Abstract
Female sperm storage is common among organisms with internal fertilization. It is important for extended fertility and, in cases of multiple mating, for sperm competition. The physiological mechanisms by which females store and manage stored sperm are poorly understood. Here, we report that the biogenic amines tyramine (TA) and octopamine (OA) in Drosophila melanogaster females play essential roles in sperm storage. D. melanogaster females store sperm in two types of organs, a single seminal receptacle and a pair of spermathecae. We examined sperm storage parameters in females mutant in enzymes required for the biochemical synthesis of tyrosine to TA and TA to OA, respectively. Postmating uterine conformational changes, which are associated with sperm entry and accumulation into storage, were unaffected by the absence of either TA or OA. However, sperm release from storage requires both TA and OA; sperm were retained in storage in both types of mutant females at significantly higher levels than in control flies. Absence of OA inhibited sperm depletion only from the seminal receptacle, whereas absence of both OA and TA perturbed sperm depletion from both storage organ types. We find innervation of the seminal receptacle and spermathecae by octopaminergic-tyraminergic neurons. These findings identify a distinct role for TA and OA in reproduction, regulating the release of sperm from storage, and suggest a mechanism by which Drosophila females actively regulate the release of stored sperm.
Keywords: reproductive tract, physiology, insects
In organisms with internal fertilization, female sperm storage is an essential process required for efficient gamete use and the subsequent maintenance of fertility. In female organisms that mate multiply, the storage of sperm from a previous mating allows for sperm competition between rival males and/or sperm preference by females. Sperm storage is a multistep process that includes sperm entrance and accumulation into, maintenance within, and regulated release from the sites of storage (1). Sperm are typically retained in specific regions of the female reproductive tract (RT) usually associated with the epithelium of the RT lumen (e.g., the oviductal reservoir in mammals) or in specialized, blind-ended structures that maintain sperm until they are used for fertilization (e.g., sperm storage tubules in birds and insects). Among different species, the duration that sperm remain in storage varies greatly, ranging from hours to several years (2–7). In animals as diverse as mammals and insects, the disruption of sperm storage has detrimental effects on fertility (8, 9). However, the physiological mechanisms required in females for sperm storage remain unclear.
There has been emphasis on the male contributions that promote sperm storage, most notably the action of seminal fluid proteins (SFPs). SFPs are transferred in the ejaculate during mating and constitute one group of factors required for successful sperm storage in numerous organisms (reviewed in 10, 11). For example, in cows, the bovine seminal plasma family of proteins coat sperm and enable them to bind oviductal epithelium, leading to the formation of the oviductal reservoir and extending sperms’ motile life (12). In the honey bee, Apis mellifera, and the leaf-cutter ant, Atta colombica, seminal secretions from the male accessory glands promote sperm viability in storage (13, 14). In Drosophila melanogaster, genetic and transgenesis tools have allowed for the dissection of the roles of SFPs in general, and of individual SFPs, in sperm storage events. Specific SFPs function at each step of the process and are required for the efficient use of sperm stored in Drosophila female sperm storage organs (SSOs; these organs are the single seminal receptacle and the paired spermathecae) (15) (Fig. S1). Disruption of any identified step drastically reduces the fertility of a mating pair (9, 16–19).
In contrast to the ample evidence that highlights the importance of SFPs in sperm storage, we know little about the molecular contributions of females to this process. Several lines of evidence suggest that females do control aspects of sperm storage. First, females of many insect species are able to regulate the depletion of their sperm stores. Indirect evidence of this includes species with long-term sperm storage capability, such as the leaf-cutter ant, A. colombica, and the honey bee, A. mellifera (≥6 y) (20, 21), and the high efficiency of fertilization in some species, such as wild Drosophila pseudoobscura (>99%) (22). Direct evidence of this is observed in D. melanogaster females, who retain significantly more sperm in storage than normal when presented with unsuitable egg-laying substrates (23, 24). Second, secretions from female RTs have positive effects on sperm viability in the honey bee, A. mellifera, and in D. melanogaster (13, 25). Third, in Drosophila, sperm continue to be maintained and released long after most SFPs are no longer detected in the female RT. Fourth, when incapacitated with anesthesia shortly after mating, females of several insect species fail to accumulate normal quantities of sperm in storage (26–28). Finally, studies in numerous species indicate that in polyandrous situations, females can exert preference or choice over which male's sperm fertilize their eggs (29).
How females exert control over sperm storage is not understood. There is evidence that the female's nervous system is important in the process, suggesting active control by the female. Innervation of the female RT potentially allows for control over specific regions, as well as for the coordination of sperm movement within the RT. In the African migratory locust, Locusta migratoria, nervous system-mediated contractions of the spermatheca are hypothesized to trigger sperm release (30–32). In D. melanogaster, females with incapacitated or masculinized central nervous systems store significantly fewer sperm than controls, with the distribution of sperm stored within the SSOs perturbed, suggesting that a functional nervous system is required to manage sperm entry into storage as well as sperm distribution between the SSOs (33, 34). Thus, female contributions to sperm storage can serve two important functions: (i) promoting the maintenance, nourishment, or stabilization of sperm (13, 25, 35, 36) and (ii) modulating the mobilization of sperm within the female RT through nervous system inputs.
