Summary
The requirement of a metalloprotease ADAM17 (a disintegrin and metalloprotease 17) for the growth of cultured vascular smooth muscle cells has been demonstrated in vitro. However, whether this metalloprotease is responsible for vascular remodeling in vivo remains unanswered. Rat carotid arteries were analyzed 2 weeks after a balloon angioplasty. The neointimal cells were strongly positive for ADAM17 immunostaining. Marked inhibition of intimal hyperplasia was observed in dominant negative ADAM17 adenovirus treated carotid artery. Proliferating cell nuclear antigen positive cells and phospho-epidermal growth factor receptor positive cells in the neointima were reduced by dominant negative ADAM17 as well. In contrast, the neointima formation, proliferating cell nuclear antigen positive cells, and phospho-epidermal growth factor receptor positive cells were markedly enhanced by wild-type ADAM17 adenovirus. In conclusion, ADAM17 activation is involved in epidermal growth factor receptor activation and subsequent neointimal hyperplasia after vascular injury. ADAM17 could be a novel therapeutic target for pathophysiological vascular remodeling.
Keywords: ADAM Proteins, Tumor Necrosis Factor-alpha Convertase, Angioplasty, Vascular Intima, Epidermal Growth Factor Receptor
Introduction
ADAMs (a disintegrin and metalloprotease)s are membrane-anchored metalloproteases implicated in the ectodomain shedding of cell surface proteins, including the ligands for epidermal growth factor receptors (EGFRs)/ErbBs 1, 2. It has been well documented that the transactivation of the EGFR plays critical roles for many cellular functions in the cardiovascular system such as hypertrophy, proliferation and migration mediated through multiple G protein-coupled receptors (GPCRs) 3.
We have demonstrated that ADAM17 is responsible for the EGFR transactivation and subsequent hypertrophy by angiotensin II in cultured vascular smooth muscle cells (VSMCs) 4. However, in vivo evidence for a role of ADAM17 in mediating cardiovascular diseases remains limited. Here, we hypothesized that targeted inactivation of ADAM17 may reduce proliferating vascular remodeling. To test the hypothesis, we have utilized a model of arterial hyperplasia in response to angioplasty together with adenoviral gene manipulation of ADAM17.
Materials and Methods
Balloon angioplasty and adenoviral gene transfer
Left common carotid artery balloon angioplasty was performed in male Sprague-Dawley rats (Charles River Breeding Laboratory) as previously reported 5. Adenoviral vectors encoding wild-type mouse ADAM17 (wtADAM17) and a catalytically inactive/dominant negative mouse ADAM17 mutant, E406A, (dnADAM17) were created using the pCMV expression vectors as the template 4. The wtADAM17 and dnADAM17 sequences were amplified by PCR and ligated into the pAdTrack-CMV vector at the BgIII/NotI site. The fragment containing the wtADAM17 or dnADAM17 with EGFP sequence was cloned into pENTR4 vector by the TOPO cloning reaction (Invitrogen) and then cloned into pAd/CMV/V5-DEST vector by a reaction with LR Clonase II (Invitrogen). The adenovirus titers were determined by Adeno-X™ Rapid Titer Kit (BD Biosciences). Subsequently, 100 μL of the adenovirus encoding wtADAM17, dnADAM17 or control GFP (2×109 pfu/mL) was delivered to the injured artery. We have confirmed protein expression of an adenoviral-encoded gene in medial VSMCs and neointimal cells 14 days after the delivery 6. The vessels were harvested 14 days later, fixed, and histology was determined as described 5. These investigations conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and Temple University 5.
Immunohistochemistry, morphometry and statistics
Immunohistochemistry was performed as described previously 5 with ADAM17 antibody (Abcam 39163), proliferating cell nuclear antigen (PCNA) antibody (Millipore P12004) and phospho-Tyr1068 EGFR (Cell Signaling 2236). For the quantification of PCNA and phospho-Tyr1068 EGFR, percentage of PCNA positive nucleus and nucleus surrounded by pEGFR positive staining were counted respectively in the neointima as described previously 5, 6. For vascular morphometry, digitized images were averaged from at least three representative stained tissue sections using Image Pro Plus (Media Cybernetics). The circumference of the lumen, the area encircled internal elastic lamina, and the external elastic lamina were quantified. The medial and intimal areas were then calculated 6. The data are presented as mean±SE. Groups were compared using ANOVA followed by student t test. The null hypothesis was rejected when p<0.05.
