using mainly neurons and chromaffin cells as models, two types of vesicle fusion have been described: (i) full fusion, where the vesicle collapses into the plasma membrane upon fusion (Figure 2D), and (ii) transient fusion, where vesicles remain attached to the plasma membrane and transiently open the fusion pore (Figure 2E). Both modes of exocytosis occur in astrocytes (Bezzi et al., 2004; Chen et al., 2005; Bowser and Khakh, 2007; Cali et al., 2008; Marchaland et al., 2008; Malarkey and Parpura, 2011). Spontaneously occurring events had a similar ratio of transient to full fusions; the preference for one fusion type over the other was stimulus-dependent (Bowser and Khakh, 2007; Malarkey and Parpura, 2011) (Figure 2E). The consequence of transient versus full fusion on release of gliotransmitters critically depends on the measurements of the fusion pore. Such measurements would allow us to assess the amount and/or type of gliotransmitter (i.e., amino acid versus peptide) that could be released during transient fusion events (Vardjan et al., 2007, 2009). In turn, such differential release properties of the two forms of vesicular release could have profound differential effects on astrocytic modulation of synaptic transmission and plasticity at the tripartite synapse (Araque et al., 1999).
Taken together, astrocytes possess functional secretory machinery for release of various gliotransmitters, which in turn can act upon neuronal receptors. The consequences of such gliotransmitter-mediated astrocyte–neuron signalling are further explored in upcoming sections below.
Mobility of secretory vesicles in astrocytes
Ca2+-dependent exocytosis not only permits the exit of vesicle lumen cargo, such as gliotransmitters into the ECS, but also modifies important signalling membrane molecules, including transporters, receptors, exchangers and ion pumps, to be integrated by exocytosis into the plasma membrane where they can take part in the signal transduction. Thus, the understanding of vesicle traffic is crucial for uncovering the role astrocytes play in signal integration in the brain. Here, we will address vesicle traffic, vesicle delivery to and removal from the site of exocytosis, a highly complex set of processes at resting and stimulated conditions.
The traffic of vesicles likely evolved when eukaryotic cells emerged 1000–2000 million years ago (Yoon et al., 2004). The evolution of these cells was associated with a cell volume increase by three to four orders of magnitude versus that of a typical prokaryotic cell. The increased cell size dictated a new organizational make-up. An important reason for this is that signalling and communication within the relatively large eukaryotic cell volume could no longer be supported mainly by diffusion-based processes. While the transport of ions and small molecules by diffusion in the sub-micrometre range is physiologically appropriate, the diffusional mobility of larger structures, such as vesicles, is predicted to be extremely slow, proportional to the aqueous diffusion coefficient and the inverse mass of the structure (Pusch and Neher, 1988).
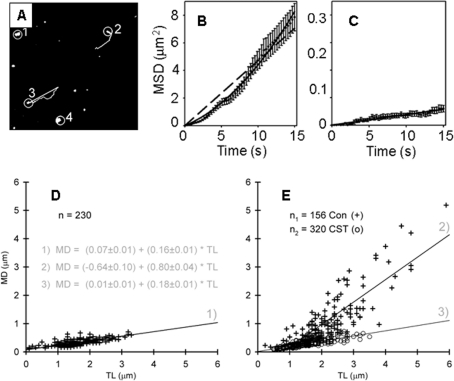
The first studies in astrocytes revealed two types of vesicular mobility, directional and non-directional (Potokar et al., 2005, 2007). Directional mobility was observed as vesicular tracks approximating straight-line appearance (Figure 3A). In contrast to the non-directional type of vesicle mobility, the directional mobility failed to exhibit a linear relationship between the mean square displacement as a function of time (Figure 3B), as is expected for diffusion-based processes, but was better described by a quadratic equation (Potokar et al., 2005). Similar types of vesicle mobility were observed in other cell types (Burke et al., 1997; Tvaruskó et al., 1999; Duncan et al., 2003; Hill et al., 2004). Vesicles exhibiting directional mobility are likely transported along the cytoskeleton: their mobility may be modulated by the dynamics of cytoskeletal elements, by different types of molecular motor proteins associated with the cytoskeleton, and by a variety of regulatory and signalling molecules (Chang and Goldman, 2004).
Figure 3. Vesicular mobility in astrocytes.
(A–C) Directional and non-directional vesicle mobility in resting astrocytes. (A) Examples of the mobility of vesicles, labelled by expressing the fluorescent ANP (pro-ANP-Emd) in astrocytes. Vesicles labelled with numbers 2 and 3 show directional mobility with elongated tracks (directional), whereas vesicles 1 and 4 exhibit non-directional mobility. Line on vesicle number 3 depicts the maximal displacement the vesicle attained in the observation time of 15 s. (B) Directional vesicles and (C) non-directional vesicles show the mean square displacement (MSD) of vesicle mobility as a function of time. Dashed line in (B) represents linear function (typical for a diffusion-based process) fitted to the data using equation: MSD (μm2) = (0.4702±0.0099)×Time(s). Upwardly curving line, which fits the data better than the linear function, represents quadratic function: MSD (μm2) = (0.2189±0.0148)×Time(s)+(0.0221±0.0013)×Time2(s2). In (C) a linear function was fitted to the data following the equation: [MSD (μm2) = (0.0038±0.0001)×Time(s)]. Reprinted from Biochem Biophys Res Commun, 329, Maja Potokar, Marko Kreft, Tina Pangršič, Robert Zorec, Vesicle mobility studied in cultured astrocytes, 678–683, Copyright 2005, with permission from Elsevier. (D, E) Post-fusion mobility of retrieved VGLUT1-positive vesicles in non-stimulated and stimulated astrocytes. Glutamatergic vesicles were fluorescently labelled by exposing the astrocytes to the extracellular antibody recognizing the vesicle luminal domain of the VGLUT1. Vesicle mobility was analysed by plotting vesicle maximal displacement (MD) as a function of the track length (TL). Numbered lines represent fits to the data (equations in left panel in grey). Stimulated cells (E) were treated with Clostridium spiroforme toxin (CST) to disintegrate actin filaments or left untreated (Con). Note that following the stimulation of cells by the addition of ionomycin to raise cytosolic calcium levels, increased vesicular mobility is seen as the steeper slope of the line (number 2). If cells were pretreated by the toxin CST the slope of the line (number 3) remained unchanged in comparison with controls (D, line number 1). (D, E) Reprinted from Experimental Cell Research, 313, Matjaž Stenovec et al., Ca2+-dependent mobility of vesicles capturing anti-VGLUT1 antibodies, 3809–3818, Copyright (2007), with permission from Elsevier.
In addition to microtubules and actin filaments as general cytoskeletal elements, astrocytes also express the IFs, such as GFAP, vimentin, nestin and synemin (Pekny et al., 1999; Pekny and Pekna, 2004; Jing et al., 2007). It was shown that all three classes of cytoskeletal elements play a role in directional vesicle mobility in astrocytes (Potokar et al., 2007). Unlike actin filaments and microtubules, IFs are devoid of enzymatic activity and are the least understood part of the cytoskeleton (Eliasson et al., 1999; Chang and Goldman, 2004; Pekny and Pekna, 2004). While the first two types of IFs, GFAP and vimentin, form the IF cytoskeleton in non-reactive astrocytes in the adult brain (Pekny and Pekna, 2004), in reactive astrocytes, an increased expression of GFAP, vimentin, nestin and synemin filaments was reported (Eliasson et al., 1999; Jing et al., 2007). Since the characteristics of single vesicle traffic was first described in primary cultured astrocytes (Potokar et al., 2005), a question often raised was how the overexpression of IFs in reactive astrocytes, under pathological conditions, affects vesicle dynamics, in particular, how the traffic of distinct types of vesicles is affected (Potokar et al., 2010, 2011).
