Abstract
The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent lung carcinogen. Previously, we have demonstrated that NNK-induced lung tumorigenesis in mice depends on target-tissue bioactivation by pulmonary cytochrome P450 (P450) enzymes. The present study was designed to test the hypothesis that mouse CYP2A5 plays an essential role in NNK bioactivation in mouse lung. The role of CYP2A5 in NNK bioactivation was studied both in vitro and in vivo, by comparing the kinetic parameters of microsomal NNK metabolism and tissue levels of O6-methylguanine (O6-mG) (the DNA adduct highly correlated with lung tumorigenesis) between wild-type (WT) and Cyp2a5-null mice. In both liver and lung microsomes, the loss of CYP2A5 resulted in significant increases in the apparent Km values for the formation of 4-oxo-4-(3-pyridyl)butanone, which represents the reactive intermediate that produces O6-mG in vivo. The loss of CYP2A5 did not change circulating levels of NNK or 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in mice treated intraperitoneally with NNK at either 20 or 100 mg/kg. However, the levels of lung O6-mG were significantly lower in Cyp2a5-null than in WT mice; the extent of the reduction was greater at the 20 mg/kg dose (∼40%) than at the 100 mg/kg dose (∼20%). These results indicate that CYP2A5 is the low-Km enzyme for NNK bioactivation in mouse lung. It is noteworthy that the remaining NNK bioactivation activities in the Cyp2a5-null mice could be inhibited by 8-methoxypsoralen, a P450 inhibitor used previously to demonstrate the role of CYP2A5 in NNK-induced lung tumorigenesis. Thus, P450 enzymes other than CYP2A5 probably also contribute to NNK-induced lung tumorigenesis in mice.
Introduction
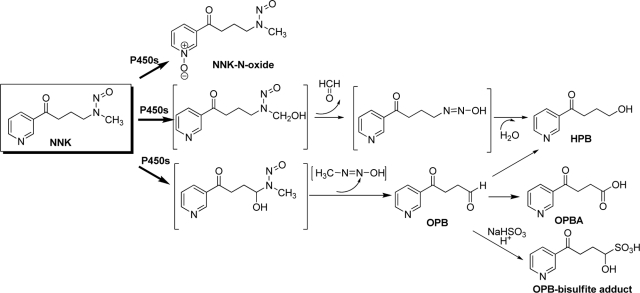
The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent lung procarcinogen in laboratory animals and a human carcinogen (Hecht et al., 1989; International Agency for Research on Cancer, 2007). The carcinogenic effects of NNK depend on cytochrome P450 (P450)-mediated NNK α-hydroxylation at the methylene group or the methyl group, generating two distinct electrophilic intermediates, which can, respectively, methylate and pyridyloxobutylate DNA (Hecht, 1998). The methylene hydroxylation and methyl hydroxylation pathways also produce 4-oxo-4-(3-pyridyl)-butanone (OPB) and 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB), respectively, which are used to represent activities of the respective α-hydroxylation pathways (Scheme 1). Multiple human and mouse P450s are active in the bioactivation of NNK in vitro, including human CYP1A, CYP2A, CYP2B, CYP2D, and CYP3A and mouse CYP1A, CYP2A, and CYP2B enzymes (Smith et al., 1993; Hecht, 1998; Jalas et al., 2005). On the other hand, NNK can be metabolized by P450 to form NNK-N-oxide, which results in detoxification (Castonguay et al., 1983). NNK is also extensively reduced by carbonyl reductase in the liver, forming 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), the major circulating metabolite of NNK. Both NNAL and NNAL glucuronide are excreted in the urine (Hecht, 1998).
Scheme 1.
P450-mediated biotransformation of NNK (modified from Jalas et al., 2005).
The formation and persistence of O6-methylguanine (O6-mG), through the methylene hydroxylation pathway, were found to be critical in the initiation of lung tumoregenesis by NNK in mice (Peterson and Hecht, 1991). The production of pyridyloxobutylated DNA, through the methyl hydroxylation pathway, also plays an important role in NNK-induced lung tumorigenesis, because the pyridyloxobutyl DNA adducts can inhibit O6-alkylguanine-DNA-alkyltransferase, the enzyme responsible for the repair of O6-mG (Peterson et al., 1993). Gene mutations caused by the formation of DNA adducts on the K-ras oncogene have been found to be characteristic of NNK-induced lung tumors in mice (Ronai et al., 1993) and smoking-related lung adenocarcinoma in humans (Westra et al., 1993; Ahrendt et al., 2001).
We have demonstrated previously that, although hepatic P450-mediated NNK metabolism influences the bioavailability of NNK in the lung, metabolic activation of NNK in the target tissue (the lung) plays an essential role in NNK-induced lung carcinogenesis (Weng et al., 2007). The specific P450 enzymes responsible for NNK bioactivation in the lung have not been identified. Human CYP2A13, known to be expressed selectively in the respiratory tract (Su et al., 2000), displayed greater enzymatic efficiency in catalyzing both methyl and methylene α-hydroxylation of NNK than did any other P450 enzymes examined (Su et al., 2000; Jalas et al., 2005). It has been proposed that CYP2A13 plays a critical role in the bioactivation of NNK and NNK-induced initiation of lung cancer in human smokers.
There is also evidence suggesting that CYP2A5, the ortholog of human CYP2A13 in mice, is essential for NNK bioactivation and tumorigenesis in the mouse lung. NNK readily induces lung adenocarcinoma after a single intraperitoneal injection of the carcinogen to mice (Hecht et al., 1989). CYP2A5, which is expressed in the lung, as well as in liver, kidney, and nasal mucosa (Su et al., 1996), is efficient in the bioactivation of NNK, as indicated by in vitro studies with recombinant CYP2A5 (Felicia et al., 2000; Jalas et al., 2003). Furthermore, NNK-induced lung tumorigenesis in mice was strongly inhibited by 8-methoxypsoralen (8-MOP) (Miyazaki et al., 2005), a compound often used as an inhibitor of CYP2A enzymes (Koenigs et al., 1997; Visoni et al., 2008; von Weymarn et al., 2005), although it is also capable of inhibiting CYP1A2 and CYP2E1 at higher concentrations (Labbe et al., 1987; Apseloff et al., 1991). However, direct proof for the role of CYP2A5 or CYP2A13 in mediating NNK bioactivation in vivo has yet to be obtained.
In the present study, we have directly determined the role of CYP2A5 in NNK bioactivation, both in vitro and in vivo, by comparing the kinetic parameters of microsomal NNK metabolism and tissue levels of O6-mG between wild-type (WT) and Cyp2a5-null mice. The Cyp2a5-null mouse model, produced through germ-line deletion of the last Cyp2a5 coding exon, has been used successfully in studies on the role of CYP2A5 in the clearance of nicotine and cotinine (Zhou et al., 2010) and the bioactivation of nasal toxicants 2,6-dichlorobenzonitrile (Xie et al., 2010) and methimazole (Xie et al., 2011). Here, we present evidence that CYP2A5 is the low-Km enzyme for NNK bioactivation in mouse lung and P450s other than CYP2A5 probably also contribute to NNK-induced lung tumorigenesis in mice.
