Abstract
A number of 3,5-bis(benzylidene)-4-piperidones 1 and some N-4-(2-aminoethoxy)phenylcarbonyl analogs 3–6 display excellent in vitro antimycobacterial properties. In particular, 1c and 6d are potent antimycobacterials which are well tolerated in mice and are identified as important lead molecules. The nature of both the benzylidene aryl rings and the terminal basic groups which affect the antimycobacterial potencies and the absence of neurotoxic side effects were identified. Several representative compounds stimulated respiration in mitochondria isolated from rat liver and this effect was not caused by the swelling of these organelles. Various guidelines for the creation of further related novel antimycobacterial agents are provided.
Keywords: 3,5-Bis(benzylidene)-4-piperidones; Antimycobacterial; Neurotoxic; Mitochondria; Rodent toxicity; Piperidones; Antitubercular
1. Introduction
Tuberculosis is an infection caused by Mycobacterium tuberculosis and is a major problem at the present time. Currently this microorganism is present in approximately two billion people.1 There are about 8 million new cases each year and at least 2 million people die annually from this disease.2 The rise in the incidence of mycobacterial infections is multifactorial including the pandemic of acquired immunodeficiency syndrome which lowers resistance to infection by M. tuberculosis.
At the present time, a number of drugs are available for the initial treatment of tuberculosis, principally rifampin, isoniazid, ethambutol and pyrazinamide. These drugs are often administered over a period of six months3 and this prolonged treatment leads on occasions to the development of drug resistance.4 Thus novel classes of antimycobacterials are urgently required which have modes of action that are divergent from those drugs used in current therapy.
A major interest in these laboratories is the synthesis and bioevaluations of Mannich bases (3-aminoketones) derived from α,β-unsaturated ketones.5 Various studies revealed that the mode of action of representative compounds includes alkylation of thiols6–8 and interference with respiration in the electron transport chain in mitochondria. 9–11 Thus the way in which these compounds exert their bioactivities is likely different from that of contemporary antimycobacterial drugs, for example, ethambutol interferes with the incorporation of mycolic acids into the bacterial cell wall.12 A number of acyclic Mannich bases possess antibacterial properties13–15 and recently a series of these molecules were described which displayed antimycobacterial activity.16 However a recurrent problem with many acyclic Mannich bases is their ability to cause neurotoxicity as well as death by respiratory depression.17,18 Thus consideration was given to attaching arylidene groups to a cyclic Mannich base, namely 4-piperidone, leading to 1a which was isolated as the hydrochloride salt. This compound showed excellent potency towards M. tuberculosis whereby the growth of the microorganism was inhibited completely at a concentration of 6.25 μg/mL.19 Intraperitoneal injection of 1a hydrochloride into mice revealed that this compound lowered hepatic thiol concentrations.20 Hence the interaction of the 1,5-diphenyl-3-oxo-1,4-pentadienyl group of 1a with cellular thiols likely accounts, at least in part, for its antimycobacterial properties. Compound 1a had been shown earlier to have cytotoxic