Results
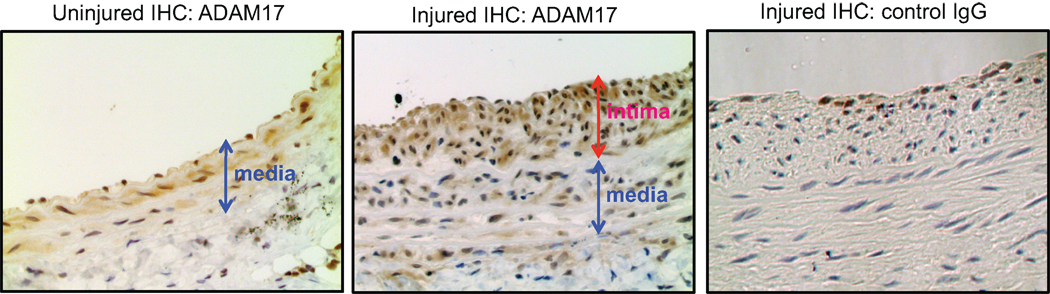
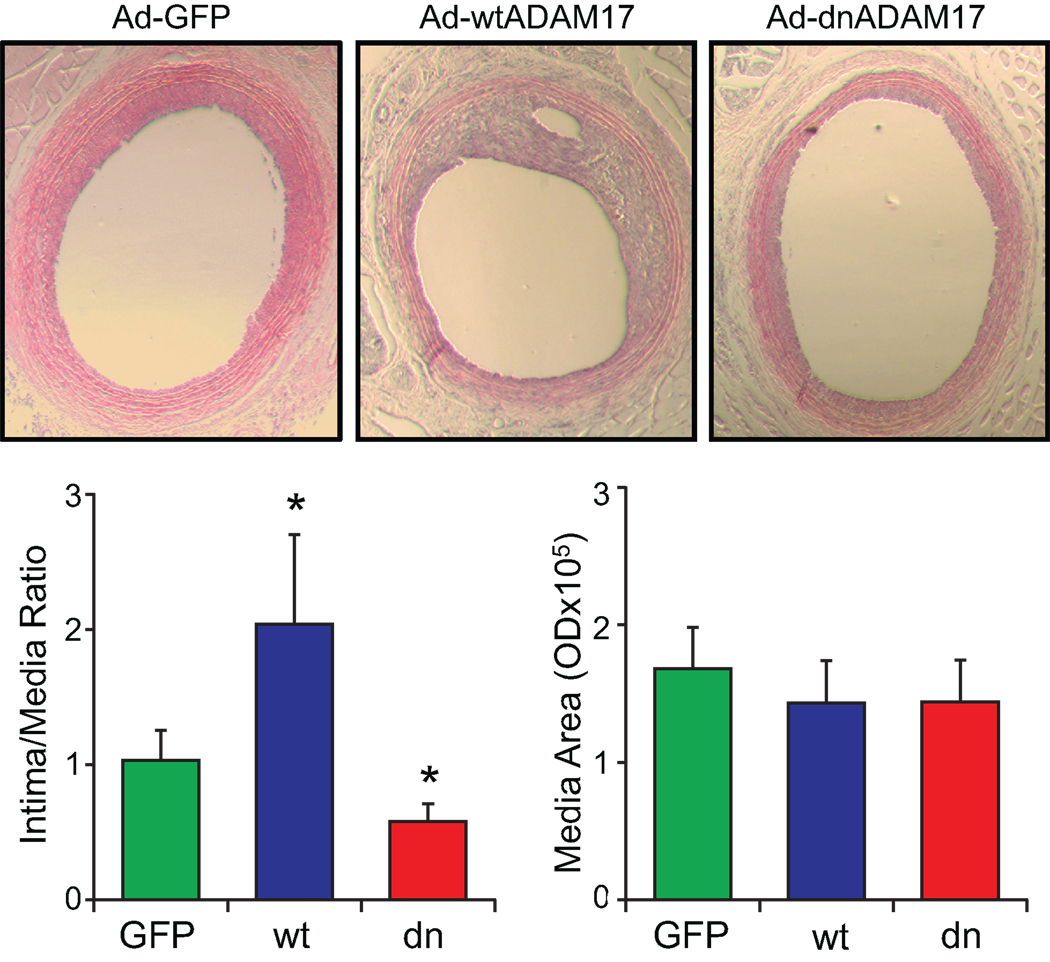
To examine the role of ADAM17 in participating vascular remodeling, expression of ADAM17 was assessed in the carotid artery after a balloon angioplasty. The presence of ADAM17-positive cells were observed in the neointima lesion compared with a control uninjured carotid artery, which has a weak staining in the medial layer (Figure 1). To study the involvement of ADAM17 in the neointima formation, wtADAM17 adenovirus or dnADAM17 adenovirus was delivered upon arterial injury. wtADAM17 adenovirus enhanced, whereas dnADAM17 adenovirus reduced the intima/media ratio compared with GFP adenovirus (Figure 2). The efficiency of gene transfer was confirmed with immunohistochemical analysis of the samples with anti-ADAM17 antibody (Figure S1, please see http://hyper.ahajournals.org).
