Abstract
Primary hyperoxaluria type 1 (PH1) and type 2 (PH2) are rare genetic diseases that result from deficiencies in glyoxylate metabolism. The increased oxalate synthesis that occurs can lead to kidney stone formation, deposition of calcium oxalate in the kidney and other tissues, and renal failure. Hydroxyproline (Hyp) catabolism, which occurs mainly in the liver and kidney, is a prominent source of glyoxylate and could account for a significant portion of the oxalate produced in PH. To determine the sensitivity of mouse models of PH1 and PH2 to Hyp-derived oxalate, animals were fed diets containing 1% Hyp. Urinary excretions of glycolate and oxalate were used to monitor Hyp catabolism and the kidneys were examined to assess pathological changes. Both strains of knockout (KO) mice excreted more oxalate than wild-type (WT) animals with Hyp feeding. After 4 wk of Hyp feeding, all mice deficient in glyoxylate reductase/hydroxypyruvate reductase (GRHPR KO) developed severe nephrocalcinosis in contrast to animals deficient in alanine-glyoxylate aminotransferase (AGXT KO) where nephrocalcinosis was milder and with a lower frequency. Plasma cystatin C measurements over 4-wk Hyp feeding indicated no significant loss of renal function in WT and AGXT KO animals, and significant and severe loss of renal function in GRHPR KO animals after 2 and 4 wk, respectively. These data suggest that GRHPR activity may be vital in the kidney for limiting the conversion of Hyp-derived glyoxylate to oxalate. As Hyp catabolism may make a major contribution to the oxalate produced in PH patients, Hyp feeding in these mouse models should be useful in understanding the mechanisms associated with calcium oxalate deposition in the kidney.
Keywords: oxalate, calcium oxalate crystals, nephrocalcinosis
the primary hyperoxalurias (PH) are rare genetic disorders with an incompletely characterized incidence that probably lies between 1 in 106 and 1 in 105 (3). The major types characterized result from deficiencies in glyoxylate metabolism. Alanine:glyoxylate aminotransferase (AGT1) is deficient in PH1 and catalyzes the transamination of glyoxylate to glycine. Glyoxylate reductase and hydroxypyruvate reductase activities (GRHPR) are deficient in PH2 and normally catalyze the reduction of glyoxylate to glycolate and hydroxypyruvate to d-glycerate, respectively (3). A deficiency in either AGT or GRHPR activities results in an inadequate removal of glyoxylate and its oxidation to oxalate by lactate dehydrogenase (LDH). The increased oxalate synthesis can lead to kidney stone formation and the deposition of calcium oxalate in tissues (3). In PH1, much of the glyoxylate is also metabolized to glycolate through GRHPR activity, accounting for the hyperglycolic aciduria. Hyperglyceric aciduria is characteristic of PH2 due to the deficient hydroxypyruvate reductase activity and the conversion of excessive hydroxypyruvate to l-glycerate by LDH (2). Apart from a minority of PH1 patients who respond to pyridoxine therapy, there are no specific therapies for the disease and an increased fluid intake, coupled with orthophosphate or citrate administration, is often utilized to limit renal calcium oxalate deposition and renal impairment (7).
Glyoxylate (CO.COO−) is the simplest α-keto acid and is generated in cells as a result of metabolism. Known sources are the breakdown of hydroxyproline (Hyp) and the oxidation of glycolate (6). In Hyp metabolism, 4-hydroxy-2-oxoglutaric acid (HOG) is hydrolyzed by HOG aldolase (HOGA) to pyruvate and glyoxylate, whereas glycolate is oxidized to glyoxylate by glycolate oxidase (GO) (6, 15). These enzymes are present in different subcellular compartments with HOGA localized in liver and kidney mitochondria, GO and AGT in liver peroxisomes, and GR in both mitochondria and the cytoplasm of all cells. Hyp is derived both from endogenous collagen turnover, which is estimated to be 2–3 g/day (15), and from dietary sources of Hyp. Collagen contains ∼15% Hyp and its turnover results in the release of 300–450 mg of Hyp. Metabolism of this Hyp results in the formation of 180–240 mg of glyoxylate per day. Meat, meat products, and gelatin-containing foods in the diet can provide additional Hyp for metabolism. This level of Hyp turnover is supported by studies of individuals with hyperhydroxyprolinemia who lack Hyp oxidase and are unable to degrade Hyp. Hyp excretion levels in these individuals range from 285 to 550 mg/24 h (15). Glyoxylate formed from Hyp is predominantly converted to glycine and glycolate by AGT and GRHPR, respectively. A small portion of the glyoxylate appears to escape metabolism in these pathways and is converted to oxalate by LDH.