properties21 and recently a project was initiated to use 1a as the lead molecule in the simultaneous development of both candidate antimycobacterial and cytotoxic agents. In both cases the hypothesis was made that the 1,5-diphenyl- 3-oxo-1,4-pentadienyl group interacts at a primary binding site while groups attached to the piperidyl nitrogen atom could interact at a complementary binding site thereby increasing potencies. Conversely, steric repulsion between the N-substituents and various groups adjacent to the primary binding site could take place. This communication outlines the efficacy of these compounds as antimycobacterial agents; the synthesis and cytotoxic properties of these molecules have recently been disclosed.22
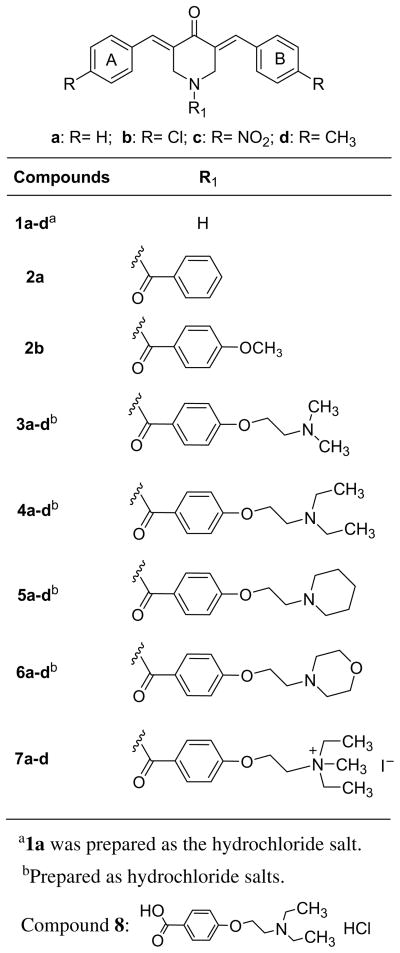
Initially van der Waals bonding between aryl ring C and a possible complementary area on a binding site was considered leading to the decision to prepare 2a. However at a concentration of 6.25 μg/mL, this compound was inactive. Notwithstanding this result, the placement onto aryl ring C of a methoxy group, which has hydrophilic and lipophilic components which would allow hydrogen and hydrophobic bonding, respectively, to occur as found in 2b, restored antimycobacterial properties vide infra. Thus the decision was made to increase the size of the group on ring C with a mixture of hydrophilic and hydrophobic components by preparing 3a, 4a, 5a, 6a and 7a. In order to compare the relative efficiencies of the different N-acyl groups to increase antimycobacterial properties, various substituents were placed in aryl rings A and B of 1a leading to 1b–d which were subsequently converted to the corresponding amides in series 3–7. In order to gain an appreciation of the possible contribution of the N-acyl group per se to antimycobacterial properties, the synthesis and bioevaluation of 8 were planned. This molecule contains most of the structural features of the N-aroyl substituent in series 4. The recent reports of the promising antimycobacterial properties of a number of aromatic compounds containing 2-aminoethoxyphenyl groups23–25 afforded a further reason for developing these compounds for assessment against M. tuberculosis (Fig. 1).
Figure 1.
Structures of the compounds in series 1–8.
A further segment of the study involved assessing the compounds for murine neurotoxicity and survival while selected compounds were planned to be examined for any toxic symptoms in rats.
In summary, the objectives of the present study were to examine a variety of 3,5-bis(benzylidene)-4-piperidones and N-aroyl analogs as candidate antimycobacterials and to assess their toxicity in rodents. The aspiration was that from the biodata generated, a decision could be reached whether a major expansion in this area was warranted or not.
2. Results
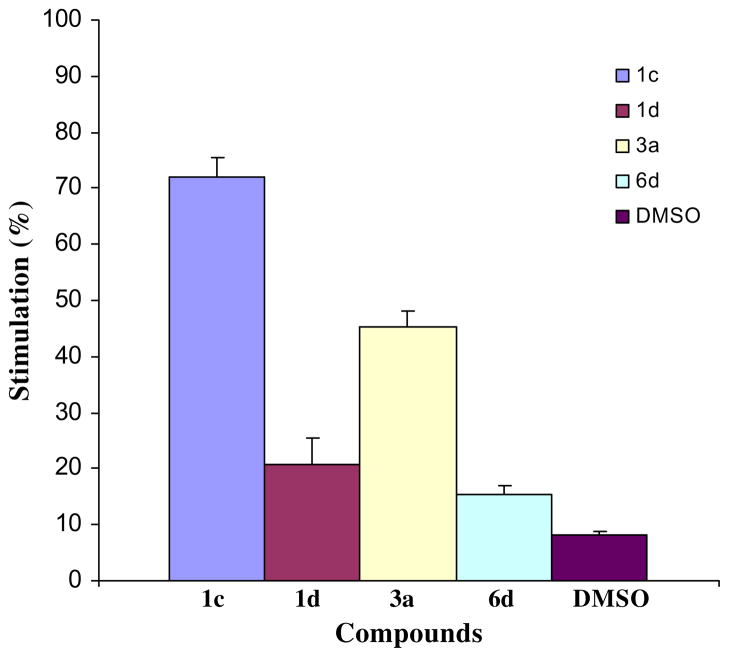
All of the compounds in series 1–8 were evaluated against M. tuberculosis H37Rv. This strain was chosen since it is drug sensitive and is more virulent than M. tuberculosis H37Ra.27 The data are presented in Table 1. The enones 1c, 1d, 3a and 6d were examined for their effect on respiration in rat liver mitochondria; these results are portrayed in Figure 2. Both 1c and 3a were assessed for their ability to cause swelling in mitochondria. All of the compounds in series 1–8 were examined for neurotoxicity and survival in mice while several compounds were assessed in rats.