Materials and Methods
Chemicals and Reagents.
HPB, (3,3,4,4-D4)-HPB (D4-HPB), NNK-N-oxide, and 4-oxo-4-(3-pyridyl)-butyric acid (OPBA) were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). 8-MOP, sodium bisulfite, and reduced NADPH were purchased from Sigma-Aldrich (St. Louis, MO). OPB was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The sources of NNK, NNAL, 4-(methylnitrosamino)-1-(3-[2,4,5,6-D4]-pyridyl)-1-butanone (D4-NNK), 4-(methylnitrosamino)-1-(3-[2,4,5,6-D4]-pyridyl)-1-butanol (D4-NNAL), O6-mG, O6-trideuteriomethyl-guanosine, O6-methyl-deoxyguanosine, and O6-trideuteriomethyl-deoxyguanosine were the same as described previously (Weng et al., 2007). All solvents (acetonitrile, methanol, and water) were of high-performance liquid chromatography (LC) grade (Thermo Fisher Scientific, Waltham, MA).
Determination of Catalytic Activity.
Lung and liver microsomes were prepared as described previously (Ding and Coon, 1990). Protein concentration was determined by the bicinchoninic acid method (Thermo Fisher Scientific) with bovine serum albumin as the standard. For the identification of microsomal NNK metabolites, assay mixtures contained 100 mM potassium phosphate buffer, pH 7.4, 10 μM NNK, 0.5 mg/ml liver or lung microsomal protein, 0 or 5 mM sodium bisulfite (as a trapping agent for OPB; Peterson et al., 1991), and 1.0 mM NADPH in a final volume of 0.2 ml. Reactions were carried out at 37°C for 30 min and terminated by the addition of 0.2 ml of acetonitrile. NADPH was omitted in negative control incubations. The apparent Km and Vmax values were determined by using a broad range of NNK concentrations (0.5, 1, 2.5, 10, 25, 50, 100, and 200 μM) with 0.25 mg/ml liver or lung microsomal protein from 2-month-old female WT A/J or B6 mice or Cyp2a5-null mice (on B6 genetic background); the reactions were carried out for 10 min (for liver) or 30 min (for lung) at 37°C, conditions under which the rates of product formation were linear with time, in the presence of 5 mM sodium bisulfite and 1 mM NADPH.
To determine the effects of 8-MOP on NNK bioactivation, 8-MOP (0, 0.5, or 2.5 μM) was first added (in 2 μl of 50% methanol in H2O) to “preincubation” mixtures consisting of 100 mM potassium phosphate buffer, pH 7.4, microsomal proteins (0.25 mg/ml) from 2-month-old Cyp2a5-null female mice, and 1 mM NADPH in a total volume of 0.2 ml, and the mixture was incubated at 30°C for 20 min. NNK and sodium bisulfite were then added to the preincubation mixture to final concentrations of 5 μM and 5 mM, respectively, and a final volume of 0.22 ml, and the incubation was continued at 30°C for another 10 min (for liver) or 30 min (for lung). A lower temperature (30°C) was used, according to a published protocol (von Weymarn et al., 2005), to minimize NADPH-dependent activity losses in the control samples (with 0 μM 8-MOP).
For all reactions, internal standards (500 pg each of D4-HPB and D4-NNK) were added after the microsomal reactions were terminated with acetonitrile. The resultant mixtures were spun to remove precipitated protein; the supernatant was dried with nitrogen and then reconstituted in 50 μl of 50% (v/v) acetonitrile and 0.05% (w/v) formic acid in water for LC-mass spectrometry (MS) analysis.
Animal Studies.
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. For determination of NNK pharmacokinetics, 2-month-old WT B6 and Cyp2a5-null female mice were given a single intraperitoneal injection (at 9:00–10:00 AM local time) of NNK (at 20 or 100 mg/kg) in saline. Blood samples (∼20 μl each) were collected from the tails of individual mice at various time points (10 min to 4 h) after the injection. The samples were centrifuged at 1000g for 5 min at 4°C for preparation of plasma. For determination of tissue levels of O6-mG DNA adduct, liver and lung were obtained at 4 h after NNK injection (at 20 or 100 mg/kg). For studies on in vivo inhibition of NNK clearance and bioactivation by 8-MOP, 2-month-old Cyp2a5-null female mice were pretreated via oral gavage with 8-MOP at 0 (vehicle control), 12.5, or 50 mg/kg in 0.2 ml of corn oil daily for 3 days, essentially as described previously (Takeuchi et al., 2003). One hour after the final dose of 8-MOP, NNK was given (at 100 mg/kg i.p.), and the blood and tissue samples were collected as described above. Plasma and tissue samples were processed for LC-MS analysis of NNK/NNAL and O6-mG, respectively, essentially as described previously (supplementary materials in Weng et al., 2007).
LC-MS Analytical Methods.
A LC-MS system composed of an Agilent 1200 Series high-performance LC and an ABI 4000 Q-Trap mass spectrometer (Applied Biosystems, Foster City, CA) was used. Analytes were resolved by using a 5-μm Gemini C18 column (100 × 2.0 mm; Phenomenex, Torrance, CA). The mass spectrometer was operated in a positive ion mode with an electrospay ionization source. The parameters for the chamber were as follows: curtain gas, 30 psi; heated nebulizer temperature, 350°C; ion spray voltage, 4500 V; nebulizer gas, 50 psi; turbo gas, 50 psi, declustering potential, 50 V; and entrance potential, 10 V.
For the identification of NNK metabolites, the mobile phase consisted of solvent A (0.05% formic acid in water) and solvent B (0.05% formic acid in acetonitrile). Samples were eluted at a flow rate of 0.3 ml/min with 100% A for 2 min followed by linear increases from 0% B to 100% B between 2 and 15 min, and then 100% B for another 2.5 min. The mass spectrometer was set for the information-dependent acquisition scan mode, using multiple ion monitoring-dependent enhanced product ion (MIM-EPI) acquisition, essentially as described by Yao et al. (2008)).
For quantitative analysis of NNK metabolites, shorter gradients (linear increase from 0% B to 90% B between 1 to 8 min, followed by a wash at 90% B for 2 min) were used, with formic acid either included (acidic condition, for OPB-bisulfite determination) or omitted (neutral condition, for HPB and NNK-N-oxide determination). Under acidic conditions, retention times for OPB-bisulfite, HPB (and D4-HBP), NNK-N-oxide, and NNK (and D4-NNK) were 5.5, 5.8, 6.2, and 6.7 min, respectively; under neutral conditions, the retention times for HPB, NNK-N-oxide, and NNK were 6.5, 6.2, and 7.2 min, respectively, whereas OPB-bisulfite was unstable as reported previously (Peterson et al., 1991).
