Abstract
Blockade of D2 family dopamine receptors (D2Rs) is a fundamental property of antipsychotics, and the degree of striatal D2R occupancy has been related to antipsychotic and motor effects of these drugs. Recent studies suggest the D2R occupancy of antipsychotics may differ in extrastriatal regions compared with the dorsal striatum. We studied this issue in macaque monkeys by using a within-subjects design. [18F]fallypride positron emission tomography scans were obtained on four different doses of risperidone and paliperidone (the 9-OH metabolite of risperidone) and compared with multiple off-drug scans in each animal. The half-life of the two drugs in these monkeys was determined to be between 3 and 4 h, and drug was administered by a constant infusion through an intragastric catheter. The D2R occupancy of antipsychotic was determined in the caudate, putamen, ventral striatum, and four prefrontal and temporal cortical regions and was related to serum and cerebrospinal fluid drug levels. Repeated 2-week treatment with risperidone or paliperidone did not produce lasting changes in D2R binding potential in any region examined. As expected, D2R binding potential was highest in the caudate and putamen and was approximately one-third that level in the ventral striatum and 2% of that level in the cortical regions. We found dose-dependent D2R occupancy for both risperidone and paliperidone in both basal ganglia and cortical regions of interest. We could not find evidence of regional variation in D2R occupancy of either drug. Comparison of D2R occupancy and serum drug levels supports a target of 40 to 80 ng/ml active drug for these two atypical antipsychotics.
Introduction
Antagonism of D2 family dopamine receptors (D2Rs) is an essential component of the pharmacology of antipsychotic drugs (Seeman et al., 1975). The relationship between D2R binding and antipsychotic action has been characterized in PET studies of patients treated with antipsychotics (Farde et al., 1992; Nordström et al., 1993; Kapur et al., 1996, 2000). Those studies examined D2R binding potential in the dorsal striatum of patients treated with antipsychotics and compared it to D2R binding potential in normal controls or patients off of medication to determine the degree of D2R occupancy of the studied antipsychotic. Clinical response and the presence of extrapyramidal side effects were correlated with the degree of occupancy of brain D2R as measured in the dorsal striatum. A threshold of 60 to 65% D2R occupancy in the dorsal striatum for antipsychotic effect and a threshold of 80% D2R occupancy for extrapyramidal side effects were reported.
The association between the Parkinsonian side effects of antipsychotics and their blockade of D2R in the dorsal striatum is consonant with the known function of dopamine in the dorsal striatum in motor control (Graybiel et al., 1994). Moreover, the caudate nucleus is a component of the circuitry involved in cognitive processes (Haber, 2003), and altered dopamine neurotransmission has been observed in the basal ganglia of patients with schizophrenia (Laruelle et al., 1996). However, other brain regions are also plausible sites of schizophrenic pathology and antipsychotic therapeutic action. The ventral striatum, including the nucleus accumbens, is part of the neural circuitry of reward and motivation, which is impaired in schizophrenia (Gold et al., 2008). In addition, cortical brain regions are thought to play a role in schizophrenia, in particular the prefrontal cortex (PFC) (Weinberger et al., 1986; Goldman-Rakic, 1999; Lewis et al., 1999; Abi-Dargham et al., 2002). The dorsolateral PFC (dlPFC) plays a critical role in higher cognitive processes, including working memory, that are disordered in schizophrenia (Abi-Dargham et al., 2002). The medial PFC (mPFC) and orbital PFC (oPFC) are also part of the circuitry involved in motivation, and dysfunction in these brain regions has been related to both decreased motivation and apathy as well as disinhibition of behavioral responses (Peters et al., 2006). Given the evidence for involvement of the ventral striatum (Crespo-Facorro et al., 2001; Lauer et al., 2001) and PFC (Weinberger et al., 1986; Selemon et al., 1995; Lewis et al., 1999) in schizophrenia, a better understanding of antipsychotic binding in these brain regions is needed.
With the development of new tracers and PET technology it has become possible to study extrastriatal D2R occupancy. Most studies of extrastriatal D2R occupancy have compared the temporal cortex (TCtx) to the dorsal striatum. Those studies have suggested that there may be regional variation in D2R occupancy, at least with some antipsychotics (Kessler et al., 2006; Agid et al., 2007). Although studies in human patients allow for the correlation of D2R occupancy with clinical symptoms, a significant limitation of the studies is they typically examine only one dose of antipsychotic in each patient, and on-drug scans are compared with off-drug scans from a separate group of patients or normal controls. To address these limitations, we undertook a study of D2R occupancy by the antipsychotics risperidone and paliperidone in nonhuman primates. They are two, separate, second-generation antipsychotics, and paliperidone is the 9-OH metabolite of risperidone. Each animal received four doses of each drug, and D2R occupancy was calculated by using multiple off-drug scans from each animal in both the basal ganglia and prefrontal regions of interest (ROIs) to test the hypothesis that the D2R occupancy of risperidone and paliperidone shows regional variation.
Materials and Methods
A total of five male rhesus monkeys (Macaca mulata) ranging in age from 4.2 to 6.3 years were selected for this study. All animals were individually housed with ad libitum access to food and water, except for being held without food or water before procedures. Diet was supplemented with a variety of food treats. All procedures and animal care were performed according to the National Institutes for Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Institutional Animal Care and Use Committee of Emory University.
For this study we used risperidone and paliperidone, both provided by Ortho-McNeil Janssen (Titusville, NJ). Risperidone has a molecular weight of 410.485 and binds to D2/3 receptors with an apparent affinity of 3.13 nM or 1.28 ng/ml (Leysen et al., 1988). Paliperidone is the 9-OH metabolite of risperidone. It has a molecular weight of 426.484 and binds to D2/3 receptors with an apparent affinity of 4.0 nM or 1.7 ng/ml (Schotte et al., 1996).
Pharmacokinetic Study.
To determine the half-life of risperidone and paliperidone in nonhuman primates a pharmacokinetic study was initially performed. Each animal was sedated with Telazol (Butler Animal Health, Dublin, GA) (3–5 mg/kg), and then a 1-mg dose of either risperidone or paliperidone was administered intravenously (animal weight averaged 9.18 kg with a range of 8.25–10.2 kg). Blood was then drawn at the following times after drug administration: 2, 10, and 30 min and 1, 2, 4, 8, and 24 h (all blood draws were done under Telazol sedation). After a 2-week washout period, the process was repeated in each animal with the second study drug. The order in which the two drugs was given was randomized for each animal. In each serum sample, the concentrations of risperidone and paliperidone were determined.
Chronic Treatment Study.
