Abstract
Calsequestrin is the most abundant Ca-binding protein of the specialized endoplasmic reticulum found in muscle, the sarcoplasmic reticulum (SR). Calsequestrin binds Ca with high capacity and low affinity and importantly contributes to the mobilization of Ca during each contraction both in skeletal and cardiac muscle. Surprisingly, mutations in the gene encoding the cardiac isoform of calsequestrin (Casq2) have been associated with an inherited form of ventricular arrhythmia triggered by emotional or physical stress termed catecholaminergic polymorphic ventricular tachycardia (CPVT). Despite normal cardiac contractility and normal resting ECG, CPVT patients present with a high risk of sudden death at a young age. Here, we review recent new insights regarding the role of calsequestrin in genetic and acquired arrhythmia disorders. Mouse models of CPVT have shed light on the pathophysiological mechanism underlying CPVT. Casq2 is not only a Ca-storing protein as initially hypothesized, but it has a far more complex function in Ca handling and regulating SR Ca release channels. The functional importance of Casq2 interactions with other SR proteins and the importance of alterations in Casq2 trafficking are also being investigated. Reports of altered Casq2 trafficking in animal models of acquired heart diseases such as heart failure suggest that Casq2 may contribute to arrhythmia risk beyond genetic forms of Casq2 dysfunction.
Keywords: catecholaminergic polymorphic ventricular tachycardia, cardiac arrhythmia, calcium handling
this article is part of a collection on Electrophysiology and Excitation-Contraction Coupling. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
In cardiac muscle, excitation-contraction coupling is regulated by voltage-gated Ca influx through the sarcolemmal L-type Ca channels (8). The resulting increase in the cytosolic Ca concentration triggers the opening of cardiac ryanodine receptor (RyR2) Ca release channels located in the terminal cisternae of the sarcoplasmic reticulum (jSR), the main intracellular Ca-storage compartment (Fig. 1A) (37). Tethered to RyR2 Ca release channel complex (108) are polymers of the cardiac isoform of calsequestrin, calsequestrin 2 (Casq2), which is a low-affinity high-capacity Ca-binding protein thought to provide much of the Ca released from the SR during each heart beat (Fig. 1A) (66).
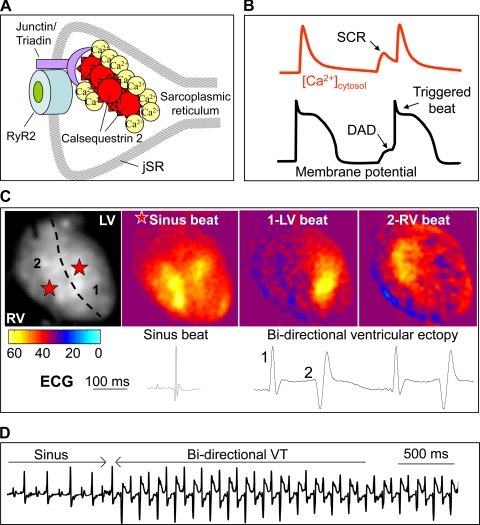
Fig. 1.
Role of the cardiac isoform of calsequestrin (Casq2) in excitation-contraction coupling and arrhythmia. A: Casq2 molecules polymerize in the junctional sarcoplasmic reticulum (jSR) and form a complex with triadin, junctin, and ryanodine receptor 2 (RyR2) Ca release channels. B: cartoon illustrating a SR spontaneous Ca release (SCR), which generates a delayed after depolarization (DAD) of the membrane potential and causes a premature triggered beat. Cardiomyocytes lacking Casq2 protein exhibit frequent spontaneous Ca release events after exposure to catecholamine stress. C: optical maps of isolated Casq2 knockout hearts demonstrate bidirectional ventricular beats that originate from the left ventricle (LV) and right ventricle (RV) in response to catecholamine stress. D: telemetry ECG recording of bidirectional ventricular tachycardia (VT) in a conscious Casq2 knockout mouse during exercise.
The released Ca binds to troponin C on the myofilaments starting the contraction. Relaxation occurs when Ca release ceases and the cytosolic Ca concentration returns to diastolic values. Ca is removed from the cytosol by three independent mechanisms: 1) reuptake of Ca into the SR via a Ca-ATPase pump (SERCA); 2) extrusion from the cell via the Na/Ca exchanger (NCX), and to a lesser degree by the sarcolemmal Ca-ATPase pump; and 3) mitochondrial uptake via the Ca uniporter. Together, these mechanisms effectively reduce the cytosolic Ca ions promoting Ca dissociation from the myofilaments (7).
Arrhythmogenic SR Ca release occurs when RyR2 Ca release channels open spontaneously without being triggered by voltage-gated Ca influx. As shown in Fig. 1B, the spontaneous SR Ca release activates the electrogenic NCX and produces a delayed afterdepolarization (DAD; Ref. 36). Premature DAD-triggered action potentials can result in life-threatening ventricular tachycardia and have been implicated as the underlying mechanism responsible for familial arrhythmia disorders caused by mutations in the genes encoding RyR2 or Casq2 (50, 75). This review focuses on Casq2 properties and physiological functions and on the role of Casq2 in the pathophysiology of genetic as well as acquired arrhythmia disorders.