Here, we explored one aspect of nervous system inputs, using Drosophila as a model. We identified roles for the biogenic amines tyramine (TA) and octopamine (OA) in sperm storage. TA and OA are potent neuromodulators, considered to be the invertebrate counterparts of vertebrate adrenergic transmitters. Both molecules have been implicated in a multitude of physiological processes and overt behaviors, exerting their actions through G-coupled protein receptors (reviewed in 37). In Drosophila, TA and OA are synthesized from tyrosine precursors. Mutation of the enzymes responsible for their biochemical synthesis results in female sterility, reportedly attributable to abnormal egg retention; specifically, mutant females are unable to lay eggs (38–40). Sterility of females lacking OA is attributable to a defect in ovulation: mature eggs are not released from the ovary (39). Females lacking TA ovulate, but the mature oocytes released from the oviduct do not normally transit through the RT and do not reach the uterus (38). These studies suggest that OA and TA have distinct and separate activities in the egg production pathway. Because of their presence in the RT and their functions in ovulation and egg laying, OA and TA are also candidates to modulate sperm storage. However, this distinct role has not yet been examined.
Measuring an early process required for sperm entry into storage and assessing the overall number of sperm stored over time allowed us to determine whether sperm accumulation into and depletion from the SSOs are affected by the absence of OA, TA, or the OA receptor OAMB (OA receptor in Mushroom Bodies). We found that the absence of TA, OA, or OAMB did not affect sperm accumulation into storage. However, sperm were retained at significantly higher levels in storage in the absence of TA, OA, and OAMB, suggesting that these molecules are required for efficient sperm depletion from storage. Further, the SSOs appear to be differentially affected by the absence of these molecules: The lack of OA only inhibited sperm release from the seminal receptacle, whereas the absence of both OA and TA suppressed sperm release from both storage organ types. The sperm retention defect is distinct from the effects of TA and OA on ovulation and egg laying, because similar egg-laying mutants depleted their total sperm stores faster than controls. Finally, we show direct and distinct innervation of both the seminal receptacle and the spermathecae by neurons that release OA and TA. These results support a newly identified role for OA and TA in Drosophila reproduction, in addition to their roles in ovulation and egg laying. Our results also identify a physiological mechanism by which females manage stored sperm.
Results and Discussion
TA and/or OA Is Not Required for the Accumulation of Sperm into Storage in Females.
The events required for the efficient storage of sperm in Drosophila begin shortly after the onset of copulation. First, a series of changes in uterine conformation are initiated during mating and continue after mating ends (41). These changes, in which the lumen of the uterus opens from its tightly compacted, premated state, require male SFPs from the accessory gland for initiation (41). Failure of these changes to complete results in submaximal sperm storage, suggesting that the changes in uterine conformation are necessary for the efficient accumulation of sperm into storage (42).
If these changes in uterine shape reflect the contraction of the circular muscles surrounding the uterus (43), it is possible that the biogenic amines OA and TA might have a role in their initiation or modulation. OA and TA are synthesized in neurons that innervate the female RT and are required for contraction of the oviduct (34, 44). To address the potential involvement of OA and TA in the uterine conformational changes and subsequent accumulation of sperm into storage, we used fly lines mutant in genes that encode the enzymes that synthesize TA from a tyrosine precursor [tyrosine decarboxylase 2 (tdc2)] and OA from a TA precursor [tyramine β-hydroxylase (tβh)], generating adult females lacking both TA and OA (tdc2RO54) or only OA (and with excess TA; tβhM18) (38). We performed uterine conformation assays in both types of females following mating to a wild-type (WT) male (41, 42).
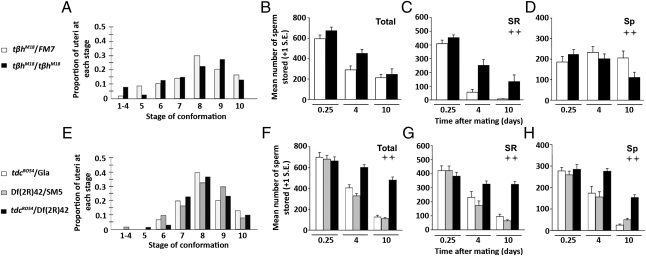
In the absence of OA, or both TA and OA, we detected no difference in the progression of the uterine conformational changes. OA-less and TA/OA-less mutant females progressed through the uterine stages at similar rates to control females (Fig. 1 A and E; Wilcoxon test (Rank sums), P = 0.88 and P = 0.92, respectively). Consistent with this, the total number of sperm stored in OA-less and TA/OA-less females at 6 h postmating, when sperm entry into storage is complete and females have begun to lay eggs, did not differ from that of the controls (Fig. 1 B and F; tβhM18: t = −1.61, df = 26, P = 0.12; tdc2RO54: F2,22 = 0.135, P = 0.87). Additionally, sperm numbers in the individual SSO types did not differ between mutant and control females at this time point (Fig. 1 C and G; tβhM18: t = −1.36, df = 26, P = 0.19; tdc2RO54: F2,22 = 0.440, P = 0.65; Fig. 1 D and H; tβhM18: t = −1.06, df = 26, P = 0.30; tdc2RO54: F2,22 = 0.521, P = 0.60). These results suggest that neither TA nor OA has a role in uterine conformational change or in the accumulation of sperm into storage.
