Abstract
Objective:
To test the hypothesis that hospitalization in old age is associated with subsequent cognitive decline.
Methods:
As part of a longitudinal population-based cohort study, 1,870 older residents of an urban community were interviewed at 3-year intervals for up to 12 years. The interview included a set of brief cognitive tests from which measures of global cognition, episodic memory, and executive function were derived. Information about hospitalization during the observation period was obtained from Medicare records.
Results:
During a mean of 9.3 years, 1,335 of 1,870 persons (71.4%) were hospitalized at least once. In a mixed-effects model adjusted for age, sex, race, and education, the global cognitive score declined a mean of 0.031 unit per year before the first hospitalization compared with 0.075 unit per year thereafter, a more than 2.4-fold increase. The posthospital acceleration in cognitive decline was also evident on measures of episodic memory (3.3-fold increase) and executive function (1.7-fold increase). The rate of cognitive decline after hospitalization was not related to the level of cognitive function at study entry (r = 0.01, p = 0.88) but was moderately correlated with rate of cognitive decline before hospitalization (r = 0.55, p = 0.021). More severe illness, longer hospital stay, and older age were each associated with faster cognitive decline after hospitalization but did not eliminate the effect of hospitalization.
Conclusion:
In old age, cognitive functioning tends to decline substantially after hospitalization even after controlling for illness severity and prehospital cognitive decline.
Cognitive decline in old age is common and is associated with increased risk of disability, dementia, and death. With the aging of the US population, this problem is expected to increase in the coming decades, underscoring the need to identify factors contributing to cognitive decline and strategies to reduce its impact. One common occurrence in old age that may be related to cognitive decline is hospitalization. Two observations suggest an association. First, hospitalization of older persons increases risk of loss of independence in daily living activities,1–3 and this effect has been associated with impairment in cognitive functioning.4–8 Second, some common medical conditions, such as diabetes9,10 and chronic obstructive pulmonary disease,11,12 and general indicators of physical health, such as frailty13,14 and body mass index,15,16 have been associated with late life cognitive decline. However, few studies have examined the relationship between hospitalization and change in cognitive function,17,18 and their ability to reliably estimate the association has been limited by insufficient longitudinal cognitive data before and after hospitalization.
In the present study, we used data from the Chicago Health and Aging Project19 to test the hypothesis that hospitalization of older people is associated with subsequent cognitive decline. Participants are 1,870 older residents of a geographically defined neighborhood in Chicago, Illinois. During a mean of more than 9 years of observation, cognitive function was assessed 3–5 times at 3-year intervals, and Medicare records of hospitalizations were obtained. We used mixed-effects regression models to test the hypothesis that cognitive decline accelerates after hospitalization.
METHODS
Participants.
As part of the Chicago Health and Aging Project,19,20 a census of a geographically defined section of the city was taken beginning in 1993. Those aged 65 years or older were invited to participate in an interview, which included brief tests of cognitive function. The interview was subsequently repeated at intervals of approximately 3 years, with 5 waves of interviews completed at the time of these analyses.
Eligibility for analyses required Medicare data to assess hospitalization and completion of at least 3 interviews (including at least one before hospitalization) to make it possible to observe a shift in the rate of cognitive change over time. As of December 31, 2007, the baseline interview had been completed by 10,052 people. Medicare records were not available for 3,894 persons (enrolled in a health maintenance organization [n = 1,902]; Medicare number not provided [n = 349], not yet submitted [n = 561], or did not match data from the Centers for Medicare and Medicaid Services [n = 891]; and age less than 65 years at baseline [n = 191]). This left 6,158 persons eligible at baseline. They were similar in age to those without Medicare data (mean of 73.2 vs 73.3 years, t[7,706] = 1.03, p = 0.304), more apt to be men (42% vs 35%, χ2[1] = 54.9, p < 0.001) and white (41% vs 29%, χ2[1] = 138.9, p < 0.001), and had more years of schooling (mean of 12.3 vs 12.0, t[9,992] = 3.95, p < 0.001) and better cognitive function at baseline (mean Mini-Mental State Examination of 26.2 vs 25.5, χ2[1] = 30.7, p < 0.001). Of those with Medicare data, 2,101 died within 6 years of baseline (the time needed to complete 2 follow-up interviews) and 1,869 had been in the study less than 6 years, leaving 2,188 eligible for follow-up. Of these, 1,870 (85.5%) completed 2 or more follow-up visits. Analyses are based on this group. Compared with the 4,288 ineligible persons (i.e., 2,101 + 1,869 + 318), they were younger at baseline (mean of 72.7 vs 73.4, t[4,578.8] = 3.66, p < 0.001), more educated (mean of 12.9 vs 12.0, t[6,139] = 9.42, p < 0.001), less apt to be men (39% vs 43%, χ2[1] = 9.3, p = 0.002) and black (51% vs 62%, χ2[1] = 68.0, p < 0.001), and had better baseline cognitive function (mean Mini-Mental State Examination of 27.2 vs 25.7, χ2[1] = 131.8, p < 0.001).