Properties of Casq2 and Its Role in Ca Handling
Protein conformation and characteristics.
Calsequestrin is a glycoprotein isolated for the first time in the early 1970s from the SR of rabbit skeletal muscle (63). A few years later the cardiac-specific isoform Casq2 was identified (11). Amino acid sequencing and crystal structure studies have shed light on the conformation of this molecule: Casq2 is composed of three domains each with negatively charged thioredoxin-like folds with four α-helix surrounding a β-sheet core. The center of each domain is hydrophobic due to high aromatic acid content, whereas the exterior domain surfaces have an electronegative potential due to a large number of acidic residues. The interdomain space and connecting loops also contain many acidic residues, rendering the core of the molecule overall hydrophilic (100).
Therefore, to stabilize the molecular core, a divalent cation such as Ca is required, inducing at the same time conformational changes that further increase its Ca-binding capability (100). Formation of a Casq2 dimer creates additional negatively charged pockets that can absorb more Ca than two single monomers (100). Initially, the intermolecular binding occurs by front to front dimer formation through the N-terminal arms of Casq2. At higher Ca concentrations, back to back binding occurs through acidic C-terminal tails, producing tetramers. Salt bridges stabilize the union. Deletion of either the N terminal or C terminal of the monomers results in the incapability of the molecule to form linear polymers (5). Polymerization can also be inhibited by K ions that compete with Ca ions in binding to Casq2, but being a monovalent cation, K fails to produce cross bridges with other monomers (4, 70). Ca-induced dimerization starts at ion concentrations in the range of 0.5–1 mM, and at 3 mM Ca there is a detectable tetramer population (69). Once the polymeric state is reached ∼40 Ca ions are bound to the molecule (66).
Cellular localization and function.
Electron microscopy images of muscle fiber thin sections show that Casq2 is localized in the terminal cisternae and is connected to the jSR membrane (24). Casq2 binds to the ryanodine receptor Ca channel (RyR2) in the SR through two proteins, triadin and junctin (Fig. 1), which, extending from the junctional face of the membrane into the lumen of the SR, form a trimeric complex with Casq2 and are involved in the regulation of Ca releases (84).
Owing to its high Ca-binding capacity (40–50 mol Ca/mol Casq2) and yet low affinity to Ca (Kd = 1 mM; Refs. 19, 87), Casq2 is not only able to buffer large quantities of Ca inside the SR, but it can also readily release Ca into the cytosol upon the opening of RyR2 channels. At the same time, the free Ca level inside the SR is maintained below the inhibitory level of the SERCA (66). Considering Casq2 properties investigators have speculated that this molecule plays a central role in Ca handling. However, as shown by in vitro studies and genetic mouse models (see sections below), Casq2 not only is an important Ca-buffering protein but it also regulates RyR2 channel open probability, likely through its interaction with triadin and junctin (28, 95).
Genetic and Clinical Evidence for Casq2 in Arrhythmogenesis
Genetics.
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmogenic condition with an estimated prevalence in the population of 1 in 10,000. An autosomal-dominant form of CPVT was initially mapped to the chromosomal locus 1q42-q43 where the RYR2 gene was colocalized (93). A later study (78) confirmed that mutations in the RYR2 gene are in fact responsible for a dominant inherited CPVT phenotype and suggested that impaired Ca handling is the underlying mechanism of this disease. In the same year, an autosomic recessive form of CPVT was observed in 7 related families of a Bedouin tribe with 9 cases of sudden cardiac death and 12 other children with recurrent syncope and seizures starting at the age of 6 ± 3 yr. The disorder was mapped, once again, on chromosome 1 but this time on the short arm, 1p13–21. Compared with individuals with RYR2 mutations described by Swan et al. (93), the recessive form of CPVT in Bedouins was more severe with an earlier onset, higher penetrance, and younger age at death (54). Responsible for this severe form of CPVT was a missense mutation in an highly conserved region of the CASQ2 gene that changed aspartic acid to the positively charged histidine at position 307 of the protein (55).
Since then, 14 other mutations in the CASQ2 gene have been associated with CPVT: seven missense mutations like the D307H described by Lahat et al. (55), three single-nucleotide polymorphisms and four nonsense, deletion, or frameshift mutations that are predicted to cause a loss of CASQ2 protein in the SR (Table 1).
Table 1.
