Abstract
Altered systemic levels of 6-formylindolo[3,2-b]carbazole (FICZ), an enigmatic endogenous ligand for the aryl hydrocarbon receptor (AHR), may explain adverse physiological responses evoked by small natural and anthropogenic molecules as well as by oxidative stress and light. We demonstrate here that several different chemical compounds can inhibit the metabolism of FICZ, thereby disrupting the autoregulatory feedback control of cytochrome P4501 systems and other proteins whose expression is regulated by AHR. FICZ is both the most tightly bound endogenous agonist for the AHR and an ideal substrate for cytochrome CYP1A1/1A2 and 1B1, thereby also participating in an autoregulatory loop that keeps its own steady-state concentration low. At very low concentrations FICZ influences circadian rhythms, responses to UV light, homeostasis associated with pro- and anti-inflammatory processes, and genomic stability. Here, we demonstrate that, if its metabolic clearance is compromised, femtomolar background levels of this compound in cell-culture medium are sufficient to up-regulate CYP1A1 mRNA and enzyme activity. The oxidants UVB irradiation and hydrogen peroxide and the model AHR antagonist 3′-methoxy-4′-nitroflavone all inhibited induction of CYP1A1 enzyme activity by FICZ or 2,3,7,8-tetrachlorodibenzo-p-dioxin, thereby subsequently elevating intracellular levels of FICZ and activating AHR. Taken together, these findings support an indirect mechanism of AHR activation, indicating that AHR activation by molecules with low affinity actually may reflect inhibition of FICZ metabolism and raising questions about the reported promiscuity of the AHR. Accordingly, we propose that prolonged induction of AHR activity through inhibition of CYP1 disturbs feedback regulation of FICZ levels, with potential detrimental consequences.
The toxicity exerted by a number of anthropogenic substances involves activation of a cytosolic transcription factor, known as the “aryl hydrocarbon receptor” (AHR) or the dioxin receptor. This receptor, which is activated by small molecules and by light, is one of the 34 known proteins that both contain a Per-Arnt-Sim domain and regulate responses to environmental changes (1–4). Both AHR itself and the first family of cytochrome P450 (CYP1) enzymes, whose expression is regulated by AHR, have been highly conserved during evolution (5, 6). This observation, together with its presence in most cell types and during all phases of development, indicate strongly that, in addition to responses to xenobiotics, the AHR and its endogenous activators are involved in vital physiological processes. Indeed, evidence is accumulating that the AHR plays important roles in xenobiotic-independent control of perinatal growth (7, 8), fertility (9, 10), hepatic and vascular development (11–13), peripheral and intestinal immunity (14–19), and hematopoiesis (13, 20), as well as in stem cell expansion (21, 22) and cancer (23, 24).
These recent insights into the physiological roles of the AHR have renewed interest in the identification of endogenous activators. Although it was postulated more than 30 y ago, on the basis of their evolutionary conservation, that the CYP1 proteins metabolize important endogenous substances (25), only recently was 6-formylindolo[3,2-b]carbazole (FICZ), a derivative of tryptophan (Trp), shown to be an ideal substrate for CYP1A1, 1A2, and 1B1, exhibiting a catalytic efficiency close to being limited by diffusion (ref. 3 and references therein). FICZ also is bound to the AHR with the highest affinity known to date and thereby demonstrates the characteristics of an endogenous signaling molecule; i.e., it stimulates an efficient receptor-mediated pathway for transcriptional activation of metabolic enzymes that, among other things, catalyze the clearance of FICZ itself, thereby generating negative feedback regulation of its action (3, 26, 27). The physiological effects of FICZ reported to date include alterations in circadian rhythms (28), adaptive responses to UV light (4, 29), induced retrotransposition of long interspersed nucleotide element-1 (30), and stimulated production of IL-22, a member of the IL-10 cytokine family that helps regulate inflammatory responses associated with many autoimmune diseases (17, 18, 31). In humans, the involvement of FICZ in IL-22 production has been demonstrated both in stimulated blood cells from the umbilical cord (32) and in intestinal tissue from patients with inflammatory bowel disease (31).
FICZ is formed from Trp in aqueous solutions, including cell-culture media, upon exposure to UV and visible light (3, 4, 33, 34) as well as in human skin cells exposed in vitro to UVB light (4). Moreover, FICZ is conjugated metabolically with sulfate, and such conjugates have been detected in human urine (3), further verifying the presence of the parent compound in vivo. In addition to its efficient activation of AHR signaling, FICZ appears to be a potent activator of signaling involving the liver X receptor or pregnane X receptor pathways (35, 36). On the basis of such findings, we propose that FICZ should be added to the list of bioactive signaling substances derived from Trp that now includes serotonin, melatonin, and the plant growth hormone indole-3-acetic acid.