Standard protocol approvals, registrations, and patient consents.
After a thorough description of the study, written informed consent was obtained from all subjects. The study was approved by the institutional review board of Rush University Medical Center.
Assessment of cognitive function.
Each interview included administration of 4 cognitive tests.20–22 Episodic memory was assessed by immediate and delayed recall of the East Boston Story. The oral version of the Symbol Digits Modalities Test was used to assess perceptual speed, a component of executive function. The Mini-Mental State Examination assessed global cognition. A composite of all 4 measures was the primary outcome. Raw scores on each measure were converted to z scores, using the baseline mean and SD of the population, and z scores were averaged to yield a composite measure of global cognition. In secondary analyses, we used the Symbol Digit Modalities Test score as a measure of executive function and a composite of immediate and delayed recall as a measure of episodic memory.23
Assessment of hospitalization and other covariates.
Data on hospital use from January 1993 through December 2007 were obtained from Part A Medicare beneficiary records. Analyses are based on the first hospitalization that occurred between the baseline interview and the last assessment of cognitive function for a given individual.
Severity of illness during hospitalization was assessed with the Charlson comorbidity index,24 a weighted measure that reflects the number and seriousness of diseases, and length of hospital stay. The definition of critical illness hospitalization was based on codes from the International Classification of Diseases, Ninth Revision, as specified in the Adult Changes in Thought study.18
Depressive symptoms were assessed at baseline with a 10-item version of the Center for Epidemiologic Studies Depression Scale.25 Disability was assessed at baseline with the Katz scale,26 a self-report measure of the ability to perform 6 basic activities of daily living (e.g., dressing and bathing).
Statistical analysis.
Mixed-effects regression models27 were used to characterize change in cognitive function. Each model allowed rate of cognitive change to shift after the first hospitalization. Random effects were included for the intercept, slope before hospitalization, and slope after hospitalization to allow for individual differences in initial level of cognitive function and rates of prehospital and posthospital change. Each model also included fixed effects for time before hospitalization (in years since baseline); time after hospitalization; and age, sex, race, education and their interactions with time before hospitalization. We examined the interactions of age, sex, race, and education with time after hospitalization and retained the only significant interaction (with age) in subsequent models. We repeated the initial analysis excluding persons hospitalized in the year before baseline cognitive assessment, excluding individuals first hospitalized after their last cognitive assessment, with terms for the interaction of time after hospitalization with Charlson score during hospitalization and length of hospital stay, and with a term for the interaction of time after hospitalization with an indicator for whether a critical illness was involved. We tested the effects of depressive symptoms and disability (in separate models) including terms for baseline level of the covariate and its interactions with time before and after hospitalization. Programming was done in SAS.28
RESULTS
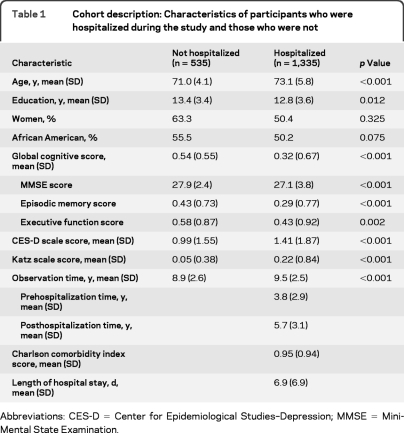
During a mean of more than 9 years of observation, 1,335 of 1,870 persons (71.4%) were hospitalized at least once. The initial hospitalization occurred a mean of 3.8 years after study onset (SD = 2.9) and lasted for a median of 5 days (interquartile range 3–8 days) with a median Charlson comorbidity Index score of 1 (interquartile range 0–2). There was a mean of 5.7 years of follow-up (SD = 3.1) after the initial hospitalization. Those who were hospitalized were older and less educated and had higher baseline levels of cognitive impairment, disability, and depression than those who were not hospitalized (table 1).