Human CASQ2 mutations
| Nucleotide Change (Reference No.) | Amino Acid Change | Mutation Type | Mutation Effect In Vitro | Zygosity | Mouse Model |
|---|---|---|---|---|---|
| 1038 G>C (53) | D307H | Missense | Impairment of Ca-buffering activity | Homozygous | Present |
| 97 C>T (75) | R33X | Nonsense | Premature stop codon with complete absence of Casq2 | Homozygous | Present |
| 532 + 1 G>A (75) | — | Splicing | Premature stop codon with complete absence of Casq2 | Homozygous | Present |
| 62delA (75) | L23fs + 14X | Deletion | Premature stop codon with complete absence of Casq2 | Homozygous | Present |
| 98 G>A (94) | R33Q | Missense | Impaired interactions of Casq2 with the RyR2 channel. Altered regulation of RyR2 by luminal Ca. Altered Casq2 polymerization | homozygous | Present |
| 339-354 del (21) | G112 + 5X | Deletion | Reduced Ca-binding properties | Homozygous | — |
| 500 T>A (21) | L167H | Missense | Reduction of SR Ca release and Ca content | Compound heterozygous | — |
| 164A>G (20) | Y55C | Missense | Cumulative effect with other mutations | Compound heterozygous | — |
| 923C>T (20) | P308L | Missense | Likely reduction of Ca-binding capacity or its interaction with RYR2 | Compound heterozygous | — |
| (61) | F189L | Missense | — | Heterozygous | — |
| 529G>C (105) | E177Q | Missense | Altered Ca-binding capacity of Casq2 domain II | Heterozygous | — |
| 196A>G (105) | T66A | SNP | no change | — | — |
| 226G>A (105) | V76M | SNP | Reduced Ca-binding, altered Casq2 polymerization | — | — |
| 1185 C>T (105) | D395D | SNP | — | — | — |
| 618 A>C (46) | K206N | Missense | Altered Ca-binding capacity and aggregation state | Heterozygous | — |
Casq2, cardiac isoform of calsequestrin; SNP, single-nucleotide polymorphism; SR, sarcoplasmic reticulum.
Since CASQ2-linked CPVT accounts for 3–5% of all CPVT cases (1), one can estimate the overall prevalence of these homozygous Casq2 mutations in the general population to be between 1:400,000 and 1:200,000 [similar to the prevalence of aortic arch interruption (106) and ∼20 times lower than LQT syndrome (83)]. The frequency of heterozygous mutation carriers is much higher (1:20,000) and may contribute as added risk in combination with other disorders that cause RyR2 dysfunction (e.g., heart failure).
Despite the many different CASQ2 mutations leading to CPVT, the clinical presentation of these patients appears to be more homogeneous than that of RYR2-linked CPVT. A possible explanation is provided by studies in knockin mice (discussed below), which showed that all mutations lead to either severe reduction or complete loss of CASQ2 protein (80, 88).
Clinical features.
Subjects affected by CPVT experience life-threatening ventricular arrhythmia induced by emotional or physical stress in the absence of any structural heart disease. These patients are frequently diagnosed at a young age (9 ± 4 yr) following single or recurrent syncope and/or seizures (75). Other symptoms are light-headedness, dizziness, and palpitations (56). The age at the onset of the first symptoms may vary, and it appears to be related to the severity of the phenotype, with early onset being associated with worse long-term prognosis. The diagnosis of CPVT is not easy, as the physical examination is negative and the resting ECG is normal with a QT interval in the physiologic range. A slight bradycardia is often the only finding observed in these subjects (76). However, that is not specific and CPVT should be suspected in individuals with a personal and/or family history of syncope, seizures, and unexplained sudden cardiac deaths triggered by physical or emotionally stressful activity. The diagnosis can be made with 24-h Holter ECG recording or exercise stress test (67). During exercise, patients develop sporadic premature ventricular beats that turn into bigeminal patterns and eventually ventricular tachycardia with higher heart rates (56, 77). A typical ECG finding during exercise or acute emotional stress is bidirectional ventricular tachycardia (BVT), characterized by a beat-to-beat 180° rotation of the QRS complex (Fig. 1D). However, it must be noted that BVT is not pathognomonic of CPVT, because BVT also occurs in acquired conditions such as digitalis toxicity, ischemic heart disease, and myocarditis (10, 27, 89).
Treatment.