Detailed structural information about the ligand-binding domain of the AHR is still lacking. This domain generally is thought to be relatively unspecific (promiscuous), because, in addition to its classical ligands such as dioxins and similar molecules, this receptor is activated by a multitude of exogenous and endogenous compounds with diverse chemical structures (ref. 37 and references therein). Here, we test the hypothesis that FICZ, an endogenous ligand that can activate the AHR at extremely low concentrations and also is metabolized readily to inactive products, is the actual ligand associated with activation by most of these compounds. For this purpose, we compiled a list of 125 compounds that up-regulate expression of target genes of AHR and/or induce CYP1 enzyme activity in vitro and/or in vivo (Table S1). This list includes endogenous substances, clinical drugs, food additives, industrial compounds, pesticides, metals, phytochemicals, and oxidants as well as compounds used to inhibit or block specific signaling pathways. Although most of these substances exhibit low or neglible affinity for binding to the AHR, they all inhibit members of the CYP1 family. Thus, they all might activate the AHR indirectly by preventing the inactivation of FICZ.
To test this hypothesis, we first examined whether UVB, hydrogen peroxide (H2O2), and 3′-methoxy-4′-nitroflavone (MNF) can inhibit the degradation of FICZ and thereby give rise to prolonged AHR signaling. The two first strong oxidants display no affinity for the AHR, whereas the flavonoid MNF is regarded as a potent AHR antagonist that also exhibits some agonist activity (38, 39).
Results
FICZ Is a Potent Systemic Activator of the AHR.
At low concentrations, FICZ causes transient elevation in the expression of the CYP1A1 gene (3, 40). Here, we found that exposure of confluent immortalized human keratinocytes (HaCaT) to 5 pM FICZ significantly enhanced their level of CYP1A1 mRNA within 2 h (Fig. S1).
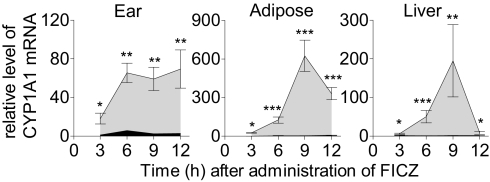
To determine whether, despite its efficient metabolic clearance, FICZ can elicit systemic effects in vivo, 10 ng of this compound was applied to one ear of female C57BL/6J mice; after this administration pronounced time-dependent induction of CYP1A1 mRNA levels was observed in all tissues examined (the ear, adipose tissue, and liver) (Fig. 1). Clearly, FICZ is distributed systemically and can act at sites distant from the point of application.
Fig. 1.
FICZ is a potent inducer of CYP1A1 gene expression in vivo. The levels of CYP1A1 mRNA in the ear, adipose tissue (perirenal visceral fat), and liver of C57BL/6J mice treated with FICZ were determined as a function of time by quantitative RT-PCR (qRT-PCR) using the β-actin housekeeping gene as an internal standard. The levels in treated animals are shown in light gray, and those in mice receiving vehicle alone are shown in black. The relative increases presented are the means ± SE for three animals. Asterisks indicate significant differences (*P < 0.05; **P < 0.01; ***P < 0.001) between FICZ- and vehicle-treated animals.
Inhibition of CYP1A1 Slows Intracellular Metabolism of FICZ.
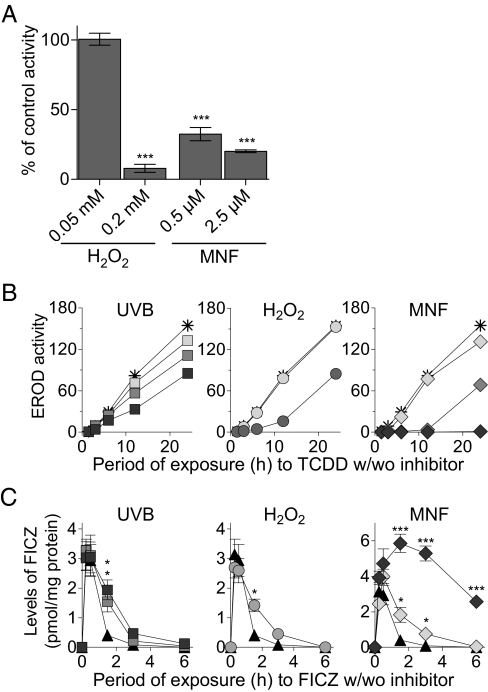
Next, using the ethoxyresorufin deethylase (EROD) activity of preparations (Supersomes) from the baculovirus-infected insect cell system containing recombinant human CYP1A1 or of cultured keratinocytes cotreated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) as a measure of CYP1A1 activity, we examined the effects of inhibitors of this activity on the autoregulatory clearance of FICZ. H2O2 and MNF were found to inhibit the basic EROD activity of the recombinant CYP1A1 enzyme (Fig. 2A), and UVB, H2O2, and MNF all inhibited TCDD-induced EROD activity in a dose-dependent manner both in HaCaT cells (Fig. 2B) and in primary human epidermal keratinocytes (HEKa cells) (Fig. S2 Middle). The viability of the HaCaT cells was not affected significantly by the cotreatments (the viability ranged from 96–102% of that of untreated cells). Furthermore, in HaCaT cells coexposed to 5 nM FICZ and any one of these three inhibitors, the clearance rate of FICZ was reduced significantly (most potently by MNF at the concentrations used) (Fig. 2C).