Table 1.
Evaluation of the compounds in series 1–8 against M. tuberculosis H37Rv in vitro and for neurotoxicity and survival in mice
| Compound | Antimycobacterial evaluationa
|
log Pb | Neurotoxicityc
|
||||
|---|---|---|---|---|---|---|---|
| % Inhibition 6.25 μg/mL | MIC (μg/mL) | 0.5 h
|
4 h
|
||||
| 100 mg/kg | 300 mg/kg | 100 mg/kg | 300 mg/kg | ||||
| 1a | 99 | 3.13 | 5.7 | — | — | — | — |
| 1b | 99 | 6.25 | 6.8 | — | — | — | — |
| 1c | 100 | 0.2 | 5.3 | — | — | — | — |
| 1d | 0 | — | 6.6 | — | — | — | — |
| 2a | 0 | — | 5.5 | 1/8 | 3/4 | 1/4 | 1/2 |
| 2b | 47 | — | 6.0 | 2/8 | — | — | — |
| 3a | 98 | 1.56 | 5.9 | — | — | — | — |
| 3b | 100 | 1.56 | 7.0 | — | — | — | — |
| 3c | 27 | — | 5.5 | — | 1/4 | — | 2/2 |
| 3d | 100 | 3.13 | 6.8 | 1/8 | — | — | — |
| 4a | 99 | — | 7.0 | 1/8 | 1/4 | — | — |
| 4b | 37 | — | 8.0 | — | — | — | — |
| 4c | 34 | — | 6.5 | 1/8 | 1/4 | 1/4 | 1/2 |
| 4d | 100 | 3.13 | 7.9 | — | — | — | — |
| 5a | 100 | 6.25 | 7.1 | 3/8 | 2/4 | 1/4 | 1/2 |
| 5b | 86 | — | 8.1 | 3/8 | 3/4 | 1/4 | 1/2 |
| 5c | 100 | 3.13 | 6.6 | 1/8 | 1/4 | — | 1/2 |
| 5d | 100 | 3.13 | 8.0 | 1/8 | — | — | — |
| 6a | 100 | 6.25 | 5.4 | — | 1/4 | — | — |
| 6b | 0 | — | 6.4 | — | — | — | — |
| 6c | 100 | 6.25 | 5.0 | — | 2/4 | — | — |
| 6d | 100 | 0.78 | 6.3 | — | — | — | — |
| 7a | 0 | — | 2.2 | d | d | d | d |
| 7b | 23 | — | 3.2 | — | — | — | — |
| 7c | 0 | — | 1.8 | 7/8 | 4/4 | 1/2 | e |
| 7d | 81 | — | 3.1 | — | 1/4 | — | f |
| 8 | 12 | — | 2.9 | — | — | — | — |
The hydrochloride salt of 1a was employed in this assay. A concentration of 12.5 μg/mL was used to obtain the % inhibition figures for 1a–c. A concentration of 6.25 μg/mL of a reference drug rifampin caused 100% inhibition of the growth of M. tuberculosis H37Rv. Rifampin has a MIC value of 0.07 (0.015–0.125) μg/mL. The single line—indicates that no attempt was made to determine the MIC value.
The log P values were calculated using the algorithm and database statistics of ACD.26
Compounds 1a, b were evaluated as the hydrochloride salts. The figures refer to the number of animals demonstrating neurotoxicity/total number of animals dosed. The single line—indicates an absence of neurotoxicity.
After 0.5 h, 8/8 and 4/4 mice receiving 100 and 300 mg/kg, respectively, of 7a were dead.
After 0.5 h, 2/8 mice receiving 100 mg/kg of 7c were dead and another animal died between 0.5 and 4 h. A dose of 300 mg/kg was fatal to 4/4 mice when observed 0.5 h after the administration of the compound.
