Abstract
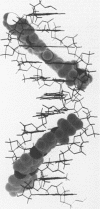
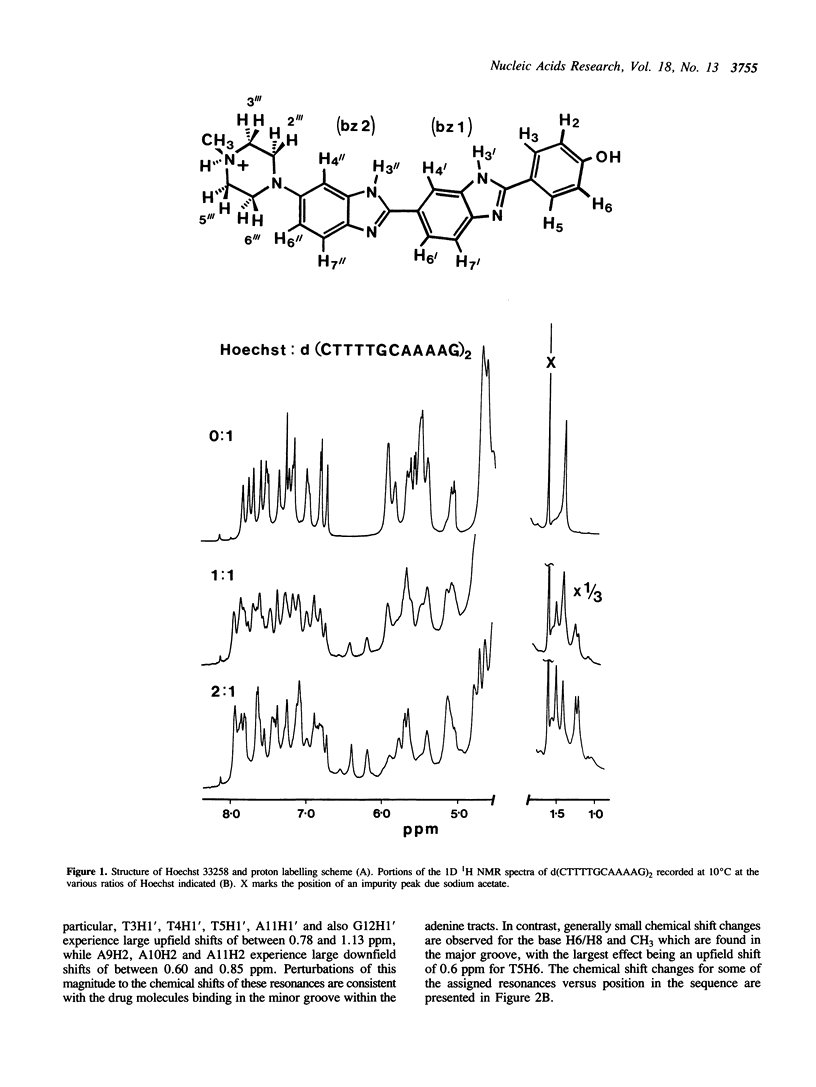
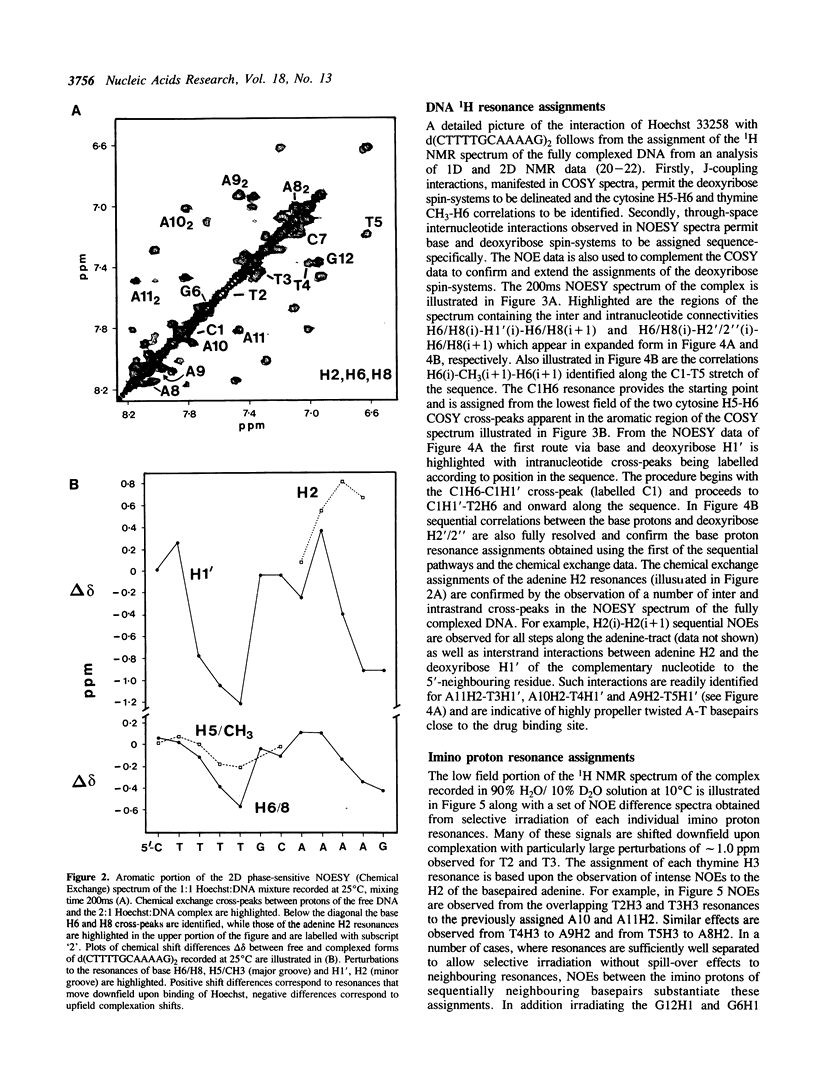
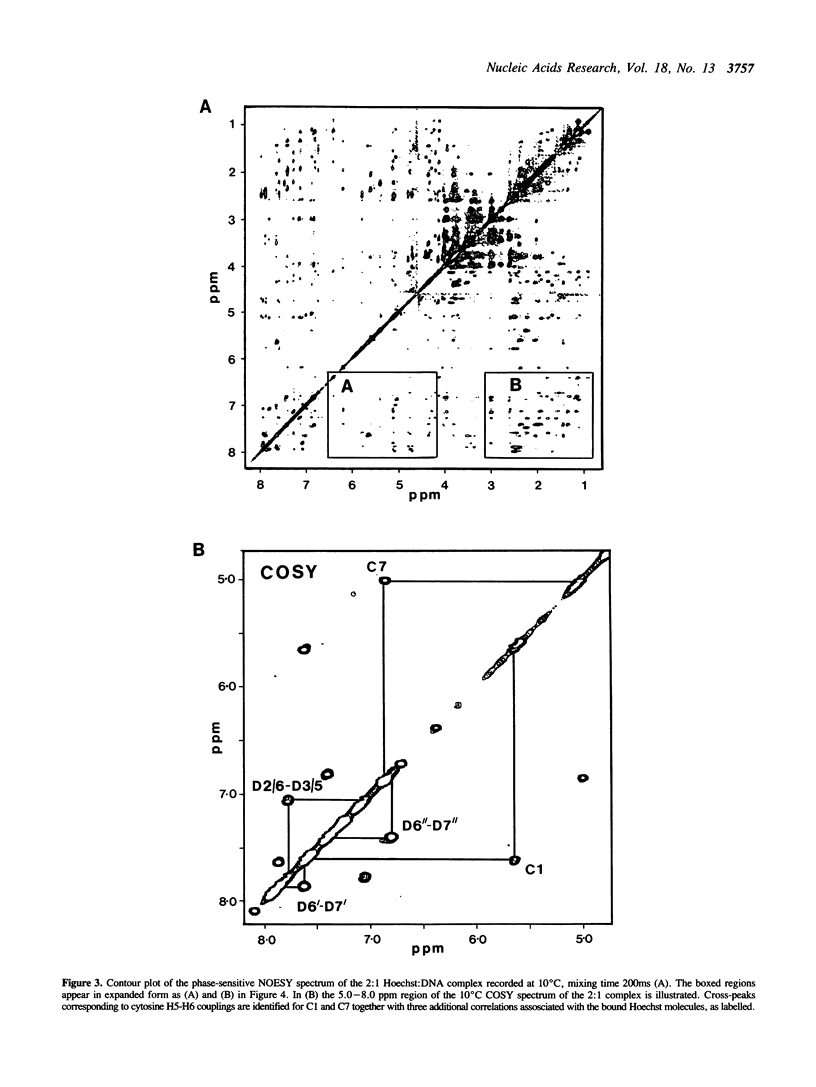
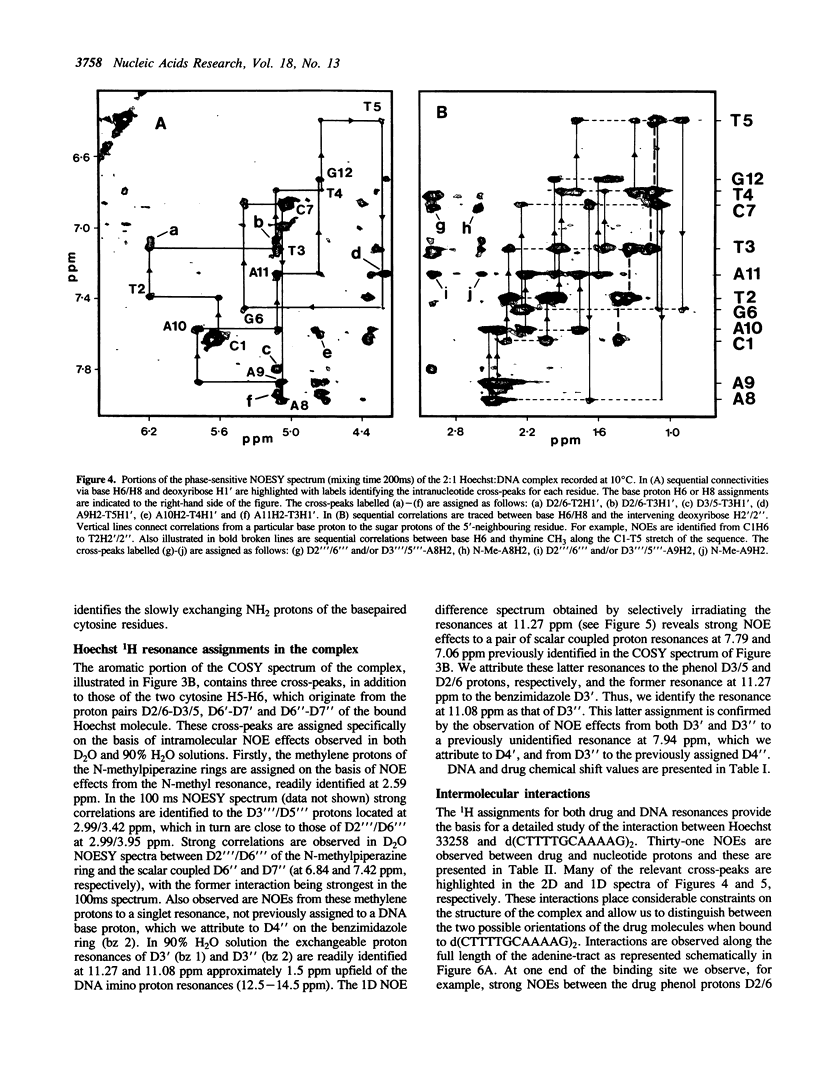
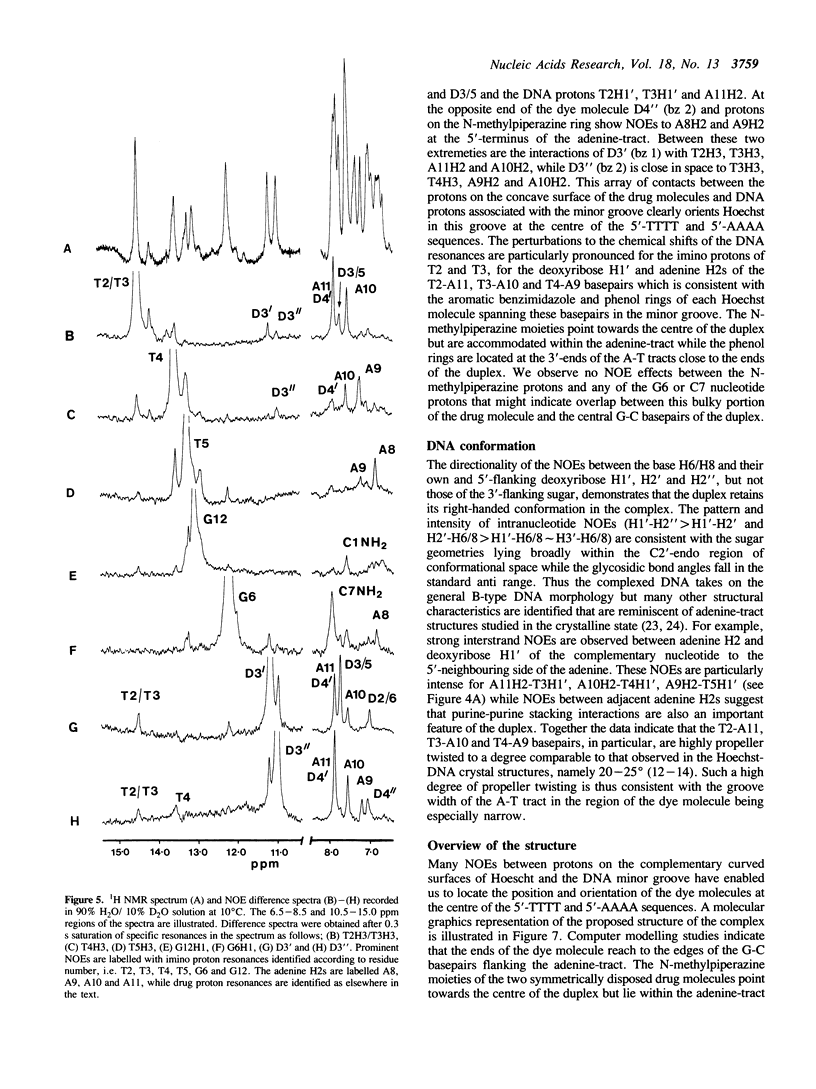
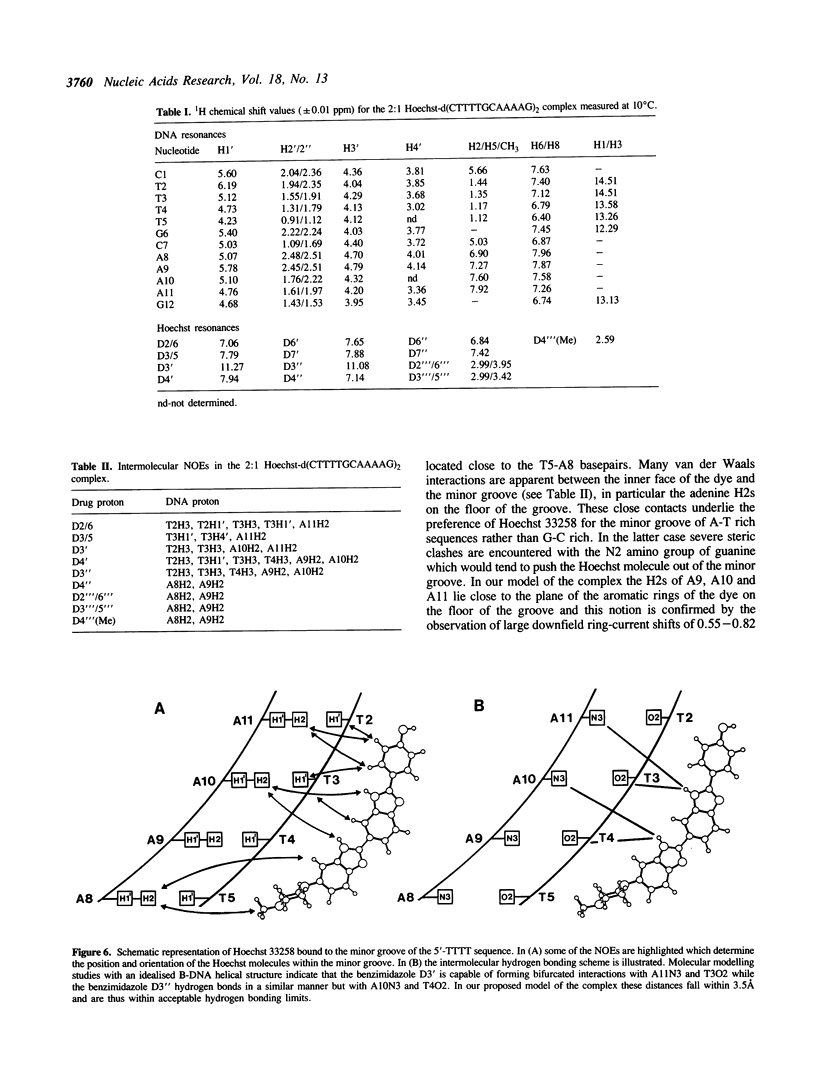
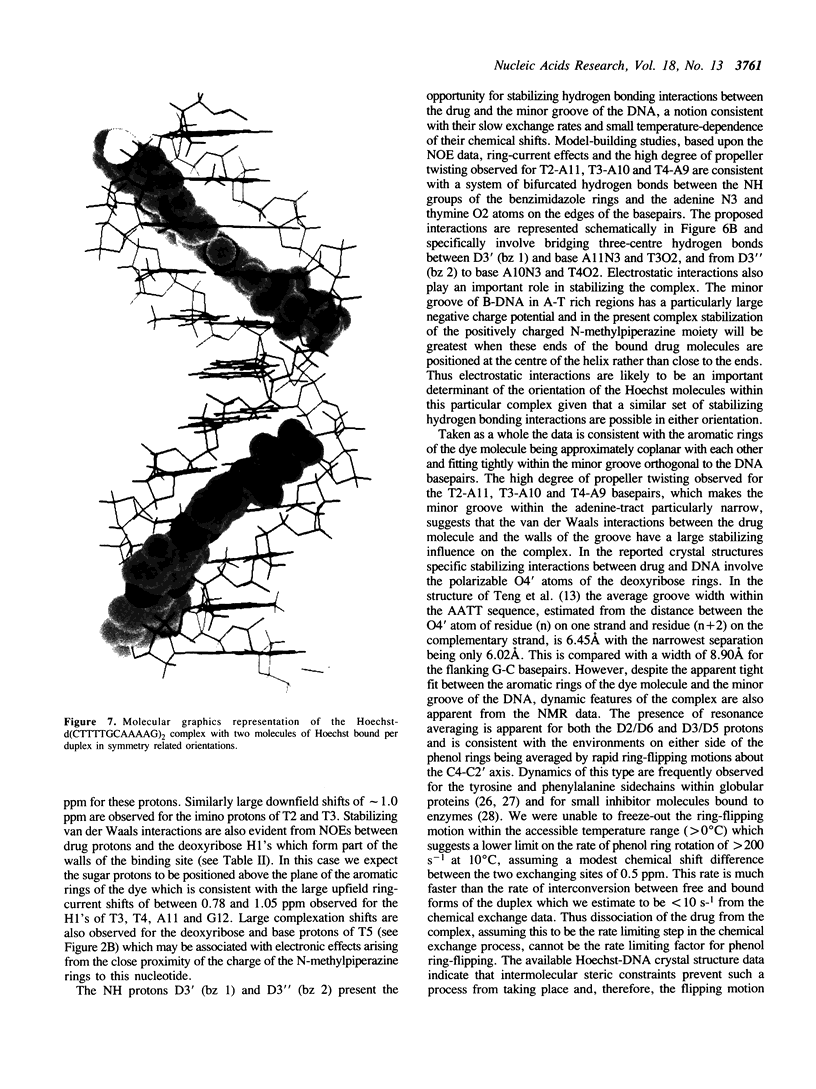
The interaction of Hoechst 33258 with the minor groove of the adenine-tract DNA duplex d(CTTTTGCAAAAG)2 has been studied in both D2O and H2O solutions by 1D and 2D 1H NMR spectroscopy. Thirty-one nuclear Overhauser effects between drug and nucleotide protons within the minor groove of the duplex, together with ring-current induced perturbations to the chemical shifts of basepair and deoxyribose protons, define the position and orientation of the bound dye molecules. Two drug molecules bind cooperatively and in symmetry related orientations at the centre of the 5'-TTTT and 5'-AAAA sequences with the binding interactions spanning only