Table 1.
Cohort description: Characteristics of participants who were hospitalized during the study and those who were not
Abbreviations: CES-D=Center for Epidemiological Studies–Depression; MMSE=Mini-Mental State Examination.
Hospitalization and cognitive decline.
At study entry, scores on the composite measure of global cognition ranged from −3.03 to 1.54 (mean = 0.36, SD = 0.66). To characterize change on this measure, we used a mixed-effects regression model that allowed the rate of cognitive change to shift after the initial hospitalization and controlled for the potentially confounding effects of age, sex, race, and education.
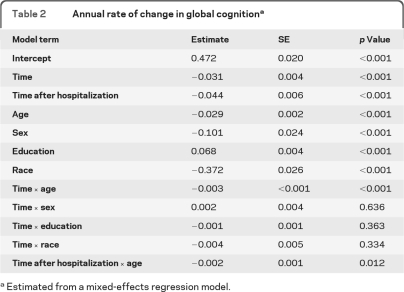
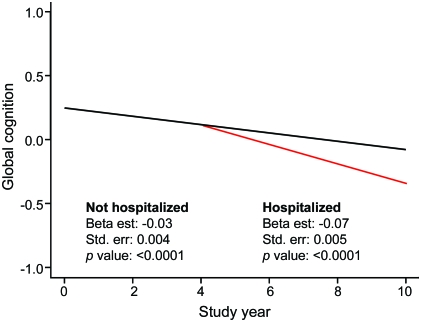
As shown in table 2, the composite measure of global cognition declined a mean of 0.031 unit per year before hospitalization and in those never hospitalized. After hospitalization, the rate accelerated by 0.044 unit to a mean loss of 0.075 unit per year, a more than 2.4-fold increase relative to the decline preceding hospitalization. This effect is shown in figure 1for a person hospitalized in year 4 (red line) compared with a person not hospitalized (solid line).
Table 2.
Annual rate of change in global cognitiona
Estimated from a mixed-effects regression model.
Figure 1. Typical path of posthospital change in global cognitive function.
Predicted 10-year paths of change in composite measure of global cognition for typical participants hospitalized in year 4 (red line) or not hospitalized (black line) from a mixed-effects model adjusted for age, sex, race, and education.
We considered the possibility that findings were biased by hospitalizations that occurred outside the cognitive data collection period. Results were essentially unchanged, however, in analyses that excluded the 222 individuals hospitalized in the year before their baseline cognitive assessment (estimate for time before hospitalization = −0.029, SE = 0.004, p < 0.001; estimate for time after hospitalization = −0.075, SE = 0.006, p < 0.001) or the 189 individuals who were hospitalized after their last cognitive assessment (estimate for time before hospitalization = −0.027, SE = 0.004, p < 0.001; estimate for time after hospitalization = −0.075, SE = 0.005, p < 0.001).
To see whether hospitalization was related to decline in some cognitive domains but not others, we repeated the analysis separately with episodic memory (mean = 0.33, SD = 0.76) and executive function (mean = 0.48, SD = 0.91) as outcome measures. There was a 3.3-fold increase in the rate of episodic memory decline, from a loss of 0.014 unit per year before hospitalization (SE = 0.005, p = 0.005) to 0.046 unit per year after hospitalization (SE = 0.006, p < 0.001) (top panel of figure e-1 on the Neurology® Web site at www.neurology.org). Decline on the executive function measure increased 1.7-fold, from a loss of 0.053 unit per year before hospitalization (SE = 0.004, p < 0.001) to 0.091 unit per year after hospitalization (SE = 0.005, p < 0.001) (bottom panel of figure e-1).