Early identification and treatment of CPVT patients are very important because of the high mortality of untreated subjects by the time they reach 20 to 30 yr of age (32). The cornerstone of pharmacological treatment of CPVT are β-adrenergic receptor blockers (76). Sequential exercise tests are used to assess the dose and to monitor the efficacy of the treatment. Ca channel blockers, such as verapamil can be used as a second-line treatment in place of a β-blocker or in addition to it in patients who are still symptomatic with the maximum tolerated dose of β-blockers (81, 92). Unfortunately β-blockers and Ca channel blockers are not effective in all the patients, and implantable cardioverter defibrillators (ICD) are frequently used as an additional measure to prevent sudden death. However, ICDs are not always protective because defibrillation shocks can cause catecholamine release and electrical storm, leading to more arrhythmic events; episodes of sudden cardiac deaths in CPVT patients with ICDs have been reported (65, 73, 76). Hence, the need for new pharmacological approaches to CPVT led to the recent observation that the class I antiarrhythmic drug flecainide effectively prevents exercise-induced CPVT in transgenic mice and patients (101), probably because of a dual effect of the drug on Na channels and RyR2 channels that reduces SR spontaneous Ca release and triggered beats (25, 59). In 2011, a clinical case series (97) of 33 CPVT patients unresponsive to conventional drug therapy showed additional antiarrhythmic benefit from adding flecainide to β-blockers. Propafenone, another class I antiarrhythmic medication that also inhibits RyR2 channels, was proposed as a possible alternative drug to flecainide after treatment of a 22-yr-old subject with CPVT drastically reduced ICD shocks during a 12-mo observation period (35). Most recently, the β-blocker carvedilol was found to also inhibit RyR2 channels and was superior to other β-blockers in a transgenic mouse model of CPVT (109). Compounds such as S107 and JTV519 reportedly increase the binding of the modulatory protein calstabin2 to the RyR channels and prevent SR Ca leak in experimental models (57, 58), although JTV519 was ineffective in vivo in a CPVT mouse model (85). Nevertheless, despite these promising results, drug efficacy in CPVT is currently based only on observational studies, hence the need for prospective clinical trials (48). An S107 derivative is currently in early phase clinical trials for CPVT in Europe. A placebo-controlled prospective trial of flecainide in CPVT is open for enrolment in the U.S. and Europe (ClinicalTrials.gov: NCT01117454).
Experimental Evidence from Casq2 Mouse Models
Casq2 gene-targeted mouse models of CPVT.
In recent years, studies in transgenic mouse models of CASQ2-linked CPVT have clarified the pathophysiological mechanisms underlying the ventricular arrhythmia as well as identified new therapeutic approaches. In contrast to mouse models of RYR2-linked CPVT, all Casq2 mutant mouse models consistently reproduce the major aspects of human CPVT: exercise and/or emotional stress-induced polymorphic ventricular tachycardia, sinus bradycardia, normal contractility, and normal ECG parameters (76). Table 2 summarizes key findings from the five mouse models currently available: Casq2 homozygous knockout mice (50), Casq2 heterozygous knockout mice (14), Casq2D307H, Casq2ΔE9 (88), and Casq2R33Q knockin mice (80). Interestingly, all the mutations studied in knockin mice result in either complete loss or severe reduction of Casq2 protein in the jSR. For example, Casq2ΔE9 is a frame shift mutation that causes a premature stop codon that leads to a truncated, nonfunctional mRNA and loss of Casq2 protein. On the other hand, Casq2D307H knockin mice have normal mRNA levels. However, mutant Casq2D307H protein is almost completely absent (>95% reduction) in the hearts of adult mice, suggesting that the D307H mutation causes enhanced protein degradation or impairs cellular trafficking in vivo (49). In vitro (34) studies suggest that the single amino acid change between the second and third domain of the molecule disrupts the formation of properly oriented dimers and impairs the interactions with triadin and junctin, either of which might contribute to reduced retention of the mature protein in the jSR. Similarly, in Casq2R33Q knockin mice, the mutant protein reaches full maturation but does not form Ca-dependent polymers at physiologic Ca concentrations (5, 45). The defective Casq2R33Q molecule is more susceptible to proteolysis (80), which may explain the drastically reduced levels of mutant Casq2 protein found in this model (Table 2).
Table 2.
Mouse models of Casq2
| Mouse Model (Reference No.) | In Vivo Phenotype | Cellular Phenotype | Casq2 Protein Expression |
|---|---|---|---|
| Homozygous knockout (50) | Slight increase in heart weight and left-ventricular wall thickness with normal contractile function: severe CPVT phenotype. | Near absence of the Casq2-binding proteins triadin-1 and junctin. Increased SR volume with preserved Ca content. Normal Ca-induced SR Ca releases, increased spontaneous SR Ca release during catecholaminergic stress. | Absent |
| Heterozygous knockout (14) | Normal cardiac morphology. Mild CPVT phenotype with 3-fold higher rates of ventricular ectopy under stress compared with wild-type littermates. | No significant change in the expression of other SR protein. Field-stimulated Ca transients, cell shortening, L-type Ca current, and SR volume not significantly different compared with wild-type myocytes. Increased SR Ca leak at the same free intra-SR Ca concentrations. | 25% reduction |