Fig. 2.
Inhibitors of CYP1A1 attenuate degradation of FICZ. (A) Inhibition of basal EROD activity in human recombinant CYP1A1 (Supersomes) by H2O2.and MNF. Activity is expressed relative to the control (DMSO). Error bars indicate SE. Asterisks denote significant differences (***P < 0.001) from the control. (B) Inhibition of EROD activity (pmol resorufin/mg protein) induced by 5 nM TCDD (star) in HaCaT cells by 5 mJ/cm2 (light gray square),10 mJ/cm2 (medium gray square), or 20 mJ/cm2 (dark gray square) UVB, by 0.2 mM (light gray circle) or 2 mM (medium gray circle) H2O2, or by 0.05 μM (light gray diamond), 0.5 μM (medium gray diamond) or 2.5 μM (dark gray diamond) MNF at the time points indicated. Statistically significant inhibition (P < 0.05 to P < 0.001) was obtained in the cells exposed to UVB at all time points except for 5 mJ/cm2, which was significant only at 6 h and later; for the cells exposed to 0.2 mM H2O2 at 1.5 h and 3 h and to 2 mM at all time points; and for the cells exposed to MNF at all exposure levels and all time points. (C) HaCaT cells were exposed for 0–6 h to 5 nM (black triangle) FICZ alone or in combination with 10 mJ/cm2 (medium gray square) or 20 mJ/cm2 (dark gray square) UVB, 0.2 mM (light gray circle) H2O2, or 0.05 μM (light gray diamond) or 2.5 μM (dark gray diamond) MNF. The cells were harvested at the time points indicated, and their FICZ content was determined by HPLC. Error bars indicate SE. Asterisks indicate significant differences (*P < 0.05; ***P < 0.001) in coexposed cells vs. cells exposed only to FICZ.
Inhibition of CYP1A1 Enhances FICZ-Dependent Transcription of the CYP1A1 Gene and Related Enzyme Activity.
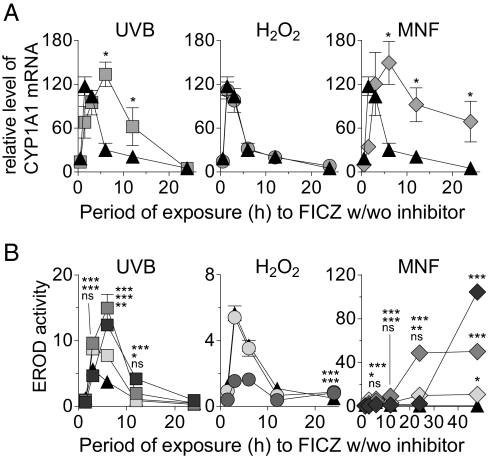
All the treatments described above inhibited the early increases in CYP1A1 gene transcription and/or related enzyme activity elicited by exposure to FICZ (Fig. 3 and Fig. S3). However, upon prolonged exposure these cotreatments influenced FICZ-dependent CYP1A1 induction to different extents. UVB caused a prolonged elevation in the levels of CYP1A1 mRNA (Fig. 3A) and EROD activity (Fig. 3B and Fig. S2 Top), with maximal responses almost threefold higher than seen with FICZ alone. H2O2 exerted no effects on CYP1A1 mRNA levels in HaCaT cells (Fig. 3A) and marginally but significantly enhanced the FICZ-dependent EROD activity in HaCaT cells and HEKa cells (Fig. 3B and Fig. S2 Top). Cotreatment with MNF potentiated FICZ-dependent CYP1A1 gene expression (Fig. 3A), initially causing a longer period of EROD inhibition that was followed by the most pronounced and sustained induction of EROD in both HaCaT and HEKa cells (Fig. 3B and Fig. S2 Top). These observations with UVB and MNF clearly demonstrate that inhibition of CYP1A1 can potentiate subsequent FICZ-dependent stimulation of CYP1A1 transcription and related synthesis of a functional protein.
Fig. 3.
Inhibitors of CYP1A1 initially delay and subsequently potentiate FICZ-dependent induction of CYP1A1 mRNA and enzyme activity. HaCaT cells were exposed to 5 nM FICZ (black triangle) alone or in combination with 5 mJ/cm2 (light gray square), 10 mJ/cm2 (medium gray square), or 20 mJ/cm2 (dark gray square) UVB, 0.2 mM (light gray circle) or 2 mM (medium gray circle) H2O2, or 0.05 μM (light gray diamond), 0.5 μM (medium gray diamond, or 2.5 μM (dark gray diamond) MNF for different periods of time. (A) Determination of CYP1A1 mRNA relative to that of the housekeeping gene β-2-microglobulin. Exposure to 10 mJ/cm2 UVB or 0.5 μM MNF caused statistically significant inhibition of FICZ-induced CYP1A1 gene expression at 1.5 h (P < 0.05) and significant induction at 6–12 h (UVB) and 6–24 h (MNF). Error bars indicate SE (n = 2). Asterisks denote significantly higher induction (*P < 0.05) in coexposed cells compared with cells exposed to FICZ alone. (B) Determination of EROD activity (pmol resorufin/mg protein). The inhibition of FICZ-induced EROD activity was statistically significant at 1.5 h and 3 h in cells exposed to UVB, H2O2, or MNF as detailed in Fig. S3. Significantly increased EROD induction was caused by UVB at 3–12 h, by H2O2 at 24 h, and by 0.05–0.5 μM MNF at 6–24 h. However, 2.5 μM MNF increased FICZ-induced EROD activity only at 48 h. Error bars indicate SE. Asterisks indicate significantly higher induction (*P < 0.05; **P < 0.01; ***P < 0.001) in coexposed cells vs. cells exposed to FICZ alone. ns, nonsignificant.