In the initial studies, single vesicles were fluorescently labelled by expressing pro-ANP-Emd in cultured astrocytes (Potokar et al., 2005). To describe vesicle mobility, parameters such as speed, step length, track length, maximal displacement and mean square displacement, were estimated. Vesicles containing recombinant peptide pro-ANP-Emd revealed directional mobility (Figures 3A–3C) in 35% of vesicles (Potokar et al., 2005), which is similar to the Sb2-EGFP tagged vesicles, where 25% of vesicles were highly mobile (Crippa et al., 2006). It is likely that the directional mobility requires a cytoskeletal network, since disintegration of microtubules, actin filaments and IFs significantly reduced the population of vesicles exhibiting directional mobility (Potokar et al., 2007). The role of IFs in vesicle traffic, where they appear to be required for long-range directional vesicle mobility, was unexpected, since these filaments were considered previously to primarily maintain the cell shape (Chang and Goldman, 2004).
There was one other unexpected finding. After the vesicle membrane fuses with the plasma membrane transiently, the vesicle is thought to be retrieved into the cytoplasm (Jahn and Südhof, 1999; Taraska et al., 2003). The mobility of retrieved vesicles was studied by labelling the vesicles with extracellular antibodies, which were captured when the vesicle lumen was temporarily exposed to the extracellular medium during transient vesicle fusion (Stenovec et al., 2007; Potokar et al., 2008); a similar approach was used to label synaptic vesicles previously (Sara et al., 2005).
The mobility of retrieved vesicles differs from the peptidergic vesicle traffic to the plasma membrane, in the post-synthetic secretory pathway. First, the speed of retrieving ANP and VGLUT1 vesicles is one order of magnitude slower (∼0.05 μm/s) than the speed of peptidergic vesicles along the post-synthetic pathway towards the plasma membrane. These velocities are similar to that of ATP-containing vesicles (Pangrsic et al., 2007; Potokar et al., 2008). Secondly, while it has been reported that an increase in [Ca2+]i does not cause a significant difference in the mean velocity of pro-ANP-Emd labelled vesicles before and after stimulation (Ng et al., 2002; Potokar et al., 2007), the mobility of retrieved vesicles was altered following astrocyte stimulation by ionomycin to increase [Ca2+]i. The mobility of VGLUT1-positive vesicles was accelerated (Figures 3D and 3E), while the mobility of ANP-positive vesicles was attenuated (Stenovec et al., 2007; Potokar et al., 2008). However, as with the vesicle traffic along the post-synthetic pathway, the mobility of retrieved vesicles was reported to be sensitive to the disintegration of microtubules, actin filaments and IFs (Stenovec et al., 2007; Potokar et al., 2008), consistent with results on the impaired traffic of vesicles carrying the GPCR (G-protein-coupled receptor) cannabiniod receptor 1 in astrocytes in which the dynamics of actin filaments or microtubules was perturbed (Osborne et al., 2009).
To test that the vesicle mobility recorded in cultured astrocytes also occurs in vivo, vesicle dynamics was studied in brain slices in which cell-to-cell contacts are preserved and tissue architecture is closer to the one present in the brain (Potokar et al., 2009). The mobility of specifically labelled recycling glutamatergic and peptidergic vesicles (immuno-positive for VGLUT1 or ANP respectively) was similar to that in cultured astrocytes (Stenovec et al., 2007; Potokar et al., 2008).
To learn whether the accelerated/inhibited vesicle mobility requires the IFs, wild-type and double IF (GFAP−/− and vimentin−/−) knock-out astrocytes were studied. The results have shown that the stimulus-accelerated mobility of VGLUT1-positive glutamatergic vesicles, and the stimulus-inhibited mobility of ANP-positive peptidergic vesicles was absent in astrocytes without IFs. Moreover, the stimulus-mediated-inhibition of endosome/lysosome mobility was absent in cells devoid of IFs (Potokar et al., 2010). Thus, IFs play an important role in vesicle mobility regulation. One can thus expect that in reactive astrocytes, where IFs are overexpressed, this may differentially affect the stimulation-dependent mobility of recycling vesicles and endosomes (Potokar et al., 2011). More direct evidence that pathological states in astrocytes may affect vesicle traffic was provided recently, where the mobility of endosomes/lysosomes in cultured astrocytes was increased upon the application of amyotrophic lateral sclerosis IgG (Stenovec et al., 2011).
In summary, recent studies of single vesicle traffic in astrocytes revealed complex properties of glutamatergic and peptidergic vesicle mobility and their specific responses to extracellular stimuli. It appears that all vesicle types in astrocytes do not respond to altered physiological and pathological conditions in the same pattern. This may represent a new cellular paradigm of how astrocytes contribute to the phenotype of brain function in physiological and pathological conditions. One of the key questions for the future is to learn about the molecular mechanisms that mediate stimulus-dependent vesicle mobility regulation. Moreover, we need further insights into the role of factors that affect vesicle mobility and whether these manifest at a more systemic level.
Astrocytes modulate synaptic transmission
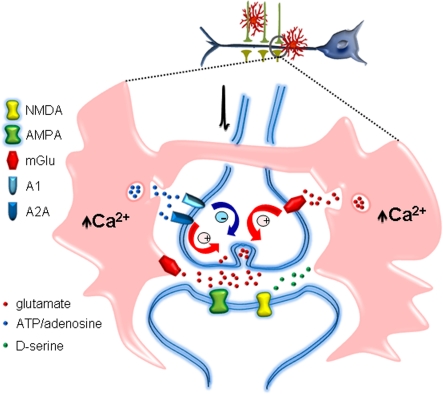
The elucidation of the functional consequences of gliotransmission on brain physiology has emerged as one of the most appealing topics in contemporary neuroscience. Astrocytes may release different neuroactive molecules, such as glutamate, d-serine, ATP, adenosine, GABA (γ-aminobutyric acid), TNFα (tumour necrosis factor α), prostaglandins, proteins and peptides that can potentially influence neuronal activity and synaptic physiology (Volterra and Bezzi, 2002; Perea et al., 2009). Glutamate, one of the first recognized gliotransmitters originally identified in culture (Parpura et al., 1994), has been shown to regulate synaptic transmission in cultured hippocampal cells (Araque et al., 1998a, b) and hippocampal slices. Glutamate released from astrocytes may exert multiple neuromodulatory actions on hippocampal synaptic transmission depending on the type of synapses, the neuronal sites of action and the subtypes of receptors activated. Astrocytic glutamate enhances the frequency of spontaneous and evoked excitatory synaptic currents through the activation of presynaptic group I mGluRs at hippocampal CA3–CA1 synapses (Newman, 2003; Liu et al., 2004b; Perea and Araque, 2007; Navarrete and Araque, 2010) or presynaptic NMDARs at mossy fibres of the hippocampal dentate gyrus (Jourdain et al., 2007). It can also affect inhibitory transmission, inducing the potentiation (Kang et al., 1998) or depression of inhibitory synaptic currents by activation of presynaptic kainate (Liu et al., 2004a) or group II/III mGluRs (Martin et al., 2007) respectively. The fact that a single gliotransmitter can exert multiple effects provides a high degree of complexity (Figure 4) to the possible impact of the astrocyte–neuron communication on network function.