| Homozygous knockin (D309 ΔE9) (88) | Structurally normal hearts at young age with stress-induced ventricular arrhythmias; aging produces cardiac hypertrophy and reduced contractile function. | Reduced total SR Ca load. Increased calreticulin and RyR2 levels. Prolonged Ca release, and delayed SR Ca re-uptake. Stress-induced diastolic Ca release. | 95% reduction |
| Homozygous knockin (R33Q) (80) | Structurally normal hearts. Ventricular ectopy at rest and on exposure to environmental stress. | Unchanged SR volume. Significant reduction of triadin and junctin. Reduced SR Ca content. Adrenergically induced delayed (DADs) and early (EADs) afterdepolarizations leading to triggered activity. | >50% reduction |
| Casq2 overexpression (38) | Severe cardiac hypertrophy, with a 2-fold increase in heart mass and cell size. | Downregulation of RyR2, triadin, and junctin. Normal levels of Ca-ATPase and phospholamban. Severe reduction of Ca-mediated SR Ca releases and frequency of spontaneous Ca sparks. | 10-fold increase |
| Casq2 overexpression (82) | Increased heart-to-body weight ratio. Mild hypertrophy in the left ventricular free walls and intraventricular septa, with depressed rates of contraction and relaxation. | Normal levels of RyR2, triadin and junctin. Increased expression of SR Ca-ATPase, phospholamban, and calreticulin. Increased SR Ca storage capacity but reduced Ca-induced Ca releases and Ca transient amplitude (45%) and reduced L-type Ca density. | 20-fold increase |
| Casq2D307H overexpression in wild-type background (23) | Structurally normal hearts. Mild arrhythmogenic phenotype: few spontaneous ventricular arrhythmias at rest and nonsustained polymorphic ventricular tachycardia during stress | Normal levels of RyR2, triadin, SERCA, and L-type Ca channel. Diminished Ca-induced Ca transient amplitude and duration. Increased Ca spark frequency. | 2- to 6-fold increase |
| Casq2D307H overexpression in Casq2 knockout background (39, 40) | Structurally normal hearts. Partial rescue of Casq2 knockout CPVT phenotype. | Expression of mutant Casq2 targeted to junctional SR. Partial recovery of SR ultrastructure compared with Casq2 knockout mice (e.g., terminal cisternae contain Casq2D307H and triadin levels are partially restored). Normal levels of calreticulin and SERCA. Altered protein conformation and increased proteolysis compared with wild type. | 2-fold increase |
RyR2, ryanodine receptor 2; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; CPVT, catecholaminergic polymorphic ventricular tachycardia.
Casq2 overexpression mouse models of CPVT.
A different approach that gives further insight on the role of Casq2 in CPVT comes from transgenic overexpression of Casq2 carrying the CPVT-linked D307H point mutation using an α-myosin heavy chain promoter either in wild-type or Casq2 knockout mice. In the first report, a chronic increase (from 2- to 6-fold) of mutant Casq2D307H protein in wild-type mice that also have wild-type Casq2 protein produces intracellular changes and in vivo phenotype of catecholamine-induced ventricular arrhythmia (23). This seems to suggest a negative dominant behavior of this mutated protein but is at odds with the human data showing that heterozygous carriers of the D307H mutant are asymptomatic. An explanation may be provided by results obtained by overexpressing Casq2D307H in Casq2 knockout mice that lack Casq2 protein, which model the situation in homozygous human carriers of the D307H mutation. Contrary to the complete loss of mature protein observed in the D307H knockin mice, in this transgenic overexpression model Casq2D307H protein is present at approximately twofold higher concentration than wild-type Casq2 found in nontransgenic littermates. This result suggests that a strong transgenic promoter (α-myosin heavy chain) may be sufficient to overcome the increased protein destruction rate. The mature Casq2D307H displays a folding pattern similar to wild-type Casq2 protein and is also successfully targeted to the jSR cisternae (39). Surprisingly, when expressed at such high levels, the mutant Casq2D307H not only restores the SR ultrastructure compromised in the Casq2 knockout mice but also prevents spontaneous Ca release events in myocytes and almost completely rescues the CPVT phenotype of Casq2 knockout mice (40). Taken together, these results are consistent with the emerging paradigm that in homozygous carriers of Casq2 point mutations, the severe reduction or complete loss of Casq2 is due to altered trafficking and/or increased protein degradation and is the primary underlying mechanism responsible for the CPVT phenotype. In heterozygous carriers, enough wild-type Casq2 is present to compensate for the high degradation rate of the mutant Casq2 mRNA or protein; hence heterozygous individuals are asymptomatic. These results also suggest that the dominant-negative effects of the mutant Casq2 protein observed in overexpression experiments, using mice or isolated myocytes, may not be relevant for the pathogenesis of human CASQ2-linked CPVT. However, this conclusion has been recently challenged by a study of induced pluripotent stem cells derived from a homozygous carrier of the CASQ2-D307H mutation, which showed that mutant D307H protein was indeed present in induced pluripotent stem cell-derived cardiomyocytes (68). Yet, it is still not known if mutant D307H protein is present in the human heart. Thus the definitive answer can only be provided by a cardiac muscle biopsy from a patient with the D307H mutation. Until one becomes available, the exact role of mutant Casq2-D307H protein in the pathogenesis of CPVT remains speculation.
How Does Casq2 Dysfunction Cause Ventricular Arrhythmia?
Cellular arrhythmia mechanisms.