Activation of the AHR by UVB, H2O2, and MNF Is Caused by the Presence of Trace Amounts of FICZ.
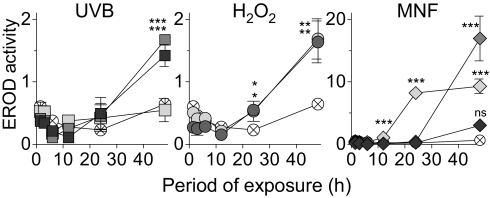
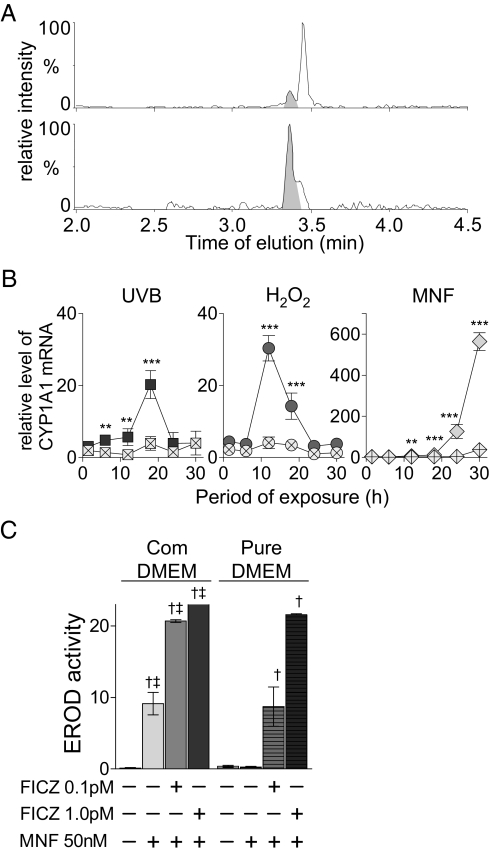
CYP1A1-catalyzed enzyme activity also was induced in HaCaT cells exposed to UVB, H2O2, or MNF alone (Fig. 4) but not when these same cells were stably transfected with shRNA sequences for AHR silencing, confirming that in all three cases this induction was AHR dependent (Figs. S4C and S5 A–C). The known formation of FICZ from Trp (3, 4, 33, 34) suggested to us that, if its metabolic degradation is suppressed, background levels of FICZ in cell-culture media (even in media that had been protected from light) might be sufficient to activate such AHR-dependent responses. Indeed, liquid chromatography (LC)-MS analysis of DMEM revealed the presence of FICZ, even when the medium had been protected from light. Comparison of the peak area with the area of a corresponding peak in medium spiked with authentic FICZ revealed that the concentration of FICZ in commercial DMEM was ∼0.1 pM (Fig. 5A). This calculation was confirmed by HPLC analysis of several light-protected bottles of DMEM, in which the FICZ concentrations ranged from 0.1–0.2 pM.
Fig. 4.
Induction of CYP1A1 activity in HaCaT cells by UVB, H2O2, and MNF. These cells were exposed to 5 mJ/cm2 (light gray square), 10 mJ/cm2 (medium gray square), or 20 mJ/cm2 (dark gray square) UVB, to 0.2 mM (light gray circle) or 2 mM (dark gray circle) H2O2; to 0.05 μM (light gray diamond), 0.5 μM (medium gray diamond), or 2.5 μM (dark gray diamond) MNF, or to DMSO alone (crossed circle), and their EROD activity (pmol resorufin/mg protein) was measured at the time points indicated. Error bars indicate SE. Asterisks denote significant differences (*P < 0.05; **P < 0.01; ***P < 0.001) from the DMSO-treated cells. ns, nonsignificant.
Fig. 5.