After 4 h, 2/2 animals receiving a dose of 300 mg/kg were dead.
Figure 2.
Effects of 10 μM of 1c, 1d, 3a and 6d as well as solvent control (dimethylsulfoxide 4 μL) on respiration in rat liver mitochondria.
3. Discussion
The initial evaluation of the compounds in series 1–8 against M. tuberculosis is presented in Table 1. A compound is considered to be active which causes at least 90% inhibition of the growth of the microorganism at a concentration of 6.25 μg/mL. This criterion was met by 75% of the compounds in series 1 and 69% of the amides 3–6.
The 4-piperidones 1a–c possess antimycobacterial properties and the minimum inhibitory concentration (MIC) figures, which are presented in Table 1, reveal that 1c in particular is a promising lead molecule. The lack of inhibition of M. tuberculosis by 1d is surprising and the evaluation was repeated in which case the inhibition was 4% at a concentration of 6.25 μg/mL, thereby confirming its inactivity. In contrast to 1a–c, the 4-methyl analog 1d has an electron-donating group in rings A and B which may have contributed to its lack of potency. Clearly, development of series 1 as candidate antimycobacterials is warranted whereby one or more electron-attracting groups are placed in the aryl rings. In addition, a null hypothesis should be evaluated by preparing analogs having negative Hammett σ values in the aryl rings; these compounds would be predicted to be weakly potent or possibly inactive antimycobacterials.
While benzoylation of 1a led to the inactive amide 2a, the 4-methoxy analog 2b displayed weak antimycobacterial properties. Expanding the hydrophilic–hydrophobic methoxy substituent in ring C led to the 1-[4-(2-aminoethoxy) phenylcarbonyl]-3,5-bis(benzylidene)-4-piperidones 3–6 which are a new cluster of antimycobacterials.
An examination of the biodata in series 3–6 was made in order to determine the optimal substitutions in aryl rings A and B and also the preferred terminal amino functions. In regard to the aryl substituents, all of the unsubstituted compounds (3a, 4a, 5a, 6a) and 4-methyl analogs (3d, 4d, 5d, 6d) were active whereas the 4-chloro and 4-nitro derivatives displayed activity in only one and two compounds, respectively. This result may be influenced by the electronic and steric properties of the R1 groups since the Hammett σ values of the hydrogen atom and the 4-methyl, 4-nitro and 4-chloro substituents are 0.00, −0.17, 0.78 and 0.23, respectively, while the molecular refractivity (MR) figures are 1.03, 5.65, 7.36 and 6.03, respectively.28 Thus amplification of the project should include the placement of small electrondonating substituents in rings A and B, for example, the 4-hydroxy group (σp = −0.66; MR = 5.42).28
In regard to the terminal basic group, the criterion for activity was met by three of the four members in series 3, 5 and 6 suggesting that there is considerable tolerance for the size of the terminal basic group at a binding site. The observation that only 4a, d displayed significant antimycobacterial properties indicates that in developing these compounds as candidate antimycobacterials, using the diethylamino substituent as the terminal basic group should be avoided. Neither the quaternary ammonium salts 7a–d nor the aromatic acid 8 displayed noteworthy antimycobacterial properties. Since M. tuberculosis contains a thick cell wall, drug penetration of this microorganism is likely to be more facile with hydrophobic molecules. In fact a number of studies have reported positive correlation between log P values and antimycobacterial properties.4,29 Hence the log P figures of the compounds in series 1–8 were calculated and are presented in Table 1. While no definite conclusions were drawn between the antimycobacterial potencies and hydrophobicity, two observations may be noted. First, those molecules causing greater than 90% inhibition at 6.25 μg/mL have log P values of ~5–8 indicating that these compounds are markedly hydrophobic. It is conceivable that this property, inter alia, contributes to the bioactivity observed. Second, the compounds in series 7 and 8 which are virtually bereft of antimycobacterial potencies have substantially lower hydrophobic properties than the other compounds listed in Table 1.