Cardiac contractility and systolic Ca releases from the SR appear normal in Casq2 mutant mice at rest. The higher arrhythmogenic risk during stress can be attributed to spontaneous Ca releases from the SR that occur during diastole. The rise in diastolic Ca concentration activates the Na/Ca exchanger, causing an inward Na current that can result in DAD of the cell membrane and triggered activity (Fig. 1B). Although all cardiomyocytes have the potential to generate a DAD, it has been proposed that DADs occurring in the Purkinje fibers (PF) of the specialized conduction system are the source of the triggered ventricular arrhythmia in CPVT. PF have several characteristics that make them particularly prone for spontaneous Ca releases: decreased T-tubular density (22), the presence of inositol 1,4,5-trisphosphate-sensitive Ca2+ channels (33), and susceptibility to Ca2+ overload (60, 98). An indirect support to this hypothesis is the observation that the drug flecainide, which is effective clinically in CPVT, preferentially suppresses arrhythmogenic Ca waves in isolated PF compared with ventricular myocytes (42).
The PF as the cellular origin of CPVT is supported by experimental evidence in vivo showing conversion of bidirectional VT to monomorphic VT in RyR2 mutant mice after chemical ablation of the right ventricular branch of the conduction system (12). Furthermore, a recent computer model of CPVT reproduced the characteristic ECG pattern of bidirectional VT with a simple “ping pong” mechanism of reciprocating bigeminy between the right and left branches of the conduction system (3). However, based on the computer model, the origin of the bidirectional VT is not restricted to the bundle branches and could also originate from more distal PF or the working myocardium. This is illustrated by recent data from our group, where isolated Casq2 knockout hearts exhibit alternating ventricular activation that originated from the right and left ventricle at sites distinct from the bundle breakthrough points during normal sinus rhythm (Fig. 1C). The resulting bidirectional QRS pattern on the ECG record of the isolated heart (Fig. 1C) was similar to that recorded in vivo using telemetry (Fig. 1D). Thus while the PF are the likely culprit, the exact cellular origin of the bidirectional ventricular arrhythmias remains unknown and is an area of active investigation.
Theoretically, the increased rate of spontaneous Ca releases in Casq2 knockout hearts could produce stochastic membrane DADs that can induce intercellular discordant electrical alternans: when neighboring groups of cells are electrically uncoupled, different repolarization gradients are created in adjacent areas of the heart and they can be a substrate for reentry especially at elevated heart rates (71, 104). However, we (51) recently demonstrated that Casq2 knockout hearts are protected against tachycardia-induced ECG T-wave alternans, which is a marker for susceptibility to reentrant ventricular arrhythmia in animal models and in clinical studies (99). Thus the results from the Casq2 knockout mice suggest that loss of Casq2 causes an increased risk for triggered arrhythmia but may paradoxically protect against reentrant-type arrhythmia.
Subcellular and molecular arrhythmia mechanisms.
At the subcellular level, we propose at least three independent mechanisms that can explain how reduced or dysfunctional calsequestrin causes the spontaneous SR Ca release events that can trigger ventricular arrhythmia: 1) loss of Ca buffering in the jSR, 2) loss of RyR2 regulation by Casq2, and 3) remodeling of SR structure and proteins. These three mechanisms are discussed in more detail next.
loss of ca buffering in the jSR.
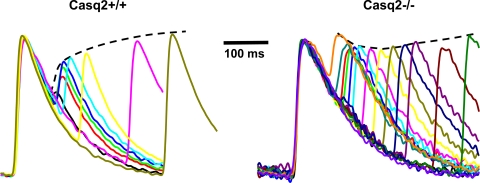
Casq2 is the main Ca-buffering protein of the SR. Both acute as well as chronic overexpression of Casq2 causes a large increase in the SR Ca content (38, 82). Surprisingly, Casq2 homozygous knockout mice display a preserved SR Ca content. Studies on isolated myocytes show that loss of Casq2 Ca-buffering activity was compensated for by a significant (∼50%) increase in the SR volume (50). Although the total SR luminal Ca content seems to be preserved, a recent report shows that the kinetics of free Ca in the lumen of the SR are different in Casq2 knockout mice. Loss of Casq2-mediated Ca buffering causes a faster rise in luminal free Ca after each beat (51). The resulting faster rise of Ca near the pore of the RyR2 Ca channels can be expected to have two effects: 1) an increased releasable amount of Ca early during the cardiac cycle, and 2) a faster recovery from SR release refractoriness. Both factors would promote accelerated recovery of SR Ca release, which has recently been confirmed in experiments with intact hearts from Casq2 null mice (51), as shown in Fig. 2. Another consequence of the faster recovery of Ca release in hearts lacking Casq2 is a protection against tachycardia-induced Ca transient alternans and T-wave alternans on ECG, which was significantly reduced in Casq2 knockout compared with wild-type hearts (51). Thus Casq2 likely plays a key role in the genesis of Ca transient alternans and T-wave alternans.
Fig. 2.
Casq2 regulates Ca release refractoriness. In wild-type mouse hearts (Casq2+/+), Ca release is strongly suppressed for very premature beats. This phenomenon is known as Ca release refractoriness. Ca release refractoriness is almost completely lost in Casq2 knockout hearts (Casq2−/−). Ca fluorescent records are from the epicardial surface of isolated perfused hearts loaded with rhod-2 AM at 37 °C (51).
loss of RyR2 regulation by casq2.