Activation of AHR in HaCaT cells by UVB, H2O2, and MNF requires the presence of Trp derivatives in the culture medium. (A) The levels of FICZ in commercial DMEM, as determined by solid-phase extraction and HPLC using in-line concentration and ultra performance liquid chromatography/quadrupole time-of-flight high-resolution MS. (Upper) The mass chromatogram [m/z 283.087, mass window: 0.05 Thomson (Th)] for the extract of untreated medium (average mass error: 4.9 mTh). (Lower) An FICZ-spiked sample (average mass error: 5.4 mTh). The shaded areas in each trace indicate the FICZ peak. (B) HaCaT cells grown at high density in commercial DMEM or in DMEM prepared with recrystallized Trp (shown by symbols containing an x) were exposed to 20 mJ/cm2 UVB (dark gray square), to 2 mM H2O2 (dark gray circle), or to 0.05 μM MNF (light gray diamond), and their levels of CYP1A1 mRNA relative that of the housekeeping gene β-2-microglobulin were determined by qRT-PCR at the time points indicated. Error bars indicate SE. Asterisks denote significant differences (**P < 0.01; ***P < 0.001) between values from cells grown in the two media. (C) HaCaT cells were exposed to MNF alone or in combination with different concentrations of FICZ in commercial DMEM (Com) or in DMEM prepared with recrystallized Trp (Pure). After 48 h their EROD activity (pmol resorufin/mg protein) was measured. Error bars indicate SD. †Significantly different (P < 0.001) from the untreated control in the same medium. ‡Significantly different (P < 0.01 to P < 0.001) from the same treatment in pure DMEM.
When cells were exposed to UVB, H2O2, or MNF in this commercial medium containing low background levels of FICZ, up-regulation of CYP1A1 gene expression was significantly higher in all three cases (again, most strongly with MNF) than in cells cultured in DMEM prepared with Trp that had been recrystallized and therefor was uncontaminated by compounds such as FICZ (Fig. 5B). Moreover, UVB, which has been found previously to stimulate the formation of FICZ and to induce CYP1A1 in human keratinocytes, had no such effects in the pure medium. The lack of effect of UVB in the present case probably can be explained by the Trp levels being too low, because in earlier studies UVB activated CYP1A1 expression only in cells loaded with high levels of Trp (2, 4), and because FICZ is produced upon exposure of HaCaT cells loaded with Trp to UVB (4).
These findings clearly demonstrate that the agonistic behavior of certain agents can depend on the presence of an AHR activator derived from Trp in the medium. To determine how much FICZ would be required to explain the results obtained with MNF in the commercial medium, different amounts of FICZ were added to DMEM prepared with recrystallized Trp. We found that 50 nM MNF, which was inactive in the FICZ-free medium, elicited the same response as seen in the commercial medium when the pure medium was supplemented with 0.1 pM FICZ (Fig. 5C). These results indicate beyond doubt that CYP1A1 induction in this system is caused by FICZ rather than by MNF itself.
Activation of AHR by Various Inhibitors of CYP1A.
The observations that UVB, H2O2, and MNF require background levels of FICZ to elicit their responses in our system led us to test the abilities of eight other compounds to elevate EROD activity in commercial and pure medium. Trioxalen, ketoconazole, ellipticine, genistein, diosmin, α-naphthoflavone (αNF), cycloheximide, and α-tocopherol inhibited TCDD-induced EROD activity to varying extents (Table 1). All the eight tested compounds evoked significantly higher cellular EROD activities in cells exposed in commercial medium than in cells in pure medium (Table 1 and Fig. S6). The observation that ellipticine induced EROD activity in the pure medium (although to a lesser degree than in the commercial medium) raises the possibility that this compound may act both as an AHR agonist and as an inhibitor of CYP1A1. TCDD produced the same EROD induction in both media. Cycloheximide and H2O2 (at the highest concentration) reduced cell viability slightly (Table S2). All other treatments did not affect cell viability, and no differences in viability were observed between treatments performed in commercial or pure media.
Table 1.
CYP1A activity of HaCaT cells exposed to inhibitors of CYP1A in commercial DMEM (Com) or in DMEM prepared with purified tryptophan (Pure)
| % inhibition† |
Induction‡ |
||
| Compound (concentration) | Com | Com | Pure |
| TCDD (1 nM) | 24.1 ± 1.48ns | 24.5 ± 2.03 | |
| Trioxalen (1 μM) | 33.0 ± 9.1* | 1.48 ± 0.18** | 0.05 ± 0.03 |
| Ketoconazole (10 μM) | 69.7 ± 1.7*** | 1.31 ± 0.22*** | 0.27 ± 0.05 |
| Ellipticine (0.05 μM) | 43.3 ± 3.4*** | 9.01 ± 1.03*** | 3.88 ± 0.32 |
| Genistein (10 μM) | 14.2 ± 5.3ns | 1.10 ± 0.07*** | 0.02 ± 0.04 |
| Diosmin (10 μM) | 40.8 ± 3.3** | 0.79 ± 0.06*** | 0.18 ± 0.04 |
| α-Naphtoflavone (0.25 μM) | 62.2 ± 2.9*** | 1.46 ± 0.14*** | 0.08 ± 0.03 |
| Cycloheximide (18 μM) | 65.8 ± 1.9*** | 3.45 ± 0.33*** | 0.27 ± 0.04 |
| α-Tocopherol (50 μM) | 14.9 ± 6.2ns | 0.54 ± 0.03*** | 0.13 ± 0.02 |
| DMSO (0.1%, vol/vol) | 0.25 ± 0.04*** | 0.05 ± 0.02 | |
| EtOH (0.1%, vol/vol) | 0.33 ± 0.04** | 0.16 ± 0.03 | |
†Percent inhibition of TCDD-induced EROD activity following 6 h of exposure in the absence of any other addition; means ± SE of triplicate measurements. Significance of inhibition compared with TCDD is depicted with asterisks (ns, non significant; *P < 0.05; **P < 0.01; ***P < 0.001).