After a minimum of 2 weeks of washout from the second pharmacokinetic study, each animal received a structural MRI scan using a 3-tesla magnet (Siemens Trio; Siemens Medical Systems, Knoxville, TN). In addition, a baseline [18F]fallypride PET scan was performed. The animals then underwent surgery to place a gastrostomy tube that was tunneled subcutaneously to a pocket on the animal's back where it was connected to an osmotic pump (Alzet, Cupertino, CA) (for details of the surgical procedure see Strait et al., 2010). Initially, the tube was connected to a 2-week pump containing study drug. After 12 days of treatment, the animal underwent an on-drug PET scan. At the time of the on-drug PET scan, each animal had blood and CSF collected for drug level determination. Two days later, the animal was sedated with Telazol (4 mg/kg i.m.), and the pump was changed for a 4-week pump containing saline. After 26 days of washout, the animal underwent an off-drug PET scan, and 2 days later the pump was changed for a 2-week pump with a different dose of drug. This was repeated until all animals received all four doses of each study drug. The treatment order is shown in Fig. 1. The choice of drug dose was made in consultation with the manufacturer. The following doses of drug were used for this study: RIS1, 0.025 mg/kg/day; RIS2, 0.05 mg/kg/day; RIS3, 0.1 mg/kg/day; RIS4, 0.3 mg/kg/day; PAL1, 0.05 mg/kg/day; PAL2, 0.1 mg/kg/day; PAL3, 0.2 mg/kg/day; and PAL4, 0.6 mg/kg/day.
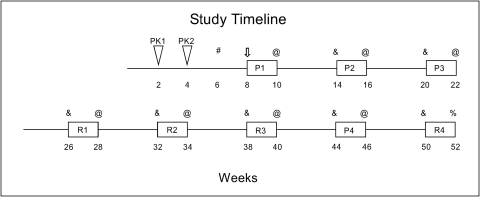
Fig. 1.
A schematic of the study time line. The time in weeks is shown below the timeline. The timing of the two pharmacokinetic studies is indicated by the triangles PK1 and PK2. The drug dosing periods are indicated by rectangles labeled R or P for risperidone and paliperidone treatment, respectively, with the numbers after the letters indicating the dose of drug administered (e.g., R1 indicates the lowest dose of risperidone, 0.025 mg/kg/day). #, when each animal received an MRI and baseline PET scan. ⇓, when each animal underwent gastric catheter surgery and drug pump placement. @, when each animal underwent blood and CSF collection and an on-drug PET scan followed by replacement of drug pump for a saline pump. &, when each animal underwent an off-drug PET scan followed by replacement of saline pump for a drug pump. %, when each animal underwent blood and CSF collection and an on-drug PET scan followed by sacrifice.
Drug Assay.
Two different drug assays were used in the study. For the pharmacokinetic experiment, drug levels were determined by using high-performance liquid chromatography as described previously (Price and Hoffman, 1997). A six-point standard curve was used with concentrations from 0.5 to 100 ng/ml of risperidone and paliperidone. Two levels of quality control were included in each run with target concentrations of 7.5 and 30 ng/ml of risperidone and its metabolite. The method has a limit of quantitation of 0.25 ng/ml for the two compounds of interest, and recoveries of both compounds averaged >98% over the entire analytical range (0.25–100 ng/ml). The overall imprecision averages 9.5% at 7.5 ng/ml and 5.6% at 30 ng/ml (n = 20). For the chronic treatment experiment, the concentration of risperidone and paliperidone was determined by liquid chromatography-tandem mass spectrometry (Remmerie et al., 2003). The following transitions were monitored: risperidone, 411.1 > 190.9; paliperidone, 427.0 > 206.9; and the internal standard, d3, 13C2-risperidone, 415.5 > 192.9. Secondary ions were also monitored to enhance specificity. An eight-point standard curve with two levels of quality control was processed in each run. The method was linear from 0.2 to 2000 ng/ml and exhibited no matrix effects. Absolute recoveries ranged from 80 to 122% at 46 ng/ml, and interassay imprecisions ranged from 4 to 10% at levels of 5, 50, and 350 ng/ml for all compounds.
PET Scan Acquisition.
Quantitative brain images were acquired by using a MicroPET Focus 220 scanner system (Siemens Medical Systems) located at the Yerkes Imaging Center. All PET imaging procedures occurred at the same time of day (11:00 AM-2:30 PM) to control for any diurnal effects. After induction of anesthesia with 1 to 2% isoflurane, an intravenous catheter was placed, and the animals were positioned with the head immobilized and fitted with an oximeter, a rectal thermistor, and a blood pressure/heart rate monitor. Initially, a transmission scan was obtained with a 57Co source. The resulting 57Co images were segmented into tissue (water), bone, and air, and substitution was made for appropriate 511-keV attenuation coefficients. These data were then foreprojected and used to correct the emission data for attenuation. Subsequently, [18F]fallypride was infused intravenously over 1 min, and emission data were collected in list mode for 120 min after injection and then binned into a histogram of 21 frames ranging from 1 to 10 min in duration (5/60, 5/180, 2/300, and 9/600 frames/s). Emission data were corrected for scatter, dead time, and attenuation and then reconstructed into images by using the manufacturer-supplied 3D FastMAP algorithm (Siemens Medical Systems).
PET Image Analysis.
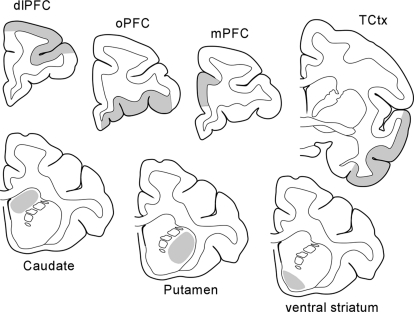
PET data were coregistered to each individual subject's MRI by minimizing mutual information with the Powell iterative method, and six ROIs were identified (Fig. 2). ROIs were manually traced for each monkey in coronal views by using custom software written in the IDL language (ITT Visual Information Solutions, Boulder, CO). The precommissural basal ganglia were divided into the caudate, putamen, and ventral striatum. In addition, four different cortical regions were identified: three prefrontal and one temporal. The dlPFC focused on areas 9 and 46 with some area 10 (Paxinos et al., 2000) in the rostral portion of the ROI. The rostral border was set as 4 mm behind the tip of the rostral pole, the caudal border was 4 mm rostral to the tip of the superior limb of the arcuate sulcus, the medial border was defined as the gray white junction of the dorsal convexity extended horizontally to the midline, and the lateral border was the gray white border of the ventral bank of the principal sulcus extended in a straight line laterally to the edge of the brain. The orbital region (oPFC) included areas 11 and 13 with a rostral border of 6 mm behind the rostral pole, a caudal border at the level of the tip of the lateral ventricles, and with the medial and lateral borders defined by lines from the medial and lateral edge of ventral white mater extended ventrally to the edge of the brain. The medial region (mPFC) focused on areas 32 and 24 rostral to the genu of the corpus callosum with a dorsal border of the cingulate sulcus extended laterally to the white matter, a ventral border set at half the distance between the cingulate sulcus, and the ventral tip of the frontal lobe and rostral and caudal borders at 8 mm rostral to, and the first section with, the corpus callosum, respectively. A temporal cortex ROI was defined as the temporal cortex from the lip of the lateral fissure to the lip of the rhinal fissure, from the level of the back of the anterior commissure caudally to the end of the amygdala. In addition, a cerebellum ROI was identified as a reference region for nonspecific [18F]fallypride binding due to the relative absence of D2R binding sites.