Various acyclic Mannich bases interfere with respiration in mitochondria.30 Thus an investigation was undertaken to determine if one of the ways in which bioactivity of these compounds is mediated involves interfering with respiration in mitochondria and if so, whether the magnitude of the responses correlated with antimycobacterial potencies. Representative compounds were chosen which have different potencies namely 1c, 3a and 6d, possessing MIC values of 0.2, 1.56 and 0.78 μg/mL, respectively, as well as 1d which is bereft of antimycobacterial properties in the bioassay employed. These compounds were also chosen in view of their being well tolerated in mice vide infra. The effects of these compounds on rat liver mitochondria are portrayed in Figure 2. At the concentrations utilized, stimulation of respiration was observed. Thus interference with mitochondrial respiration is one way whereby antimycobacterial properties are mediated and, as mentioned previously, it is markedly divergent from the mechanisms of action of contemporary antimycobacterial drugs. The percentage stimulation is in order of 1c > 3a > 1d, 6d. Since the relative antimycobacterial potencies are 1c > 6d > 3a > 1d, there is no correlations between this observation and the percentage of stimulation of respiration.
Stimulation of respiration in mitochondria may be caused by different biochemical mechanisms including the swelling of these organelles. Concentrations of 5, 10 and 100 μM of 1c and 3a did not demonstrate this effect. Hence the increases in respiration noted are caused in other ways which may include uncoupling of oxidative phosphorylation.
A major factor in considering whether to develop antimycobacterial drugs is their tolerability in animals. In particular, an investigation was conducted to ascertain whether neurotoxicity and/or lethality were found in these cyclic Mannich bases. All of the compounds in series 1–8 were administered intraperitoneally to mice using doses of 30, 100 and 300 mg/kg and the animals were observed after 0.5 and 4 h. These data are presented in Table 1. The following observations were made regarding structure–toxicity relationships. First, in terms of survival, mice tolerated the maximum dose of 300 mg/kg of each compound in series 1–6 and 8. For series 7, deaths were noted using 100 mg/kg (7a and c) and 300 mg/kg (7d); this observation coupled with their relatively low inhibitory properties towards M. tuberculosis indicate that these quaternary ammonium compounds do not serve as prototypes for further development as candidate antimycobacterials. Second, neurotoxicity was absent in approximately half of the compounds. No neurological deficit was observed when a dose of 30 mg/kg was administered to mice except in the case of 4a (in 2/4 animals after 0.5 h). In general, neurotoxicity was more pronounced at the end of 0.5 than 4 h. Third, neurotoxicity was absent in series 1 and bearing in mind the promising antimycobacterial properties of 1a–c, expansion of this series is clearly warranted. Fourth, an assessment was made of the optimal aryl substituents and terminal basic group in regard to the absence of neurotoxicity of the compounds in series 3– 6. Three compounds containing a 4-chloro group (3b, 4b, 6b), two with a 4-methyl substituent (4d and 6d) and in one unsubstituted analog (3a) did not display neurotoxicity. Thus 4-chloro and 4-methyl groups (and other halogens and alkyl substituents) are recommended for placement in the aryl rings A and B when developing these compounds. Two 4-piperidones in each of the series 3, 4 and 6 did not display neurotoxicity revealing that the dimethylamino, diethylamino and 4-morpholinyl groups are preferable to the 1-piperidino substituent.
Fifth, a dose of 50 mg/kg of 3a, 4a and 6b was injected intraperitoneally into rats and a similar result to the murine screens was observed, that is, only 4a showed neurological deficit. However when 50 mg/kg of 4a was administered to rats by the oral route, no neurotoxicity was observed indicating that the appropriate route of administration may eliminate the side effect of neurotoxicity. In addition, 3a, 3c, 6b and 7c were given orally to rats using a dose of 30 mg/kg and no neurotoxicity was noted. The observation with rats was generally made 0.25, 0.5,1, 2 and 4 h after administering the compound.