‡Mean EROD activity (pmol resorufin/mg protein) ± SE following 12 h of exposure. Differences in EROD induction for respective treatment in the commercial compared with the pure media were tested. ns, non significant; *P < 0.05; **P < 0.01; ***P < 0.001. EROD induction at several time points is shown in Fig. S6.
Discussion
The findings documented here corroborate and extend our previous observations and the observations published by others that very low concentrations of FICZ can activate the AHR directly, both in vitro and in vivo (3, 4, 28, 40, 41). We demonstrate activation of the AHR at the site of application and at remote sites if FICZ is applied topically on the ear (Fig. 1). These observations suggest that FICZ can be transported to remote sites and thereby work as a chemical messenger. They also suggest participation of specific binding proteins or lipoproteins that protect against degradation of FICZ during circulation. Transient up-regulation of CYP1A1 transcription was observed in adipose and liver tissues; more sustained expression was seen in the ear. The prolonged expression at the site of application probably is the result of inhibited clearance of FICZ, because at higher concentrations this compound itself is an inhibitor of CYP1 enzymes (3, 40). Furthermore, the present investigation offers a relatively straightforward explanation for the reported ability of numerous structurally diverse chemicals to activate the AHR; such reports have been used to argue that the AHR binds ligands promiscuously (37). Our results also may explain why the addition of fresh Trp-containing medium to cell cultures (42), oxidative stress [e.g., by hyperoxia (43)], and hydrodynamic shear that gives rise to oxidized LDL (44, 45), as well as why the addition of various complex mixtures, such as extracts of paper and ink, can activate the AHR (46, 47).
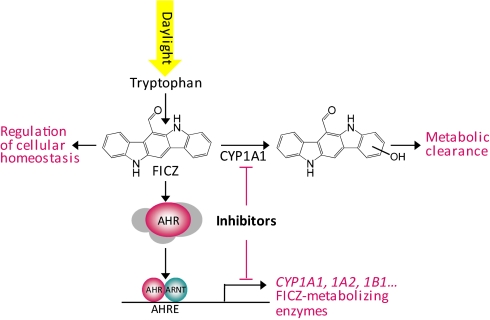
On the basis of our present observations with UVB, H2O2, and MNF and the previously demonstrated activation of AHR by αNF, a potent inhibitor of CYP1A1 (3), we propose an indirect mechanism of AHR activation involving the endogenous ligand FICZ (illustrated in Fig. 6). Such an indirect mechanism also is supported by the observation that a range of CYP1A1-inhibiting compounds, most of which also have been shown to activate the AHR (Table S1), enhanced EROD activity to a greater extent in commercial than in pure medium (Table 1). MNF and αNF are used commonly as AHR antagonists in mechanistic studies, because these substances effectively inhibit TCDD-mediated up-regulation of CYP1A1 transcription and enzyme activity. Here, MNF and αNF efficiently inhibited the metabolism of FICZ, thereby activating AHR signaling in medium containing background levels of this ligand. Interestingly, in the presence of elevated levels of FICZ, MNF up-regulated CYP1A1 to an extent similar to that seen with the high-affinity, metabolically inert ligand TCDD (compare Figs. 2B and 3B). Accordingly, potent inhibitors of CYP1A1 should be used as AHR antagonists only with extreme caution.
Fig. 6.
Schematic illustration of indirect activation of the AHR by the endogenous ligand FICZ when the catalytic function of CYP1A1 is reduced or inhibited.
In most reports on the AHR-activating capacity of natural and synthetic molecules, activation has been assessed by determining CYP1A1 mRNA levels (originating either from endogenous or reporter gene expression), EROD activity, or binding of AHR to labeled synthetic response elements in DNA. Many of the AHR activators listed in Table S1 undoubtedly induce EROD activity in the liver or lung tissue of rodents, both in vitro and in vivo. The specificity of AHR involvement in CYP1A1 induction is supported routinely by the demonstration that no such up-regulation is observed with reporter constructs carrying mutations in the AHR response elements, after inactivation of AHR by siRNA, and/or in the presence of antagonists considered to be specific for AHR, such as αNF and MNF. Such controls indeed confirm that the AHR is required for the responses obtained but do not rule out the possibility that FICZ in the cultured cells or the culture medium is involved. Even when the commercial DMEM we used was protected from light, it still contained 0.1–0.2 pM FICZ, an amount that was sufficient to explain the agonistic effect of MNF (Figs. 4 and 5). Furthermore, it can be assumed that background levels of FICZ are present in all Trp-containing media.