Fig. 1.
OA and TA do not contribute to early sperm storage events but are required for sperm depletion from storage. Sperm storage events are shown in OA-less females (A–D) and in OA/TA-less females (E–H). Distribution of uterine conformational stages, shown as proportions, at 35 min after the start of mating in tβhM18 (A) and tdc2RO54/Df(2R)42 mutant females (E) compared with their sibling controls (A: NM18 = 40, NFM7 = 50; E: NRO54/Df(2R)42 = 30, NGla = 30, NSM5 = 42). Total sperm stored in the SSOs (B and F), only in the seminal receptacle (C and G), and only in the spermathecae (D and H) in tβhM18 and tdc2RO54/Df(2R)42 mutant females and their sibling controls. Differences between/among female genotypes in the depletion of stored sperm over time were analyzed using two-factor ANOVA. The significance of the genotype factor, indicating differences in sperm depletion between/among mutant and control females, is reported in the figure as follows: ++ = P < 0.005 (an additional explanation of the statistical analysis is provided in Materials and Methods). Sp, spermathecae; SR, seminal receptacle. Sample sizes for sperm counts range from n = 7–20 (Table S1).
TA and OA Are Required for the Depletion of Sperm from Storage in Females.
Although no significant differences were observed in uterine conformation or sperm accumulation within storage in the absence of OA or TA/OA, sperm release from storage was perturbed in the absence of each neuromodulator. Examination of the total number of stored sperm in OA-less females did not reveal differences in sperm retention relative to their controls over time (Fig. 1B; F1,47 = 0.146, P = 0.71). However, a retention phenotype became obvious when the SSOs were examined separately. OA-less females retained significantly more sperm in the seminal receptacle than did the controls (Fig. 1C; F1,47 = 11.94, P = 0.001). In contrast, although control females maintained a similar number of stored spermathecal sperm over 10 d, OA-less females depleted nearly half of their spermathecal sperm, resulting in a statistically significant difference between the two groups (Fig. 1D; F1,47 = 12.24, P = 0.001). Together, these results suggest that either OA is necessary for normal sperm depletion from the seminal receptacle or that the elevated TA levels present in tβh mutants inhibit sperm depletion there.
To distinguish between these two possibilities, we examined sperm depletion in tdc2 mutants, which lack both TA and OA. TA/OA-less females retained significantly more sperm than controls (Fig. 1F; F2,66 = 116.1, P < 0.0005; least significant difference (LSD) post hoc test, mutant < controls, P < 0.0005), and this effect was seen in both types of SSO (Fig. 1 G and H; seminal receptacle: F2,68 = 57.15, P < 0.0005 and LSD post hoc test, mutant < controls, P < 0.0005; and spermathecae: F2,66 = 22.97, P < 0.0005 and LSD post hoc test, mutant < controls, P < 0.0005). These results are consistent with the idea that OA is necessary for normal sperm depletion from storage. Differences between tβhM18 and tdc2RO54 females in sperm storage dynamics indicate that TA may also influence sperm retention.
To compare sperm storage between these two groups more directly, we normalized sperm storage values of mutant females by their controls and examined depletion over time. A significant genotype × time interaction (F1,45 = 10.19, P = 0.003) was further examined to determine whether or not tβhM18 and tdc2RO54 females differed at 4 d and 10 d after mating. At each time point, females lacking OA but producing TA retained relatively fewer sperm than females lacking both OA and TA (4 d: separate variances t test, t = 3.64, df = 20.41, P = 0.002; 10 d: t = 7.01, df = 16.0, P < 0.0005). Thus, the retention phenotype we observe is significantly more pronounced in TA/OA-less females than in OA-less females within the seminal receptacle (ANOVA mutant genotype effect: F1,45 = 8.83, P = 0.005) and spermathecae (F1,45 = 91.75, P < 0.0005). The sperm retention is not attributable to the lack of egg laying in our mutants. Total sperm release from storage in an egg-retention mutant (logjam) (45) was faster than in controls (Fig. S2). In this mutant, sperm release from the seminal receptacle occurred at a significantly greater rate than in controls. Sperm depletion from the spermathecae of logjam-mutant females was significantly greater than in controls only on day 10 postmating. These results suggest that the defects in sperm release in OA and OA/TA-less females is not simply a consequence of egg retention but, rather, that both OA and TA have roles in promoting sperm depletion from storage.
Female OAMB Mutants Retain Sperm.
To dissect the role of OA and TA in sperm release further, we examined sperm storage parameters in females mutant for oamb (oamb286), the G protein-coupled receptor responsible for mediating OA's effects on ovulation in the female RT (46). OAMB has also been shown to bind TA, but cAMP accumulation occurs ∼100-fold more efficiently when OA acts as the ligand (47). Several TA and OA receptors have been identified (48–52), but OAMB is the only receptor known thus far to affect female fertility. OAMB activity is required for ovulation: Mated oamb mutant females retain mature eggs in their RT (46). We expected that if OA and/or TA mediates its actions on sperm release through OAMB, female oamb mutants should recapitulate the sperm retention phenotypes of tβhM18 mutants, and perhaps tdc2RO54 mutants.