Our results of studies on gelatin ingestion in normal subjects suggested that the glyoxylate formed from Hyp is efficiently converted to glycine. The ingestion of 500 mg of dietary Hyp (∼7 g gelatin) increased urinary output of glycolate by 10–20 mg and oxalate by 1–3 mg (13). This amount of oxalate would represent 3–10% of the oxalate normally excreted in urine. In PH1 and PH2, this efficient metabolism of glyoxylate is perturbed, due to the deficiencies in AGT and GRHPR activities. It is striking that in many PH1 patients the sum of the glycolate and oxalate excreted in urine is ∼300 mg/day and close to the expected size of the Hyp-derived glyoxylate pool (7), suggesting Hyp metabolism in PH patients could account for the majority of the oxalate produced.
To increase our understanding of the pathology and the altered metabolism associated with PH and to test new treatment strategies, animal models will be potentially valuable tools. A mouse model of PH1 that lacks AGT activity and retains many of the features of the PH1 phenotype has been previously described (16). In this report, we describe the phenotype of GRHPR KO mice and compare how Hyp is metabolized in both mouse models.
MATERIALS AND METHODS
Chemicals.
Reagent grade chemicals were obtained from either Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Trans-4-l-hydroxyproline for feeding studies was purchased from Sigma.
Animals.
GRHPR heterozygous mice with mixed 129/Sv and C57Bl6 background were developed by Lexicon Genetics (Woodlands, TX) from an ES clone (OST383093) with the trapping retroviral vector (VICTR37) inserted in the first intron of the GRHPR gene. GRHPR heterozygous mice were intercrossed to generate GRHPR KO and wild-type mice, and these animals of mixed background were used to obtain phenotypic data. To place the mutation in a homogenous genetic background, male mice heterozygous for GRHPR were backcrossed with C57BL6 females for 12 generations, and they were finally intercrossed to produce homozygous GRHPR KO mice. Mice were genotyped by tail snip DNA, using a PCR with primers Gr-F2: 5′-CTTGCCTCCTTGGATACTCG-3′, Gr-R: 5′-CACTCTCACCTCCTCCCAAA-3′, and LTR2: 5′-ATAAACCCTCTTGCAGTTGCATC-3′. Agarose electrophoresis of PCR products yields a 289-bp product for the wild-type allele (primers F2&R) and a 173-bp product for the trapped allele (primers F2<R2). The development and genotyping of AGXT KO mice have previously been described (16). AGXT KO mice with the pure C57Bl6 background were used for these studies. Mice were maintained in a barrier facility with a 12:12-h light-dark cycle and an ambient temperature of 23 ± 1°C and had free access to food and water. All animal studies were approved by the Institutional Animal Use and Care Committee.
Metabolic cage collections.
Animals were equilibrated to metabolic cages for 1 wk. Any animals that did not equilibrate to the metabolic cage in this time, as measured by inconsistent food intake and weight gain, were not used for further experiments. After equilibration, three 24-h urines were collected. The mean of these three collections was used to characterize the urinary phenotype of each animal. Urines were collected on mineral oil to limit evaporation and to prevent gaseous exchange, which can alter the pH of the urine. Urine collections with creatinine values 25% lower/higher than mean values for each animal were considered incomplete/over collections and were discarded. Mice were fed a purified casein/lactalbumin-based test diet. The nutrient composition of this diet has been previously described (1) and contains 2.56 calories/g wet wt, 19.5% protein, 16% lipid, 64.6% carbohydrate, 10% fiber, 5 mg/g calcium, 0.04 mg/g oxalate, 0.06 mg/g glycolate, and no Hyp.