It has been demonstrated repeatedly that expression of the CYP1A1 gene is autoregulated by the action of its product protein and that this feedback mechanism is essential for the appropriate timing, duration, and magnitude of normal cellular functions regulated by AHR (ref. 26 and references therein). Activation of AHR is commonly inhibited by agents and conditions that cause cellular stress, such as hypoxia and oxidative stress, as well as by infectious and inflammatory stimuli. In addition, repression of transcription is well known to be associated with epigenetic events (ref. 48 and references therein). Although we have yet to unravel the precise mechanisms underlying transcriptional regulation of the CYP1A1 gene, the inhibition by the oxidants UVB, H2O2, and MNF observed here in cells cotreated with FICZ or TCDD is likely to involve chromatin remodeling via epigenetic regulatory processes. In rats and mice expression of CYP1A1 exhibits diurnal rhythmicity (49, 50), and it is tempting to speculate that inhibition of CYP1A1 could be of physiological significance by providing periods with elevated intracellular levels of FICZ that promote AHR-dependent and/or -independent adaptive responses to oxidative stress or inflammatory stimuli.
To elucidate in greater detail the impact of agents that interfere with the degradation of FICZ, the consequences of modulating systemic levels of this ligand on intrinsic biological functions must be examined. This highly bioactive compound may influence multiple targets via binding to several nuclear receptors and possibly by affecting the stability of the genome (reviewed in ref. 51). In light of the vast number and variety of agents that inhibit the CYP1 enzymes (only some of which are listed in Table S1), this proposal may imply the existence of a unique mechanism of toxicity. Recent studies suggest that the effects of FICZ on various cells of the immune system, both as a stimulus and suppressor of growth (17, 18, 32), may exert a profound impact on human health. Indeed, FICZ and the AHR may play fundamental roles in modulating the immune response. In this connection, TCDD was shown recently to polarize CD4+ T cells specifically to produce IL-22, resulting in a long-lasting effect on the human adaptive immune system (52). Available evidence also supports a central role for AHR signaling in connection with systemic inflammatory disorders whose pathogenesis is not yet fully understood, such as rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease (17–19, 31, 53).
Materials and Methods
Detailed descriptions of the cells, media, reagents, recombinant enzymes, UV irradiation protocols, animal treatments, reverse transcription and real-time PCR, EROD assay, determination of cell viability, recrystallization of Trp, and analyses by HPLC and LC-MS are provided in SI Materials and Methods.
Cell Culturing.
In differentiated HaCaT cells grown to high confluence and expressing involucrin and transglutaminase 1 at high levels (Fig. S4 A and B), expression of the AHR is much greater than in low-density cultures (Fig. S4C). Accordingly, cells in this state were treated with various agents either alone or in combination with FICZ or TCDD. These treatments were initiated by exposure to fresh medium containing the compound(s) of interest or by irradiation with UVB.
Statistical Analysis.
All measurements were performed with replicates (n = 3–6, unless otherwise indicated). Dose- and time-course studies of EROD activity, CYP1A1 expression, and clearance of FICZ were performed at least in two independent experiments. The tests of the overall null hypotheses were performed using one-way ANOVA tests with Dunnett´s post test for comparing three or more groups or by multiple unpaired, two-tailed t tests with Bonferroni correction, adjusting for the number of tests for comparisons of two groups. If the overall null hypothesis was rejected, then continued comparisons were performed by comparing individual P values with the uncorrected α = 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Fedor Moncek for performing the animal experiments, Kristoffer Spricer for statistical consultation and calculations, Anna Smirnova for technical assistance, and Dr. Joe de Pierre for valuable comments on the manuscript. This study was financed by grants from the Swedish Research Council for the Environment, Agricultural Sciences and Spatial Planning and by the Sven and Lilly Lawski Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118467109/-/DCSupplemental.