Fig. 2.
The location of ROIs for basal ganglia and the cortical regions are indicated in gray on line drawings of representative brain sections.
Regions were transferred to the PET data, and quantitative (nCi/ml) time activity curves were generated. Regional measures of D2R distribution volume ratio (DVR) were determined by using the method of Logan et al. (1996). In all cases, the cerebellum was used as the reference region. The analysis assumes that the reference region has negligible specific D2R binding and the equilibrium measure of fallypride is the same as in the ROIs, and in this case the DVR is related to the binding potential (BPND) as: DVR = 1 + BPND. Hence, values reported are the slope of the Logan plot minus 1. We first determined whether there were significant differences between the left and right sides of the brain for our ROIs. We calculated a laterality z statistic [left-right/sqrt(error of left Logan slope2 + error of right Logan slope2)]. For each animal we took the average of this z statistic across the off-drug scans and then performed a one-sample t test for each ROI of the values in the five animals. For all seven ROIs, the value was not significantly different from 0 (p > 0.05), and for subsequent analyses the volume-weighted average of the left and right BP for each ROI was used. To confirm that D2R binding in cortical regions was significant, t tests were performed to test whether the value was significantly different from 0. Each animal had a total of eight off-drug scans and eight on-drug scans. The percentage occupancy of D2R in each brain region for a particular drug and dose and ROI was calculated as: D2Rocc(drug,dose) = 100 × (BPND (off-drug) − BPND (on-drug))/BPND (off-drug). For BPND (off-drug) the average of eight off-drug scans was used.
Statistical Analyses.
The half-life for each drug was calculated by plotting drug concentration as a function of time and fitting an exponential curve to the data. From this curve the τ constant was derived and the T1/2 was calculated as the natural log of one-half divided by τ. For two animals, the raw paliperidone data could not be fit because of a lost sample. Therefore, before curve fitting the data were smoothed by using the formula: ValueXcor = 0.7 × ValueX + 0.3 × ValueX-1. For each drug, the half-life was then taken as the mean of values across all five animals and reported as mean ± S.E., and these values were compared by using a paired t test.
PET data were analyzed by using one- and two-way repeated measures ANOVA with factors of ROI and ROI and drug dose, respectively. Post hoc testing for significant effects was performed by using Tukey tests. For off-drug PET scans, outliers were sought by using the Grubbs Outlier analysis; however, no outliers were detected.
Results
An initial pharmacokinetic study was undertaken in all five animals to determine the half-life of each drug in rhesus monkeys. Serum was collected from 2 min to 24 h after intravenous injection of 1 mg of each drug. As expected, risperidone-treated animals showed high levels of risperidone in their serum, which decayed rapidly, and a delayed peak of the 9-OH metabolite, paliperidone, followed by a decay. In paliperidone-treated animals, only paliperidone was detected at each time point, confirming that no back metabolism of paliperidone occurred. The half-life of risperidone was found to be 3.03 ± 0.39 h and that of paliperidone was found to be 3.75 ± 1.04 h; these values did not differ significantly (t = 0.566; p = 0.59). These results confirm that at the time we obtained on-drug PET scans on our animals the drug levels would have long since reached equilibrium (∼88 half-lives), and at the time we obtained off-drug PET scans, the drug levels would be negligible (∼180 half-lives).
Blood and CSF Levels of Drug.
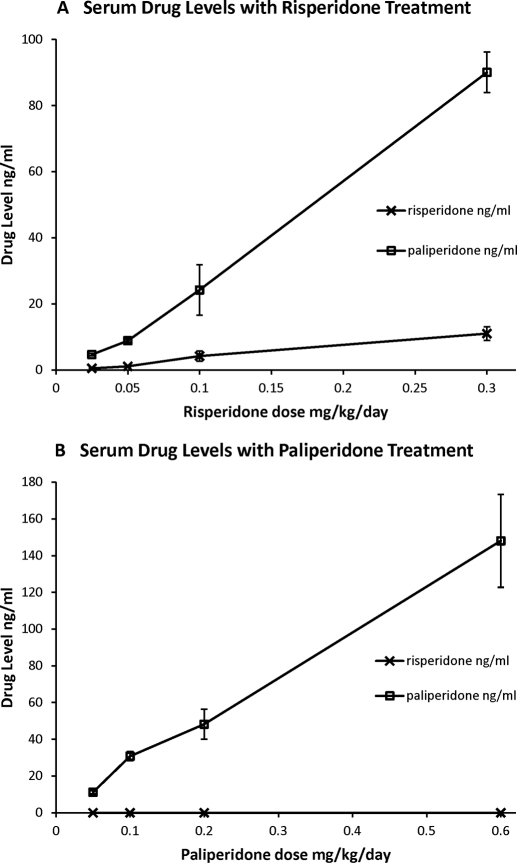
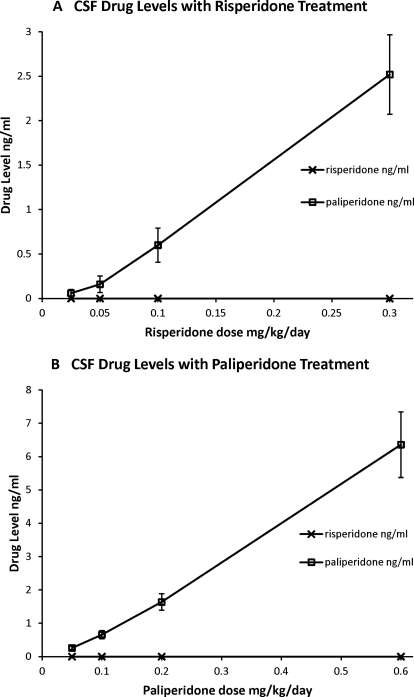
Chronic treatment with the four doses of both risperidone and paliperidone resulted in dose-dependent increases in serum drug levels (Fig. 3). In samples drawn after treatment with risperidone, both the parent drug and the 9-OH metabolite were detected. The concentration of risperidone and its metabolite paliperidone were highly correlated (r = 0.861; p < 0.0001). Intriguingly, in these chronic risperidone-treated animals, paliperidone was the predominant form of drug found in the serum (Fig. 3A). In samples taken after treatment with paliperidone, only paliperidone was observed (Fig. 3B). There were similar dose-dependent increases in drug levels in the CSF (Fig. 4). In particular, paliperidone was observed in the CSF of animals treated with both risperidone (Fig. 4A) and paliperidone (Fig. 4B). Unexpectedly, samples from risperidone-treated animals did not show measurable risperidone in the CSF (Fig. 4A). Risperidone levels were undetectable in all samples of the first three doses, and only one sample at the highest dose of risperidone showed detectable risperidone. In paliperidone-treated animals, the levels of paliperidone in the CSF were strongly correlated with serum levels of drug (r = 0.983; p < 0.0001). In risperidone-treated animals, the levels of paliperidone in the CSF were strongly correlated with serum levels of risperidone (r = 0.75; p < 0.0001) and paliperidone (r = 0.885; p < 0.0001). These results confirm that our drug treatment resulted in effective levels of drug in the serum and CSF at the time of our PET scans.