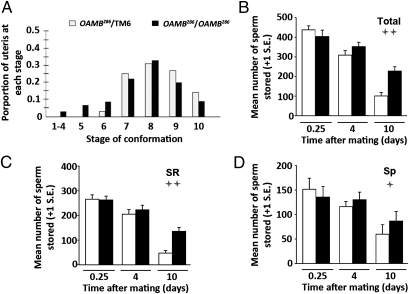
As in females lacking OA and TA/OA, uterine conformation [Fig. 2A; Wilcoxon test (Rank sums), P = 0.12] and subsequent sperm accumulation into storage (Fig. 2 B–D; total sperm: separate variances t = −0.832, df = 17.36, P = 0.42) were unaffected by the absence of OAMB. Sperm stored in the seminal receptacle and spermathecae in oamb286 females were similar to those in controls at 6 h postmating (Fig. 2 C and D; t = −0.051, df = 28, P = 0.96 and t = −0.48, df = 23, P = 0.634, respectively). However, defects in sperm depletion from storage were observed (Fig. 2B; F1,43 = 30.58, P < 0.0005), with sperm in the seminal receptacle and spermathecae retained at significantly higher numbers than in controls at 10 d postmating (Fig. 2 C and D; F1,51 = 12.34, P = 0.001 and F1,43 = 4.88, P = 0.033, respectively). These results further support a role for OA (and possibly TA) in the efficient release of sperm from storage. The magnitude of sperm retention in oamb mutants is less severe than that seen in tdc2 and tβh mutants. This may be because OA has several receptors (49, 50, 52), only one of which was nonfunctional in these experiments. It is also possible that the milder phenotype of oamb mutant females relative to tβh and tdc2 mutant females reflects OA and/or TA exerting its effects through alternative receptors in the nervous system. Additionally, TA may exert its RT effects through specific TA receptors. A role for these receptors in Drosophila female fertility has yet to be examined.
Fig. 2.
OAMB is not required for sperm entry into storage but is required for the depletion of sperm from the seminal receptacle and spermathecae. (A) Distribution of the uterine conformational stages at 35 min after the start of mating in oamb286 mutant females compared with their sibling controls (NOAMB = 36, NTM6 = 36). Total sperm stored in the SSOs (B), in the seminal receptacle (C), and in the spermathecae (D) in oamb286 mutant females compared with their sibling controls. The difference between female genotypes in the depletion of stored sperm over time was analyzed using two-factor ANOVA. The significance of the genotype factor, indicating differences in sperm depletion between oamb286 mutant and control females, is reported in the figure as follows: + = 0.005 < P < 0.05 or ++ = P < 0.005 (an additional explanation of the statistical analysis is provided in Materials and Methods). Sp, spermathecae; SR, seminal receptacle. Sample sizes for sperm counts range from n = 11–15 (Table S1).
Innervation of the SSOs by tdc Neurons.
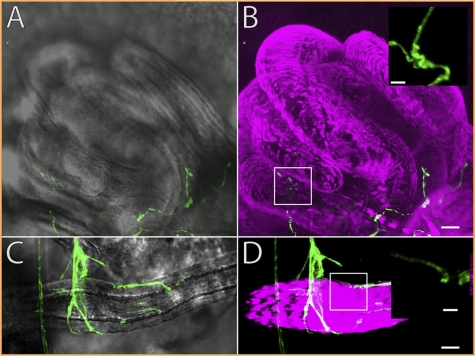
Because our genetic analysis uncovered a role for OA, TA, and OAMB in the release of sperm from storage, we examined whether the SSOs are innervated by motor neurons that release OA and TA and determined OAMB expression patterns in these tissues. To address SSO innervation, we expressed a mouse CD8-GFP (mCD8-GFP) reporter construct using a tdc2-GAL4 driver, which allowed for the visualization and identification of putative octopaminergic-tyraminergic nerve terminals (38). Neurotransmitter release occurs at boutons, axonal swellings that contain synaptic release machinery. OA and TA are released from a subset of boutons, morphologically discernible by their small size and “wandering” appearance, indicative of type II nerve terminals (53).
In the seminal receptacle, we noted innervation by type II terminals in 8 of 8 preparations using only GFP fluorescence and in 24 of 27 preparations combining GFP fluorescence and anti-GFP immunostaining (Materials and Methods and Fig. 3 A and B). Innervation by type II terminals was limited to compartments of the folded seminal receptacle most proximal to the uterus. It is possible the limited innervation selectively activates specific compartments of seminal receptacle, such as the proximal half of the receptacle, which is morphologically distinct from the distal half and contains the organ's entrance (43, 54).
Fig. 3.
Innervation of the SSOs by octopaminergic and tyraminergic neurons. The seminal receptacle (A and B) and spermathecae (C and D) are innervated by neurons marked with mCD8-GFP (green) driven by tdc2-GAL4. Nomarski (A and C) and phalloidin labeling muscles (magenta) (B and D) are shown. (Insets) Higher resolution boutons are illustrated. (Scale bar: main panels, 10 μm; Insets, 2 μm.) Although arborizations on the seminal receptacle are often limited to the proximal portion, extensive axonal arborizations are visible on the spermathecae.