Figure 4. Astrocytes modulate synaptic transmission.
Astrocyte calcium signal triggered by synaptic activity stimulates the release of different gliotransmitters, such as glutamate, ATP/adenosine or d-serine. These gliotransmitters act on specific glutamate α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), NMDAR and mGluR and adenosine (A1 and A2A) receptor types localized at the pre- or post-synaptic neurons. The consequently activated intracellular signalling pathways lead to depression or potentiation of synaptic transmission.
Besides glutamate, other gliotransmitters such as ATP and its metabolic product adenosine have also been shown to modulate synaptic transmission. The astrocytic release of ATP, which is converted into adenosine by extracellular nucleotidases, is responsible for the presynaptic A1 receptor activation that tonically depresses neurotransmission (Serrano et al., 2006) and mediates heterosynaptic depression in hippocampal CA3–CA1 synapses (Zhang et al., 2003; Panatier et al., 2006; Serrano et al., 2006). Adenosine itself directly released from astrocytes under hypoxic conditions has also been shown to down-regulate synaptic transmission in those synapses through activation of presynaptic A1 receptors (Martin et al., 2007). Furthermore, a more recent study has shown that basal synaptic transmission in CA3–CA1 synapses is up-regulated by the release of ATP/adenosine that tonically activates A2A receptors (Panatier et al., 2011). Again, as in the case of glutamate, these studies exemplify how a single gliotransmitter may have multiple and contrasting effects on synaptic transmission and, hence, in neural network function.
The fact that astrocytes may release different gliotransmitters and that a single gliotransmitter may act on different receptors and in different neuronal elements could provide apparent contradictory results. However, the existence of different regulatory effects should rather be regarded as complementary mechanisms that provide a high degree of complexity in the astrocytic control of the synaptic physiology. Experimental studies are generally designed to reveal the involvement of astrocytes in particular synaptic phenomena. However, the consequences of the astrocytic regulation of synaptic function can be much more complex in an actual neural network that is dynamically active because different neuromodulatory mechanisms can be simultaneously present. The influence of astrocytes, which are capable of releasing various gliotransmitters on synaptic transmission and neural network function, could be extremely diverse depending on the type of gliotransmitter released, the nature of the synapses involved, the type of neuronal receptors targeted as well as their pre- or post-synaptic localization. The temporal properties of these regulatory mechanisms may also be important. For example, sustained astrocytic purinergic signalling, which tonically up- or down-regulates synaptic activity through activation of A1 or A2A receptors (Pascual et al., 2005; Serrano et al., 2006; Panatier et al., 2011) may co-exist with a phasic glutamate signalling evoked by acute astrocytic calcium elevations that transiently potentiate synaptic transmission by activating presynaptic mGluRs (Fiacco and McCarthy, 2004; Perea and Araque, 2007; Navarrete and Araque, 2010) or NMDARs (Santello et al., 2011), which would provide higher degrees of freedom to the neural network activity.
Taken together, the functional consequences of astrocyte activity on synaptic function depend on the magnitude and spatial and temporal properties of the astrocyte calcium signal responsible for gliotransmitter release (i.e. which, when and where a type of gliotransmitter is released), as well as on the neuronal elements involved (i.e. what type of synapses, what type of receptors are activated and where in the pre- or post-synaptic membrane). At present we are gaining a better understanding of astrocyte involvement in synaptic physiology by revealing the possible regulatory mechanisms that may contribute to the astrocyte neuromodulation, but a detailed characterization of the physiological conditions, the astrocytic cellular code and the specific intercellular signalling that lead to the particular neuromodulation is necessary to coherently understand the actual consequences of synaptic transmission modulation by astrocyte activity on neural network function.
The astrocytic release of the gliotransmitter d-serine has also important consequences for the modulation of synaptic transmission properties. d-serine has been shown to be an endogenous ligand of the glycine-binding site of the NMDARs that acts as a co-agonist for these receptors and has important implications in glutamatergic transmission. In the hypothalamic supraoptic nucleus, changes in the astrocytic coverage of synapses under different physiological conditions result in changes in the ambient levels of d-serine released by astrocytes and its availability for NMDAR activation, thus influencing NMDAR-mediated synaptic responses (Panatier et al., 2006).
Long-term synaptic changes, considered to represent the cellular mechanisms underlying learning and memory, can also be influenced in multiple forms by different gliotransmitters released from astrocytes. Some mechanisms are based on a tonic mode of action of astrocytic gliotransmitters that tonically and persistently regulate synaptic transmission (Serrano et al., 2006; Stellwagen and Malenka, 2006; Henneberger et al., 2010; Panatier et al., 2011). As a consequence of the above described modulation of NMDAR activity by astrocytic d-serine, astrocytes regulate the amplitude and direction of the synaptic plasticity in the supraoptic nucleus (Panatier et al., 2006). Likewise, astrocytes in the CA1 area of the hippocampus control NMDAR-mediated synaptic plasticity through the release of d-serine (Henneberger et al., 2010). ATP/adenosine released from astrocytes has also been shown to govern the strength of the basal hippocampal synaptic activity by tonic suppression of neurotransmission, which results in an increase in the dynamic range for LTP (long-term potentiation) (Pascual et al., 2005). In addition, astrocytes also participate in the generation of LTP in hippocampal CA3–CA1 synapses through a phasic signalling process, in which the temporal coincidence of the astrocyte calcium signal and the postsynaptic neuronal activity induces LTP through the activation of presynaptic group I mGluRs by calcium-dependent glutamate release from astrocytes (Perea and Araque, 2007).
Although most of the studies on synaptic transmission modulation by astrocytes have been performed on hippocampal slices because of their suitability as an experimental model to study synaptic transmission, synaptic regulation by astrocytes can be considered as a general phenomenon in the nervous system, as indicated by its presence in other brain areas, such as the retina, supraoptic nucleus and cerebellum, as well as in the PNS (peripheral nervous system) (for reviews, see Pascual et al., 2005; Jourdain et al., 2007; Perea et al., 2009). However, to fully understand the role of astrocytes in brain function, it should be considered that astrocytes are largely interconnected cells through the expression of connexins, the membrane protein constituents of gap junction channels and hemichannels. Therefore, the existence of astroglial networks adds further complexity to the neuroglial networks underlying brain function (Giaume, 2010).
In conclusion, accumulating evidence obtained during recent years has shown that astrocytes not only respond to neuronal activity but also actively modulate synaptic transmission and plasticity, demonstrating the existence of reciprocal communication between neurons and astrocytes. This evidence has led to the establishment of the concept for the tripartite synapse (Araque et al., 1999; Perea et al., 2009), which represents a novel functional view of synaptic physiology where astrocytes are relevant elements actively involved in synaptic function and neurophysiology.
