Abstract
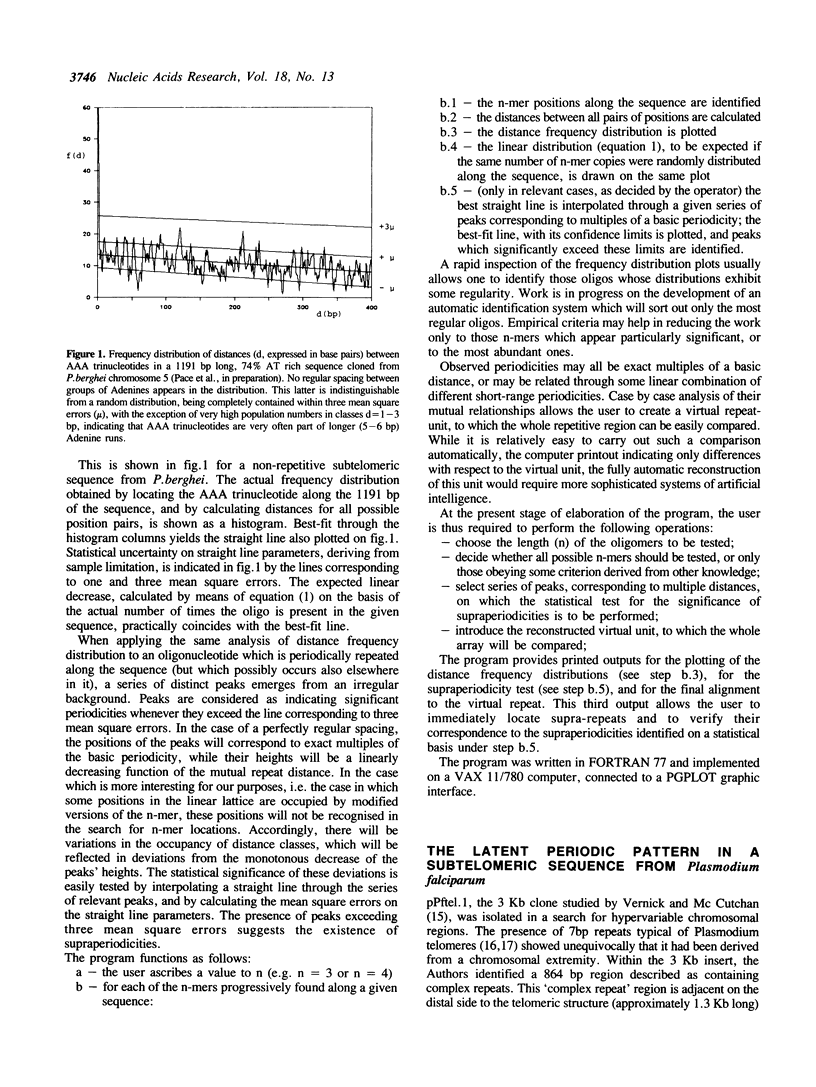
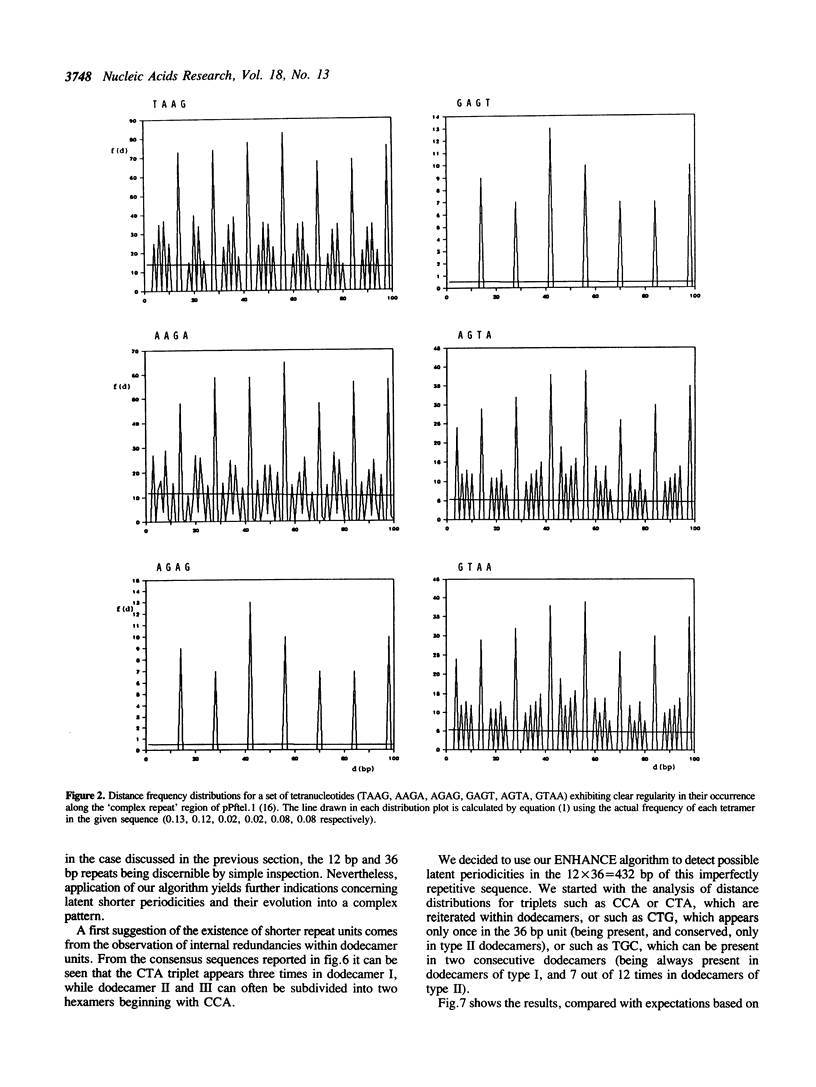
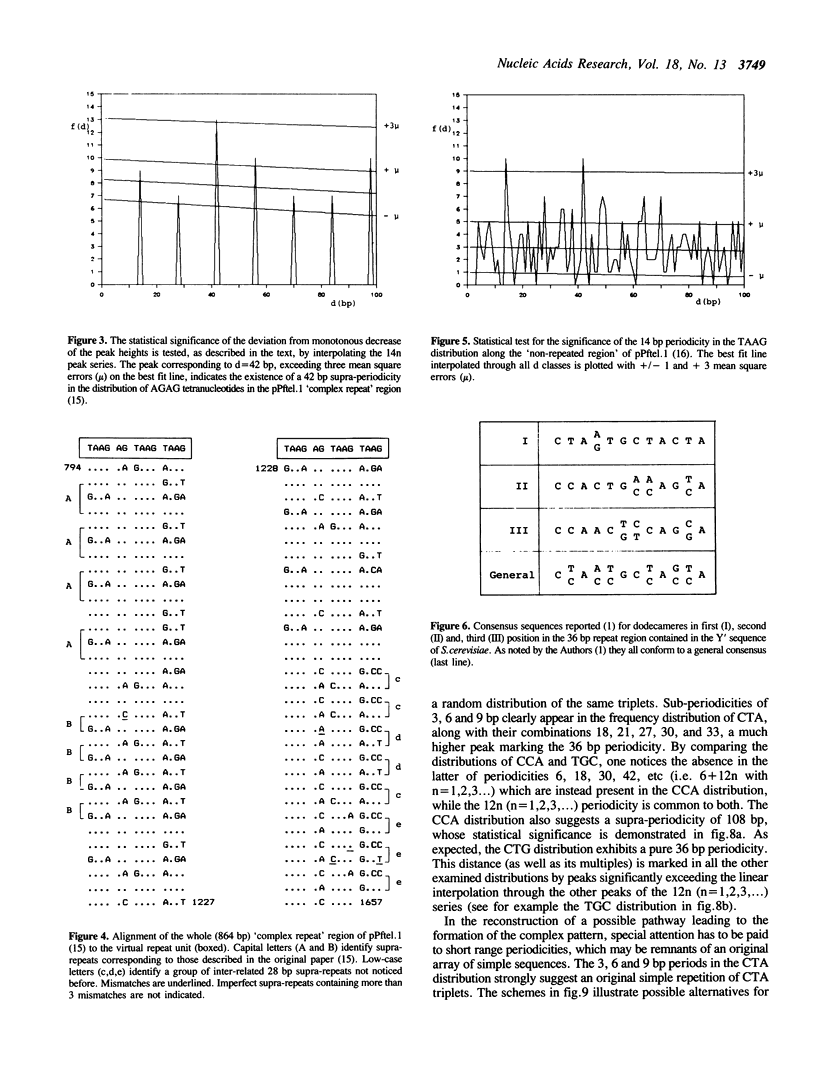
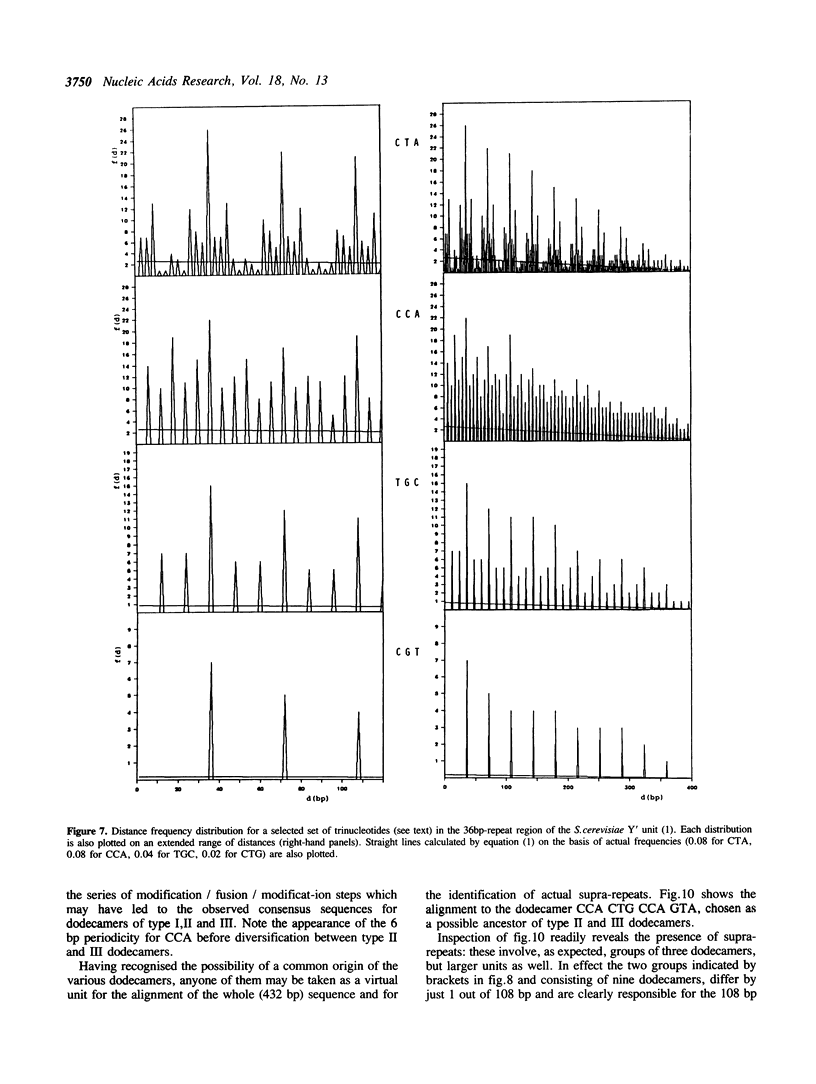
A method is proposed for the automatic detection of serial periodicities in a linear sequence. Its application to DNA subtelomeric sequences from two lower eukaryotes, P.falciparum and S.cerevisiae, reveals ordered patterns organised in hierarchical periodicities, not easily recognizable by other methods. The possible implications concerning the evolution of tandemly repetitive arrays are discussed in light of a model which involves, as successive steps, random repeat modification, the fusion of differently modified repeat versions into longer units, and the amplification of (and/or homogenization to) the more recent repeat units.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barnwell J. W., Tam J. P., Nussenzweig V., Nussenzweig R. S., Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985 Nov 15;230(4727):815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Dore E., Pace T., Ponzi M., Scotti R., Frontali C. Homologous telomeric sequences are present in different species of the genus Plasmodium. Mol Biochem Parasitol. 1986 Nov;21(2):121–127. doi: 10.1016/0166-6851(86)90015-0. [DOI] [PubMed] [Google Scholar]