Modifying factors.
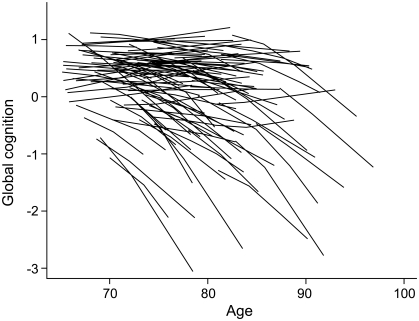
To examine individual differences in paths of cognitive change after hospitalization, we plotted the model-estimated paths for a 7.5% random sample of the cohort (figure 2). Much variability is evident, with posthospital cognitive function stable in some individuals but declining at rates ranging from gradual to rapid in others.
Figure 2. Individual differences in posthospital change in global cognitive function.
Person-specific paths of change in composite measure of global cognition estimated for a 7.5% random sample of participants from a mixed-effects model adjusted for age, sex, race, and education.
To determine whether prehospital cognitive functioning affected results, we examined the correlations among the person-specific deviations from the intercept and slopes estimated in the initial mixed-effects model. Posthospital rate of cognitive decline was not associated with baseline level of cognition (r = 0.01, p = 0.88) but was associated with prehospital cognitive decline (r = 0.56, p = 0.021). To further examine this effect, we plotted the predicted 10-year paths of cognitive change for 2 individuals hospitalized in year 4, one with average prehospitalization cognitive decline (50th percentile, black line) and the other with moderate decline (10th percentile, red line). Figure e-2 suggests that posthospital cognitive decline partly reflects an exacerbation of prehospital cognitive decline.
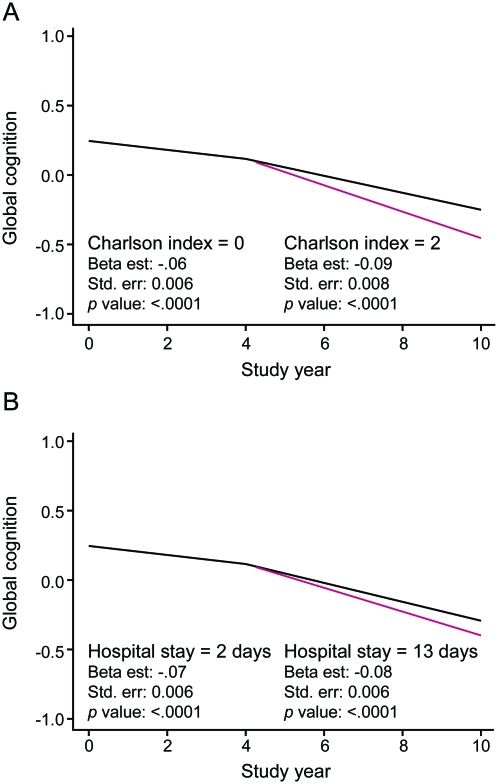
The Charlson comorbidity index score for the hospitalized participants ranged from a low of 0 to a high of 5 (median = 1, interquartile range = 0–2) and duration of hospitalization ranged from 1 to 92 days (median = 5, interquartile range = 3–8). To see whether these indicators of illness severity modified the relation of hospitalization to cognitive functioning, we repeated the initial analysis with terms for the interactions of time after hospitalization with illness severity and with duration of hospitalization. A higher Charlson score (estimate of interaction = −0.016, SE = 0.005, p < 0.001) and longer hospital stay (estimate of interaction = −0.0014, SE = 0.0006, p = 0.019) were each associated with more rapid cognitive decline after hospitalization. The overall impact of these variables on cognitive decline was modest, however, as shown by the predicted paths of cognitive change associated with high vs low Charlson scores (top panel of figure 3) and durations of hospitalization (bottom panel of figure 3).
Figure 3. Modification of posthospital change in global cognitive function.
Predicted 10-year paths of change in composite measure of global cognition for typical participants hospitalized in year 4 with a Charlson score of 0 (black line) vs 2 (red line) (A) or a hospital stay of 2 days (black line) vs 13 days (red line) (B), from mixed-effects models adjusted for age, sex, race, and education.