Hyp feeding.
Male wild-type, AGXT KO, and GRHPR KO animals (n = 6) were fed the purified diet containing 1% Hyp for 1 wk, and 24-h urines were collected on the last 3 days. Animals were then killed for blood, kidney tissue, and bladder stone enumeration. Another group of animals (n = 6) was maintained for 4 wk on this Hyp diet and blood was collected for plasma cystatin C measurements at weeks 0, 1, 2, and 4 wk. Cystatin C serum concentration is an indicator of renal function (17). Mouse plasma cystatin C was measured by ELISA, according to the manufacturer's instructions (R&D Systems). After 4 wk, animals were killed for kidney tissue analysis and bladder stone enumeration.
Histological analysis.
Tissues were either fixed in 4% buffered paraformaldehyde and embedded in paraffin or snap-frozen in liquid nitrogen and sectioned with a cryostat. All tissues were stained with hematoxylin and eosin, and calcium (Yasue) and collagen (sirius red) staining were performed on kidney slides (19). Kidney sections from a PH1 patient with nephrocalcinosis were used as a positive control for the calcium oxalate staining.
Sample preparation.
For oxalate determination, part of the urine collection was acidified to pH < 1.0 with hydrochloric acid before storage at −80°C to prevent any possible oxalate crystallization that can occur with cold storage and/or oxalogenesis associated with alkalinization. The remaining urine was frozen at −80°C for the measurement of other urinary parameters. Plasma was stored at −80°C. Plasma preparations were filtered through Nano-sep centrifugal filters (VWR International, Batavia, IL) with a 10,000 nominal molecular weight limit, and the ultrafiltrate was diluted twofold with 0.8 M boric acid before ion chromatography (IC). Centrifugal filters are contaminated with oxalate (5) and were thus washed with 0.1 M HCl before sample filtration. Liver and kidney tissue were freeze-clamped in liquid nitrogen immediately after death and stored at −80°C. Liver tissue was extracted with 7% perchloric acid (PCA) before glyoxylate analysis. For renal oxalate measurements, kidney tissue was homogenized in 2 M HCl.
Analytical methods.
Creatinine, calcium, magnesium, sodium, potassium, urea-N, and uric acid levels in urine were measured on a Beckman C5E Analyzer. Sodium and potassium in urine were measured by ion-specific electrodes, and other analytes using commercial kits, following protocols provided by the manufacturer. Glyoxylate was measured in liver PCA extract by reversed phase HPLC after derivatization with phenylhydrazine, as previously described (12). Oxalate was determined in urine and plasma by IC, as previously described (11). Reagent-free IC coupled with negative electrospray mass spectrometry (Dionex) was used to measure glycolate and glycerate in urine and plasma, as previously described (11).
A Coomassie Plus protein assay kit (Pierce, Rockford, IL), with BSA as the standard, was used to determine protein concentrations in tissue preparations.
d-Glycerate dehydrogenase activity.
d-Glycerate dehydrogenase (DGDH) activity was used as an index of glyoxylate reductase activity and was measured using a sensitive HPLC method, as previously described (12).
Western blotting.
SDS-PAGE of tissue lysates was performed with a Bio-Rad Ready Gel System (Bio-Rad Laboratories, Hercules, CA) under reducing conditions using a 6% stacking gel and a 12% resolving gel of acrylamide. After electrophoresis, samples were electroblotted from 12% (wt/vol) acrylamide gels onto Hybond-C nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Membranes were incubated for 1 h in 5% (wt/vol) nonfat dried milk dissolved in PBS, pH 7.4, containing 1% (vol/vol) Tween-20, followed by incubation for 1.5 h at room temperature with rabbit anti-sera to mouse recombinant GRHPR (1:8,000 dilution). The membrane was then washed and incubated for 1 h at room temperature with peroxidase-conjugated goat anti-rabbit immunoglobulin G (0.2 μg/ml; Dako, Carpenteria, CA). GRHPR was visualized by using enhanced chemiluminescence (Amersham Biosciences). Antibodies against mouse GRHPR were raised in rabbits injected with His-tagged recombinant GRHPR protein, expressed in Escherichia coli using vector pET15 (Novagen, Madison, WI), purified by nickel-affinity chromatography and mixed with Freund's adjuvant (Sigma).