The parent/product ion pairs of m/z 166/106 (for HPB), m/z 224/177 (for NNK-N-oxide), m/z 246/164 (for bisulfite-trapped OPB), m/z 170/106 (for D4-HPB), and m/z 212/126 (for D4-NNK) (each representing transitions of the molecular ion to the most abundant product ion) were monitored in the multiple reaction monitoring (MRM) scan mode. For preparation of calibration curves, boiled microsomal preparations were mixed with increasing amounts of OPB, HPB, and NNK-N-oxide standards in the presence of 5 mM sodium bisulfite. The standard mixtures were incubated, and then processed as described under Determination of Catalytic Activity for assay samples. The detection limits for OPB-bisulfite (246/164), HPB (166/106), and NNK-N-oxide (224/177) were 0.3, 0.03, and 0.05 pmol (on column), respectively. The recoveries for metabolite standards were >80%.
The same LC-MS system was used for determination of the levels of NNK, NNAL, and O6-mG, except that a shorter Gemini C18 column (5-μm, 50 × 2.0 mm; Phenomenex) was used, and the samples were eluted at a flow rate of 0.25 ml/min with solvent A (water) and solvent B (acetonitrile). The column was equilibrated with 100% A for 1 min, and the solvent gradient consisted of linear increases from 0% B to 100% B between 1 and 5 min, followed by a wash at 100% B for 2.5 min. The parent/product ion pairs of m/z 208/122 (for NNK), m/z 210/93 (for NNAL), m/z 212/126 (for D4-NNK), m/z 214/97 (for D4-NNAL), m/z 166/149 (for O6-mG), and m/z 169/152 (for O6-trideuteriomethyl-guanosine) were monitored in the MRM scan mode. The retention time was 6.1 min for O6-mG and O6-trideuteriomethyl-guanosine, 6.7 min for NNK and D4-NNK, and 7.3 min for NNAL and D4-NNAL. Calibration curves for NNK, NNAL, and O6-mG were prepared, and the levels of total guanine were determined as described previously (Weng et al., 2007). The detection limits for NNK, NNAL, and O6-mG were 0.05, 0.05, and 0.03 pmol (on column), respectively.
Statistics.
Nonlinear regression and enzyme kinetic analyses were performed with GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). Student's t test was used for comparisons between two groups. One-way ANOVA (or one-way ANOVA on ranks when data failed normality test), followed by Tukey's post hoc test (when p was less than 0.05), was used for comparisons among multiple groups.
Results
Analysis of NNK Metabolite Formation Using LC-MS.
Details of the LC-MS analysis for the identification of NNK metabolites formed by mouse liver and lung microsomes (with or without the addition of sodium bisulfite for trapping the unstable OPB and preventing conversion of OPB to HPB) are shown in the Supplemental Material, including total ion chromatograms of the MIM scans (Supplemental Fig. 1) and the MS/MS spectra obtained in the EPI scan mode for the detected metabolites (Supplemental Fig. 2). Identification of the parent compound (NNK, m/z = 208) and the stable metabolites (NNAL, m/z = 212; HPB, m/z = 166; OPBA, m/z = 180; and NNK-N-oxide, m/z = 224) was confirmed by coelution with authentic standards and matching of MS/MS spectra. A standard for OPB-bisulfite was not available. The MS/MS spectrum of the assumed OPB-bisulfite molecular ion, at m/z 246, revealed two major product ions, at m/z 164 (loss of a sulfur dioxide and a water) and m/z 148 (loss of a sulfur trioxide and a water) (Supplemental Fig. 2E), both indicative of cleavage of the bisulfite moiety. The identification of OPB-bisulfite was further supported by the disappearance of OPBA (the oxidative product of OPB) upon inclusion of sodium bisulfite in the reaction mixture (Supplemental Fig. 1) and the bisulfite-dependent and OPB concentration-dependent formation of the m/z 246 ion in incubations of OPB with liver microsomes (data not shown).
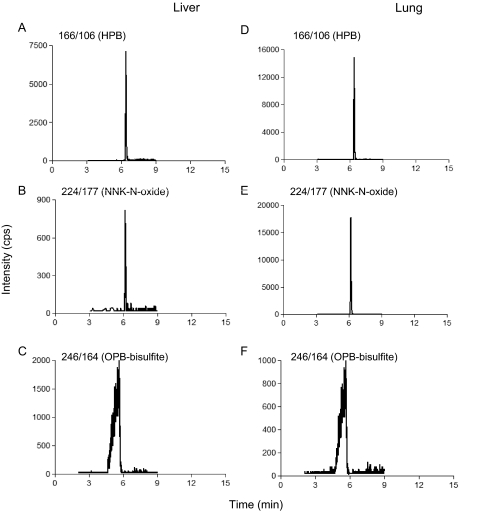
Based on the qualitative data obtained in the MIM-EPI scan mode, methods for the quantitative analysis of NNK-N-oxide, HPB, and OPB-bisulfite were established in the MRM scan mode. Typical LC-MS chromatograms for the detection of the NNK metabolites are shown in Fig. 1. It is notable that OPB-bisulfite, although barely detected in mouse lung microsomal incubations in the MIM-EPI scan mode (Supplemental Fig. 1), was readily detectable in the more sensitive MRM mode (Fig. 1). To test the validity of the LC-MS method, the apparent Km and Vmax values for the formation of OPB and HPB by liver and lung microsomes from B6 and A/J female mice were determined (Supplemental Table 1); the kinetic parameters determined, which were nearly identical between A/J and B6 mice, were comparable with reported values (Peterson et al., 1991; Nunes et al., 1998; Jalas et al., 2003).
Fig. 1.
Typical LC-MS chromatograms for detection of NNK metabolites. Extracted LC-MS chromatograms for individual metabolites detected in reactions with liver (A–C) and lung (D–F) microsomes from WT mice, obtained in the MRM scan mode for quantitative analysis of metabolite levels, are shown for HPB (A and D), NNK-N-oxide (B and E), and OPB-bisulfite (C and F), with NNK substrate concentration at 2.5 μM. The chromatograms for OPB-bisulfite were obtained under acidic conditions, whereas those for HPB and NNK-N-oxide were obtained under neutral conditions. Microsomal incubations were performed without (negative controls) or with NADPH, and sodium bisulfite was added to some samples to trap OPB, as described under Materials and Methods. No signal was detected when NADPH was omitted from the incubations (data not shown). The parent/product ion pairs of m/z 166/106 (for HPB), m/z 224/177 (for NNK-N-oxide), and m/z 246/164 (for bisulfite-trapped OPB) were monitored. Metabolite identification using the MIM-EPI acquisition mode and the resultant MS/MS product-ion spectra of the detected metabolites are shown in Supplemental Figs. 1 and 2.
Role of CYP2A5 in NNK Bioactivation In Vitro.