Fig. 3.
Dose-dependent increases in serum drug concentrations are seen after treatment with risperidone (A) or paliperidone (B). After risperidone treatment both risperidone and paliperidone are found in serum samples. After paliperidone treatment only paliperidone is found in serum.
Fig. 4.
Dose-dependent increases in CSF drug concentrations are seen after treatment with risperidone (A) or paliperidone (B). Only paliperidone was observed in CSF after either risperidone or paliperidone treatment.
Baseline Regional D2R Binding Potential.
Each animal received eight off-drug PET scans (Fig. 5) comprised of an initial baseline scan and seven subsequent scans after drug washouts. Repeated antipsychotic treatment did not significantly alter off-drug D2R BPND in any of the ROIs examined (tested by one-way repeated measures ANOVAs; p > 0.05 for all ROIs, corrected for multiple comparisons). We determined the mean off-drug D2R BPND for each brain region (Table 1). As expected, the highest level of D2R BPND was in the dorsal striatal regions, the caudate and putamen. The ventral striatum had levels of D2R approximately one-third those of the dorsal striatum. Levels in the four cortical regions, the dlPFC, oPFC, mPFC, and the lateral TCtx, were markedly lower than the three basal ganglia regions with binding potentials approximately 2% of that seen in the dorsal striatum. Nevertheless, signal above that in the cerebellum was consistently seen in these cortical regions and was significantly above cerebellum values for all four of them (dlPFC, t = 22.076, p < 0.0001; oPFC, t = 24.502, p <0.0001; mPFC, t = 21.695, p < 0.0001; TCtx, t = 28.316, p < 0.0001).
Fig. 5.
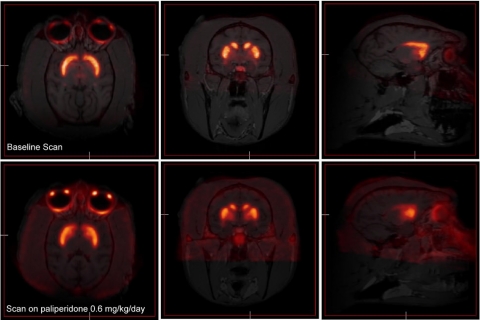
Sample [18F]fallypride PET scans coregistered with the animal's structural MRI scan are shown. An off-drug scan is shown in the top three images above, and the bottom three images depict a scan of an animal while the animal was receiving paliperidone. Three representative images are shown from each scan in the horizontal, coronal, and sagittal planes (from left to right). In both cases strong labeling in the caudate and putamen is easily identified.
TABLE 1.
D2R binding potential in different regions of interest
The binding potential of [18F]fallypride at D2R in different brain regions is shown. Binding is given in arbitrary units and is the average of 40 off-drug PET scans taken across the five animals (mean ± S.E.). There was a significant main effect of brain region on D2R binding (F6,28 = 394.064; P < 0.0001), and post hoc Scheffe tests showed all pairwise comparisons were significant at the P < 0.0001 level except for caudate/putamen (P = 0.73) and all six comparisons between the four cortical regions (P > 0.999).
| ROI | Baseline D2R Binding |
|---|---|
| Caudate | 43.83 ± 2.35 |
| Putamen | 46.64 ± 1.23 |
| Ventral striatum | 15.72 ± 0.84 |
| dlPFC | 1.20 ± 0.11 |
| oPFC | 1.09 ± 0.04 |
| mPFC | 0.89 ± 0.04 |
| TCtx | 0.79 ± 0.06 |
D2R Occupancy of Risperidone and Paliperidone.
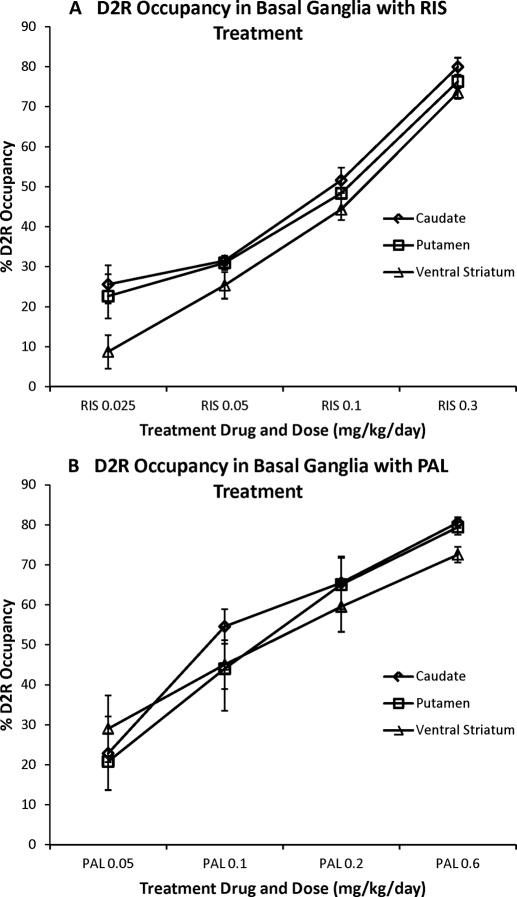
We next determined the degree to which our antipsychotic study drugs occupied central D2R at various doses. We examined D2R occupancy in seven ROIs: three basal ganglia regions (caudate, putamen, and ventral striatum) and four cortical regions (dlPFC, mPFC, oPFC, and TCtx). As expected, in the basal ganglia we saw dose-dependent D2R binding of both risperidone and paliperidone in each of the three ROIs (Fig. 6). We tested this observation by using one-way repeated measures ANOVA of the average D2R occupancy across the three basal ganglia regions for each drug treatment separately. We found significant main effects of dose for both risperidone treatment (F3,12 = 92.129; p < 0.001) and paliperidone treatment (F3,12 = 23.977; p < 0.001).