We observed spermathecal innervation by type II terminals in 10 of 11 preparations using GFP fluorescence and in 26 of 27 preparations altogether (Fig. 3 C and D). Most branching was observed in the distal one-third of the stalk, although processes were sometimes observed further from the stalk base. Longitudinal muscle fibers, visualized using phalloidin staining, were observed to stretch the entire length of the stalk. No circular fibers were observed, suggesting that neural-evoked muscle contraction may allow “pumping” of the spermathecae to extrude sperm or the products from secretory cells associated with the organ (55). Our results identify octopaminergic-tyraminergic innervation at SSO neuromuscular junctions, but it is currently unknown whether the SSOs also receive input from other neurotransmitters (e.g., glutamate).
Because we identified a role for OA and TA in sperm storage, we examined the expression pattern of OAMB in the lower female RT. We expressed mCD8-GFP using an oamb-GAL4 driver (46). Surprisingly, we were unable to find substantial oamb-driven fluorescence in tissues of the spermathecae or seminal receptacle (Fig. S3 A and B), although the occasional spermathecal epithelial cell showed a low level of expression (Fig. S3 A and B). Interestingly, we detected high levels of OAMB expression in the parovaria (Figs. S1 and S3C). Assuming that oamb-GAL4 faithfully recapitulates OAMB expression, these results suggest that OAMB may be expressed at low levels in SSO tissues (beyond our detection limit) or that OA is signaling through an as yet unidentified alternative receptor. Additionally, OAMB function may be required not in the female RT but, rather, upstream in the central nervous system. Alternatively, OAMB expression from the parovaria may be influencing sperm storage. Although the function of these structures remains ambiguous, mutations that perturb development of the spermathecae in Drosophila also affect parovaria formation (55, 56).
SSOs Differ in Their Sperm Storage Dynamics.
We noted striking differences between the SSOs in the magnitude of sperm retention in the absence of OA and/or TA. The retention phenotype, when normalized by the appropriate control, was consistently greater in the seminal receptacle than in the spermathecae in females lacking OA and TA/OA at 4 d postmating (paired t test: t = 9.42, df = 14, P < 0.0005 and t = 2.84, df = 8, P = 0.022, respectively) and 10 d postmating (t = 3.81, df = 10, P = 0.003 and t = 4.00, df = 13, P = 0.002, respectively). These findings are consistent with the role of the seminal receptacle as the primary SSO (57). Surprisingly, we observed a greater degree of octopaminergic-tyraminergic innervation of the spermathecae, a result that may suggest greater female control over organs that not only store sperm but secrete molecules essential to manage stored sperm in both types of SSO (25, 55, 56).
Our observation of the differences in neuromodulator roles between the SSOs is of interest in light of previous studies in D. melanogaster that reported other differences between the two organ types. For example, although females with masculinized nervous systems accumulate fewer sperm in storage overall, the defect is particularly pronounced in the spermathecae (33). Also, a study using WT flies showed that females retain sperm preferentially within the spermathecae when exposed infrequently to fresh medium (23). The recent identification of wasted mutants reported that sperm release is severely defective in these mutants, with sperm rapidly lost during each ovulation. However, sperm depletion from the seminal receptacle occurs much more rapidly than from the spermathecae in these mutants (58). In other cases, only storage in the seminal receptacle is disrupted. Removal from the male ejaculate of the SFP sex peptide, or of a network of SFPs required for sex peptide localization to sperm, results in sperm retention within the seminal receptacle but does not impair sperm release from the spermathecae (17, 19, 59). The results of these studies showcase the differences in sperm use patterns from the individual SSOs. Finally, phylogenetic analysis of SSO use across 113 Drosophila species has shown that not all species use both SSO types to store sperm. Although the use of both organs to store sperm represents the ancestral state, loss of sperm storage in one organ, almost always the spermathecae, has occurred multiple times (60). In Drosophila species that have lost sperm storage ability in the spermathecae, the organ still exists, likely attributable to the importance of the organ's secretion products in female fertility (55, 61). These results suggest that the SSO types are under different selective pressures, potentially explaining how distinct mechanisms regulating their sperm use patterns may have arisen over time.
Our results show that sperm storage dynamics in the seminal receptacle and spermathecae are modulated differently by the female. This physiological difference is reflected in differences in innervation and is attributable, in part, to the distinct actions of the two neuromodulators studied here.
Conclusions.
Although organism-level sperm use patterns have been described in vertebrates and invertebrates, much remains to be learned about the physiological mechanisms that control the process. Despite its ubiquity across phyla, few female-derived molecules involved in sperm storage have been identified. The results we report here show that the two neuromodulators, OA and TA, are necessary for the proper release of stored sperm in D. melanogaster females, identifying a physiological mechanism by which the female actively modulates sperm release. These newly identified roles for OA and TA differ from their described roles in ovulation and egg laying. Because accelerated egg laying initiates within hours after the start of mating, it is intriguing that sperm release from storage would be influenced by the same molecules required for egg laying, potentially coordinating the two processes and maximizing sperm use efficiency. It is currently unknown how ovulation, egg laying, and sperm storage are coordinated at the neural circuit level, and these processes may indeed involve an identical or overlapping set of neurons. A role for the nervous system in coordinating sperm release and egg laying has been shown in the African migratory locust L. migratoria, where egg laying activates sensory neurons of the genital chamber, thereby increasing spermathecal contraction, which is hypothesized to cause sperm release from this organ, a neural loop that couples sperm release to egg presence in the genital chamber (30–32).