Astrocytes modulate sleep homoeostasis through their control of adenosine
A significant challenge in studying the role of the astrocyte in brain function has been the development of strategies that allow one to determine how these glial cells regulate synapses, neural networks and ultimately behaviour. Towards these goals, it was necessary to develop various lines of astrocyte specific transgenic mice that allow the evaluation of the role of glial signalling pathways in the control of brain function (Pascual et al., 2005; Fellin et al., 2009). One such line in the dnSNARE (dominant negative SNARE) mouse expressing a cytosolic domain of Sb2 in astrocytes in the adult brain is due to conditional and selective transgenic targeting. Initial studies in situ using hippocampal slices revealed that the expression of dnSNARE in astrocytes led to an increase in the magnitude of excitatory synaptic transmission at the CA3–CA1 synapse (Pascual et al., 2005). By performing pharmacological studies it was determined that this enhancement of synaptic transmission resulted from a reduction in the activation of presynaptic A1 receptors (Figure 5) (Pascual, 2005). It is well known that there is a basal level of extracellular adenosine that tonically exerts a presynaptic inhibition of synaptic transmission. Consequently, these results led to conclusion that astrocytic dnSNARE expression removed the source of adenosine, thereby enhancing synaptic transmission (Pascual et al., 2005). Independent work using entirely different strategies (Serrano et al., 2006) provided significant evidence in support of the notion that astrocytes can regulate extracellular adenosine.
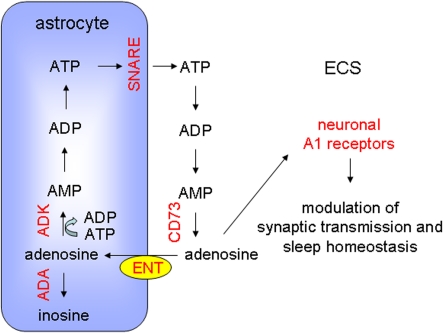
Figure 5. Pathways for synthesis, uptake and release of purines that may contribute to sleep homoeostasis.
Astrocytes release ATP through a SNARE-dependent mechanism. In the ECS, ectonucleotidases, such as CD73, can convert ATP into adenosine, which can be taken up by astrocytes via ENT. Intracellular adenosine can be converted into AMP or inosine by the action of ADK and ADA respectively. Astrocyte-mediated accumulation of extracellular adenosine leads to the activation of neuronal adenosine (A1) receptors resulting in modulation of synaptic transmission and sleep homoeostasis. Experimentally manipulated entities that support this model are shown in red. See text for additional details.
There are many pathways that regulate extracellular adenosine (Figure 5). One possible pathway is mediated through the diffusion of adenosine down concentration gradients that is facilitated by plasma membrane ENTs (equilibrative nucleoside transporters). Pascual et al. (2005) tested the importance of this pathway by pharmacologically inhibiting the transporter and determining whether it led to altered adenosine-dependent signalling. Following inhibition, adenosine-mediated presynaptic inhibition was enhanced, a result that is consistent with the transporter normally being used to uptake adenosine from the extracellular to intracellular milieu. Pascual et al. (2005) therefore sought the identification of a different pathway of extracellular adenosine accumulation. One potential pathway is mediated by the release of ATP. Once released into the ECS ATP can be hydrolysed to adenosine by extracellular nucleotidases. Extracellular bioluminescence imaging studies demonstrated that astrocytic dnSNARE expression caused a reduction in extracellular ATP. Moreover, the addition of exogenous ATP rescued the normal adenosine tone in slices obtained from dnSNARE mice. Collectively, these results suggest that via a SNARE sensitive mechanism, presumably exocytosis, astrocytes release ATP which is hydrolysed in the ECS to adenosine. This adenosine modulates synaptic transmission and is cleared via uptake through ENTs (Figure 5).
Having demonstrated that the astrocyte is critical for the control of adenosine, one can turn attention to potential behavioural phenotypes that result. It is well known that adenosine can modulate sleep (Basheer et al., 2000, 2004). For example, during wakefulness adenosine levels rise. Sleep deprivation leads to sustained adenosine elevations and infusion of adenosine promotes sleep. Additionally, adenosine receptor antagonists, including caffeine, promote wakefulness. The ability to selectively express dnSNARE only in astrocytes and thereby regulate the glial source of adenosine permitted investigations of whether astrocytes might modulate sleep systems.
There are two predominant modulators of sleep: the circadian oscillator that controls the timing of sleep and the sleep homoeostat that integrates the time awake and promotes sleep drive. Adenosine is implicated in the control of sleep homoeostasis. Using electroencephalography, Halassa et al. (2009) asked whether the astrocyte contributes to sleep homoeostasis. The drive to sleep can be assessed by measuring the power of SWA (slow wave activity) during non-rapid eye movement sleep. Following sleep deprivation, for example, SWA is enhanced. Under baseline conditions the power of SWA was reduced in dnSNARE mice and dnSNARE expression attenuated the responses to sleep deprivation. Following a period of sleep deprivation there is a compensatory increase in sleep time. Mice expressing dnSNARE in their astrocytes do not exhibit this increase of sleep time. Pharmacology performed in vivo demonstrated that inhibition of A1 receptors, the target of astrocyte-derived adenosine, phenocopied the dnSNARE phenotype (Halassa et al., 2009) (Figure 5).
This astrocyte adenosine mediated concept of the control of sleep homoeostasis is supported by additional studies. For example, mice with pan-cell knockout of the enzyme ecto-5′-nucleotidase, also known as CD73, necessary for extracellular hydrolysis of AMP to adenosine (Mochizuki et al., 2008), as well as mice with conditional neuron-specific A1 receptor deletion both show similar sleep phenotypes to dnSNARE mice (Bjorness et al., 2009) (Figure 5). Overexpression of ADK (adenosine kinase), an intracellular enzyme that is normally enriched in astrocytes and is responsible for the intracellular phosphorylation of adenosine to AMP, with concomitant conversion of ATP to ADP, leads to sleep phenotypes (Palchykova et al., 2010) (Figure 5). Interestingly, humans with specific mutations in ADA (adenosine deaminase), which leads to reduced intracellular metabolism of adenosine to inosine, have an increased depth of sleep (Rétey et al., 2005).
These results demonstrate the importance of adenosine and the astrocytic control of this purine in the control of sleep homoeostasis and point to the importance of these glial cells in the control of brain circuits underlying behaviour. Since many disorders of the brain have co-morbid sleep disorders it will be intriguing to determine the contribution of astrocytes to such disorders and whether targeting astrocytes could act as potential therapeutic strategies.
Astrocyte calcium and network modulation – epilepsy
Although we are beginning to understand how deep and wide the impact of astrocyte-to-neuron signalling might be on neuronal functions in physiological conditions (Halassa and Haydon, 2010), an involvement of astrocytes in certain brain disorders is also gradually emerging (Maragakis and Rothstein, 2006; Seifert et al., 2006, 2010; Wetherington et al., 2008; Blackburn et al., 2009). One of the brain disorders in which astrocytes have been proposed to play a relevant role is epilepsy, a family of multifactorial brain diseases affecting at least 50 million people worldwide (Baulac and Pitkanen, 2009). The clinical manifestation of epilepsy is the seizure, a highly synchronous electrical discharge arising in a population of neurons from a relatively small region (i.e. the epileptogenic focus) that secondarily spreads across the brain by a progressive recruitment of other neuronal populations (Traub and Wong, 1982; Jefferys, 1990; Avoli et al., 2002; Pinto et al., 2005; Trevelyan et al., 2006). Epilepsy can be considered a disorder of excessive synchronization of neurons, fundamentally linked to an imbalance between excitatory and inhibitory activities that produces a hyperexcitable neural network.