Fig. 6.
The D2R occupancy of risperidone (A) and paliperidone (B) in the basal ganglia. The D2R occupancy is plotted for the four different doses of study drug in three different basal ganglia ROIs: caudate, putamen, and ventral striatum.
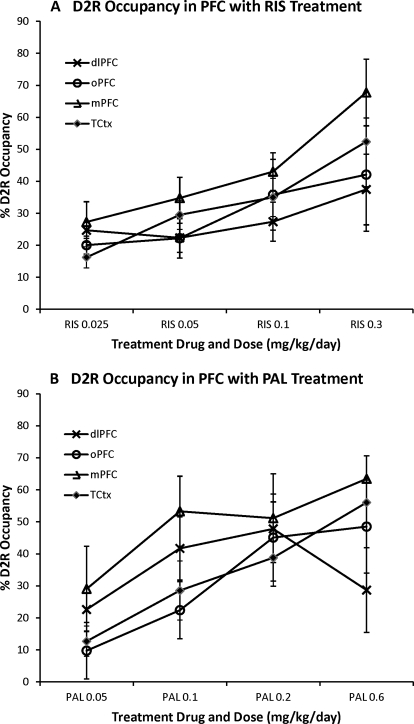
The combination of the high-resolution MicroPET scanner and the [18F]fallypride tracer allowed us to reproducibly measure D2R BPND in cortical regions. Dose-dependent binding of antipsychotic medication to cortical D2R is generally assumed but has not previously been demonstrated. Accordingly, we examined these ROIs to determine whether we could observe dose-dependent D2R occupancy of risperidone and paliperidone in the neocortex. As might be expected from the low D2R BP in the cortex, there was greater variability in the drug occupancy measures in the cortical regions compared with the basal ganglia regions; however, we did observe dose-dependent increases in cortical D2R occupancy for both risperidone and paliperidone (Fig. 7). As for the subcortical regions, we tested this observation by using one-way repeated-measures ANOVA of the average D2R occupancy across the four cortical regions for each drug treatment separately. We found a significant main effect of dose for risperidone treatment (F3,12 = 8.251; p = 0.003) and a strong trend toward a dose effect for paliperidone treatment (F3,12 = 3.309; p = 0.057).
Fig. 7.
The D2R occupancy of risperidone (A) and paliperidone (B) in the prefrontal cortex. The D2R occupancy is plotted for the four different doses of study drug in four different cortical ROIs: dlPFC, oPFC, mPFC, and TCtx.
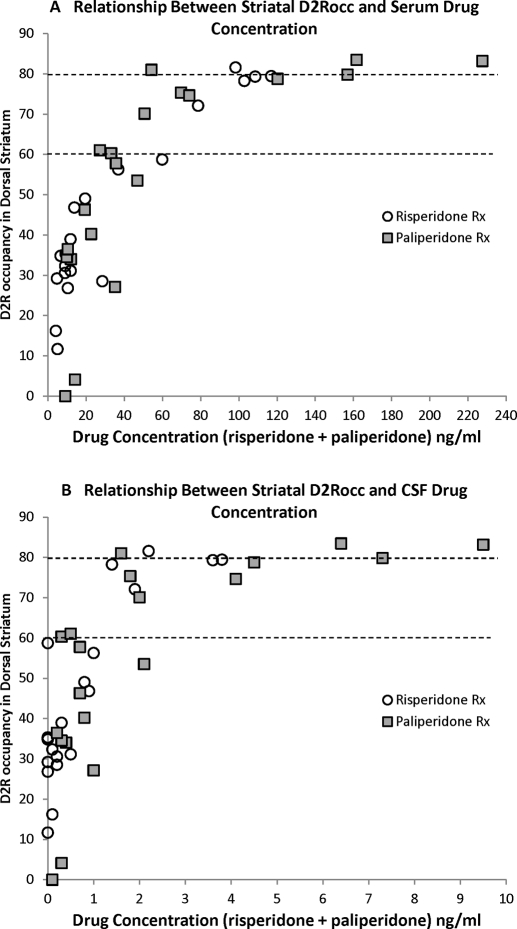
Finally, the dataset obtained in this study allows us to compare serum drug levels to D2R occupancy and relate them to thresholds for antipsychotic efficacy and extrapyramidal side effects suggested by previous research in the dorsal striatum of patients with schizophrenia (Farde et al., 1992; Nordström et al., 1993; Kapur et al., 2000; Nord and Farde, 2011). To better compare our data with those studies in patients, for each PET scan we averaged the occupancy in caudate and putamen to give a value for the dorsal striatum and plotted this value compared with active drug concentration, the sum of risperidone and 9-hydroxyrisperidone, in serum (Fig. 8A) and CSF (Fig. 8B). We found that for risperidone treatment D2R occupancy of 60 to 80% was obtained with serum drug concentrations of 60 to 80 ng/ml; for paliperidone treatment, serum drug concentrations were 45 to 80 ng/ml and gave D2R occupancy in this window. For both drugs, the CSF active drug level associated with D2R occupancy of 60 to 80% was 1 to 2.5 ng/ml. Note that the affinity of paliperidone for D2R has been reported to be 4.0 nM (Schotte et al., 1996), which corresponds to a concentration of 1.7 ng/ml. This is similar to what our graph indicates to be the concentration at which 50% D2R occupancy is achieved (Fig. 8B).
Fig. 8.
Scatter plots showing the relationship between D2R occupancy in the dorsal striatum and active drug concentration in the serum (A) and CSF (B). For this analysis, D2R occupancy was averaged between the caudate and putamen to obtain a single dorsal striatum value and plotted with the sum of risperidone and 9-hydroxyrisperidone concentrations in the serum at the time of the PET scan. Values obtained after risperidone (○) and paliperidone (■) treatment are plotted.