To see whether hospitalization involving a critical illness disproportionately contributed to results,18 we tested for an interaction between a critical illness at first hospitalization (n = 43) and time after hospitalization. There was no interaction (estimate = −0.009, SE = 0.020, p = 0.67).
Older age was associated with lower level of cognition at baseline and more rapid decline before and after hospitalization (table 2). This effect is shown in figure e-3 for a younger participant (baseline age 67, 10th percentile, solid line) compared with an older one (baseline age 81, 90th percentile, dotted line). Sex, education, and race were related to level of cognitive function but not to change before or after hospitalization (data not shown).
Because depression has been associated with both hospitalization29 and cognitive decline,30,31 we conducted an additional analysis with terms added for baseline score on the Center for Epidemiologic Studies Depression scale and its interactions with time. In this analysis, depressive symptoms were robustly related to baseline cognition (estimate = −0.032, SE = 0.007, p < 0.001), marginally related to prehospital cognitive decline (estimate = −0.003, SE = 0.002, p = 0.053) and unrelated to posthospital cognitive decline (estimate = −0.003, SE = 0.003, p = 0.26). Disability has also been linked to hospitalization1–3 and cognitive decline.32,33 In a subsequent analysis, baseline disability on the Katz scale was associated with cognition at baseline (estimate = −0.175, SE = 0.016, p < 0.001) but not with rate of cognitive decline before (estimate = 0.001, SE = 0.005, p = 0.90) or after (estimate = 0.002, SE = 0.008, p = 0.84) hospitalization.
DISCUSSION
As part of a longitudinal population-based study, change in cognitive function was assessed in more than 1,800 older people and Medicare records of hospitalization were obtained. During a mean of more than 9 years of observation, 71.4% of the participants were hospitalized at least once, and there was a more than 2.4-fold increase in the rate of cognitive decline after hospitalization. This finding persisted after controlling for severity of illness and prehospital cognitive decline. The results support the hypothesis that hospitalization in old age is associated with accelerated cognitive decline.
The association between hospitalization and cognitive decline has not been extensively investigated. One population-based study of older persons found that hospitalization was associated with increased decline on a brief mental status test during a 3-year period,17 consistent with the results of the present study. Yet decline was not observed on other outcome measures and was estimated from 2 measurement points, making it difficult to distinguish from level of function or to know whether it preceded or followed hospitalization. Another population-based study found that hospitalization of older people was associated with a lower level of cognitive function but not with cognitive decline.18 However, the power to detect an association between hospitalization and cognitive change was limited, possibly because of insufficient posthospitalization data (i.e., mean of 1.9 years of posthospital observation with cognitive testing at 2-year intervals). Thus, these studies may not have had enough longitudinal cognitive data to securely estimate the relation of hospitalization to cognitive decline.
The bases of the association of hospitalization with accelerated cognitive decline are uncertain. Cognitive dysfunction has been identified as a complication of critical illnesses34 and surgical procedures.35 Delirium is common in older patients with these conditions, affecting about 75% of those with a critical illness36 and 25%–65% of those undergoing surgery,37 and it has been associated with chronic cognitive impairment.38 In the present study, only 3% of hospitalizations involved a critical illness, but 15%–20% of older general medical inpatients are estimated to meet the criteria for delirium,39 and it is likely that many more exhibit subsyndromal signs of delirium.40 These data suggest that confusion and inattention due to acute brain dysfunction during hospitalization contributed to posthospital cognitive decline.
Individuals differed in the rate of posthospital cognitive decline. Those who were sicker and had longer hospital stays experienced more rapid decline after hospitalization, suggesting that the severity of the medical conditions that necessitated hospitalization may have contributed to accelerated cognitive decline. That these illness severity effects were relatively weak, however, suggests that other factors were also involved. Posthospital cognitive decline was also related to older age and more rapid prehospital rate of cognitive decline. Thus, individuals at elevated risk for cognitive decline or already experiencing it tended to experience a relatively greater posthospital increase in cognitive decline, suggesting that hospitalization may unmask cognitive symptoms in vulnerable older persons and perhaps that hospitalization is associated with declining cognitive competence. That more severe illness, older age, and prehospital cognitive impairment are also risk factors for delirium supports the idea that delirium partially mediates the association of hospitalization with subsequent cognitive decline.