A role for both OA and TA has now been shown in two essential processes in Drosophila reproduction: egg laying and sperm release. Because changes in the neuronal architecture of the D. melanogaster oviduct occur postmating (strengthening of the synapse at neuromuscular junctions) (62), it would be interesting to see if a similar process occurs in the SSO-associated octopaminergic-tyraminergic neurons we identified here. Because SFP receipt affects vesicle release from neurons of the female RT in D. melanogaster (63), SFPs with known roles in sperm storage may mediate changes in axon connectivity at SSO neuromuscular junctions. Another not mutually exclusive possibility is that SFPs act upstream of octopaminergic-tyraminergic neuron vesicle release. Both hypotheses await examination.
Materials and Methods
Flies.
Matings were carried out by crossing 3- to 5-d-old mutant or control females to 3- to 5-d-old unmated males of the Canton-S (CS) strain of D. melanogaster. Fly lines used in our experiments were provided by the following: Patricia Rivlin [Cornell University, New York, NY (tβhM18/FM7 and tdc2RO54/Gla)], Kyung-An Han [University of Texas at El Paso, TX (oamb286/TM6 and oamb-GAL4)], Ginger Carney [Texas A&M University, College Station, TX (loj00898/TM3 and loj04026/TM3)], and the Bloomington Stock Center [Df(2R)42/SM5, tdc2-GAL4, and mCD8-GFP; stock nos. 3367, 9313, and 5137, respectively]. All flies were maintained on a standard yeast-glucose medium at room temperature (22 ± 1 °C) and a 12:12 light/dark cycle.
Uterine Conformational Assay.
Uterine conformation of mated females was determined as in the study by Avila and Wolfner (42). Briefly, an unmated male and a virgin female were placed together in an empty glass vial containing moistened Whatman filter paper. Their time of mating initiation was recorded, and mating pairs were frozen at −20 °C at 35 min after the start of mating. RTs from mated females were dissected in 0.7% NaCl and visualized using an Olympus SZ61 dissection microscope. Uteri were staged as in the study by Adams and Wolfner (41). Stage distribution of mutant vs. control mates was analyzed by means of a Wilcoxon rank sum test using Jmp-In software (version 5.1.2; SAS Institute).
Sperm Counts.
The number of sperm stored within the individual SSOs was counted at three time points postmating corresponding to maximal sperm accumulation (6 h) and various levels of storage depletion (4 d and 10 d) (17). Briefly, a null-mutant, virgin female [tβhM18/tβhM18, tdc2RO54/Df(2R)42, oamb286/oamb286, and loj00898/loj04026, respectively] or a control female [tβhM18/FM7, tdc2RO54/SM5, Df(2R)42/Gla, oamb286/TM6, and loj00898/TM3, respectively] and a virgin CS male were placed together in a vial containing standard medium until mating occurred. Males were removed shortly after mating to ensure that females mated only once. Females remained alone in their vials for the predetermined time postmating, and were then frozen and processed for sperm counts. To visualize sperm, female RTs were isolated and stained with orcein as previously described (9, 23). Sperm in the SSOs were counted using transillumination microscopy at a magnification of 1,000×. We avoided treatment biases in counting by blind-coding samples before counting sperm. We had a repeatability of >93% for each experiment, based on duplicate counts of a subset of samples.
To determine whether sperm accumulated normally in mutant females, we used t tests or ANOVA to compare the mean number of sperm stored in the SSOs together (total sperm) and separately (seminal receptacle and spermathecae) at 6 h after mating between or among female groups. To determine whether sperm were retained in storage, we calculated the magnitude of sperm depletion: Sperm counts of individual females at 4 d and 10 d were subtracted from the mean number of sperm stored at 6 h after mating for the corresponding female genotype and storage organ. We then analyzed differences in the depletion of stored sperm over time (4 d and 10 d) and among female groups using two-factor ANOVA (we report F values from the genotype effects). This approach was preferable to one consisting of a series of paired comparisons (mutant vs. control at each time point for each storage compartment) because it has a higher statistical power for detecting differences in depletion dynamics between mutants and their controls, and because it minimizes type I statistical errors (i.e., false-positive results). To examine retention phenotypes between thM18 and tdc2RO54 mutant females, we analyzed depletion of control-normalized stored sperm (mean number of stored sperm in control group at time t − sperm stored in each mutant at time t) over time (4 d and 10 d) and between female genotypes using two-factor ANOVA. The t tests were used as planned comparisons between female genotypes at 4 d and 10 d after mating. Assumptions of parametrical statistical tests were not violated. Analyses were performed using SPSS Statistics software (version 18.0.0; IBM SPSS, Inc.). For clarity, absolute numbers of stored sperm at each time point are presented in the figures.
Histology and Microscopy.