The contribution of CYP2A5 to NNK bioactivation in liver and lung microsomal reactions was determined by comparing activities between Cyp2a5-null mice (on B6 genetic background) and WT B6 mice. Female mice were studied, given the preferred use of female mice in NNK lung tumorigenesis bioassays (Hecht et al., 1989); in vitro NNK bioactivation by mouse lung and liver microsomes did not display obvious sex difference in the WT or Cyp2a5-null mice (data not shown). As shown in Table 1, the apparent Km values for the formation of OPB by lung microsomes from Cyp2a5-null mice were significantly higher than those of WT mice, although the Vmax values were not different. In contrast, there was no significant difference in the apparent Km and Vmax values for the formation of HPB or the detoxification product, NNK-N-oxide. Higher Km and lower Vmax values were observed for the formation of HPB by liver microsomes from Cyp2a5-null mice than the values for WT B6 mice (Table 1). Higher Km values were also observed for the formation of OPB by liver microsomes from Cyp2a5-null mice, although the Vmax values remained unchanged. In contrast, no difference was observed in the kinetic parameters for the formation of NNK-N-oxide by liver microsomes from the WT and the Cyp2a5-null mice.
TABLE 1.
Kinetic parameters for the formation of NNK metabolites by mouse lung and liver microsomes
Apparent Km and Vmax values for the microsomal formation of three major NNK metabolites (HPB, NNK-N-oxide, and sodium bisulfite-trapped OPB) were determined as described under Materials and Methods. Values represent means ± S.D. of values determined for three separate microsomal samples, each prepared from tissues pooled from five 2-month-old female mice.
| Metabolite | Strain | Lung |
Liver |
||||
|---|---|---|---|---|---|---|---|
| Km | Vmax | Vmax/Km | Km | Vmax | Vmax/Km | ||
| μM | pmol/min/mg | μM | pmol/min/mg | ||||
| OPB | WT (B6) | 28.0 ± 1.9 | 57.3 ± 1.8 | 2.0 | 29 ± 4 | 220 ± 10 | 7.6 |
| Cyp2a5-null | 40.2 ± 2.1a | 61.5 ± 2.0 | 1.5 | 55 ± 5a | 210 ± 10 | 4.0 | |
| HPB | WT (B6) | 2.8 ± 0.3 | 24.3 ± 0.5 | 8.7 | 18 ± 3 | 105 ± 5 | 5.9 |
| Cyp2a5-null | 2.9 ± 0.3 | 24.4 ± 1.0 | 8.4 | 65 ± 15a | 70 ± 8a | 1.1 | |
| NNK-N-oxide | WT (B6) | 1.6 ± 0.2 | 35.8 ± 3.1 | 22.4 | 84 ± 18 | 19 ± 2 | 0.2 |
| Cyp2a5-null | 1.9 ± 0.3 | 38.7 ± 2.2 | 20.4 | 83 ± 16 | 20 ± 2 | 0.2 | |
p < 0.01, compared with WT mice, Student's t test.
Pharmacokinetic Studies of Plasma NNK and NNAL in WT and Cyp2a5-Null Mice.
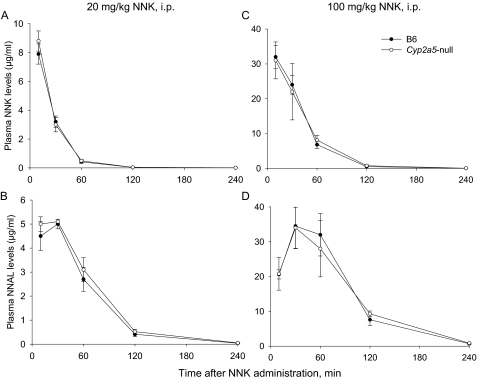
To determine whether the loss of CYP2A5 in the Cyp2a5-null mice affects systemic elimination of NNK and its major circulating metabolite NNAL, plasma levels of NNK and NNAL were measured in WT and Cyp2a5-null mice after NNK administration at either 20 mg/kg (Fig. 2, A and B) or 100 mg/kg (Fig. 2, C and D). As expected, the pharmacokinetic profiles of plasma NNK or NNAL were not different between the two mouse strains at either of the tested doses. The result indicated that neither hepatic nor lung CYP2A5 contributes significantly to the systemic clearance of NNK or NNAL.
Fig. 2.
Comparisons of NNK and NNAL clearance between WT and Cyp2a5-null mice. WT B6 and Cyp2a5-null mice (2 months old; female) were injected with 20 mg/kg NNK (A and B) or 100 mg/kg NNK (C and D) intraperitoneally, and levels of NNK (A and C) and NNAL (B and D) were determined in the plasma at various times after the injection. Data represent means ± S.D. (n = 4). No significant difference was found between the two strains, at any time point, for either NNK or NNAL (Student's t test).
Role of CYP2A5 in the Bioactivation of NNK In Vivo.
The levels of O6-mG in the lung, which have been reported to correlate with incidences of lung tumors induced by NNK administration in mice (Peterson and Hecht, 1991), were determined as the genotoxic endpoint representing the extent of NNK's in vivo bioactivation, and its lung tumorigenic potential, in WT and Cyp2a5-null mice. The DNA adduct levels were determined at 4 h after NNK injection, a time point that was previously shown to display changes in adduct levels resulting from decreased NNK bioactivation in the lungs of the lung-Cpr-null mice (Weng et al., 2007). After NNK treatment at 100 mg/kg, a standard dose used in lung tumorigenesis bioassays in mice (e.g., Weng et al., 2007), the levels of O6-mG were found to be ∼20% lower in the lungs of Cyp2a5-null than in the lungs of WT mice (Table 2). At a lower (20 mg/kg) NNK dose, the O6-mG levels were >40% lower in the lungs of Cyp2a5-null than in the lungs of WT mice. These results indicate that CYP2A5 contributed to NNK bioactivation in the mouse lung in vivo, and CYP2A5's contribution was much greater at the lower NNK dose tested, consistent with the results of in vitro studies showing that CYP2A5 is a low-Km enzyme for catalyzing the formation of OPB in lung microsomes (Table 1).
TABLE 2.
NNK-induced O6-mG adduct formation in the liver and lung
Two-month-old WT B6 and Cyp2a5-null female mice were injected with NNK at a dose of 20 or 100 mg/kg i.p., and tissue levels of O6-mG and total guanine were determined at 4 h after the injection, as described under Materials and Methods. Data represent means ± S.D. (n = 8).
| NNK Dose | Strain | O6-mG Level |
|
|---|---|---|---|
| Lung | Liver | ||
| pmol/μmol guanine | |||
| 20 mg/kg | WT (B6) | 5.4 ± 0.5 | 115 ± 13 |
| Cyp2a5-null | 3.3 ± 0.8a | 108 ± 18 | |
| 100 mg/kg | WT (B6) | 23.6 ± 1.6 | 594 ± 70 |
| Cyp2a5-null | 18.8 ± 1.7b | 604 ± 48 | |
p < 0.01, compared with WT mice; Student's t test.
p < 0.05, compared with WT mice; Student's t test.