Figure 1.
ADAM17 expression in response to arterial injury. Histological analysis of ADAM17 expression in arterial cross-sections obtained after balloon injury. Arterial sections obtained on day 14 after injury were stained with ADAM17 antibody or with control IgG (×200 magnification). Representative sections (each from n=3) are shown.
Figure 2.
ADAM17 is involved in neointima formation in response to arterial injury. The effect of ADAM17 adenovirus on arterial neointima formation after balloon injury was analyzed. Representative sections (×40 magnification) are shown. 14 days after injury, the common carotid artery was stained and the area of neointima and media were quantified. Data are mean±SE of sections from 4–6 rats. *p<0.05 compared to the GFP adenovirus-infected control.
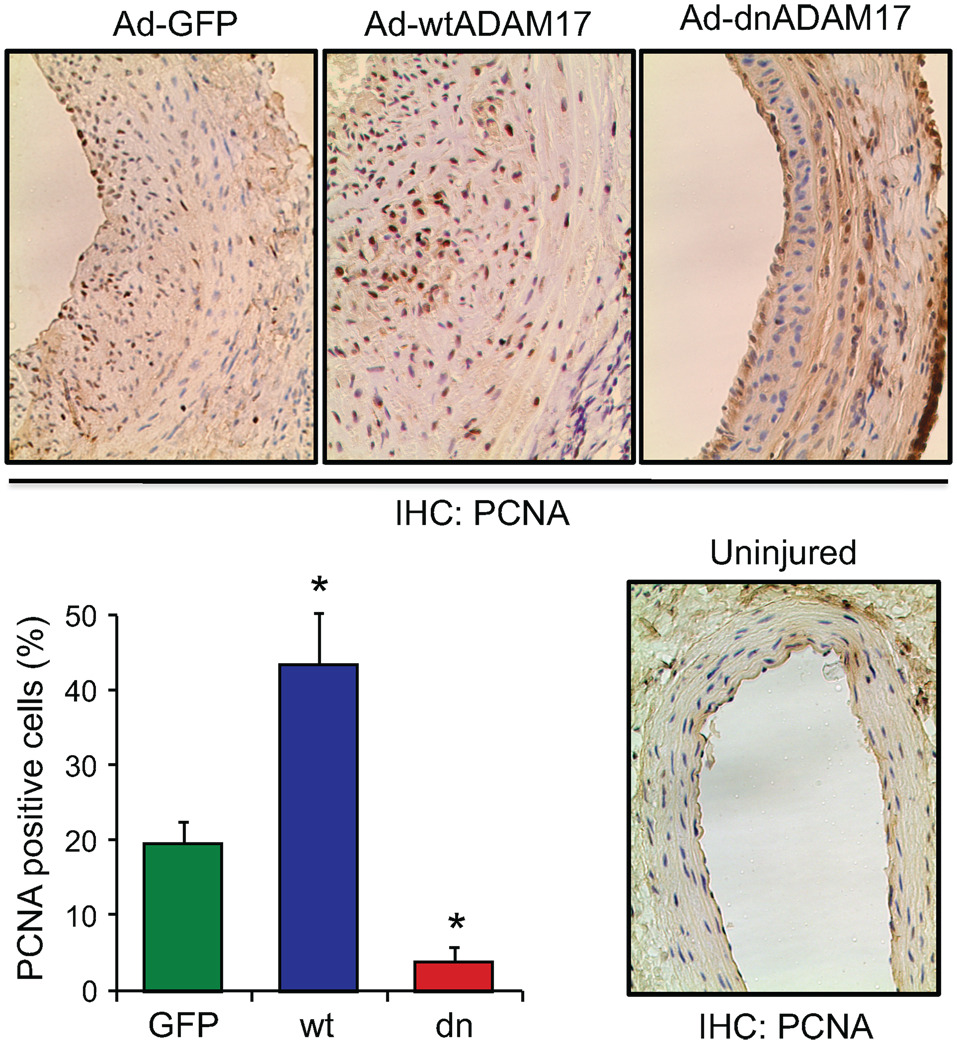
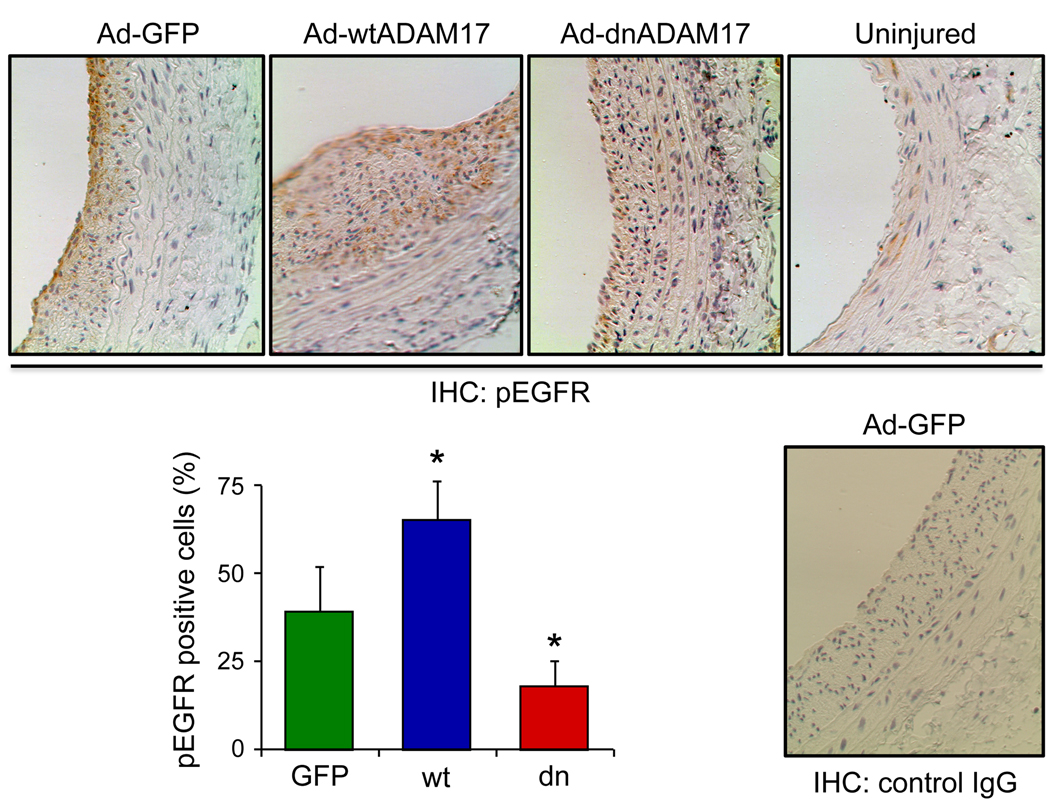
To study the role of ADAM17 in regulating VSMC proliferation in response to arterial injury, PCNA positive cells were evaluated in the above conditions. PCNA positive cells were more abundant in the neointima with the wtADAM17 gene transfer, and were less abundant with the dnADAM17 gene transfer compared with control GFP delivered arteries (Figure 3). To assess EGFR activation in the neointima in response to arterial injury, phospho-EGFR staining was evaluated. Uninjured media was faintly stained with phospho-EGFR antibody. Compared to the GFP control, neointimal phospho-EGFR positive cell numbers were enhanced with wtADAM17 and reduced with dnADAM17 (Figure 4).