Discussion
This study provides the first information on antipsychotic occupancy in striatal and extrastriatal brain regions by using a within-subject design. We used nonhuman primates to facilitate multiple doses of different antipsychotics, repeated PET scans, and CSF sampling. The applicability of a nonhuman primate model to understanding human drug treatment is supported by several lines of evidence. First, the rhesus monkey brain has greater homology to human brain than other animal models, especially in the PFC (Preuss, 2007). The half-lives of risperidone in our monkeys were similar to those in humans with the extensive metabolizer cytochrome P450 phenotype (Huang et al., 1993), consistent with an apparent lack of poor metabolizer phenotype monkeys (Wu et al., 1993). In humans (Spina et al., 2001), as in our monkeys, chronic risperidone treatment results in higher levels of the metabolite paliperidone than of the parent compound. Finally, the relationship between drug concentration and striatal D2R occupancy we find is strikingly similar to that in risperidone-treated humans (Uchida et al., 2011). While using monkeys we cannot address any effect of illness on regional D2R BP or antipsychotic occupancy;, this possibility remains an open question because the bulk of human studies have used baseline D2R binding levels obtained from normal controls.
The drugs examined here, risperidone and paliperidone, are closely related with paliperidone being the 9-OH metabolite of risperidone. Both gave 70 to 80% occupancy in caudate and putamen at the highest dose, despite paliperidone being administered at twice the dose of risperidone. Both drugs are substrates for P-glycoprotein, a transporter that limits brain penetration of a variety or drugs. Risperidone inhibits P-glycoprotein transport, whereas paliperidone is much less potent (Zhu et al., 2007), suggesting risperidone treatment could inhibit P-glycoprotein, allowing higher brain levels of paliperidone than would otherwise be expected.
Using [18F]fallypride we were able to examine regional D2R binding. We found that D2R binding in the ventral striatum was one-third that of the dorsal striatum, consistent with previous reports in humans using PET (Okubo et al., 1999; Kessler et al., 2009) and postmortem epidepride binding (Joyce et al., 1991; Hall et al., 1996). Intriguingly, an in situ hybridization study in humans found higher levels of D2 mRNA in the ventral striatum than the dorsal striatum (Meador-Woodruff et al., 1996), suggesting less D2 message translation in the ventral striatum. Likewise, our finding that D2R binding in the monkey cortex is approximately 2% of that in the dorsal striatum is consistent with previous PET studies in the human prefrontal or temporal cortex (Kessler et al., 2009) or monkey frontal cortex (Mukherjee et al., 2001) and human epidepride binding postmortem studies (Joyce et al., 1991); although values from 12% (Okubo et al., 1999) to 0.2% (Hall et al., 1996) have been reported. The general agreement between our data and previous PET and postmortem drug binding studies gives us confidence that the D2R binding we observed, although low in the cortex, is an accurate reflection of D2R distribution and that the monkey is a useful model for studies of antipsychotic drugs.
Animal studies have found that treatment with antipsychotic drugs increase D2R binding (Florijn et al., 1997; Joyce, 2001) and mRNA expression (Bernard et al., 1991; Fishburn et al., 1994; Lidow and Goldman-Rakic, 1997). Likewise, patients treated chronically with antipsychotics show increased D2R binding potential using [11C]raclopride PET scanning compared with a different group of drug-naive patients (Silvestri et al., 2000). We found no up-regulation of [18F]fallypride signal from repeated treatments with risperidone or paliperidone in any ROI examined. Although the effect on D2 receptors may depend on the antipsychotic used (Florijn et al., 1997; Sakai et al., 2001), risperidone has previously been shown to up-regulate D2 mRNA in monkey cortex and striatum (Lidow and Goldman-Rakic, 1997). The effect on D2 mRNA expression can occur in as little as 7 days (Bernard et al., 1991). One possible reason for the discrepancy between our results and previous reports is that most studies examine D2R levels 3 days or less after the last dose of antipsychotic, whereas our off-drug scans were 26 days after the end of treatment. Thus reported antipsychotic induced changes in D2R levels may be reversible, supporting the use of postmortem studies of patients off-drug for defined periods of time as a means to evaluate neuropathological features of schizophrenia. However, although we saw no significant effect of repeated 2-week antipsychotic treatments, it is possible that longer treatment periods might induce persistent changes in D2R levels (Joyce, 2001).
Earlier studies of D2R occupancy by antipsychotic drugs have led to the hypothesis of a threshold for antipsychotic effect of 60 to 70% occupancy in the dorsal striatum, whereas extrapyramidal side effects were associated with occupancy of 80% or higher (Farde et al., 1992; Nordström et al., 1993; Kapur et al., 2000; Nord and Farde, 2011). We cannot compare occupancy to clinical effect in our monkeys; however, the relationship between serum drug concentration and striatal D2R occupancy we found in monkeys is similar to that reported in a review of human risperidone studies (Uchida et al., 2011). Based on our data, we would predict that serum drug levels from 45 to 60 and up to 80 ng/ml would give D2R occupancy in the desired therapeutic window of 60 to 80%. These results are consistent with a human PET study (Xiberas et al., 2001) and a clinical study of risperidone-treated patients showing a plasma drug level of 65 resulted in significant improvement in psychotic symptoms (Riedel et al., 2005).
The central finding of this study is the demonstration of dose-dependent D2R occupancy across various cortical and basal ganglia ROIs for two atypical antipsychotics by using a within-subjects design. Dose-dependent binding in the basal ganglia was similar for the two drugs and consistent with previous studies of human patients individually treated with various doses of atypical antipsychotics. Dose-dependent binding of antipsychotics to cortical D2R has been generally assumed, but not previously demonstrated. The occupancy values we found in the cortical regions were similar to the range seen in the basal ganglia ROIs, although they tended to be somewhat lower, especially at higher doses. There was substantially more variability in our determination of D2R occupancy in cortical ROIs, probably related to the 50-fold lower D2R binding potential in the cortex compared with the dorsal striatum. This may have made it difficult to measure higher occupancies in the cortex. When the lowest doses of the two drugs were examined, cortical and basal ganglia D2R occupancy was similar for both drugs.