Bladder stone analysis.
Stones representative of the morphological types observed were crushed and analyzed with a powder X-ray diffractometer (D8 Advance, Bruker). Stone composition was identified with the Eva software of the Diffrac Plus suite (Bruker), using reference compounds.
Statistical analysis.
Analysis of data was performed using either a paired or unpaired t-test for the comparison of two means. Two-way ANOVA was used to test whether plasma cystatin C changed over time with 1% Hyp feeding and whether any changes were different among the different strains. Differences were considered significant if P ≤ 0.05.
RESULTS
Growth, development, and histology of GRHPR KO mice.
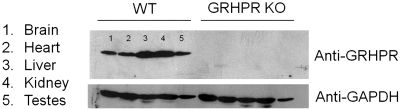
Western blot analysis showed GRHPR present in all tissues from wild-type animals that were examined with expression in the liver and kidney greater than in other tissues (Fig. 1). No expression of GRHPR was detected in tissues from GRHPR KO mice (Fig. 1). No differences were observed in the growth and development of GRHPR KO mice compared with their heterozygous or wild-type littermates. No differences were observed in the histological analyses of brain, lung, heart, stomach, liver, spleen, pancreas, small and large bowel, gonads, and bone in the various genotypes. Kidneys from GRHPR KO mice were more thoroughly studied, with a total of 20, 6-mo-old males and four consecutive sections observed per mouse. Mild nephrocalcinosis, defined as occasional deposits of calcium oxalate in cortical and medullary tubules without significant tubular atrophy or interstitial fibrosis, was observed in five animals (25%). One animal showed moderate nephrocalcinosis, with mild tubular atrophy and interstitial fibrosis. Urine bladder stones were observed in 12 of the 20 animals (60%), similar to the incidence reported in AGXT KO mice in the same mixed genetic background (16). Eight mice had multiple, round stones, 0.5–1.5 mm in size, consisting of calcium oxalate monohydrate (COM) and mixed COM and calcium oxalate dihydrate (COD) upon powder X-ray crystallography analysis. Four animals showed single, larger stones, 2–4 mm in diameter, and consisted of COD. The bladders of animals with stones showed marked signs of inflammation, with abundant mononuclear cell infiltrates in mucosa and muscle layers, and numerous epithelial erosions.
Fig. 1.
Western blot of mouse tissues incubated with polyclonal rabbit antibodies against mouse glyoxylate reductase and hydroxypyruvate reductase (GRHPR) and mouse GAPDH. WT, wild-type; KO, knockout.
Biochemical analysis of GRHPR KO mice.
Biochemical analyses of plasma, urine, and tissue from GRHPR KO and wild-type mice are shown in Table 1. Significant differences between wild-type and GRHPR KO animals were observed in liver DGDH activity, plasma glycerate, and the urinary excretions of oxalate and glycerate. GRHPR KO animals had ∼40% higher plasma oxalate compared with wild-type animals, but this difference was not significant. There were no differences between wild-type and GRHPR KO animals in liver glyoxylate. Significant sex differences were observed in urinary glycolate, oxalate, and glycerate excretions. No change was detected in urine parameters in wild-type or GRHPR KO animals at 3, 6, or 12 mo of age (data not shown).
Table 1.