References
- 1.McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–645. doi: 10.1146/annurev-physiol-021909-135922. [DOI] [PubMed] [Google Scholar]
- 2.Wei YD, Rannug U, Rannug A. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 1999;118:127–140. doi: 10.1016/s0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 3.Wincent E, et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche E, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci USA. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstone JV, et al. Cytochrome P450 1 genes in early deuterostomes (tunicates and sea urchins) and vertebrates (chicken and frog): Origin and diversification of the CYP1 gene family. Mol Biol Evol. 2007;24:2619–2631. doi: 10.1093/molbev/msm200. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Salguero P, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 8.Mimura J, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 9.Abbott BD, et al. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol. 1999;155:62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- 10.Baba T, et al. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahvis GP, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol Appl Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chmill S, Kadow S, Winter M, Weighardt H, Esser C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs stable establishment of oral tolerance in mice. Toxicol Sci. 2010;118:98–107. doi: 10.1093/toxsci/kfq232. [DOI] [PubMed] [Google Scholar]
- 15.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Casado FL, Singh KP, Gasiewicz TA. The aryl hydrocarbon receptor: Regulation of hematopoiesis and involvement in the progression of blood diseases. Blood Cells Mol Dis. 2010;44:199–206. doi: 10.1016/j.bcmd.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Fan Y, Puga A. Dioxin exposure disrupts the differentiation of mouse embryonic stem cells into cardiomyocytes. Toxicol Sci. 2010;115:225–237. doi: 10.1093/toxsci/kfq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boitano AE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu Y, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Y, et al. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010;70:212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez FJ. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988;40:243–288. [PubMed] [Google Scholar]
- 26.Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol. 2007;72:1369–1379. doi: 10.1124/mol.107.038968. [DOI] [PubMed] [Google Scholar]
- 27.Ma Q. Influence of light on aryl hydrocarbon receptor signaling and consequences in drug metabolism, physiology and disease. Expert Opin Drug Metab Toxicol. 2011;7:1267–1293. doi: 10.1517/17425255.2011.614947. [DOI] [PubMed] [Google Scholar]
- 28.Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: Searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 2007;95:172–181. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- 29.Luecke S, et al. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment Cell Melanoma Res. 2010;23:828–833. doi: 10.1111/j.1755-148X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 30.Okudaira N, et al. Induction of long interspersed nucleotide element-1 (L1) retrotransposition by 6-formylindolo[3,2-b]carbazole (FICZ), a tryptophan photoproduct. Proc Natl Acad Sci USA. 2010;107:18487–18492. doi: 10.1073/pnas.1001252107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteleone I, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–248. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 33.Diani-Moore S, et al. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo. Toxicol Sci. 2006;90:96–110. doi: 10.1093/toxsci/kfj065. [DOI] [PubMed] [Google Scholar]
- 34.Oberg M, Bergander L, Håkansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85:935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- 35.Reschly EJ, et al. Ligand specificity and evolution of liver X receptors. J Steroid Biochem Mol Biol. 2008;110:83–94. doi: 10.1016/j.jsbmb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekins S, Reschly EJ, Hagey LR, Krasowski MD. Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol Biol. 2008;8:103. doi: 10.1186/1471-2148-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu YF, Santostefano M, Cunningham BD, Threadgill MD, Safe S. Identification of 3′-methoxy-4′-nitroflavone as a pure aryl hydrocarbon (Ah) receptor antagonist and evidence for more than one form of the nuclear Ah receptor in MCF-7 human breast cancer cells. Arch Biochem Biophys. 1995;316:470–477. doi: 10.1006/abbi.1995.1062. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Gasiewicz TA. 3′-methoxy-4′-nitroflavone, a reported aryl hydrocarbon receptor antagonist, enhances Cyp1a1 transcription by a dioxin responsive element-dependent mechanism. Arch Biochem Biophys. 2003;416:68–80. doi: 10.1016/s0003-9861(03)00274-1. [DOI] [PubMed] [Google Scholar]
- 40.Wei YD, Helleberg H, Rannug U, Rannug A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 1998;110:39–55. doi: 10.1016/s0009-2797(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 41.Jönsson ME, et al. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem Biol Interact. 2009;181:447–454. doi: 10.1016/j.cbi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paine AJ. Induction of benzo[a]pyrene Mono-oxygenase in liver cell culture by the photochemical generation of active oxygen species. Evidence for the involvement of singlet oxygen and the formation of a stable inducing intermediate. Biochem J. 1976;158:109–117. doi: 10.1042/bj1580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y. Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem Biophys Res Commun. 1993;197:878–885. doi: 10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- 44.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci USA. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mufti NA, Bleckwenn NA, Babish JG, Shuler ML. Possible involvement of the Ah receptor in the induction of cytochrome P-450IA1 under conditions of hydrodynamic shear in microcarrier-attached hepatoma cell lines. Biochem Biophys Res Commun. 1995;208:144–152. doi: 10.1006/bbrc.1995.1316. [DOI] [PubMed] [Google Scholar]
- 46.Bohonowych JE, et al. Newspapers and newspaper ink contain agonists for the ah receptor. Toxicol Sci. 2008;102:278–290. doi: 10.1093/toxsci/kfn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanematsu M, Hayashi A, Denison MS, Young TM. Characterization and potential environmental risks of leachate from shredded rubber mulches. Chemosphere. 2009;76:952–958. doi: 10.1016/j.chemosphere.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Huang P, Ceccatelli S, Rannug A. A study on diurnal mRNA expression of CYP1A1, AHR, ARNT, and PER2 in rat pituitary and liver. Environ Toxicol Pharmacol. 2002;11:119–126. doi: 10.1016/s1382-6689(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 50.Mukai M, Lin TM, Peterson RE, Cooke PS, Tischkau SA. Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Rhythms. 2008;23:200–210. doi: 10.1177/0748730408316022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rannug A. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole helps genes jump. Proc Natl Acad Sci USA. 2010;107:18239–18240. doi: 10.1073/pnas.1013653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brembilla NC, et al. In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS ONE. 2011;6:e18741. doi: 10.1371/journal.pone.0018741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakahama T, et al. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci USA. 2011;108:14222–14227. doi: 10.1073/pnas.1111786108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.