The hyperexcitable neuronal network that characterizes the epileptic brain is generally believed to originate from abnormalities intrinsic to neurons (McNamara et al., 2006), but non-neuronal mechanisms may also contribute (Konnerth et al., 1986; Dudek et al., 1998; Jefferys, 2003). A role of astrocytes as modulators of epileptogenesis was initially proposed over 20 years ago and for a long period it focused on the ability of astrocytes to buffer extracellular K+ or neurotransmitters (released in excess during epileptic discharges). Studies performed over the last decade, in both animal models and human epilepsy strengthened the view that a dysregulation of K+ buffering in the astrocyte network can predispose to neuronal hyperexcitability and contribute to establish an epileptic condition (Bordey and Sontheimer, 1998; Hinterkeuser et al., 2000; Kivi et al., 2000; Schroder et al., 2000; Wallraff et al., 2006).
A more direct role of astrocytes in the generation of epileptiform activities was proposed only recently. The first clue for such a role came from demonstration that through the Ca2+-dependent glutamate release, and possibly d-serine (Mothet et al., 2005; Panatier et al., 2006), astrocytes can directly excite groups of neighbouring neurons and favour NMDAR-dependent synchronized activities (Fellin et al., 2004). Following this observation, studies in brain slice preparations and in vivo reported a significant increase in the Ca2+ oscillation frequency in astrocytes during epileptiform activity (Tian et al., 2005; Fellin et al., 2006), and its reduction in the presence of anticonvulsant drugs (Tian et al., 2005). The Ca2+-dependent glutamate release in astrocytes, and the consequent activation of extracellular NMDARs in neurons, was then found to contribute to cell loss in cortical and hippocampal regions during experimentally induced status epilepticus (Ding et al., 2007). In support of a direct astrocyte role in focal epilepsies, it was recently revealed that in the presence of the proconvulsant 4-aminopyridine astrocytes exhibited massive Ca2+ elevations upon an episode of intense neuronal activity (Losi et al., 2010) and by signalling back to neurons they generated a recurrent excitatory loop with neurons that had the potential to promote a focal seizure (Gomez-Gonzalo et al., 2010). Altogether, these results suggest that a hyperexcitable neuronal network may arise in the brain as a product of a dysregulation of local neuron-astrocyte reciprocal signalling and generate a focal site of seizure initiation.
Although these observations represent significant advances in our knowledge of the possible role of astrocytes in epilepsy, many important issues need to be defined. For example, the effect on epileptiform activities of other gliotransmitters, such as ATP, d-serine and GABA, or of inflammatory agents, such as cytokines and chemokines that are potentially released by activated astrocytes, has been not specifically addressed. Notably, not necessarily all these factors have proconvulsant effects. The observations that under physiological conditions adenosine derived from astrocytic ATP leads to inhibition of transmitter release and heterosynaptic depression by acting on presynaptic A1 receptors are, indeed, consistent with an anticonvulsant role of astrocytes. Also noteworthy is that seizure induction in experimental epilepsy was found to decrease adenosine extracellular concentrations through an up-regulation of astrocytic enzyme ADK that controls ambient adenosine levels (Boison, 2005), while genetic reduction of ADK was observed to prevent seizures (Li et al., 2008). The overexpression of astrocytic ADK recently observed in hippocampal and cortical specimens from patients with temporal lobe epilepsy strengthens the hypothesis that a dysregulation of this enzyme in astrocytes is a hallmark of the chronic epileptic brain tissue (Aronica et al., 2011).
The potential impact on seizure activity of astrocytes can be even more complex if we take into account that a gliotransmitter, such as glutamate, may affect not only excitatory but also inhibitory transmission (Kang et al., 1998; Benedetti et al., 2011). It follows that the overall effect of gliotransmission on the neuronal network excitability may ultimately depend not only on the type of gliotransmitter predominantly or selectively released but also on the distinct functional connectivity between astrocytes and different types of neurons from a specific brain region. As an excessive neuronal synchrony can arise as a result of inhibitory transmission impairment, it would be of great interest to understand whether a distinct signalling pathway exists between astrocytes and different GABAergic interneurons, and how it eventually changes in the epileptic brain tissue.
Astrocytes may contribute to epileptogenesis also through a number of different mechanisms. For example, a malfunction of the astrocytic plasma membrane glutamate transporters that critically control extracellular glutamate concentrations is a common feature in temporal lobe epilepsy. A recent study also revealed that the astrocytic plasma membrane GAT-1 (GABA transporter-1) likely has a central role in absence epilepsy (Cope et al., 2009). This form of non-convulsive epilepsy is characterized by synchronous spike-and-wave discharges arising in thalamo-cortical networks. In various well-established experimental models, the enhanced tonic inhibition, which in the ventrobasal thalamus crucially controls absence seizure generation, was regularly found to develop as a consequence of GAT-1 malfunction (Cope et al., 2009). As GAT-1 expression in the thalamus is mainly, if not exclusively, confined to astrocytes (De Biasi et al., 1998; Vitellaro-Zuccarello et al., 2003), these cells may be potential targets for the development of a new therapy in absence epilepsy.
A full clarification of the functional significance of the multiple signals that astrocytes exchange with neurons represents an intriguing challenge in the years to come. With a better understanding of astrocyte roles in both brain physiology and pathology, gliotransmission might be recognized as a target for developing new anti-epileptic therapies.
It is also worth underlining that the changes occurring in astrocyte signalling in the epileptic tissue may resemble the dysregulation of molecules and pathways that occur in other brain disorders. Indeed, astroglial GluRs and transporters, water channels (aquaporins), inward rectifier K+ channels and connexins were found to be dysregulated not only in epilepsy but also in motor-neuron disease, stroke, hepatic encephalopathy, schizophrenia, Huntington's disease and Alzheimer's disease (Blackburn et al., 2009; Maragakis and Rothstein, 2006; Seifert et al., 2006). All in all, these studies suggest that astrocytes might hold the key to understand the pathogenesis of these brain disorders.
To improve our understanding we need, however, the results obtained from in vitro models to be validated in the intact brain. With the recent development of optogenetic tools to stimulate astrocytes selectively (Airan et al., 2009; Figueiredo et al., 2011; Gourine et al., 2010), and of new more sensitive, genetically encoded Ca2+ sensors, such as GCaMP3 (Shigetomi et al., 2011), we have now the opportunity to study how astrocyte Ca2+ signalling can affect network activities in the living brain.
A full clarification of the functional significance of the multiple signals that astrocytes exchange with neurons represents an intriguing challenge in the years to come. With a better understanding of the astrocyte roles in both brain physiology and pathology, gliotransmission might be recognized as a target for developing new anti-epileptic therapies.