Tissue, 24-h urine, and plasma measurements from 3-mo-old wild-type and GRHPR KO mice
| Wild-Type |
GRHPR KO |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Body wt, g | 29.8 (3.0)* | 22.7 (2.1) | 29.1 (3.9) | 25.3 (4.9) |
| Urine volume, ml | 1.37 (0.31) | 1.63 (0.33) | 1.74 (0.48) | 1.27 (0.48) |
| Urine creatinine, mg | 0.627 (0.092)* | 0.403 (0.121) | 0.484 (0.105)* | 0.377 (0.121) |
| Urine pH | 5.9 (0.07) | 5.88 (0.19) | 5.87 (0.06) | 5.74 (0.09) |
| Urine oxalate, μmol | 0.51 (0.21)* | 0.30 (0.08) | 1.52 (0.26)*† | 0.73 (0.18)† |
| Urine glycolate, μmol | 0.44 (0.10)* | 0.54 (0.13) | 0.39 (0.10)* | 0.56 (0.15) |
| Urine glycerate, μmol | 0.010 (0.006) | 0.019 (0.020) | 0.016 (0.018)* | 0.57 (0.20)† |
| Urine calcium, μmol | 2.48 (0.73) | 3.4 (1.08) | 2.96 (1.10) | 3.64 (1.38) |
| Plasma oxalate, μM | 3.8 (1.7) | 3.4 (2.0) | 5.3 (1.7) | 5.4 (2.7) |
| Plasma glycolate, μM | 12.9 (2.1) | 11.0 (1.6) | 10.6 (2.5) | 9.6 (2.3) |
| Plasma glycerate, μM | 6.6 (3.1) | 4.6 (2.7) | 16.6 (5.4)† | 16.7 (7.5)† |
| Liver glyoxylate, pmol/mg protein | 59 (15) | ND | 55 (13) | ND |
| Liver DGDH activity, pmol · min−1 · mg protein−1 | 9.8 (2.4) | ND | <1.1 | ND |
Data are means ± SD, n = 8 animals per sex. A t-test was used to determine significant differences between sexes and genotype. P values <0.05 were considered significant.
Significant differences between sexes within each genotype group.
Significant differences between wild-type and glyoxylate reductase and hydroxypyruvate reductase knockout (GRHPR KO) animals. ND, not determined. For each animal, the mean value of 3 24-h urine collections was used.
Hyp metabolism.
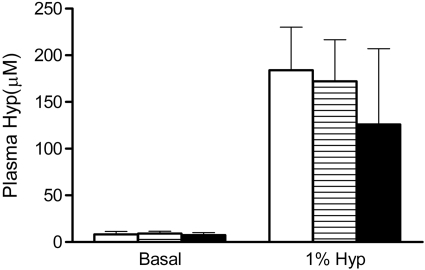
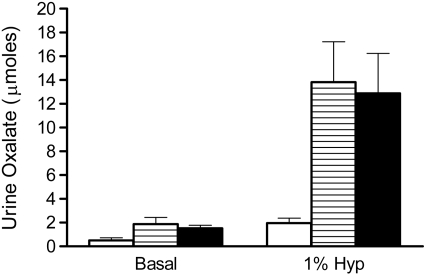
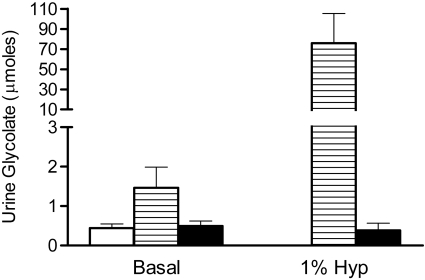
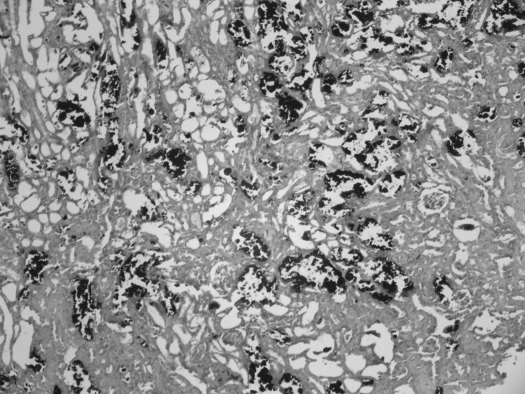
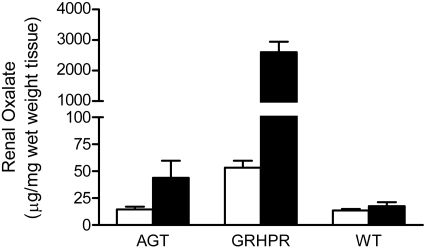
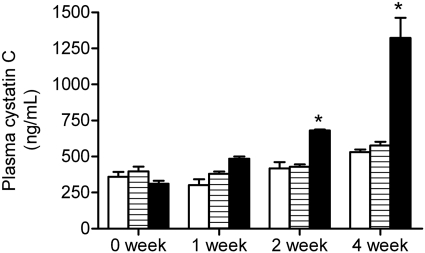
To determine how a deficiency in GRHPR and AGT modifies the metabolism of Hyp to oxalate and glycolate, 3-mo-old animals were fed diets containing 1% Hyp. Mean plasma Hyp levels increased ∼20-fold above basal levels and were similar in all three groups (Fig. 2). Both GRHPR and AGXT KO animals converted more of the Hyp metabolized to oxalate than wild-type animals. Urine oxalate excretion with Hyp feeding in wild-type, AGXT KO, and GRHPR KO animals increased 3.9-, 7.3-, and 8.5-fold above baseline, respectively (Fig. 3). Baseline urinary glycolate excretion was three- to sixfold higher in AGXT KO animals compared with wild-type and GRHPR KO animals, and it increased substantially with Hyp feeding (Fig. 4). Urine glycolate, however, was unchanged in the GRHPR KO mice. Following 1 mo of Hyp feeding, none of the wild-type animals