Figure 3.
Histological analysis of cell proliferation in arterial cross-sections obtained after balloon injury. Arterial sections obtained on day 14 after injury with infection of adenovirus encoding GFP, wtADAM17, or dnADAM17 were stained with the antibody for PCNA. Representative sections (each from 3 rats, ×200 magnification) are shown. The graph shows quantitative analysis of PCNA positive cells in the neointima from the 3 high-powered fields (mean±SE). *p<0.05 compared to the GFP adenovirus-infected control.
Figure 4.
Histological analysis of the EGFR signal transduction in arterial cross-sections obtained after balloon injury. Arterial sections obtained on day 14 after injury with infection of adenovirus encoding GFP, wtADAM17, or dnADAM17 were stained with the antibody for Tyr1068-phosphorylated EGFR (pEGFR) or with control IgG. Representative sections (each from 3 rats, ×200 magnification) are shown. The graph shows quantitative analysis of pEGFR positive cells in the neointima from the 3 high-powered fields (mean±SE). *p<0.05 compared to the GFP adenovirus-infected control.
Discussion
Although reduced ADAM17 mRNA expression in the liver of atherosclerosis resistant mice has been recently reported 7, our data demonstrate a critical role of ADAM17 for neointimal hyperplasia in response to an arterial injury. In line with our observation of the enhanced ADAM17 expression in neointima, strong ADAM17 expression has been detected in intimal lesions of apoE−/− mice and human atherosclerotic plaque 8. ADAM17 expression was also higher in patients with acute myocardial infarction than those with stable angina pectoris 9. Moreover, single-nucleotide polymorphisms of ADAM17 are associated with increased serum tissue necrosis factor-α (TNFα) and the risk of cardiovascular death in patients with coronary artery disease 10. Therefore, enhanced ADAM17 expression/activity could be a novel predictor of ongoing lesion formation in the vasculature.
EGFR activation has long been implicated in experimental models of restenosis 11–13, however the mechanism through which this occurs in vivo is ill defined. In this regard, there have been many mechanisms proposed to mediate EGFR activation associated with vascular remodeling including intracellular mechanisms without the participation of any EGFR ligand (whose precursor needs to be processed by a metalloprotease) 14. As such, we believe our non-pharmacological data supporting a critical role for ADAM17 in EGFR activation leading to neointimal hyperplasia will move the field forward.
At present, the identity of the EGFR ligand(s) shed by ADAM17 responsible for the in vivo EGFR activation remains unknown. ADAM17 is a major convertase of certain EGFR ligands including, heparin-binding EGF-like growth factor (HB-EGF), transforming growth factor-α, amphiregulin, and epiregulin in mouse embryonic cells 15. In cultured VSMCs, HB-EGF has been reported to be responsible for EGFR transactivation and subsequent ERK activation induced by angiotensin II and other GPCR agonists 3, 16, 17. Moreover, it has been reported that low flow-induced vascular remodeling was prevented in HB-EGF−/− mice 18. Epiregulin produced by ADAM17 could also be involved in the neointimal hyperplasia. It is required for the EGFR transactivation and proliferation of VSMCs stimulated by fractalkine (CX3CL1) 19. Moreover, epiregulin is a potent VSMC-derived mitogen induced by angiotensin II or endothelin 20 and is expressed in rat carotid artery after angioplasty and in human atherosclerotic arteries 21. Likewise, there is the potential for ADAM17-dependent production of transforming growth factor-α and/or amphiregulin in mediating vascular neointima formation as they are both implicated in pathological vascular remodeling 22. Therefore, it is likely that ADAM17 mediates EGFR transactivation in response to arterial injury through multiple EGFR ligands rather than through one single ligand.