Regulation of the RyR2 channel permeability appears to be very complex. Both cytosolic and SR luminal free Ca concentration affect the open probability of this channel and influence the duration of the local Ca releases from the SR (52). A large body of evidence supports the concept that SR Ca leak rate is proportional to SR luminal Ca concentration in a nonlinear fashion (62, 86). Loss of Casq2 in Casq2 knockout myocytes makes this relationship much steeper (Fig. 3): in other words, in the absence of Casq2, the SR Ca load threshold at which spontaneous Ca release happens is much lower, increasing the overall probability of these events. The same phenomenon is observed in heterozygous Casq2 knockout mice despite the small reduction (∼25%) of protein levels in this model and no change in free Ca concentrations in the SR (14, 15). Measurements of free Ca concentrations in the SR lumen of intact hearts lacking Casq2 show that larger SR Ca release can be triggered at the same free intra-SR Ca concentration (51). This result further demonstrates a direct inhibitory role of Casq2 on SR Ca release, which is independent of Casq2's action as a Ca chelator. The direct inhibitory action of Casq2 on RyR2 is also supported by single RyR2 channel experiments in artificial lipid bilayers (79), and results from studies (38) in cardiomyocytes overexpressing Casq2 that show a significant reduction of both Ca-induced and spontaneous Ca release despite the much higher total SR Ca pool available.
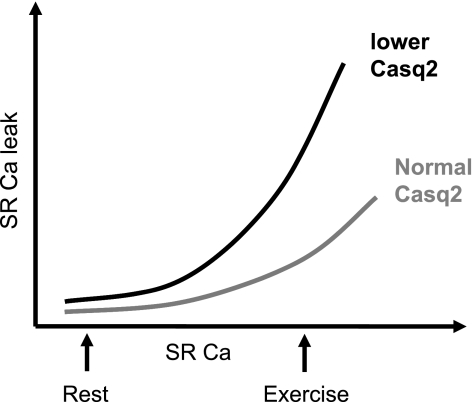
Fig. 3.
Relationship between SR Ca load and SR Ca leak: during a physical or emotional stress SR Ca load is increased and spontaneous SR Ca leak occurs more often, displaying a proportional, although nonlinear, relationship with luminal SR [Ca]. In the presence of reduced levels of Casq2, this relationship is steeper with increased Ca leaks for a given SR [Ca] compared with control levels of Casq2.
Controversy remains as to whether lack of Casq2 lowers the threshold for luminal or cytosolic activation of Ca release from the RyR2 channel complex (91). In addition, it is uncertain whether this loss of RyR2 regulation is attributable to reduction of Casq2 itself or of the proteins mediating Casq2 binding to RyR2 channels (28). Regardless of the exact mechanism, the net effect is an increased probability of spontaneous Ca release for any given luminal free Ca concentration (Fig. 3).
In this context, any catecholaminergic stress puts further strain on the intracellular Ca handling in CPVT patients. The protein kinase A-mediated effects of the β-adrenergic receptor stimulation lead to an increase in SR Ca load and spontaneous SR releases (43).
remodeling of SR proteins and SR ultrastructure.
Almost all Casq2 mutant mouse models studied to date display a significant reduction of the Casq2 binding proteins triadin and junctin (Table 2). Downregulation of triadin and junctin has also been reported in a mouse model of Casq2 overexpression, indicating a possible interdependence of Casq2 and its binding protein expression both in physiologic and pathologic conditions (38). Moreover, gene-targeted deletion of triadin or junctin also generates an arrhythmogenic phenotype in the absence of Casq2 mutations (17, 107). Loss of triadin leads to profound structural modification of the SR (50% reduction of RyR2, Casq2, and junctin) and of the terminal cisternae (up to 50% reduction in the contacts between jSR and t tubules) causing impaired excitation-contraction coupling (17). On the other hand, SR protein expression is not altered in junctin knockout mice although junctin ablation is associated with altered Ca-cycling parameters, and isoproterenol induced DADs (107). Taken together, these findings suggest that the reduction of triadin and junctin may independently contribute to the arrhythmia risk associated with Casq2 mutations (47). Increased protein expression of RyR2, calreticulin, and histidine-rich Ca-binding protein has also been reported in some but not in all of the mouse models (29, 66, 88). However, the relevance of these protein expression changes for the pathogenesis of CPVT remains to be determined. Casq2 knockout mice also compensate for the loss of Casq2 Ca buffering by an increase in SR volume but without significant changes in the jSR cisternae and dyad morphology (50). To what extent the associated changes in SR ultrastructure (e.g., SR volume increase in Casq2 null mice; Ref. 50) contribute to the arrhythmogenic Ca release is not known.
Potential Role of Casq2 in Acquired Arrhythmias
So far, we described the role of Casq2 in CPVT, an inherited arrhythmogenic condition caused by mutations in the Casq2 gene. However, secondary Casq2 dysfunction may contribute to an increased arrhythmia risk in other inherited and acquired cardiac diseases characterized by Ca-handling abnormalities: heart failure, muscular dystrophy, mitochondrial dysfunctions, and drug cardiotoxicity.