had developed bladder stones. In contrast, all GRHPR KO and AGXT KO animals had bladder stones after 1 mo of Hyp feeding, but not after 1 wk. Renal histology showed no evidence of tissue crystal deposition in wild-type animals, and only two of AGXT KO animals (20%) had mild nephrocalcinosis (occasional small deposits both within the lumen and the epithelium/interstitium) after 1 mo of Hyp feeding. Previous work with AGXT KO animals showed mild nephrocalcinosis in ∼10% of males not fed Hyp (16). In contrast, all GRHPR KO animals had severe nephrocalcinosis after 4 wk, but not 1 wk of Hyp feeding. Two of the six GRHPR KO animals died on week 3 of Hyp feeding and had severe nephrocalcinosis on necropsy (Fig. 5). The bulk of calcium oxalate deposits in the 4-wk Hyp-fed GRHPR KO animals was located intraluminally, with some smaller interstitial deposits as well. Renal oxalate levels were measured in mice after 1 and 4 wk of Hyp feeding (Fig. 6). GRHPR KO animals showed approximately 4- and 150-fold increases in renal oxalate levels following 1 and 4 wk, respectively, of 1% Hyp feeding. Renal oxalate was lower in AGXT KO mice after 1 mo 1% Hyp feeding, but 2.5-fold higher than that in wild-type mice. Renal oxalate levels were measured in GRHPR KO mice in both the mixed genetic background and the pure C57BL6 background and there was no difference between the two backgrounds with either 1 wk (P = 0.24) or 4 wk (P = 0.71) of 1% Hyp feeding. Plasma cystatin C measurements (Fig. 7) in AGXT KO and wild-type animals increased 1.5-fold after 4 wk of Hyp feeding. However, this increase was not significant. In contrast, GRHPR KO showed a significant increase in plasma cystatin C after 2 wk of Hyp feeding (1.6-fold increase compared with baseline) and by 4 wk plasma cystatin C was 4.3-fold higher than baseline levels.
Fig. 2.
Plasma hydroxyproline (Hyp) levels with and without 1% Hyp feeding for 7 days in WT (open bar), AGXTKO (hatched bar), and GRHPR KO (filled bar) mice (n = 6 per group). Data are expressed as means ± SD.
Fig. 3.
Urinary oxalate 24-h excretion with and without 1% Hyp feeding for 7 days in WT (open bar), AGXT KO (hatched bar), and GRHPR KO (filled bar) mice (n = 6 per group). Data are expressed as means ± SD.
Fig. 4.
Urinary glycolate 24-h excretion with and without 1% Hyp feeding for 7 days in WT (open bar), AGXT KO (hatched bar), and GRHPR KO (filled bar) mice (n = 6 per group). Data are expressed as means ± SD.
Fig. 5.
Representative histology after Yasue staining in a GRHPR KO mouse after 4 wk of 1% Hyp feeding. Black deposits represent calcium crystal deposits. The bulk of calcium crystal deposits was found intraluminally, with some smaller interstitial deposits as well.