- Dover G. A. DNA turnover and the molecular clock. J Mol Evol. 1987;26(1-2):47–58. doi: 10.1007/BF02111281. [DOI] [PubMed] [Google Scholar]
- Dover G. A., Flavell R. B. Molecular coevolution: DNA divergence and the maintenance of function. Cell. 1984 Oct;38(3):622–623. doi: 10.1016/0092-8674(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Dover G. A. Three into two won't go. Nature. 1988 Jan 14;331(6152):121–122. doi: 10.1038/331121a0. [DOI] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Enea V., Galinski M., Schmidt E., Gwadz R., Nussenzweig R. S. Evolutionary profile of the circumsporozoite gene of the Plasmodium cynomolgi complex. J Mol Biol. 1986 Apr 20;188(4):721–726. doi: 10.1016/s0022-2836(86)80017-1. [DOI] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Ellis J., Svec P., Schlesinger D. H., Nussenzweig V. Identification and chemical synthesis of a tandemly repeated immunogenic region of Plasmodium knowlesi circumsporozoite protein. Nature. 1983 Sep 1;305(5929):29–33. doi: 10.1038/305029a0. [DOI] [PubMed] [Google Scholar]
- Horowitz H., Haber J. E. Subtelomeric regions of yeast chromosomes contain a 36 base-pair tandemly repeated sequence. Nucleic Acids Res. 1984 Sep 25;12(18):7105–7121. doi: 10.1093/nar/12.18.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Thorburn P., Haber J. E. Rearrangements of highly polymorphic regions near telomeres of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2509–2517. doi: 10.1128/mcb.4.11.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi M., Pace T., Dore E., Frontali C. Identification of a telomeric DNA sequence in Plasmodium berghei. EMBO J. 1985 Nov;4(11):2991–2995. doi: 10.1002/j.1460-2075.1985.tb04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint R. B., Coppel R. L., Cowman A. F., Brown G. V., Shi P. T., Barzaga N., Kemp D. J., Anders R. F. Changes in repeat number, sequence, and reading frame in S-antigen genes of Plasmodium falciparum. Mol Cell Biol. 1987 Aug;7(8):2968–2973. doi: 10.1128/mcb.7.8.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Tautz D., Trick M., Dover G. A. Cryptic simplicity in DNA is a major source of genetic variation. Nature. 1986 Aug 14;322(6080):652–656. doi: 10.1038/322652a0. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., Walliker D., McCutchan T. F. Genetic hypervariability of telomere-related sequences is associated with meiosis in Plasmodium falciparum. Nucleic Acids Res. 1988 Jul 25;16(14B):6973–6985. doi: 10.1093/nar/16.14.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley R. M. Yeast telomeres: the end of the chromosome story? Yeast. 1987 Sep;3(3):139–148. doi: 10.1002/yea.320030302. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Blanton H. M. Distribution of telomere-associated sequences on natural chromosomes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 May;8(5):2257–2260. doi: 10.1128/mcb.8.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Sequence variation in putative functional domains of the circumsporozoite protein of Plasmodium falciparum. Implications for vaccine development. J Biol Chem. 1987 Sep 5;262(25):11935–11939. [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Variation among circumsporozoite protein genes from rodent malarias. Mol Biochem Parasitol. 1988 Feb;28(1):31–38. doi: 10.1016/0166-6851(88)90176-4. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., Welsh J. A., McCutchan T. F. Evolution of the immunodominant domain of the circumsporozoite protein gene from Plasmodium vivax. Implications for vaccines. J Biol Chem. 1987 May 15;262(14):6464–6467. [PubMed] [Google Scholar]