This study adds to a growing literature on the binding of antipsychotics to extrastriatal D2R. Patients treated with haloperidol or unspecified typical antipsychotics showed no significant difference between binding in temporal cortex and dorsal striatum (Pilowsky et al., 1997; Talvik et al., 2001; Xiberas et al., 2001; Kessler et al., 2005). Generally higher D2R binding in the temporal cortex than in the dorsal striatum has been reported for atypical antipsychotics including clozapine (Pilowsky et al., 1997; Kessler et al., 2006), risperidone (Xiberas et al., 2001; Bressan et al., 2003), quetiapine (Stephenson et al., 2000; Kessler et al., 2006), ziprasidone (Vernaleken et al., 2008), and olanzapine (Bigliani et al., 2000; Xiberas et al., 2001). However, this has been contradicted by some studies of clozapine (Talvik et al., 2001), risperidone (Ito et al., 2009), paliperidone (Arakawa et al., 2008), olanzapine (Kessler et al., 2005), aripiprazole (Kegeles et al., 2008), and olanzapine or risperidone (Agid et al., 2007). Comparisons between the dorsal and ventral striatum have been reported less frequently, but no significant difference has been found (Kessler et al., 2005, 2006; Kegeles et al., 2008). Furthermore, a single-dose study in normal controls of risperidone or olanzapine found no significant difference in D2R occupancy in the dorsal striatum and thalamus for either drug, although the study used different scans with different tracers to evaluate striatal and thalamic occupancy (Tauscher et al., 2002). Finally, a study of risperidone D2R occupancy in the amygdala, hippocampus, and temporal and cingulate cortex found no significant regional differences and reported that drug levels corresponding to 70 to 80% D2R occupancy in these extrastriatal areas were similar to those previously reported for the striatum (Yasuno et al., 2001). A limitation of most of these studies is the use of single on-drug scans in patients with calculation of drug occupancy based on off-drug scans in a different cohort of healthy controls and/or patients. Indeed, the few studies that used the subject's own off-drug scans to calculate D2R occupancy have, with one exception (Mukherjee et al., 2001), not supported regional differences in antipsychotic drug binding (Talvik et al., 2001; Yasuno et al., 2001; Tauscher et al., 2002; Kessler et al., 2005; Kegeles et al., 2008; Ito et al., 2009). The results presented here are consistent with the majority of these within-subjects studies, indicating that atypical antipsychotics do not show preferential D2R occupancy in limbic or cortical brain regions.
Acknowledgments
We thank Jack Orkin and Karen Strait for monkey surgical procedures and Juliet Brown and Marcelia Maddox for excellent technical assistance.
This work was supported by an Investigator Initiated Study Award from Ortho-McNeil Janssen Scientific Affairs, LLC; a Merit Award from the Office of Research and Development, Department of Veterans Affairs (to E.C.M.); the National Institutes of Health National Institute on Drug Abuse [Grant K02 DA0000517] (to L.L.H.); and the National Institutes of Health National Center for Research Resources [Grant P51-RR000165] (to Yerkes National Primate Research Center).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- D2R
- D2 family dopamine receptor
- ANOVA
- analysis of variance
- BP
- binding potential
- CSF
- cerebrospinal fluid
- DVR
- distribution volume ratio
- MRI
- magnetic resonance imaging
- PAL
- paliperidone
- PET
- positron emission tomography
- PFC
- prefrontal cortex
- dlPFC
- dorsolateral PFC
- oPFC
- orbital PFC
- mPFC
- medial PFC
- RIS
- risperidone
- ROI
- region of interest
- TCtx
- temporal cortex.
Authorship Contributions
Participated in research design: Muly and Howell.
Conducted experiments: Muly and Ritchie.
Performed data analysis: Muly and Votaw.
Wrote or contributed to the writing of the manuscript: Muly, Votaw, and Howell.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, et al. (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, Kapur S. (2007) Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response–a double-blind PET study in schizophrenia. Neuropsychopharmacology 32:1209–1215 [DOI] [PubMed] [Google Scholar]
- Arakawa R, Ito H, Takano A, Takahashi H, Morimoto T, Sassa T, Ohta K, Kato M, Okubo Y, Suhara T. (2008) Dose-finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 197:229–235 [DOI] [PubMed] [Google Scholar]
- Bernard V, Le Moine C, Bloch B. (1991) Striatal neurons express increased level of dopamine D2 receptor mRNA in response to haloperidol treatment: a quantitative in situ hybridization study. Neuroscience 45:117–126 [DOI] [PubMed] [Google Scholar]
- Bigliani V, Mulligan RS, Acton PD, Ohlsen RI, Pike VW, Ell PJ, Gacinovic S, Kerwin RW, Pilowsky LS. (2000) Striatal and temporal cortical D2/D3 receptor occupancy by olanzapine and sertindole in vivo: a [123I]epidepride single photon emission tomography (SPET) study. Psychopharmacology (Berl) 150:132–140 [DOI] [PubMed] [Google Scholar]
- Bressan RA, Erlandsson K, Jones HM, Mulligan RS, Ell PJ, Pilowsky LS. (2003) Optimizing limbic selective D2/D3 receptor occupancy by risperidone: a [123I]-epidepride SPET study. J Clin Psychopharmacol 23:5–14 [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD. (2001) Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA 286:427–435 [DOI] [PubMed] [Google Scholar]
- Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544 [DOI] [PubMed] [Google Scholar]
- Fishburn CS, David C, Carmon S, Fuchs S. (1994) The effect of haloperidol on D2 dopamine receptor subtype mRNA levels in the brain. FEBS Lett 339:63–66 [DOI] [PubMed] [Google Scholar]
- Florijn WJ, Tarazi FI, Creese I. (1997) Dopamine receptor subtypes: differential regulation after 8 months treatment with antipsychotic drugs. J Pharmacol Exp Ther 280:561–569 [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. (2008) Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull 34:835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. (1999) The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry 46:650–661 [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. (1994) The basal ganglia and adaptive motor control. Science 265:1826–1831 [DOI] [PubMed] [Google Scholar]
- Haber SN. (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26:317–330 [DOI] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G. (1996) Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse 23:115–123 [DOI] [PubMed] [Google Scholar]
- Huang ML, Van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, Zylicz Z, Visscher HW, Jonkman JH. (1993) Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther 54:257–268 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Ito H, Arakawa R, Takahashi H, Takano H, Okumura M, Otsuka T, Ikoma Y, Shidahara M, Suhara T. (2009) No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol 12:667–675 [DOI] [PubMed] [Google Scholar]
- Joyce JN. (2001) D2 but not D3 receptors are elevated after 9 or 11 months chronic haloperidol treatment: influence of withdrawal period. Synapse 40:137–144 [DOI] [PubMed] [Google Scholar]
- Joyce JN, Janowsky A, Neve KA. (1991) Characterization and distribution of [125I]epidepride binding to dopamine D2 receptors in basal ganglia and cortex of human brain. J Pharmacol Exp Ther 257:1253–1263 [PubMed] [Google Scholar]
- Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S, Zipursky R. (1996) High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiatry 153:948–950 [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. (2000) Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157:514–520 [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, Gonzales R, Kim JH, Alvarez B, Gil R, et al. (2008) Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology 33:3111–3125 [DOI] [PubMed] [Google Scholar]
- Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer HY. (2005) Occupancy of striatal and extrastriatal dopamine D2/D3 receptors by olanzapine and haloperidol. Neuropsychopharmacology 30:2283–2289 [DOI] [PubMed] [Google Scholar]
- Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer HY. (2006) Occupancy of striatal and extrastriatal dopamine D2 receptors by clozapine and quetiapine. Neuropsychopharmacology 31:1991–2001 [DOI] [PubMed] [Google Scholar]
- Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, Dawant B, Zald D, Meltzer HY. (2009) Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry 65:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, et al. (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 93:9235–9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M, Senitz D, Beckmann H. (2001) Increased volume of the nucleus accumbens in schizophrenia. J Neural Transm 108:645–660 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. (1999) Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry 46:616–626 [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Janssen PA. (1988) Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther 247:661–670 [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. (1997) Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. J Pharmacol Exp Ther 283:939–946 [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840 [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ. (1996) Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 15:17–29 [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Narayanan TK, Shi B, Mantil J. (2001) Evaluation of dopamine D-2 receptor occupancy by clozapine, risperidone, and haloperidol in vivo in the rodent and nonhuman primate brain using 18F-fallypride. Neuropsychopharmacology 25:476–488 [DOI] [PubMed] [Google Scholar]
- Nord M, Farde L. (2011) Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci Ther 17:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G. (1993) Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 33:227–235 [DOI] [PubMed] [Google Scholar]
- Okubo Y, Olsson H, Ito H, Lofti M, Suhara T, Halldin C, Farde L. (1999) PET mapping of extrastriatal D2-like dopamine receptors in the human brain using an anatomic standardization technique and [11C]FLB 457. Neuroimage 10:666–674 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. (2000) The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press, San Diego [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, Pupi A, Degueldre C, Lemaire C, Collette F, et al. (2006) Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord 21:373–379 [DOI] [PubMed] [Google Scholar]
- Pilowsky LS, Mulligan RS, Acton PD, Ell PJ, Costa DC, Kerwin RW. (1997) Limbic selectivity of clozapine. Lancet 350:490–491 [DOI] [PubMed] [Google Scholar]
- Preuss TM. (2007) Evolutionary specializations of primate brain systems, in Primate Origins and Adaptations (Ravoso MJ, Dagosto M. eds) pp 625–675, Springer, New York [Google Scholar]
- Price MC, Hoffman DW. (1997) Therapeutic drug monitoring of risperidone and 9-hydroxyrisperidone in serum with solid-phase extraction and high-performance liquid chromatography. Ther Drug Monit 19:333–337 [DOI] [PubMed] [Google Scholar]
- Remmerie BM, Sips LL, de Vries R, de Jong J, Schothuis AM, Hooijschuur EW, van de Merbel NC. (2003) Validated method for the determination of risperidone and 9-hydroxyrisperidone in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 783:461–472 [DOI] [PubMed] [Google Scholar]
- Riedel M, Schwarz MJ, Strassnig M, Spellmann I, Müller-Arends A, Weber K, Zach J, Müller N, Möller HJ. (2005) Risperidone plasma levels, clinical response and side-effects. Eur Arch Psychiatry Clin Neurosci 255:261–268 [DOI] [PubMed] [Google Scholar]
- Sakai K, Gao XM, Hashimoto T, Tamminga CA. (2001) Traditional and new antipsychotic drugs differentially alter neurotransmission markers in basal ganglia-thalamocortical neural pathways. Synapse 39:152–160 [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 124:57–73 [DOI] [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. (1975) Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A 72:4376–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. (1995) Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 52:805–818; discussion 819–820 [DOI] [PubMed] [Google Scholar]
- Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, Kapur S, Zipursky RB, Wilson AA, Christensen BK, et al. (2000) Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 152:174–180 [DOI] [PubMed] [Google Scholar]
- Spina E, Avenoso A, Facciolà G, Salemi M, Scordo MG, Ancione M, Madia AG, Perucca E. (2001) Relationship between plasma risperidone and 9-hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology (Berl) 153:238–243 [DOI] [PubMed] [Google Scholar]
- Stephenson CM, Bigliani V, Jones HM, Mulligan RS, Acton PD, Visvikis D, Ell PJ, Kerwin RW, Pilowsky LS. (2000) Striatal and extra-striatal D2/D3 dopamine receptor occupancy by quetiapine in vivo. [123I]-epidepride single photon emission tomography(SPET) study. Br J Psychiatry 177:408–415 [DOI] [PubMed] [Google Scholar]
- Strait KR, Orkin JL, Anderson DC, Muly EC. (2010) Chronic, constant-rate, gastric drug infusion in nontethered rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 49:207–214 [PMC free article] [PubMed] [Google Scholar]
- Talvik M, Nordström AL, Nyberg S, Olsson H, Halldin C, Farde L. (2001) No support for regional selectivity in clozapine-treated patients: a PET study with [11C]raclopride and [11C]FLB 457. Am J Psychiatry 158:926–930 [DOI] [PubMed] [Google Scholar]
- Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S. (2002) Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 7:317–321 [DOI] [PubMed] [Google Scholar]
- Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. (2011) Predicting dopamine D2 receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol 31:318–325 [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Fellows C, Janouschek H, Bröcheler A, Veselinovic T, Landvogt C, Boy C, Buchholz HG, Spreckelmeyer K, Bartenstein P, et al. (2008) Striatal and extrastriatal D2/D3-receptor-binding properties of ziprasidone: a positron emission tomography study with [18F]Fallypride and [11C]raclopride (D2/D3-receptor occupancy of ziprasidone). J Clin Psychopharmacol 28:608–617 [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. (1986) Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43:114–124 [DOI] [PubMed] [Google Scholar]
- Wu D, Otton SV, Morrow P, Inaba T, Kalow W, Sellers EM. (1993) Human hepatic cytochrome P450 2D6-like activity in nonhuman primates: catalytic characterization in vitro. J Pharmacol Exp Ther 266:715–719 [PubMed] [Google Scholar]
- Xiberas X, Martinot JL, Mallet L, Artiges E, Loc'H C, Mazière B, Paillère-Martinot ML. (2001) Extrastriatal and striatal D2 dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry 179:503–508 [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T, Tanada S. (2001) Dose relationship of limbic-cortical D2-dopamine receptor occupancy with risperidone. Psychopharmacology (Berl) 154:112–114 [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, DeVane CL. (2007) Risperidone and paliperidone inhibit p-glycoprotein activity in vitro. Neuropsychopharmacology 32:757–764 [DOI] [PubMed] [Google Scholar]