Fig. 6.
Renal oxalate levels in AGTKO, GRHPR KO, and WT mice after feeding with 1% Hyp for 1 wk (open bar) or 4 wk (filled bar). Data expressed as means ± SE, n = 6.
Fig. 7.
Plasma cystatin C levels over time with 1% Hyp feeding in WT (open bar), AGXT KO (hatched bar), and GRHPR KO (filled bar) mice (n = 5 per group). Data expressed as means ± SE. *Significant increase (P ≤ 0.05) compared with 0 wk.
DISCUSSION
The phenotype of the GRHPR KO mouse indicates that it shares similar characteristics to individuals with PH2 in synthesizing elevated amounts of oxalate and glycerate in plasma. Whereas urinary glycerate excretion was elevated in female GRHPR KO mice, it was normal in male mice. This difference in urinary excretion of glycerate in the face of similar, elevated plasma glycerate suggests that glycerate was reabsorbed and metabolized in the kidney of male mice but not in females. The elevated syntheses of oxalate and glycerate are consistent with glyoxylate and hydroxypyruvate concentrations increasing in tissues due to the GRHPR deficiency, and their subsequent conversion to oxalate and l-glycerate, respectively, by LDH. Similarly, oxalate and glycolate excretion have been reported to increase in AGXT KO mice, consistent with the changes observed in individuals with PH1 (16). Thus, these KO mice should be good models for examining the consequences of AGT1 and GRHPR deficiencies in PH1 and PH2, respectively, and for testing new therapies that seek to overcome these reduced enzymatic activities.
Gender differences in wild-type and GRHPR KO animals were noted in oxalate excretion, with males excreting more oxalate than females. The increased oxalate production in the GRHPR KO males suggests that they may be the preferable sex to use where the induction of profound hyperoxaluria is desired. Notably, wild-type and GRHPR KO animals excreted similar amounts of urinary glycolate, suggesting that the GRHPR-catalyzed conversion of glyoxylate to glycolate is not the most significant pathway for glycolate synthesis in mice. This observation supports our proposal that the glyoxalase-catalyzed conversion of the peroxidation product, glyoxal, to glycolate is the major source of glycolate synthesis in mammals (18).
Supplementing the diets of experimental animals with Hyp has been used as a model for the study of urolithiasis. Such models have included pigs, mice, and rats (8–10, 14). The mouse as a model has added benefits due to the wide range of available genetically modified animals that can be used to identify genes involved in calcium oxalate deposition. Both KO models responded to Hyp feeding with a substantial increase in oxalate synthesis. After 1 wk of Hyp ingestion, the urinary excretion of oxalate was similar in both the AGXT and GRHPR KO models. However, the oxalate concentration in renal tissue was three to five times higher in GRHPR KO mice compared with AGXT KO mice with 1 wk of Hyp feeding. Plasma cystatin C measurements in wild-type and AGXT KO animals showed a nonsignificant 1.5-fold increase, indicating renal function was not impaired with Hyp feeding. However, the observed increase could represent an increase in oxidative stress, as cystatin C has been reported to increase during chronic low-level inflammation (4). In contrast, plasma cystatin C levels in GRHPR KO animals increased significantly after only 2 wk of 1% Hyp feeding, indicating renal function was impaired. This greater sensitivity of the GRHPR KO mice to Hyp feeding implies that the presence of GRHPR activity in renal tissue of AGXT KO mice provides some protection against calcium oxalate deposition. Calcium oxalate deposition was assessed at 1 and 4 wk of Hyp feeding, and only after 4 wk was substantial crystal deposition observed in GRHPR KO animals, suggesting that an increase in the oxalate concentration in tissues may precede the formation of large crystal aggregates that can be visualized histologically. Our results reported here illustrate that Hyp-fed GRHPR KO mice will potentially be useful models for the study of crystals within renal tissue and their impact on inflammatory responses and renal function.