4. Conclusions
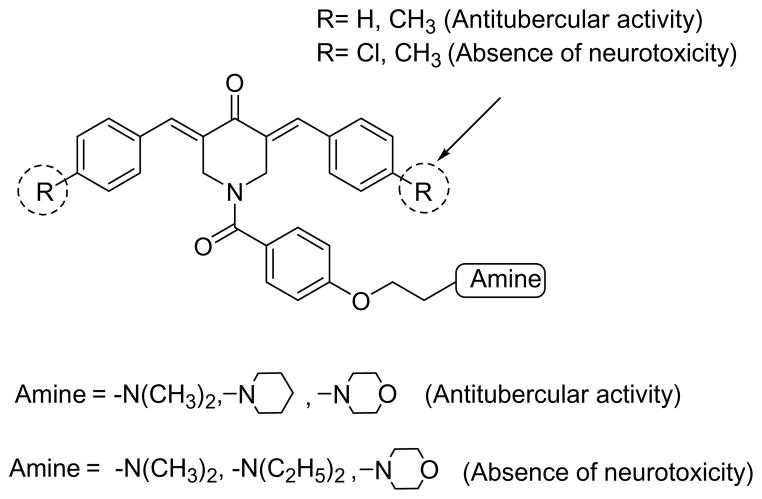
There is a crucial need to develop agents to combat tuberculosis whose structures and mode of action are divergent from contemporary drugs. This study has demonstrated that some 3,5-bis(benzylidene)-4-piperidones 1 have excellent antimycobacterial properties and are well tolerated in mice. The 1-[4-(2-aminoethoxy) phenylcarbonyl]-3,5-bis(benzylidene)-4-piperidones 3–6 are a new group of potent antimycobacterial agents representative of which stimulate respiration in mitochondria. In general these compounds are well tolerated in mice. Various guidelines have been formulated for expansion of the project based on the correlations noted between variations in the structures and the bioactivities observed which are summarized in Figure 3.
Figure 3.
Optimal aromatic ring substituents and amino groups for displaying antimycobacterial activity and absence of neurotoxicity.
5. Experimental
5.1. Synthesis of compounds 1–8
The preparation of the compounds 1–8 has been described previously.22
5.2. Antimycobacterial evaluations
All of the compounds were evaluated initially against M. tuberculosis H37Rv (ATCC 27294) using concentrations of 12.5 μg/mL (1a–c) and 6.25 μg/mL for the remaining compounds. The methodology for conducting this assay, as well as the determination of the MIC values, was undertaken in BACTEC 12B medium using the broth microdilution assay.27 The MIC values are the lowest concentrations which cause a 90% reduction in fluorescence relative to controls.
5.3. Evaluation of various compounds on respiration and swelling in rat liver mitochondria
Rats were euthanized by isoflurane anesthesia and then decapitated. A literature procedure was used for isolating the hepatic mitochondria by differential centrifugation. 31,32 A homogenization buffer of 250 mM sucrose, 10 mM Hepes, 1 mM EDTA, pH 7.2 and a wash buffer of 250 mM sucrose, 10 mM Hepes, 0.3 mM EGTA, pH 7.2 were employed. The final mitochondrial pellet was resuspended in 1–2 mL of 250 mM sucrose, 10 mM Hepes, pH 7.2 to yield 50–80 mg protein/mL. The mitochondrial preparations displayed respiratory control ratios of 5–7.
Mitochondrial oxygen consumption was measured polargraphically using a respirometer system (Hansatech Instruments Ltd, Norfolk, UK). Fresh mitochondria (0.5 mg protein/mL) were incubated at 30 °C in a respiratory buffer of 125 mM sucrose, 65 mM potassium chloride, 10 mM Hepes, 5 mM potassium phosphate, 1 mM magnesium chloride, pH 7.2 containing 5 mM succinate as respiratory substrate.