In contrast to the situation in the lung, the O6-mG levels in the livers of WT and Cyp2a5-null mice were not different at 4 h after NNK treatment at either dose. Thus, under the dosing conditions used, CYP2A5 does not contribute substantially to NNK bioactivation in the liver in vivo, despite the in vitro data showing that CYP2A5 is the low-Km enzyme for catalyzing the formation of both OPB and HPB in liver microsomes (Table 1). This result is also consistent with the lack of an impact of the CYP2A5 loss on NNK systemic clearance in our pharmacokinetics study (Fig. 2).
Effects of 8-MOP on the Clearance and Bioactivation of NNK.
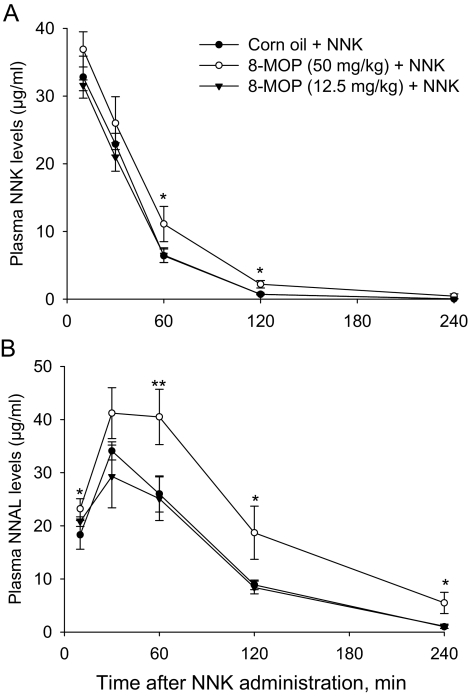
The ability of orally administered 8-MOP (at 50 or 12.5 mg/kg; Takeuchi et al., 2003) to inhibit NNK-induced lung tumorigenesis in female A/J mice was attributed to the inhibition by 8-MOP of CYP2A5-mediated NNK bioactivation (Miyazaki et al., 2005). Given our finding of a relatively high remaining NNK bioactivation activity in the lungs of the Cyp2a5-null mice, we further tested whether the remaining bioactivation activities were also inhibited by 8-MOP, with NNK given at 100 mg/kg, as was in the original study (Takeuchi et al., 2003). Contrary to the lack of effects of CYP2A5 loss on NNK clearance (Fig. 2), the rates of NNK clearance were decreased in the Cyp2a5-null mice by pretreatment of mice with 8-MOP at 50 mg/kg, as indicated by the increased plasma NNK and NNAL levels at various time points after NNK administration (Fig. 3). However, despite the increased NNK bioavailability, the levels of O6-mG in the lungs and livers were decreased, by ∼70%, in the Cyp2a5-null mice pretreated with 8-MOP at 50 mg/kg (Table 3). In Cyp2a5-null mice pretreated with 8-MOP at the lower (12.5 mg/kg) dose, which did not result in a change in NNK clearance (Fig. 3), the levels of O6-mG were also reduced, by 40 and 30% in the livers and lungs, respectively (Table 3). These results, which indicate that the 8-MOP treatment in WT mice (Takeuchi et al., 2003; Miyazaki et al., 2005) inhibited not only CYP2A5, but also other P450s in the lungs and livers, strongly suggest that P450 enzymes other than CYP2A5 also contribute to NNK-induced lung tumorigenesis in the standard NNK lung tumor bioassay.
Fig. 3.
Effects of 8-MOP pretreatment on the clearance of NNK (A) and NNAL (B) in Cyp2a5-null mice. Mice (2–3 months old, female) were pretreated with 8-MOP (at 0, 12.5, or 50 mg/kg, in 0.2 ml of corn oil, via oral gavage) once daily for 3 consecutive days. NNK was administered (at 100 mg/kg i.p.) 1 h after the final dose of 8-MOP. Data represent means ± S.D. (n = 4). **, p < 0.01; *, p < 0.05, compared with the 0-mg/kg 8-MOP (vehicle alone) group (one-way ANOVA, followed by Tukey's post hoc test).
TABLE 3.
Effects of 8-MOP on NNK-induced O6-mG adduct formation in the liver and lung of Cyp2a5-null mice
Two-month-old female Cyp2a5-null mice were pretreated with 8-MOP (at 0, 12.5, or 50 mg/kg, p.o.) in 0.2 ml of corn oil once daily for 3 consecutive days. One hour after the final injection of 8-MOP, NNK was given at a dose of 100 mg/kg i.p., and tissue levels of O6-mG and total guanine were determined at 4 h after the NNK injection. Data represent means ± S.D. (n = 4).
| 8-MOP Dose | O6-mG Level |
|
|---|---|---|
| Lung | Liver | |
| pmol/μmol guanine | ||
| 0 mg/kg | 19.0 ± 1.8 | 590 ± 21 |
| 12.5 mg/kg | 13.4 ± 1.5a | 337 ± 70b |
| 50 mg/kg | 6.2 ± 3.0b | 170 ± 99b |
p < 0.05, compared with the 0 mg/kg 8-MOP (vehicle control) group (one-way ANOVA followed by Tukey's post hoc test).
p < 0.01, compared with the 0 mg/kg 8-MOP (vehicle control) group (one-way ANOVA followed by Tukey's post hoc test).
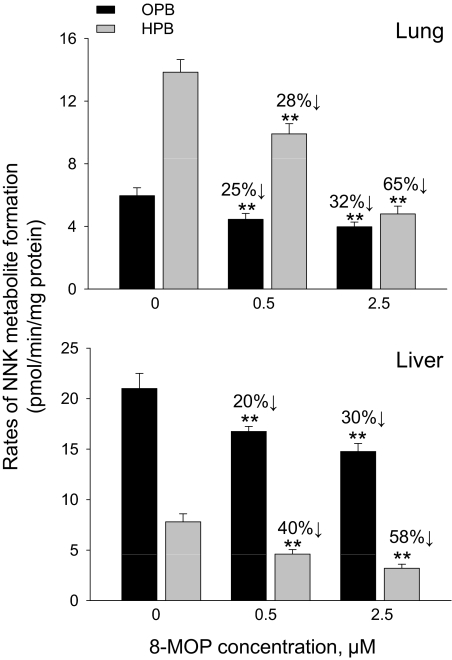
The effects of 8-MOP on NNK bioactivation were further investigated in vitro, using lung and liver microsomes from Cyp2a5-null mice, and a 8-MOP preincubation scheme to mimic the pretreatment paradigm used in vivo, given the potential role of 8-MOP as mechanism-based, competitive, and noncompetitive P450 inhibitors (Visoni et al., 2008). A pilot study (data not shown) indicated that 20-min preincubation with 8-MOP led to maximal inhibition of the activities toward NNK in either lung or liver microsomes from Cyp2a5-null mice. As shown in Fig. 4, the formation of both OPB and HPB was inhibited in a 8-MOP dose-dependent manner.
Fig. 4.