the four A-T basepairs. The positively charged N-methylpiperazine moieties point towards the centre of the duplex while the phenol groups are disposed towards the 3'-ends of the sequence. Resonance averaging is apparent for both the D2/D6 and D3/D5 phenol protons and D2"'/D6"' and D3"'/D5"' of the N-methylpiperazine ring and is consistent with these groups being involved in rapid rotation or ring-flipping motions in the bound state. Interstrand NOEs between adenine H2s and deoxyribose H1' are consistent with a high degree of propeller twisting of the A-T basepairs at the binding site of the aromatic benzimidazole and phenol rings of Hoechst. The data imply that the minor groove is particularly narrow with many contacts between the complementary curved surfaces of the drug and DNA indicating that strong van der Waals interactions, involving the floor and the walls of the minor groove, stabilize the complex. In our model the NH groups of the benzimidazole rings are positioned to make a pair of bifurcated hydrogen bonds with the adenine N3 and thymine O2 on the floor of the minor groove.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bontemps J., Houssier C., Fredericq E. Physico-chemical study of the complexes of "33258 Hoechst" with DNA and nucleohistone. Nucleic Acids Res. 1975 Jun;2(6):971–984. doi: 10.1093/nar/2.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Moore G. R., Perkins S. J., Williams R. J. Temperature dependent molecular motion of a tyrosine residue of ferrocytochrome C. FEBS Lett. 1976 Nov;70(1):96–100. doi: 10.1016/0014-5793(76)80734-x. [DOI] [PubMed] [Google Scholar]
- Carrondo M. A., Coll M., Aymami J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Binding of a Hoechst dye to d(CGCGATATCGCG) and its influence on the conformation of the DNA fragment. Biochemistry. 1989 Sep 19;28(19):7849–7859. doi: 10.1021/bi00445a047. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M. Sequence-dependent structural variations in two right-handed alternating pyrimidine-purine DNA oligomers in solution determined by nuclear Overhauser enhancement measurements. EMBO J. 1983;2(12):2109–2115. doi: 10.1002/j.1460-2075.1983.tb01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham D. A., Suswillo R. R., Rogers R., McGreevy P. B., Andrews B. J. Studies on Brugia pahangi. 13. The anthelmintic effect of compounds F151 (Friedheim), HOE 33258 (Hoechst) and their reaction product. J Helminthol. 1976 Dec;50(4):243–250. doi: 10.1017/s0022149x00026663. [DOI] [PubMed] [Google Scholar]
- DiGabriele A. D., Sanderson M. R., Steitz T. A. Crystal lattice packing is important in determining the bend of a DNA dodecamer containing an adenine tract. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1816–1820. doi: 10.1073/pnas.86.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Harshman K. D., Dervan P. B. Molecular recognition of B-DNA by Hoechst 33258. Nucleic Acids Res. 1985 Jul 11;13(13):4825–4835. doi: 10.1093/nar/13.13.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Wohlleb J. C. Optical studies of the interaction of 33258 Hoechst with DNA, chromatin, and metaphase chromosomes. Chromosoma. 1975 Nov 11;52(4):297–316. doi: 10.1007/BF00364015. [DOI] [PubMed] [Google Scholar]
- Marcus M., Goitein R., Gropp A. Condensation of all human chromosomes in phase G2 and early mitosis can be drastically inhibited by 33258-Hoechst treatment. Hum Genet. 1979 Sep 2;51(1):99–105. doi: 10.1007/BF00278298. [DOI] [PubMed] [Google Scholar]
- Marcus M., Sperling K. Condensation--inhibition by 33258-Hoechst of centromeric heterochromatin in prematurely condensed mouse chromosomes. Exp Cell Res. 1979 Oct 15;123(2):406–411. doi: 10.1016/0014-4827(79)90488-9. [DOI] [PubMed] [Google Scholar]
- Martin R. F., Holmes N. Use of an 125I-labelled DNA ligand to probe DNA structure. 1983 Mar 31-Apr 6Nature. 302(5907):452–454. doi: 10.1038/302452a0. [DOI] [PubMed] [Google Scholar]