The increased production of oxalate from Hyp metabolism in GRHPR and AGXT KO mice has potential implications for the metabolism that occurs in PH1 and PH2. It has been estimated that in humans daily collagen turnover releases 240–420 mg Hyp/day that would result in the formation of 140–240 mg glyoxylate/day (13). Additional Hyp would also be obtained from dietary sources. The increased production of oxalate in the Hyp-fed mice in this study suggests that metabolism of this amino acid could make a substantial contribution to the excessive oxalate synthesis that occurs in individuals with PH1 and PH2. The quantification of the contribution of Hyp metabolism to oxalate in individuals with PH warrants examination to evaluate whether blocking this catabolic pathway would be a useful therapeutic strategy in this disease. Furthermore, our results suggest that individuals with PH should avoid the ingestion of Hyp-rich foods to limit the contribution of Hyp catabolism to oxalate synthesis.
GRANTS
This work was supported by National Institutes of Health Grants DK54468 (to R. P. Holmes) and DK069331 (to S. D. Cramer) and SAF07-62343 (to E. Salido) from the Spanish Ministry of Science.
T. Takayama was supported in part from a grant from the Japanese Ministry of Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.K., R.P.H., S.D.C., and E.C.S. conception and design of research; J.K., T.T., and E.C.S. performed experiments; J.K., R.P.H., and E.C.S. analyzed data; J.K., R.P.H., and E.C.S. interpreted results of experiments; J.K. and E.C.S. prepared figures; J.K., R.P.H., and E.C.S. drafted manuscript; J.K., R.P.H., S.D.C., T.T., and E.C.S. edited and revised manuscript; J.K., R.P.H., S.D.C., T.T., and E.C.S. approved final version of manuscript.
REFERENCES
- 1. Adams MR, Golden DL, Franke AA, Potter SM, Smith HS, Anthony MS. Dietary soy beta-conglycinin (7S globulin) inhibits atherosclerosis in mice. J Nutr 134: 511–516, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with Primary Hyperoxaluria Type II. Hum Mol Genet 11: 2063–2069, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Danpure CJ. Primary hyperoxaluria. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Vallee D, Childs B, Kinzler KW, Vogelstein B. New York: McGraw-Hill, 2001, p. 3323–3367 [Google Scholar]
- 4. Frendeus KH, Wallin H, Janciauskiene S, Abrahamson M. Macrophage responses to interferon-gamma are dependent on cystatin C levels. Int J Biochem Cell Biol 41: 2262–2269, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Harris AH, Freel RW, Hatch M. Serum oxalate in human beings and rats as determined with the use of ion chromatography. J Lab Clin Med 144: 45–52, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and its influence on oxalate synthesis. J Urol 160: 1617–1624, 1998 [PubMed] [Google Scholar]
- 7. Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int 75: 1264–1271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaplon DM, Penniston KL, Darriet C, Crenshaw TD, Nakada SY. Hydroxyproline-induced hyperoxaluria using acidified and traditional diets in the porcine model. J Endourol 24: 355–359, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Khan SR, Glenton PA. Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol 184: 1189–1196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-l-proline. Kidney Int 70: 914–923, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Knight J, Assimos DG, Easter L, Holmes RP. Metabolism of fructose to oxalate and glycolate. Horm Metab Res 42: 868–873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knight J, Holmes RP. Mitochondrial hydroxyproline metabolism: implications for primary hyperoxaluria. Am J Nephrol 25: 171–175, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int 70: 1929–1934, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mandel NS, Henderson JD, Hung LY, Wille DF, Wiessner JH. A porcine model of calcium oxalate kidney stone disease. J Urol 171: 1301–1303, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Vallee D, Childs B, Kinzler KW, Vogelstein B. New York: McGraw-Hill, 2001, p. 1821–1838 [Google Scholar]
- 16. Salido EC, Li XM, Lu Y, Wang X, Santana A, Roy-Chowdhury N, Torres A, Shapiro LJ, Roy-Chowdhury J. Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci USA 103: 18249–18254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seronie-Vivien S, Delanaye P, Pieroni L, Mariat C, Froissart M, Cristol JP. Cystatin C: current position and future prospects. Clin Chem Lab Med 46: 1664–1686, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Thornalley PJ. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31: 1343–1348, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Yasue T. Histochemical identification of calcium oxalate. Acta Histochem Cytochem 2: 83–95, 1969 [Google Scholar]