In addition to the EGFR ligand precursors mentioned above, ADAM17 participates in the ectodomain shedding of over 40 cell surface proteins whose processing will produce mature cytokines/chemokines and other bioactive factors or lead to inactivation or modulation of the receptors or adhesion molecules 1, 23, 24. Therefore, beside EGFR activation, other ADAM17-dependent shedding/modulation events may collaboratively contribute to the initiation and/or progression of the neointimal remodeling. For example, among the known ADAM17 substrates, the production of TNFα 25, fractalkine/CX3CL1 26, stem cell factor/kit ligand 27, or macrophage colony-stimulating factor/CSF-1 28, and inactivation of p75 TNF receptor-2 29 or p75 neurotrophin receptor 30 appear to be relevant for pathological vascular remodeling. Also, the effects of ADAM17 on various cell adhesion molecules should be considered 23 when trying to evaluate the mechanisms through which ADAM17 influences neointimal remodeling.
Limitations of the current study include the lack of identification of the responsible ADAM17 substrate(s) as mentioned above. Addressing this critical issue is expected to significantly advance knowledge about ADAM involvement within the cardiovascular system. Whilst ADAM substrate identification and involvement have been assessed in in vitro experiments (biochemical assays with the recombinant protease and candidate substrates, reporter-based shedding assays in cultured cells, flow-cytometer to detect loss-of cell surface precursor, or culture medium detection of the cleaved products 31), the list of ADAM17 substrates continues to grow. Indeed, recently developed “degradomics” approaches are anticipated to expand the list of potential substrates even further 31. In combination, the sheer number of ADAM17 substrates that are likely to be involved, as well as a lack of technology to reliably measure ADAM17-dependent shedding in vivo makes this question extremely difficult to resolve at present and may require the development of novel in vivo measurement technology. In addition, the molecular mechanism by which ADAM17 is induced and activated in response to arterial injury awaits further investigations.
Perspectives.
A potential contribution of ADAM17 to obesity and metabolic syndrome has been reported 32, 33. ADAM17 is also implicated in hypertension, cardiac hypertrophy and fibrosis 34, 35. Endothelial ADAM17 appears to be involved in pathological angiogenesis 36. Our data presented here suggests that ADAM17 plays an important role in neointima formation following arterial injury and could be a novel therapeutic target against vascular remodeling associated with cardiovascular diseases. Further expansion of research is therefore expected to determine global as well as tissue specific roles of ADAM17 activity in regulating cardiovascular physiology and pathophysiology.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health Grants, HL076770 (S.E.), HL063810 (M.A.), HL090885 (M.A.), and by American Heart Association Established Investigator Award, 0740042N (S.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 2.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–e137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 5.Hinoki A, Kimura K, Higuchi S, Eguchi K, Takaguri A, Ishimaru K, Frank GD, Gerthoffer WT, Sommerville LJ, Autieri MV, Eguchi S. p21-activated kinase 1 participates in vascular remodeling in vitro and in vivo. Hypertension. 2010;55:161–165. doi: 10.1161/HYPERTENSIONAHA.109.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommerville LJ, Xing C, Kelemen SE, Eguchi S, Autieri MV. Inhibition of allograft inflammatory factor-1 expression reduces development of neointimal hyperplasia and p38 kinase activity. Cardiovasc Res. 2009;81:206–215. doi: 10.1093/cvr/cvn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holdt LM, Thiery J, Breslow JL, Teupser D. Increased ADAM17 mRNA expression and activity is associated with atherosclerosis resistance in LDL-receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1097–1103. doi: 10.1161/ATVBAHA.108.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, Alessi MC, Juhan-Vague I, Nalbone G. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Satoh M, Ishikawa Y, Itoh T, Minami Y, Takahashi Y, Nakamura M. The expression of TNF-alpha converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur J Clin Invest. 2008;38:97–105. doi: 10.1111/j.1365-2362.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 10.Morange PE, Tregouet DA, Godefroy T, Saut N, Bickel C, Rupprecht HJ, Lackner K, Barbaux S, Poirier O, Peiretti F, Nalbone G, Juhan-Vague I, Blankenberg S, Tiret L. Polymorphisms of the tumor necrosis factor-alpha (TNF) and the TNF-alpha converting enzyme (TACE/ADAM17) genes in relation to cardiovascular mortality: the AtheroGene study. J Mol Med. 2008;86:1153–1161. doi: 10.1007/s00109-008-0375-6. [DOI] [PubMed] [Google Scholar]