- Murray V., Martin R. F. Sequence specificity of 125I-labelled Hoechst 33258 in intact human cells. J Mol Biol. 1988 May 20;201(2):437–442. doi: 10.1016/0022-2836(88)90150-7. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Hare D. Sequence-dependent conformations of DNA duplexes: the TATA segment of the d(G-G-T-A-T-A-C-C) duplex in aqueous solution. Biopolymers. 1986 Apr;25(4):693–706. doi: 10.1002/bip.360250412. [DOI] [PubMed] [Google Scholar]
- Pjura P. E., Grzeskowiak K., Dickerson R. E. Binding of Hoechst 33258 to the minor groove of B-DNA. J Mol Biol. 1987 Sep 20;197(2):257–271. doi: 10.1016/0022-2836(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Raether W., Lämmler G. The filaricidal effect of basically substituted 2,6-bis-benzimidazoles in Litomosoides carinii infection of the cotton rat (Sigmodon hispidus). Ann Trop Med Parasitol. 1971 Mar;65(1):107–115. doi: 10.1080/00034983.1971.11720801. [DOI] [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Forster M. J., Birdsall B., Roberts G. C., Feeney J., Cheung H. T., Kompis I., Geddes A. J. Dynamics of trimethoprim bound to dihydrofolate reductase. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3787–3791. doi: 10.1073/pnas.85.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke T., Steen H. B. Multiple binding modes for Hoechst 33258 to DNA. J Histochem Cytochem. 1985 Apr;33(4):333–338. doi: 10.1177/33.4.2579998. [DOI] [PubMed] [Google Scholar]
- Teng M. K., Usman N., Frederick C. A., Wang A. H. The molecular structure of the complex of Hoechst 33258 and the DNA dodecamer d(CGCGAATTCGCG). Nucleic Acids Res. 1988 Mar 25;16(6):2671–2690. doi: 10.1093/nar/16.6.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K., Wagner G. NMR investigations of the dynamics of the aromatic amino acid residues in the basic pancreatic trypsin inhibitor. FEBS Lett. 1975 Feb 1;50(2):265–268. doi: 10.1016/0014-5793(75)80504-7. [DOI] [PubMed] [Google Scholar]