Virgin w; tdc2-GAL4, UAS-mCD8-GFP females (tdc2-GAL4, stock no. 9313; mCD8-GFP, stock no. 5137; Bloomington Stock Center) or w; oamb-GAL4/UAS-mCD8-GFP females (46) were isolated and aged for 5–7 d and mated to 5- to 7-d-old CS males. Females were briefly transferred to ice at 2.5–4 h after mating for processing. Females were dissected open in Ikeda's buffer (64) and fixed in 3.5% paraformaldehyde (wt/vol) in Ikeda's buffer for 20 min at room temperature. Samples were washed six times in PBS and blocked for 2 h with 10% goat serum (vol/vol) in PBS with 0.2% Triton-X (PBST). Samples were incubated with mouse α-GFP at 1:500 in block solution for 2 h at room temperature. After washing eight times with PBST, samples were incubated overnight at 4 °C in 1:250 rhodamine-phalloidin and 1:2,000 Alexa 488-conjugated goat α-mouse in PBST. Samples were mounted and imaged with a Leica confocal microscope at the Cornell University Life Sciences Core Laboratories Center. Reported results of innervation using α-GFP were further confirmed by observing endogenous GFP fluorescence without immunocytochemistry. In addition to GFP expression, identification of type II motor neuron terminals was based on two further criteria idiosyncratic to type II terminals: wandering axons and swellings ≤2 μm (53).
Supplementary Material
Acknowledgments
We thank members of the M.F.W. laboratory for helpful discussion in the preparation of the manuscript. Special thanks to Patricia Rivlin, Kyung-An Han, and Ginger Carney for providing fly lines used in this study, and to the Cornell Microscopy and Imaging Facility. This research was supported by National Institutes of Health Grant R01-HD038921 (to M.F.W.). For this research, M.C.B.Q. was supported, in part, by an American Recovery and Reinvestment Act (ARRA) supplement for educators training to Grant R01-HD038921 and, in part, by The William and Marilyn Ryerse Faculty Sabbatical Endowment Fund from Gustavus Adolphus College. For part of this work, F.W.A. was supported by National Institutes of Health Training Grant TG 5T32HD052471-04.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117689109/-/DCSupplemental.
References
- 1.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 2.Neubaum DM, Wolfner MF. Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Curr Top Dev Biol. 1999;41:67–97. doi: 10.1016/s0070-2153(08)60270-7. [DOI] [PubMed] [Google Scholar]
- 3.Holt WV, Fazeli A. The oviduct as a complex mediator of mammalian sperm function and selection. Mol Reprod Dev. 2010;77:934–943. doi: 10.1002/mrd.21234. [DOI] [PubMed] [Google Scholar]
- 4.Birkhead TR, Moller AP. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol J Linn Soc. 1993;50:295–311. [Google Scholar]
- 5.Holt WV, Lloyd RE. Sperm storage in the vertebrate female reproductive tract: How does it work so well? Theriogenology. 2010;73:713–722. doi: 10.1016/j.theriogenology.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52:455–462. doi: 10.1387/ijdb.072527ss. [DOI] [PubMed] [Google Scholar]
- 7.Talevi R, Gualtieri R. Molecules involved in sperm-oviduct adhesion and release. Theriogenology. 2010;73:796–801. doi: 10.1016/j.theriogenology.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Hunter RH, Léglise PC. Polyspermic fertilization following tubal surgery in pigs, with particular reference to the rôle of the isthmus. J Reprod Fertil. 1971;24:233–246. doi: 10.1530/jrf.0.0240233. [DOI] [PubMed] [Google Scholar]
- 9.Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: Identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poiani A. Complexity of seminal fluid: A review. Behav Ecol Sociobiol. 2006;60:289–310. [Google Scholar]
- 12.Gwathmey TM, Ignotz GG, Mueller JL, Manjunath P, Suarez SS. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol Reprod. 2006;75:501–507. doi: 10.1095/biolreprod.106.053306. [DOI] [PubMed] [Google Scholar]
- 13.den Boer SPA, Boomsma JJ, Baer B. Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J Insect Physiol. 2009;55:538–543. doi: 10.1016/j.jinsphys.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 14.King M, Eubel H, Millar AH, Baer B. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J Insect Physiol. 2011;57:409–414. doi: 10.1016/j.jinsphys.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Wolfner MF. Precious essences: Female secretions promote sperm storage in Drosophila. PLoS Biol. 2011;9:e1001191. doi: 10.1371/journal.pbio.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong A, et al. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 2008;180:921–931. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram KR, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 2007;3:e238. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol. 2003;206:3521–3528. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- 19.Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186:595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.den Boer SP, et al. Prudent sperm use by leaf-cutter ant queens. Proc Biol Sci. 2009;276:3945–3953. doi: 10.1098/rspb.2009.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baer B. Sexual selection in Apis bees. Apidologie. 2005;36:187–200. [Google Scholar]
- 22.Snook RR, Markow TA. Efficiency of gamete usage in nature: Sperm storage, fertilization and polyspermy. Proc Biol Sci. 2002;269:467–473. doi: 10.1098/rspb.2001.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch Qazi MC, Hogdal L. Hold on: Females modulate sperm depletion from storage sites in the fly Drosophila melanogaster. J Insect Physiol. 2010;56:1332–1340. doi: 10.1016/j.jinsphys.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Olivieri G, Tanzarella C, Micheli A. On the relationship between stored sperm and progeny produced in females of Drosophila melanogaster. Genetica. 1972;43(1):98–105. doi: 10.1007/BF00122503. [DOI] [PubMed] [Google Scholar]