CONCLUDING REMARKS
The intent of this review was to summarize the findings describing novel roles of astrocytes in the brain. Astrocytes can exocytotically release a variety of gliotransmitters under physiological and pathological conditions. This gliotransmission in turn can modulate synaptic transmission and plasticity and can also contribute to sleep behaviour. Although astrocytes can play a role in various disease states of the brain, as reviewed elsewhere (Verkhratsky and Parpura, 2010), we solely focused on epilepsy in which astrocytic Ca2+ homoeostasis and gliotransmission can contribute to the aetiology of this disorder. Although these exciting findings shed new light on the brain operation in health and disease, featuring astrocytes as an integral component of brain's functions, they also raise many new issues that remain to be addressed. For example, how various release mechanisms for gliotransmitters operate under (patho)physiological conditions need to be systemically investigated. For vesicular gliotransmitter release alone, it would have to be determined how various gliotransmitters, individually and/or in blend(s), affect neurotransmission in different brain regions and account for different behaviours. It is clear at this juncture that such a colossal task would require the use of various levels of analysis, from single cells to the complex network in the living brain, and comprehensive utilization of a battery of experimental approaches, including molecular genetics, imaging, electrophysiology and behavioural testing.
ACKNOWLEDGEMENTS
The writing of this review was facilitated by the discussions the authors have had during the International Society for Neurochemistry Satellite Meeting ‘Glia in (patho)physiology’, held in August, 2011 in Ljubljana, Slovenia.
Footnotes
Work of the authors was supported by the Slovenian Research Agency [grant numbers P3 310, J3 4051, J3 3632 and J3 4146], Center of Excellence CipKeBip and EduGlia ITN EU grant to R.Z.; Ministerio de Ciencia e Innovación [grant numbers BFU2010-15832, CSD2010-00045] and Cajal Blue Brain to A.A.; European Union [grant number HEALTH-F2-2007-202167] to A.A. and G.C.; Telethon Italy [grant numbers GGP07278, GGP10138B] and Cariparo Foundation to G.C.; NINDS [grant number NS037585] and NIDA [grant number DA025967] to P.G.H.; the Alzheimer's Research Trust (UK) Programme Grant [grant number ART/PG2004A/1], the Grant Agency of the Czech Republic [grant number GACR 305/08/1381 and GACR 305/08/1384] and IKERBASQUE, The Basque Foundation for Science to A.V.; and National Science Foundation [grant number CBET 0943343] and IKERBASQUE to V.P.
REFERENCES
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998b;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Aronica E, Zurolo E, Iyer A, de Groot M, Anink J, Carbonell C, van Vliet EA, Baayen JC, Boison D, Gorter JA. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–1655. doi: 10.1111/j.1528-1167.2011.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Basheer R, Porkka-Heiskanen T, Strecker RE, Thakkar MM, McCarley RW. Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biol Signals Recept. 2000;9:319–327. doi: 10.1159/000014655. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Baulac M, Pitkanen A. Research priorities in epilepsy for the next decade-a representative view of the European scientific community. Epilepsia. 2009;50:571–578. [Google Scholar]
- Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Matyash V, Kettenmann H. Astrocytes control GABAergic inhibition of neurons in the mouse barrel cortex. J Physiol. 2011;589:1159–1172. doi: 10.1113/jphysiol.2010.203224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositoltrisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function and role in motor neuron disease: a future therapeutic target? Glia. 2009;57:1251–1264. doi: 10.1002/glia.20848. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci USA. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke NV, Han W, Li D, Takimoto K, Watkins SC, Levitan ES. Neuronal peptide release is limited by secretory granule mobility. Neuron. 1997;19:1095–1102. doi: 10.1016/s0896-6273(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem. 1999;274:22539–22547. doi: 10.1074/jbc.274.32.22539. [DOI] [PubMed] [Google Scholar]
- Cali C, Marchaland J, Regazzi R, Bezzi P. SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: imaging analysis at the single-vesicle level with TIRF microscopy. J Neuroimmunol. 2008;198:82–91. doi: 10.1016/j.jneuroim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Camello C, Lomax R, Petersen OH, Tepikin AV. Calcium leak from intracellular stores – the enigma of calcium signalling. Cell Calcium. 2002;32:355–361. doi: 10.1016/s0143416002001926. [DOI] [PubMed] [Google Scholar]
- Capogna M, Gahwiler BH, Thompson SM. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42:345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–613. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. 'Kiss-and-run' glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nature Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G. Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol. 2006;570:567–582. doi: 10.1113/jphysiol.2005.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus. A light and electron-microscopic immunolocalization. Neuroscience. 1998;83:815–828. doi: 10.1016/s0306-4522(97)00414-4. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Verkhratsky AJ, Lohr C. Calcium signalling in glial cells. Cell Calcium. 1998;24:405–416. doi: 10.1016/s0143-4160(98)90063-x. [DOI] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci. 2007;27:10674–10684. doi: 10.1523/JNEUROSCI.2001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Benz B, Sorg O, Pellerin L, Magistretti PJ. beta-Adrenergic stimulation promotes homocysteic acid release from astrocyte cultures: evidence for a role of astrocytes in the modulation of synaptic transmission. J Neurochem. 1997;68:2386–2394. doi: 10.1046/j.1471-4159.1997.68062386.x. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2 × 7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Yasumura T, Rash JE. ‘Non-synaptic’ mechanisms in seizures and epileptogenesis. Cell Biol Int. 1998;22:793–805. doi: 10.1006/cbir.1999.0397. [DOI] [PubMed] [Google Scholar]
- Duncan RR, Greaves J, Wiegand UK, Matskevich I, Bodammer G, Apps DK, Shipston MJ, Chow RH. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- Fabbretti E, Sokolova E, Masten L, D'Arco M, Fabbro A, Nistri A, Giniatullin R. Identification of negative residues in the P2 × 3 ATP receptor ectodomain as structural determinants for desensitization and the Ca2+-sensing modulatory sites. J Biol Chem. 2004;279:53109–53115. doi: 10.1074/jbc.M409772200. [DOI] [PubMed] [Google Scholar]
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 2006;26:9312–9322. doi: 10.1523/JNEUROSCI.2836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci USA. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo M, Lane S, Tang F, Liu BH, Hewinson J, Marina N, Kasymov V, Souslova EA, Chudakov DM, Gourine AV, Teschemacher AG, Kasparov S. Optogenetic experimentation on astrocytes. Exp Physiol. 2011;96:40–50. doi: 10.1113/expphysiol.2010.052597. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SM. Glial calcium. Glia. 1993;9:83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- Galione A, Ruas M. NAADP receptors. Cell Calcium. 2005;38:273–280. doi: 10.1016/j.ceca.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- Giaume C. Astroglial wiring is adding complexity to neuroglial networking. Front Neuroenergetics. 2010;2:129. doi: 10.3389/fnene.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J Neurosci. 1994;14:5834–5843. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C. Vol. 33. Lombardia: Gazzetta Medica Italiana; 1873. Suella struttura della sostanza grigia del cervello (comunicazione preventiva). pp. 244–246. [Google Scholar]
- Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, Pozzan T, de Curtis M, Ratto GM, Carmignoto G. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010;8:e1000352. doi: 10.1371/journal.pbio.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca2+]i oscillations and is not involved in capacitative Ca2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SL. Ryanodine receptors. Cell Calcium. 2005;38:253–260. doi: 10.1016/j.ceca.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzi V, MacDermott AB. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46:251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- Hill DB, Plaza MJ, Bonin K, Holzwarth G. Fast vesicle transport in PC12 neurites: velocities and forces. Eur Biophys J. 2004;33:623–632. doi: 10.1007/s00249-004-0403-6. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, Schramm J, Steinhauser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte 'hemichannels'. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Basic mechanisms of focal epilepsies. Experimental Physiology. 1990;75:127–162. doi: 10.1113/expphysiol.1990.sp003390. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR. Models and mechanisms of experimental epilepsies. Epilepsia. 2003;44((Suppl. 12)):44–50. doi: 10.1111/j.0013-9580.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Jing R, Wilhelmsson U, Goodwill W, Li L, Pan Y, Pekny M, Skalli O. Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J Cell Sci. 2007;120:1267–1277. doi: 10.1242/jcs.03423. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kastritsis CH, Salm AK, McCarthy K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem. 1992;58:1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Faissner A, Trotter J. Neuron–glia interactions in homeostasis and degeneration. In: Greger R, Windhorst U, editors. In: Comprehensive Human Physiology. Berlin, Heidelberg: Springer-Verlag; 1996. pp. 533–543. [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochem Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol. 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by α1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci. 1996;8:1198–1208. doi: 10.1111/j.1460-9568.1996.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Kivi A, Lehmann TN, Kovacs R, Eilers A, Jauch R, Meencke HJ, von Deimling A, Heinemann U, Gabriel S. Effects of barium on stimulus-induced rises of [K+]o in human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur J Neurosci. 2000;12:2039–2048. doi: 10.1046/j.1460-9568.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U, Yaari Y. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. I. Development of seizurelike activity in low extracellular calcium. J Neurophysiol. 1986;56:409–423. doi: 10.1152/jn.1986.56.2.409. [DOI] [PubMed] [Google Scholar]
- Kostyuk P, Verkhratsky A. Calcium stores in neurons and glia. Neuroscience. 1994;63:381–404. doi: 10.1016/0306-4522(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Kreft M, Krizaj D, Grilc S, Zorec R. Properties of exocytotic response in vertebrate photoreceptors. J Neurophysiol. 2003;90:218–225. doi: 10.1152/jn.01025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci. 2003;23:1580–1583. doi: 10.1523/JNEUROSCI.23-05-01580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Lund PE, Akerman KE. 2-aminoethoxydiphenyl borate reveals heterogeneity in receptor-activated Ca2+ discharge and store-operated Ca2+ influx. Cell Calcium. 2001;30:117–129. doi: 10.1054/ceca.2001.0219. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: what is the role of 'excitable' molecules in non-excitable cells. Biochim Biophys Acta. 2011;1813:992–1002. doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Lenhossek Mv. Zur Kenntnis der Neuroglia des menschlichen Ruckenmarkes. Verh Anat Ges. 1891;5:193–221. [Google Scholar]
- Li D, Herault K, Oheim M, Ropert N. FM dyes enter via a store-operated calcium channel and modify calcium signaling of cultured astrocytes. Proc Natl Acad Sci USA. 2009;106:21960–21965. doi: 10.1073/pnas.0909109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Kang J, Nedergaard M. Astrocyte activation of presynaptic metabotropic glutamate receptors modulates hippocampal inhibitory synaptic transmission. Neuron Glia Biol. 2004a;1:307–316. doi: 10.1017/S1740925X05000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci USA. 2004b;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G, Cammarota M, Chiavegato A, Gomez-Gonzalo M, Carmignoto G. A new experimental model of focal seizures in the entorhinal cortex. Epilepsia. 2010;51:1493–1502. doi: 10.1111/j.1528-1167.2009.02472.x. [DOI] [PubMed] [Google Scholar]
- Maienschein V, Marxen M, Volknandt W, Zimmermann H. A plethora of presynaptic proteins associated with ATP-storing organelles in cultured astrocytes. Glia. 1999;26:233–244. doi: 10.1002/(sici)1098-1136(199905)26:3<233::aid-glia5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J Physiol. 2011;589:4271–4300. doi: 10.1113/jphysiol.2011.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Marchaland J, Cali C, Voglmaier SM, Li H, Regazzi R, Edwards RH, Bezzi P. Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J Neurosci. 2008;28:9122–9132. doi: 10.1523/JNEUROSCI.0040-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia. 2008;56:1271–1284. doi: 10.1002/glia.20696. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Takuma K, Nishiguchi E, Hashimoto H, Azuma J, Baba A. Involvement of Na+-Ca2+ exchanger in reperfusion-induced delayed cell death of cultured rat astrocytes. Eur J Neurosci. 1996;8:951–958. doi: 10.1111/j.1460-9568.1996.tb01582.x. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, Salm AK. Pharmacologically-distinct subsets of astroglia can be identified by their calcium response to neuroligands. Neuroscience. 1991;41:325–333. doi: 10.1016/0306-4522(91)90330-q. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- Mochizuki T, Clark EL, Thompson LF, Scammell TE. Washington, DC: Program No 285.24. 2008 Neuroscience Meeting Planner; 2008. Ecto-5′-nucleotidase (CD73) knockout mice have less rebound NREM sleep after sleep deprivation. [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Iga K, Shibata T, Shudo M, Kataoka K. Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia. 1993;9:48–56. doi: 10.1002/glia.440090107. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Ng YK, Lu X, Levitan ES. Physical mobilization of secretory vesicles facilitates neuropeptide release by nerve growth factor-differentiated PC12 cells. J Physiol. 2002;542:395–402. doi: 10.1113/jphysiol.2002.021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne KD, Lee W, Malarkey EB, Irving AJ, Parpura V. Dynamic imaging of cannabinoid receptor 1 vesicular trafficking in cultured astrocytes. ASN Neuro. 2009;1 doi: 10.1042/AN20090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk YV, Wakui M, Yule DI, Gallacher DV, Petersen OH. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl− current recording in single pancreatic acinar cells. Embo J. 1990;9:697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Shen HY, Boison D, Gerling A, Tobler I. Manipulation of adenosine kinase affects sleep regulation in mice. J Neurosci. 2010;30:13157–13165. doi: 10.1523/JNEUROSCI.1359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Grubisic V, Verkhratsky A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta. 2011;1813:984–991. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Parpura V, Baker BJ, Jeras M, Zorec R. Regulated exocytosis in astrocytic signal integration. Neurochem Int. 2010;57:451–459. doi: 10.1016/j.neuint.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte–neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pasantes Morales H, Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988;20:503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Pascual O. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman MC, Hill SJ. Adenosine A1 receptor-mediated changes in basal and histamine-stimulated levels of intracellular calcium in primary rat astrocytes. Br J Pharmacol. 1995;115:801–810. doi: 10.1111/j.1476-5381.1995.tb15004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204:428–437. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallén A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisén J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Petersen CC, Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Patrick SL, Huang WC, Connors BW. Initiation, propagation, and termination of epileptiform activity in rodent neocortex in vitro involve distinct mechanisms. J Neurosci. 2005;25:8131–8140. doi: 10.1523/JNEUROSCI.2278-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivneva T, Haas B, Reyes-Haro D, Laube G, Veh RW, Nolte C, Skibo G, Kettenmann H. Store-operated Ca2+ entry in astrocytes: different spatial arrangement of endoplasmic reticulum explains functional diversity in vitro and in situ. Cell Calcium. 2008;43:591–601. doi: 10.1016/j.ceca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Pangrsic T, Zorec R. Vesicle mobility studied in cultured astrocytes. Biochem Biophys Res Commun. 2005;329:678–683. doi: 10.1016/j.bbrc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Potokar M, Stenovec M, Kreft M, Kreft ME, Zorec R. Stimulation inhibits the mobility of recycling peptidergic vesicles in astrocytes. Glia. 2008;56:135–144. doi: 10.1002/glia.20597. [DOI] [PubMed] [Google Scholar]