The effects of 8-MOP on NNK bioactivation in lung and liver microsomes from Cyp2a5-null mice. The rates of formation of OPB and HPB were determined for lung (top) and liver (bottom) microsomal samples that had been preincubated with 0, 0.5, or 2.5 μM 8-MOP, as described under Materials and Methods, with NNK at 5 μM. Values represent means ± S.D. of data from three separate microsomal samples, each prepared from pooled tissues from five 2-month-old female Cyp2a5-null mice. **, p < 0.01, compared with the 0-μM 8-MOP group (one-way ANOVA followed by Tukey's post hoc test). The extents of inhibition (%) are indicated.
Discussion
The present study was designed to test the hypothesis that mouse CYP2A5 plays an essential role in NNK bioactivation in mouse lung, by comparing the kinetic parameters of microsomal NNK metabolism and tissue levels of O6-mG between WT and Cyp2a5-null mice. Recombinant CYP2A5 is very efficient in the bioactivation of NNK, producing both OPB and HPB (Felicia et al., 2000; Jalas et al., 2003). In the Cyp2a5-null mouse, the expression of CYP2A5, which is abundant in liver, lung, and olfactory mucosa from WT mice, was abolished, and there was no compensatory change in the expression of other biotransformation enzymes examined, including multiple P450s and phase II enzymes (Zhou et al., 2010, 2011). The suitability of the mouse model for studying the specific role of CYP2A5 in NNK bioactivation in vivo was further confirmed by the finding that the loss of CYP2A5 expression did not alter the rate of systemic clearance of NNK or its major circulating metabolite NNAL, a result indicating that the lungs of WT and Cyp2a5-null mice were exposed to similar levels of NNK and NNAL after the NNK treatment. Therefore, differences between the two mouse strains in the levels of lung DNA adducts detected were not caused by differences in substrate (NNK) availability. In that connection, although CYP2A5 was abolished in all tissues of the Cyp2a5-null mice, the impact of the CYP2A5 loss on DNA adduct formation in the lung can be attributed to the contribution of lung CYP2A5, given our previous finding in a study on a lung-specific Cpr-null mouse that NNK-induced lung DNA adduct (O6-mG) formation and tumorigenesis depends on target-tissue bioactivation by pulmonary P450 enzymes.
NNK metabolism was analyzed previously using radiometric LC assays with [3H]NNK as the substrate (e.g., Zhang et al., 2007). An LC-MS protocol was developed here, to improve specificity and avoid the use of radioisotopes. The kinetic parameters were nearly identical between A/J and B6 mice for both liver and lung microsomal NNK metabolism, which was consistent with previous findings that the difference in sensitivity to NNK-induced lung tumorigenesis between A/J and B6 mice was not derived from a difference in NNK bioactivation (Devereux et al., 1993).
The contribution of CYP2A5 to the formation of O6-mG in vivo was tissue- and NNK dose-dependent. The levels of lung O6-mG were significantly lower in Cyp2a5-null than WT mice; the extent of the reduction was greater at the 20 mg/kg dose (∼40%) than at the 100 mg/kg dose (∼20%). These results, which are consistent with in vitro data showing significant increases in the apparent Km values for the formation of OPB, the reactive intermediate that produces O6-mG in vivo, in lung microsomes from Cyp2a5-null mice, indicate that CYP2A5 is the low-Km enzyme for NNK bioactivation in mouse lung. Intriguingly, although significant increases in the apparent Km values for the formation of OPB were also observed in liver microsomes from Cyp2a5-null mice, hepatic levels of O6-mG in NNK-treated mice were not decreased or changed by the loss of CYP2A5 expression at either of the NNK doses tested. A possible explanation for the apparent disparity between the in vitro and in vivo results is that, with NNK given via the intraperitoneal route in the in vivo experiments, the levels of NNK in the liver (the portal-of-entry organ), but not the lung, were much higher than the Km values of multiple hepatic P450s capable of bioactivating NNK, to the extent that a loss of the low-Km enzyme (CYP2A5) would not be noticed. On the other hand, the NNK concentrations tested in vitro included those similar to the Km values for CYP2A5, thus making it possible to reveal CYP2A5's activity in liver microsomes.
Our previous studies using a liver-specific Cpr-null mouse model showed that hepatic P450 enzymes were protective against NNK-induced lung tumorigenesis (Weng et al., 2007). NNK can be detoxified via formation of NNK-N-oxide; although the specific mouse P450 enzymes responsible for NNK N-oxide formation have not been identified, rat CYP2B1 and CYP2C6 have been found to be active in NNK N-oxide formation (for a review see Jalas et al., 2005). It is noteworthy that our present data showed that the loss of CYP2A5 did not affect the rates of NNK-N-oxide formation in either liver or lung microcosms, indicating that CYP2A5 is not involved in this detoxification pathway.
The remaining activity of NNK bioactivation in the lung of Cyp2a5-null mice, which is rather high, may reflect the contributions by other P450 enzymes; members of several CYP subfamilies have been reported to be active in NNK bioactivation, including CYP1A, CYP2B, and CYP2F (Smith et al., 1993; Hecht, 1998). In addition, CYP2A4, although expressed in the lung at levels much lower than that of CYP2A5 (Su et al., 1996), displayed activity for NNK bioactivation (Jalas et al., 2005). In that connection, it is notable that, although CYP2A5 is a low-Km enzyme in the formation of HPB (Hecht, 1998), the contribution of CYP2A5 to the formation of HPB in mouse lung microsomes was negligible in our study. Thus, other low-Km enzymes, which are probably more abundant than CYP2A5 is in mouse lung microsomes, may play a more pivotal role in catalyzing the formation of HPB. Preliminary studies comparing lung microsomes from WT and Cyp2f2-null mice (Li et al., 2011) seem to indicate that mouse CYP2F2, which is highly abundant in lung microsomes, is another low-Km enzyme for the formation of HPB (X. Zhou and X. Ding, unpublished work).
Our finding that the remaining NNK bioactivation activities in the Cyp2a5-null mice could be inhibited by 8-MOP, a P450 inhibitor used previously to demonstrate the role of CYP2A5 in NNK-induced lung tumorigenesis (Miyazaki et al., 2005), further supports the notion that P450 enzymes other than CYP2A5 also contribute to NNK-induced lung tumorigenesis in mice. The ability of 8-MOP to act as a mechanism-based inhibitor of CYP2A enzymes has been well documented (Koenigs et al., 1997; von Weymarn et al., 2005); 8-MOP was also found to inhibit mouse CYP2A5 through additional (competitive and noncompetitive) mechanisms (Visoni et al., 2008). However, the specificity of inhibition of CYP2A enzymes by 8-MOP is not absolute, particularly at high 8-MOP concentration or doses; e.g., 8-MOP inhibited mouse hepatic microsomal metabolism of carbon tetrachloride, a CYP2E1 substrate, and caffeine, a CYP1A2 substrate, both in vitro and in vivo (Labbe et al., 1987; Apseloff et al., 1991).