- 25.Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9:e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMunyon CW, Eisner T. Postcopulatory sexual selection in an arctiid moth (Utetheisa ornatrix) Proc Natl Acad Sci USA. 1993;90:4689–4692. doi: 10.1073/pnas.90.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloch Qazi MC, Aprille JR, Lewis SM. Female role in sperm storage in the red flour beetle, Tribolium castaneum. Comp Biochem Physiol. 1998;120(Part A):641–647. [Google Scholar]
- 28.Hellriegel B, Bernasconi G. Female-mediated differential sperm storage in a fly with complex spermathecae, Scatophaga stercoraria. Anim Behav. 2000;59:311–317. doi: 10.1006/anbe.1999.1308. [DOI] [PubMed] [Google Scholar]
- 29.Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton Univ Press; 1996. [Google Scholar]
- 30.Clark J, Lange AB. The neural control of spermathecal contractions in the locust, Locusta migratoria. J Insect Physiol. 2000;46:191–201. doi: 10.1016/s0022-1910(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 31.Clark J, Lange AB. Evidence of a neural loop involved in controlling spermathecal contractions in Locusta migratoria. J Insect Physiol. 2001;47:607–616. doi: 10.1016/s0022-1910(00)00149-9. [DOI] [PubMed] [Google Scholar]
- 32.Lange AB. Neural mechanisms coordinating the female reproductive system in the locust. Front Biosci. 2009;14:4401–4415. doi: 10.2741/3536. [DOI] [PubMed] [Google Scholar]
- 33.Arthur BI, Hauschteck-Jungen E, Nothiger R, Ward PI. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: A masculinized nervous system is as good as none. Proc R Soc Lond B Biol Sci. 1998;265:1749–1753. [Google Scholar]
- 34.Rodríguez-Valentín R, et al. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 2006;209:183–198. doi: 10.1002/jcp.20722. [DOI] [PubMed] [Google Scholar]
- 35.Baer B, Eubel H, Taylor NL, O'Toole N, Millar AH. Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biol. 2009;10:R67. doi: 10.1186/gb-2009-10-6-r67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.den Boer SP, Baer B, Boomsma JJ. Seminal fluid mediates ejaculate competition in social insects. Science. 2010;327:1506–1509. doi: 10.1126/science.1184709. [DOI] [PubMed] [Google Scholar]
- 37.Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 38.Cole SH, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: Distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 39.Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264(1):38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams EM, Wolfner MF. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol. 2007;53:319–331. doi: 10.1016/j.jinsphys.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA. 2009;106:15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. New York: Wiley; 1950. pp. 420–534. [Google Scholar]
- 44.Middleton CA, et al. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carney GE, Taylor BJ. Logjam encodes a predicted EMP24/GP25 protein that is required for Drosophila oviposition behavior. Genetics. 2003;164:173–186. doi: 10.1093/genetics/164.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HG, Seong CS, Kim YC, Davis RL, Han KA. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264(1):179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ. A new family of insect tyramine receptors. Biochem Biophys Res Commun. 2005;338:1189–1196. doi: 10.1016/j.bbrc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 49.Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94:547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- 50.Arakawa S, et al. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990;4:343–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- 51.Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balfanz S, Strünker T, Frings S, Baumann A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2005;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]
- 53.Johansen J, Halpern ME, Keshishian H. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J Neurosci. 1989;9:4318–4332. doi: 10.1523/JNEUROSCI.09-12-04318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heifetz Y, Rivlin PK. Beyond the mouse model: Using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology. 2010;73:723–739. doi: 10.1016/j.theriogenology.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–321. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- 56.Anderson RC. A Study of the Factors Affecting Fertility of Lozenge Females of Drosophila Melanogaster. Genetics. 1945;30:280–296. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert DG. Ejaculate esterase 6 and initial sperm use by female Drosophila melanogaster. J Insect Physiol. 1981;27:641–650. [Google Scholar]
- 58.Ohsako T, Yamamoto MT. Sperm of the wasted mutant are wasted when females utilize the stored sperm in Drosophila melanogaster. Genes Genet Syst. 2011;86(2):97–108. doi: 10.1266/ggs.86.97. [DOI] [PubMed] [Google Scholar]
- 59.Ram KR, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci USA. 2009;106:15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pitnick S, Markow T, Spicer GS. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. [DOI] [PubMed] [Google Scholar]
- 61.Prokupek AM, Kachman SD, Ladunga I, Harshman LG. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 2009;18:465–475. doi: 10.1111/j.1365-2583.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 62.Kapelnikov A, Rivlin PK, Hoy RR, Heifetz Y. Tissue remodeling: A mating-induced differentiation program for the Drosophila oviduct. BMC Dev Biol. 2008;8:114. doi: 10.1186/1471-213X-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heifetz Y, Wolfner MF. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc Natl Acad Sci USA. 2004;101:6261–6266. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.