Research on hospitalization and cognitive decline has focused mainly on global cognition, but one previous study found no association between hospitalization and change on 2 measures of episodic memory,17 suggesting that hospitalization might affect some cognitive functions and spare others. In the present study, however, hospitalization was robustly related to decline on a measure of executive function and on one of the same measures of episodic memory used in the previous study, supporting the idea that cognitive decline after hospitalization is global in nature.
Because late life loss of cognitive function is a substantial and growing public health problem, understanding its link to an event as common as hospitalization is extremely important. Further research may lead to the development of strategies to prevent or reduce effects of hospitalization on cognition, possibly by more effective primary prevention of medical problems leading to late life hospitalization or by changes to inpatient and discharge policies and procedures relating to cognitively impaired older people.
The strengths of these data should be noted. Participants were drawn from a geographically defined population, making it likely that a broad spectrum of hospital experiences and paths of cognitive change were represented and that results are generalizable. Cognition was assessed with psychometrically sound measures on 3–5 occasions with good participation in follow-up. This made it possible to estimate person-specific patterns of change in cognitive function and observe a shift in rate of change conditional on hospitalization.
The main shortcoming of the study is the 3-year interval between cognitive assessments. This limited our ability to track short-term changes in cognition around the time of hospitalization. Individuals needed to survive at least 6 years to be included in analyses, and data were only available for Medicare hospitalizations. These factors may have led us to underestimate the association of hospitalization with cognitive decline. In addition, lack of detailed data about the course of hospitalization made it difficult to discern the basis of the association.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study; Ann Marie Lane for community development and oversight of project coordination; Michelle Bos, Holly Hadden, Flavio LaMorticella, and Jennifer Tarpey for coordination of the study; Todd Beck for analytic programming; and the staff of the Rush Institute for Healthy Aging.
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Drafting/revising the manuscript for content: Dr. Wilson, Dr. Hebert, Dr. Scherr, Dr. Dong, Dr. Evans. Study concept or design: Dr. Wilson, Dr. Hebert, Dr. Dong, Dr. Leurgans. Analysis or interpretation of data: Dr. Wilson, Dr. Hebert, Dr. Dong, Dr. Leurgans. Acquisition of data: Dr. Evans. Statistical analysis: Dr. Hebert, Dr. Leurgans. Study supervision or coordination: Dr. Evans. Obtaining funding: Dr. Evans.
DISCLOSURE
Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging; has served as a consultant to Pain Therapeutics, Inc; and receives research support from the NIH/NIA. Dr. Hebert receives research support from the NIH/NIA. Dr. Scherr reports no disclosures. Dr. Dong receives support from the NIH/NIA. Dr. Leurgans receives research support from the NIH/NIA. Dr. Evans has served on a data monitoring committee for Eli Lily and Company and received research support from the NIH.