Mitochondrial swelling was observed using fresh mitochondria incubated at 30 °C in a respiratory buffer composed of 125 mM sucrose, 65 mM potassium chloride, 10 mM Hepes, 5 mM potassium phosphate, 1 mM magnesium chloride, pH 7.2 and 5 mM succinate. The mitochondrial swelling was followed by the loss of absorbance at 520 nm.33
5.4. Toxicity and neurotoxicity screens
The toxicity and neurotoxicity tests were carried out by previously published method.34 Specific details of the toxicity and neurological deficit are as follows. Mortalities in mice were caused by three compounds (number of animals died/number of animals tested, dose in mg/kg, time of observation in h), namely: 7a (8/8, 100, 0.5; 4/4, 300, 0.5), 7c (2/8, 100, 0.5; 1/8, 100, 0.5–4; 4/4, 300, 0.5) and 7d (2/2, 300, 4). Neurotoxicity in mice is presented in Table 1. In the case of rats injected intraperitoneally with 50 mg/kg of 4a, neurological deficit was noted in the following number of animals/total number of rats tested (time in h in parentheses), namely 3/8 (0.25), 5/8 (0.5), 6/8 (1), 4/8 (2), 2/8 (4), 0/8 (6) and 0/8 (24). The ataxia was mild but all animals were reluctant to move unless stimulated by a tail pinch up to and including 6 h after the administration of 4a. All animals behaved in a normal fashion after 24 h. The following compounds (dose in mg/kg in parentheses) were administered to rats by the oral route and the animals examined after 0.25, 0.5, 1,2 and 4 h, namely 3a (30), 3c (30), 4a (50), 6b (30) and 7c (30). No neurotoxicity was observed. Four animals were employed in all cases, except in the case of 3c after 0.5 h, only three rats were observed. The animals were handled, fed and housed following the procedures outlined in the National Research Council Publication ‘Guide for the Care and use of Laboratory Animals’. Euthanasia of the mice and rats used in this study was consistent with the policies of the Institute of Laboratory Resources which describe the humane care of laboratory animals.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for an operating grant to J. R. Dimmock as well as the Tuberculosis Antimicrobial Acquisition and Coordinating Facility who, through a research and development contract with the U.S. National Institute of Allergy and Infectious Diseases, undertook the antimycobacterial evaluations. The U.S. National Institute of Neurological Diseases and Stroke carried out the tolerability studies in rodents which is gratefully acknowledged. Ms. B. McCullough is thanked for typing various drafts describing this study.
References and notes
- 1.Nayyar A, Jain R. Curr Med Chem. 2005;12:1873– 1886. doi: 10.2174/0929867054546654. [DOI] [PubMed] [Google Scholar]
- 2.Gadad AK, Noolvi MN, Karpoormath RV. Bioorg Med Chem. 2004;12:5651–5659. doi: 10.1016/j.bmc.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 3.Bass JB, Jr, Farer LS, Hopewell PC, O’Brien R, Jacobs RF, Ruben F, Snider DE, Jr, Thornton G. Am J Respir Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 4.Sriram D, Yogeeswari P, Reddy SP. Bioorg Med Chem Lett. 2006;16:2113–2116. doi: 10.1016/j.bmcl.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 5.Dimmock JR, Kumar P. Curr Med Chem. 1997;4:1–22. [Google Scholar]
- 6.Mutus B, Wagner JD, Talpas CJ, Dimmock JR, Phillips OA, Reid RS. Anal Biochem. 1989;177:237– 243. doi: 10.1016/0003-2697(89)90045-6. [DOI] [PubMed] [Google Scholar]
- 7.Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Eur J Med Chem. 1983;18:248–254. [Google Scholar]
- 8.Pati HN, Das U, Sharma RK, Dimmock JR. Mini-Rev Med Chem. 2007;7:131–139. doi: 10.2174/138955707779802642. [DOI] [PubMed] [Google Scholar]
- 9.Dimmock JR, Shyam K, Hamon NW, Patil SA, Smith PJ. Neoplasma. 1985;32:85–91. [PubMed] [Google Scholar]