Additional studies to directly demonstrate the role of CYP2A5, as well as other P450 enzymes, including CYP2F2 and CYP2B, in NNK-induced lung tumorigenesis are warranted. In that regard, the current Cyp2a5-null mouse is on the B6 genetic background, which is resistant to lung tumorigenesis. However, we have confirmed that there is no strain difference in NNK bioactivation in liver and lung microsomes between B6 and A/J mice; the latter is an established mouse strain for studies of NNK-induced lung tumorigenesis. Therefore, future studies for the role of CYP2A5 in NNK-induced lung tumorigenesis can be performed using Cyp2a5-null mice on the A/J background. In that connection, a recent study of a mouse model with targeted deletion of Akt, a putative oncogene closely linked to the Cyp2a locus on mouse chromosome 7, has already yielded indirect evidence that genetic variations in CYP2A5 can affect susceptibility to NNK-induced lung tumorigenesis in mice (Hollander et al., 2011).
Our demonstration of the role of CYP2A5 in NNK bioactivation in the mouse lung in vivo lends further support to the idea that human CYP2A13, which is even more efficient than mouse CYP2A5 is in NNK bioactivation (Jalas et al., 2005), is a susceptibility gene for NNK-induced lung carcinogenesis in humans. CYP2A13 protein is expressed in human lung (Zhu et al., 2006; Zhang et al., 2007). Levels of CYP2A13 protein expression correlated with rates of lung microsomal NNK bioactivation (Zhang et al., 2007). Consistent with the evidence indicating that CYP2A13 is the most efficient enzyme for NNK bioactivation (Su et al., 2000; Jalas et al., 2005), a CYP2A13 genetic variation (CYP2A13*2), featuring a R257C change, which results in decreases both in CYP2A13 gene expression and in CYP2A13 metabolic activity toward NNK (Zhang et al., 2002; Schlicht et al., 2007; D'Agostino et al., 2008), has been associated with decreased incidences of lung adenocarcinoma in light smokers (Wang et al., 2003).
In conclusion, we proved that CYP2A5 is the low-Km enzyme for NNK bioactivation in the mouse lung, although P450 enzymes other than CYP2A5 also contribute. Our findings lay an important foundation for further demonstration of the ability of human CYP2A13 to catalyze NNK bioactivation in vivo and mediate NNK-induced lung tumorigenesis, through studies of a CYP2A13-transgenic mouse on a Cyp2a5-null genetic background.
Supplementary Material
Acknowledgments
We thank Yi Zhu for help with animal dosing; Weizhu Yang for technical assistance; and Dr. Qing-Yu Zhang for helpful discussions.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA092596].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- NNK
- 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- D4-NNK
- 4-(methylnitrosamino)-1-(3-[2,4,5,6-D4]-pyridyl)-1-butanone
- P450
- cytochrome P450
- LC
- liquid chromatography
- MS
- mass spectrometry
- HPB
- 4-hydroxy-1-(3-pyridyl)-1-butanone
- D4-HPB
- (3,3,4,4-D4)-HPB
- OPB
- 4-oxo-4-(3-pyridyl)butanone
- OPBA
- 4-oxo-4-(3-pyridyl)butyric acid
- NNAL
- 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- D4-NNAL
- 4-(methylnitrosamino)-1-(3-[2,4,5,6-D4]-pyridyl)-1-butanol
- O6-mG
- O6-methylguanine
- 8-MOP
- 8-methoxypsoralen
- WT
- wild type
- MIM-EPI
- multiple ion monitoring-dependent enhanced product ion
- MRM
- multiple reaction monitoring
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Zhou, D'Agostino, and Ding.
Conducted experiments: Zhou, D'Agostino, and Xie.
Performed data analysis: Zhou and Ding.
Wrote or contributed to the writing of the manuscript: Zhou and Ding.
References
- Ahrendt SA, Decker PA, Alawi EA, Zhu YR, Sanchez-Cespedes M, Yang SC, Haasler GB, Kajdacsy-Balla A, Demeure MJ, Sidransky D. (2001) Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer 92:1525–1530 [DOI] [PubMed] [Google Scholar]
- Apseloff G, Hilliard JB, Gerber N, Mays DC. (1991) Inhibition and induction of drug metabolism by psoralens: alterations in duration of sleep induced by hexobarbital and in clearance of caffeine and hexobarbital in mice. Xenobiotica 21:1461–1471 [DOI] [PubMed] [Google Scholar]
- Castonguay A, Stoner GD, Schut HA, Hecht SS. (1983) Metabolism of tobacco-specific N-nitrosamines by cultured human tissues. Proc Natl Acad Sci U S A 80:6694–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino J, Zhang X, Wu H, Ling G, Wang S, Zhang QY, Liu F, Ding ×. (2008) Characterization of CYP2A13*2, a variant cytochrome P450 allele previously found to be associated with decreased incidences of lung adenocarcinoma in smokers. Drug Metab Dispos 36:2316–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux TR, Belinsky SA, Maronpot RR, White CM, Hegi ME, Patel AC, Foley JF, Greenwell A, Anderson MW. (1993) Comparison of pulmonary O6-methylguanine DNA adduct levels and Ki-ras activation in lung tumors from resistant and susceptible mouse strains. Mol Carcinog 8:177–185 [DOI] [PubMed] [Google Scholar]
- Ding X, Coon MJ. (1990) Immunochemical characterization of multiple forms of cytochrome P-450 in rabbit nasal microsomes and evidence for tissue-specific expression of P-450s NMa and NMb. Mol Pharmacol 37:489–496 [PubMed] [Google Scholar]
- Felicia ND, Rekha GK, Murphy SE. (2000) Characterization of cytochrome P450 2A4 and 2A5-catalyzed 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism. Arch Biochem Biophys 384:418–424 [DOI] [PubMed] [Google Scholar]
- Hecht SS. (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 11:559–603 [DOI] [PubMed] [Google Scholar]
- Hecht SS, Morse MA, Amin S, Stoner GD, Jordan KG, Choi CI, Chung FL. (1989) Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis 10:1901–1904 [DOI] [PubMed] [Google Scholar]
- Hollander MC, Zhou X, Maier CR, Patterson AD, Ding X, Dennis PA. (2011) A Cyp2a polymorphism predicts susceptibility to NNK-induced lung tumorigenesis in mice. Carcinogenesis 32:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2007) IARC Monograph on the Evaluation of Carcinogenic Risks to Humans: Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamine. International Agency for Research on Cancer, Lyon, France [Google Scholar]
- Jalas JR, Ding X, Murphy SE. (2003) Comparative metabolism of the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol by rat cytochrome P450 2A3 and human cytochrome P450 2A13. Drug Metab Dispos 31:1199–1202 [DOI] [PubMed] [Google Scholar]