REFERENCES
- 1. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003; 51: 451– 458 [DOI] [PubMed] [Google Scholar]
- 2. Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004; 292: 2115– 2124 [DOI] [PubMed] [Google Scholar]
- 3. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA 2010; 304: 1919– 1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd CM, Xue Q-L, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med 2005; 118: 1225– 1231 [DOI] [PubMed] [Google Scholar]
- 5. Inouye SK, Wagner DR, Acampora D, et al. A predictive index for functional decline in hospitalized elderly medical patients. J Gen Intern Med 1993; 8: 645– 652 [DOI] [PubMed] [Google Scholar]
- 6. Sands LP, Yaffe K, Covinsky K, et al. Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders. J Gerontol A Biol Sci Med Sci 2003; 58: 37– 45 [DOI] [PubMed] [Google Scholar]
- 7. Pedone C, Ercolani S, Catani M, et al. Elderly patients with cognitive impairment have a high risk for functional decline during hospitalization: the GIFA Study. J Gerontol A Biol Sci Med Sci 2005; 60: 1576– 1580 [DOI] [PubMed] [Google Scholar]
- 8. Volpato S, Onder G, Cavalieri M, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med 2007; 22: 668– 674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med 2000; 160: 174– 180 [DOI] [PubMed] [Google Scholar]
- 10. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus, and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004; 61: 661– 666 [DOI] [PubMed] [Google Scholar]
- 11. Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142: 1470– 1476 [PubMed] [Google Scholar]
- 12. Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180: 134– 137 [DOI] [PubMed] [Google Scholar]
- 13. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med 2007; 69: 483– 489 [DOI] [PubMed] [Google Scholar]
- 14. Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc 2008; 56: 1845– 1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buchman AS, Wilson RS, Biennas JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology 2005; 65: 892– 897 [DOI] [PubMed] [Google Scholar]
- 16. Sturman MT, Mendes de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology 2008; 70: 360– 367 [DOI] [PubMed] [Google Scholar]
- 17. Chodosh J, Seeman TE, Keeler E, et al. Cognitive decline in high-functioning older persons is associated with an increased risk of hospitalization. J Am Geriatr Soc 2004; 52: 1456– 1462 [DOI] [PubMed] [Google Scholar]
- 18. Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010; 303: 763– 770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis 2003; 5: 349– 355 [DOI] [PubMed] [Google Scholar]
- 20. Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003; 61: 812– 816 [DOI] [PubMed] [Google Scholar]
- 21. Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined community. J Gerontol B Psychol Sci Soc Sci 1999; 54: P155– P160 [DOI] [PubMed] [Google Scholar]
- 22. Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology 2005; 30: 11– 17 [DOI] [PubMed] [Google Scholar]
- 23. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in population-based sample of older persons. Neurology 2002; 59: 1910– 1914 [DOI] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373– 383 [DOI] [PubMed] [Google Scholar]
- 25. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntly J. Two shorter forms of the CES-D depression symptoms index. J Aging Health 1993; 5: 179– 193 [DOI] [PubMed] [Google Scholar]
- 26. Katz S. Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976; 6: 493– 508 [DOI] [PubMed] [Google Scholar]
- 27. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38: 963– 974 [PubMed] [Google Scholar]
- 28. SAS Institute, Inc SAS 9.3 Help and Documentation. Cary, NC: SAS Institute Inc., 2002–2011. [Google Scholar]
- 29. Laudisio A, Marzetti E, Pagano F, Pozzi G, Bernabel R, Zuccala G. Depressive symptoms are associated with hospitalization, but not with mortality in the elderly: a population-based study. Aging Ment Health 2010; 14: 955– 961 [DOI] [PubMed] [Google Scholar]
- 30. Sachs-Ericsson N, Joiner T, Plant EA, Blazer DG. The influence of depression on cognitive decline in community-dwelling elderly persons. Am J Geriatr Psychiatry 2005; 5: 402– 408 [DOI] [PubMed] [Google Scholar]
- 31. Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older person. J Neurol Neurosurg Psychiatry 2004; 75: 126– 129 [PMC free article] [PubMed] [Google Scholar]
- 32. Njegovan V, Hing MM, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older person. J Gerontol A Biol Sci Med Sci 2001; 56: M638– M643 [DOI] [PubMed] [Google Scholar]
- 33. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc 2010; 58: 889– 894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj 2006; 20: 263– 271 [DOI] [PubMed] [Google Scholar]
- 35. Newman M, Kirchner J, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001; 344: 395– 402 [DOI] [PubMed] [Google Scholar]
- 36. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an underrecognized syndrome of organ dysfunction. Semin Respir Crit Care Med 2001; 22: 115– 126 [DOI] [PubMed] [Google Scholar]
- 37. O'Keefe ST, Chonchubhair AN. Postoperative delirium in the elderly. Br J Anaesth 1994; 73: 673– 687 [DOI] [PubMed] [Google Scholar]
- 38. Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev 2004; 14: 87– 98 [DOI] [PubMed] [Google Scholar]
- 39. Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152: 334– 340 [DOI] [PubMed] [Google Scholar]
- 40. Ouimet S, Riker R, Bergeon N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care 2007; 33: 1007– 1013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.