- 11.Pastore CJ, Isner JM, Bacha PA, Kearney M, Pickering JG. Epidermal growth factor receptor-targeted cytotoxin inhibits neointimal hyperplasia in vivo. Results of local versus systemic administration. Circ Res. 1995;77:519–529. doi: 10.1161/01.res.77.3.519. [DOI] [PubMed] [Google Scholar]
- 12.Trieu VN, Narla RK, Myers DE, Uckun FM. EGF-genistein inhibits neointimal hyperplasia after vascular injury in an experimental restenosis model. J Cardiovasc Pharmacol. 2000;35:595–605. doi: 10.1097/00005344-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Chan AK, Kalmes A, Hawkins S, Daum G, Clowes AW. Blockade of the epidermal growth factor receptor decreases intimal hyperplasia in balloon-injured rat carotid artery. J Vasc Surg. 2003;37:644–649. doi: 10.1067/mva.2003.92. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 15.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res. 2000;87:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Chalothorn D, Jackson LF, Lee DC, Faber JE. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res. 2004;95:989–997. doi: 10.1161/01.RES.0000147962.01036.bb. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Sunnarborg SW, McNaughton KK, Johns TG, Lee DC, Faber JE. Heparin-binding epidermal growth factor-like growth factor signaling in flow-induced arterial remodeling. Circ Res. 2008;102:1275–1285. doi: 10.1161/CIRCRESAHA.108.171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res. 2010;85:825–835. doi: 10.1093/cvr/cvp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor DS, Cheng X, Pawlowski JE, Wallace AR, Ferrer P, Molloy CJ. Epiregulin is a potent vascular smooth muscle cell-derived mitogen induced by angiotensin II, endothelin-1, and thrombin. Proc Natl Acad Sci U S A. 1999;96:1633–1638. doi: 10.1073/pnas.96.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi M, Hayashi K, Yoshida K, Ohkawa Y, Komurasaki T, Kitabatake A, Ogawa A, Nishida W, Yano M, Monden M, Sobue K. Epiregulin as a major autocrine/paracrine factor released from ERK- and p38MAPK-activated vascular smooth muscle cells. Circulation. 2003;108:2524–2529. doi: 10.1161/01.CIR.0000096482.02567.8C. [DOI] [PubMed] [Google Scholar]
- 22.Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186:38–53. doi: 10.1016/j.atherosclerosis.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol. 2006;79:1105–1116. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- 24.Reiss K, Saftig P. The "a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Takeda R, Suzuki E, Satonaka H, Oba S, Nishimatsu H, Omata M, Fujita T, Nagai R, Hirata Y. Blockade of endogenous cytokines mitigates neointimal formation in obese Zucker rats. Circulation. 2005;111:1398–1406. doi: 10.1161/01.CIR.0000158482.83179.DB. [DOI] [PubMed] [Google Scholar]
- 26.Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101:17795–17800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CH, Verma S, Hsieh IC, Hung A, Cheng TT, Wang SY, Liu YC, Stanford WL, Weisel RD, Li RK, Cherng WJ. Stem cell factor attenuates vascular smooth muscle apoptosis and increases intimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:540–547. doi: 10.1161/01.ATV.0000257148.01384.7d. [DOI] [PubMed] [Google Scholar]
- 28.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, Peppel K. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol. 2008;28:284–289. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer R. Reduced apoptosis and increased lesion development in the flow-restricted carotid artery of p75(NTR)-null mutant mice. Circ Res. 2002;91:494–500. doi: 10.1161/01.res.0000035245.83233.2a. [DOI] [PubMed] [Google Scholar]
- 31.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 32.Serino M, Menghini R, Fiorentino L, Amoruso R, Mauriello A, Lauro D, Sbraccia P, Hribal ML, Lauro R, Federici M. Mice heterozygous for tumor necrosis factor-alpha converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes. 2007;56:2541–2546. doi: 10.2337/db07-0360. [DOI] [PubMed] [Google Scholar]
- 33.Gelling RW, Yan W, Al-Noori S, Pardini A, Morton GJ, Ogimoto K, Schwartz MW, Dempsey PJ. Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology. 2008;149:6053–6064. doi: 10.1210/en.2008-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z, Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 35.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C. MMP-2 Mediates Angiotensin II-Induced Hypertension Under the Transcriptional Control of MMP-7 and TACE. Hypertension. 57:123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 36.Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.