- 10.Dimmock JR, Hamon NW, de Gooijer CA, Grant GF, Jonnalagadda SS, Hancock DS. Pharmazie. 1984;44:560–562. [PubMed] [Google Scholar]
- 11.Hamon NW, Kirkpatrick DL, Chow EWK, Dimmock JR. J Pharm Sci. 1982;71:25–29. doi: 10.1002/jps.2600710106. [DOI] [PubMed] [Google Scholar]
- 12.Martin AR. In: Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry. 10. Delgado JN, Remers WA, editors. Lippincott-Raven Publishers; Philadelphia: 1998. p. 207. [Google Scholar]
- 13.Khachatourians GG, Holmlund PK, Dimmock JR. J Pharm Sci. 1984;73:803–808. doi: 10.1002/jps.2600730624. [DOI] [PubMed] [Google Scholar]
- 14.Dimmock JR, Nyathi CB, Smith PJ. J Pharm Sci. 1979;68:1216–1221. doi: 10.1002/jps.2600681006. [DOI] [PubMed] [Google Scholar]
- 15.Lorand T, Koesis B, Sohar P, Nagy G, Kispal G, Krane HG, Schmitt H, Weckert E. Eur J Med Chem. 2001;36:705–717. doi: 10.1016/s0223-5234(01)01264-8. [DOI] [PubMed] [Google Scholar]
- 16.Dimmock JR, Kandepu NM, Das U, Zello GA, Nienaber KH. Pharmazie. 2004;59:502–505. doi: 10.1002/chin.200447216. [DOI] [PubMed] [Google Scholar]
- 17.Dimmock JR, Patil SA, Shyam K. Pharmazie. 1991;46:538–539. [PubMed] [Google Scholar]
- 18.Dimmock JR, Jonnalagadda SS, Phillips OA, Erciyas E, Shyam K, Semple HA. J Pharm Sci. 1992;81:436–440. doi: 10.1002/jps.2600810509. [DOI] [PubMed] [Google Scholar]
- 19.Jha A, Dimmock JR. Pharmazie. 2006;61:562–563. [PubMed] [Google Scholar]
- 20.Dimmock JR, Arora VK, Wonko SL, Hamon NW, Quail JW, Jia Z, Warrington RC, Fang WD, Lee JS. Drug Des Deliv. 1990;6:183–194. [PubMed] [Google Scholar]
- 21.Dimmock JR, Padmanilyam MP, Puthucode RN, Nazarali AJ, Motaganahalli NL, Zello GA, Quail JW, Oloo EO, Kraatz HB, Prisciak JS, Allen TM, Santos CL, Balzarini J, De Clercq E, Manavathu EK. J Med Chem. 2001;44:586–593. doi: 10.1021/jm0002580. [DOI] [PubMed] [Google Scholar]
- 22.Das U, Alcorn J, Shrivastav A, Sharma RK, De Clercq E, Balzarini J, Dimmock JR. Eur J Med Chem. 2007;42:71–80. doi: 10.1016/j.ejmech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, Panda G, Chaturvedi V, Manju YS, Gaikwad AK, Sinha S. Bioorg Med Chem Lett. 2007;17:5586–5589. doi: 10.1016/j.bmcl.2007.07.089. [DOI] [PubMed] [Google Scholar]
- 24.Panda G, Parai MK, Das SK, Shagufta, Sinha M, Chaturvedi V, Srivastava AK, Manju YS, Gaikwad AN, Sinha S. Eur J Med Chem. 2007;42:410–419. doi: 10.1016/j.ejmech.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Panda G, Shagufta, Mishra JK, Chaturvedi V, Srivastava AK, Srivastava R, Srivastava BS. Bioorg Med Chem. 2004;12:5269–5276. doi: 10.1016/j.bmc.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 26.ACD/Labs release 10.00, version 10.02. http://www.acdlabs.com.
- 27.Collins L, Franzblau SG. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu KC. In: Burger’s Medicinal Chemistry, Part I. 4. Wolff ME, editor. John Wiley and Sons; New York: 1980. p. 401. [Google Scholar]
- 29.Sivakumar PM, Seenivasan SP, Kumar V, Doble M. Bioorg Med Chem Lett. 2007;17:1695–1700. doi: 10.1016/j.bmcl.2006.12.112. [DOI] [PubMed] [Google Scholar]
- 30.Dimmock JR, Shyam K, Hamon NW, Logan BM, Raghavan SK, Harwood DJ, Smith PJ. J Pharm Sci. 1983;72:887–894. doi: 10.1002/jps.2600720812. [DOI] [PubMed] [Google Scholar]
- 31.Johnson D, Lardy H. Methods Enzymol. 1967;10:94– 96. [Google Scholar]
- 32.Schneider WC. J Biol Chem. 1948;176:259–266. [PubMed] [Google Scholar]
- 33.Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJH, Vercesi AE. J Biol Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- 34.Stables JP, Kupferberg HJ. In: Molecular and Cellular Targets for Antiepileptic Drugs. Vanzini GA, Tanganelli P, Avoli M, editors. John Libby and Company Ltd; London: 1997. pp. 191–198. [Google Scholar]