- Jalas JR, Hecht SS, Murphy SE. (2005) Cytochrome P450 enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Chem Res Toxicol 18:95–110 [DOI] [PubMed] [Google Scholar]
- Koenigs LL, Peter RM, Thompson SJ, Rettie AE, Trager WF. (1997) Mechanism-based inactivation of human liver cytochrome P450 2A6 by 8-methoxypsoralen. Drug Metab Dispos 25:1407–1415 [PubMed] [Google Scholar]
- Labbe G, Descatoire V, Letteron P, Degott C, Tinel M, Larrey D, Carrion-Pavlov Y, Geneve J, Amouyal G, Pessayre D. (1987) The drug methoxsalen, a suicide substrate for cytochrome P-450, decreases the metabolic activation, and prevents the hepatotoxicity, of carbon tetrachloride in mice. Biochem Pharmacol 36:907–914 [DOI] [PubMed] [Google Scholar]
- Li L, Wei Y, Van Winkle L, Zhang QY, Zhou X, Hu J, Xie F, Kluetzman K, Ding X. (2011) Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. J Pharmacol Exp Ther 339:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Yamazaki H, Takeuchi H, Saoo K, Yokohira M, Masumura K, Nohmi T, Funae Y, Imaida K, Kamataki T. (2005) Mechanisms of chemopreventive effects of 8-methoxypsoralen against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced mouse lung adenomas. Carcinogenesis 26:1947–1955 [DOI] [PubMed] [Google Scholar]
- Nunes MG, Desai D, Koehl W, Spratt TE, Guengerich FP, Amin S. (1998) Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in human hepatic microsomes by ipomeanol analogs–an exploratory study. Cancer Lett 129:131–138 [DOI] [PubMed] [Google Scholar]
- Peterson LA, Hecht SS. (1991) O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res 51:5557–5564 [PubMed] [Google Scholar]
- Peterson LA, Liu XK, Hecht SS. (1993) Pyridyloxobutyl DNA adducts inhibit the repair of O6-methylguanine. Cancer Res 53:2780–2785 [PubMed] [Google Scholar]
- Peterson LA, Mathew R, Hecht SS. (1991) Quantitation of microsomal α-hydroxylation of the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 51:5495–5500 [PubMed] [Google Scholar]
- Ronai ZA, Gradia S, Peterson LA, Hecht SS. (1993) G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis 14:2419–2422 [DOI] [PubMed] [Google Scholar]
- Schlicht KE, Michno N, Smith BD, Scott EE, Murphy SE. (2007) Functional characterization of CYP2A13 polymorphisms. Xenobiotica 37:1439–1449 [DOI] [PubMed] [Google Scholar]
- Smith TJ, Guo Z, Li C, Ning SM, Thomas PE, Yang CS. (1993) Mechanisms of inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation in mouse by dietary phenethyl isothiocyanate. Cancer Res 53:3276–3282 [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X. (2000) Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 60:5074–5079 [PubMed] [Google Scholar]
- Su T, Sheng JJ, Lipinskas TW, Ding X. (1996) Expression of CYP2A genes in rodent and human nasal mucosa. Drug Metab Dispos 24:884–890 [PubMed] [Google Scholar]
- Takeuchi H, Saoo K, Yokohira M, Ikeda M, Maeta H, Miyazaki M, Yamazaki H, Kamataki T, Imaida K. (2003) Pretreatment with 8-methoxypsoralen, a potent human CYP2A6 inhibitor, strongly inhibits lung tumorigenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Cancer Res 63:7581–7583 [PubMed] [Google Scholar]
- Visoni S, Meireles N, Monteiro L, Rossini A, Pinto LF. (2008) Different modes of inhibition of mouse Cyp2a5 and rat CYP2A3 by the food-derived 8-methoxypsoralen. Food Chem Toxicol 46:1190–1195 [DOI] [PubMed] [Google Scholar]
- von Weymarn LB, Zhang QY, Ding X, Hollenberg PF. (2005) Effects of 8-methoxypsoralen on cytochrome P450 2A13. Carcinogenesis 26:621–629 [DOI] [PubMed] [Google Scholar]
- Wang H, Tan W, Hao B, Miao X, Zhou G, He F, Lin D. (2003) Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco-specific carcinogen NNK. Cancer Res 63:8057–8061 [PubMed] [Google Scholar]
- Weng Y, Fang C, Turesky RJ, Behr M, Kaminsky LS, Ding X. (2007) Determination of the role of target tissue metabolism in lung carcinogenesis using conditional cytochrome P450 reductase-null mice. Cancer Res 67:7825–7832 [DOI] [PubMed] [Google Scholar]
- Westra WH, Slebos RJ, Offerhaus GJ, Goodman SN, Evers SG, Kensler TW, Askin FB, Rodenhuis S, Hruban RH. (1993) K-ras oncogene activation in lung adenocarcinomas from former smokers. Evidence that K-ras mutations are an early and irreversible event in the development of adenocarcinoma of the lung. Cancer 72:432–438 [DOI] [PubMed] [Google Scholar]
- Xie F, Zhou X, Behr M, Fang C, Horii Y, Gu J, Kannan K, Ding X. (2010) Mechanisms of olfactory toxicity of the herbicide 2,6-dichlorobenzonitrile: essential roles of CYP2A5 and target-tissue metabolic activation. Toxicol Appl Pharmacol 249:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Zhou X, Genter MB, Behr M, Gu J, Ding X. (2011) The tissue-specific toxicity of methimazole in the mouse olfactory mucosa is partly mediated through target-tissue metabolic activation by CYP2A5. Drug Metab Dispos 39:947–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Ma L, Humphreys WG, Zhu M. (2008) Rapid screening and characterization of drug metabolites using a multiple ion monitoring-dependent MS/MS acquisition method on a hybrid triple quadrupole-linear ion trap mass spectrometer. J Mass Spectrom 43:1364–1375 [DOI] [PubMed] [Google Scholar]
- Zhang X, D'Agostino J, Wu H, Zhang QY, von Weymarn L, Murphy SE, Ding X. (2007) CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Pharmacol Exp Ther 323:570–578 [DOI] [PubMed] [Google Scholar]
- Zhang X, Su T, Zhang QY, Gu J, Caggana M, Li H, Ding X. (2002) Genetic polymorphisms of the human CYP2A13 gene: identification of single-nucleotide polymorphisms and functional characterization of an Arg257Cys variant. J Pharmacol Exp Ther 302:416–423 [DOI] [PubMed] [Google Scholar]
- Zhou X, Wei Y, Xie F, Laukaitis CM, Karn RC, Kluetzman K, Gu J, Zhang QY, Roberts DW, Ding X. (2011) A novel defensive mechanism against acetaminophen toxicity in the mouse lateral nasal gland: role of CYP2A5-mediated regulation of testosterone homeostasis and salivary androgen-binding protein expression. Mol Pharmacol 79:710–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, Ding X. (2010) Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a Cyp2a5-null mouse model. J Pharmacol Exp Ther 332:578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, Wang SL, Yang CS, He XY, Hong JY. (2006) CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos 34:1672–1676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.