- Potokar M, Stenovec M, Kreft M, Gabrijel M, Zorec R. Physiopathologic dynamics of vesicle traffic in astrocytes. Histol Histopathol. 2011;26:277–284. doi: 10.14670/HH-26.277. [DOI] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Lee SY, Takano H, Haydon PG, Zorec R. Trafficking of astrocytic vesicles in hippocampal slices. Biochem Biophys Res Commun. 2009;390:1192–1196. doi: 10.1016/j.bbrc.2009.10.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potokar M, Kreft M, Li L, Daniel Andersson J, Pangrsic T, Chowdhury H, Pekny M, Zorec R. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007;8:12–20. doi: 10.1111/j.1600-0854.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- Potokar M, Stenovec M, Gabrijel M, Li L, Kreft M, Grilc S, Pekny M, Zorec R. Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia. 2010;58:1208–1219. doi: 10.1002/glia.21000. [DOI] [PubMed] [Google Scholar]
- Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia. 2008;56:38–49. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rétey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci USA. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Perry G, Lesort M, Parpura V. Immunophilin deficiency augments Ca2+-dependent glutamate release from mouse cortical astrocytes. Cell Calcium. 2011;49:23–34. doi: 10.1016/j.ceca.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN NEURO 4(1):art:e00075.doi:10.1042/AN20110059. 2012 doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas H, Ramos M, Benaim G, Caputo C, DiPolo R. The activity of the Na+/Ca2+ exchanger largely modulates the Ca2+i signal induced by hypo-osmotic stress in rat cerebellar astrocytes. The effect of osmolarity on exchange activity. J Physiol Sci. 2008;58:277–279. doi: 10.2170/physiolsci.RP009208. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Knowles R, Knowles KP, Li Y. Beta-adrenergic receptor-mediated regulation of extracellular adenosine in cerebral cortex in culture. J Neurosci. 1994;14:2953–2965. doi: 10.1523/JNEUROSCI.14-05-02953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol Heart Circ Physiol. 1989;256:H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deák F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Schroder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, Steinhauser C. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia. 2000;41(Suppl 6):S181–S184. doi: 10.1111/j.1528-1157.2000.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. Neuroscience: Settling the great glia debate. Nature. 2010;468:160–162. doi: 10.1038/468160a. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Gallo V. News on glutamate receptors in glial cells. Trends Neurosci. 1996;19:339–345. doi: 10.1016/0166-2236(96)10043-6. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Grilc S, Potokar M, Kreft ME, Pangrsic T, Zorec R. Ca2+-dependent mobility of vesicles capturing anti-VGLUT1 antibodies. Exp Cell Res. 2007;313:3809–3818. doi: 10.1016/j.yexcr.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Milošević M, Petrušić V, Potokar M, Stević Z, Prebil M, Kreft M, Trkov S, Andjus PR, Zorec R. Amyotrophic lateral sclerosis immunoglobulins G enhance the mobility of Lysotracker-labelled vesicles in cultured rat astrocytes. Acta Physiol (Oxf) 2011;203:457–471. doi: 10.1111/j.1748-1716.2011.02337.x. [DOI] [PubMed] [Google Scholar]
- Stigliani S, Zappettini S, Raiteri L, Passalacqua M, Melloni E, Venturi C, Tacchetti C, Diaspro A, Usai C, Bonanno G. Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J Neurochem. 2006;96:656–668. doi: 10.1111/j.1471-4159.2005.03631.x. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Takuma K, Matsuda T, Hashimoto H, Asano S, Baba A. Cultured rat astrocytes possess Na+-Ca2+ exchanger. Glia. 1994;12:336–342. doi: 10.1002/glia.440120410. [DOI] [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nature Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Moller T, Kettenmann H, Verkhratsky A. Long-term activation of capacitative Ca2+ entry in mouse microglial cells. Neuroscience. 1998;86:925–935. doi: 10.1016/s0306-4522(98)00123-7. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neuroscie. 2006;26:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, Kettenmann H. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium. 1997;21:409–419. doi: 10.1016/s0143-4160(97)90052-x. [DOI] [PubMed] [Google Scholar]
- Tvaruskó W, Bentele M, Misteli T, Rudolf R, Kaether C, Spector DL, Gerdes HH, Eils R. Time-resolved analysis and visualization of dynamic processes in living cells. Proc Natl Acad Sci USA. 1999;96:7950–7955. doi: 10.1073/pnas.96.14.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Grilc S, Zorec R. The fusion pore and vesicle cargo discharge modulation. Ann NY Acad Sci. 2009;1152:135–144. doi: 10.1111/j.1749-6632.2008.04007.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V. Recent advances in (patho)physiology of astroglia. Acta Pharmacol Sin. 2010;31:1044–1054. doi: 10.1038/aps.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Verret F, Wheeler G, Taylor AR, Farnham G, Brownlee C. Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytol. 2010;187:23–43. doi: 10.1111/j.1469-8137.2010.03271.x. [DOI] [PubMed] [Google Scholar]
- Virchow R. Zwanzig Vorlesungen gehalten während der Monate Februar, März und April 1858 im pathologischen Institut zu Berlin, First edition. Berlin: 1858. Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre. [Google Scholar]
- Vitellaro-Zuccarello L, Calvaresi N, De Biasi S. Expression of GABA transporters, GAT-1 and GAT-3, in the cerebral cortex and thalamus of the rat during postnatal development. Cell Tissue Res. 2003;313:245–257. doi: 10.1007/s00441-003-0746-9. [DOI] [PubMed] [Google Scholar]
- Volterra A, Bezzi P. Release of transmitters from glial cells. In: Volterra A, Magistretti PJ, Haydon PG, editors. In: The Tripartite Synapse: Glia in Synaptic Transmission. Oxford: Oxford University Press; 2002. pp. 164–184. [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wakui M, Osipchuk YV, Petersen OH. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2+-induced Ca2+ release. Cell. 1990;63:1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol. 1999;514:783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Drejer J, Schousboe A, Sonnewald U. Evaluation of the importance of transamination versus deamination in astrocytic metabolism of [U-13C]glutamate. Glia. 1996;17:160–168. doi: 10.1002/(SICI)1098-1136(199606)17:2<160::AID-GLIA7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wu LG. Protein kinase c increases the apparent affinity of the release machinery to Ca2+ by enhancing the release machinery downstream of the Ca2+ sensor. J Neurosci. 2001;21:7928–7936. doi: 10.1523/JNEUROSCI.21-20-07928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol Biol Evol. 2004;21:809–818. doi: 10.1093/molbev/msh075. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]