Casq2 in heart failure.
Heart failure is associated with profound alterations in intracellular Ca handling (9, 102). One consistent finding is an increased SR Ca leak probably facilitated by increased open probability of RyR2 Ca release channels, which, similar to CPVT, increases the risk for spontaneous Ca release under conditions of β-adrenergic stimulation (9, 102). Once spontaneous Ca releases occur, a DAD might be triggered. However, due to an upregulation of the Na/Ca exchanger in failing myocytes, any rise in intracellular Ca results in a greater arrhythmogenic inward current, reducing the quantity of Ca release required to trigger a DAD (74). Oxidative, PKA and/or CaMKII-dependent modifications of the RyR2 channels during the disease progression have also been implicated in the arrhythmogenic potential of failing hearts by reducing the ability of the RyR2 channels to become refractory after a Ca release (6, 102). Interestingly, Casq2 protein levels are usually unchanged in cardiac tissue from humans and animal models of heart failure. However, investigators have identified posttranscriptional modifications of the Casq2 protein in pacing-induced heart failure models, with a significant change in the mannose content of glycans in Casq2 molecules compared with controls. The structure of the glycan residues observed in this model differs from the one expected in the jSR of healthy cells, suggesting a possible alteration in the metabolism or in the trafficking of Casq2 protein through the secretory compartments (44). These results suggest that Casq2 might be altered by posttranscriptional modifications and never reaches its subcellular target in the jSR (64). Consistent with this hypothesis, mass spectrometry analysis of Casq2 suggests that higher amounts of Casq2 protein may be retained in the rough ER of the perinuclear cisternae of cardiac myocytes during disease states such as heart failure (44, 64). Furthermore, the reduction of triadin and junctin levels, observed in failing hearts, might also play a role in a subcellular redistribution of Casq2 in the myocyte (8, 26). Taken together, these results raise the intriguing possibility that in failing hearts, even in the presence of a normal intracellular concentration of Casq2, the protein may be differently distributed in the cellular organelles, with reduced levels in the proximity of the RyR2, where Casq2 plays its pivotal role in the regulation of RyR2-mediated Ca releases and SR Ca release refractoriness (51).
Casq2 in muscular dystrophy.
Duchenne (DMD) and Becker's muscular dystrophy are X-linked inherited diseases resulting from mutations in the dystrophin gene. They are characterized by high susceptibility to contraction-induced muscle damage that leads to severe progressive muscle deterioration (18, 96). Although the skeletal muscle is primarily affected, cardiac involvement is frequently reported especially in subjects with DMD (96). Dilated cardiomyopathy with extensive fibrosis is usually diagnosed during the second decade of life (90). Mouse models reproducing the human phenotype of DMD display impaired Ca handling mostly due to reduction of two proteins: CaM and Casq2. Before any histological sign of cardiomyopathy can be detected in these mice, SR protein levels are still in the physiological range. As the condition progresses, CaM and Casq2 are downregulated with a net loss of Ca buffering activity. Investigators (72) have suggested that the consequent rise in the intracellular free Ca levels could activate proteases and profibrotic signals promoting structural remodeling.
Casq2 in mitochondrial dysfunction.
Although the precise pathophysiological mechanism is still not clear, subjects with mitochondrial alterations can develop cardiomyopathy with an abnormal Ca-handling function. Impairment in the fatty acid oxidation pathway, as observed in individuals with mutations in the very long-chain acyl-CoA dehydrogenase gene, puts individuals at high risk for cardiac disease and sudden death. Mice with the same mutation display increased incidence of polymorphic VT during isoproterenol challenge. The L-type Ca current characteristics were not different compared with controls but the SR function was altered with an increase in the SR Ca load (up to 48%) and Ca transients amplitude, similar to the mouse models of Casq2 overexpression. Protein assays of the very long-chain acyl-CoA dehydrogenase mutant mice show an upregulation of RyR2, Casq2, and phospholamban (103).
Mitochondrial dysfunction can also be observed during oxidative stress. Mouse models of reactive oxygen species-induced mitochondrial damage have been obtained by administration of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, an uncoupler of oxidative phosphorylation. This oxidative damage eventually leads to a mitochondrial cardiomyopathy associated with a reduction of Casq2 protein levels and alterations in the SR function. Coexposure of the myocytes to both carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone and a reactive oxygen species scavenger such as N-acetylcystein attenuates the Casq2 downregulation and improves Ca handling (31).
Casq2 as target for drug binding.
Cardiotoxicity can be a serious side effect of commonly used drugs such as tricyclic antidepressants, antipsychotic drugs, and anthracyclins. In vitro studies (41) suggest that many of these drugs bind to Casq2 and reduce its Ca-buffering capacity. One of the first experimental evidence suggesting an involvement of Ca-handling abnormalities in the pathogenesis of the drug-induced cardiac damage comes from a rabbit model of doxorubicin-induced cardiomyopathy. After 8 wk of chronic treatment, these animals have reduced levels of SR proteins including RyR2 and Casq2 in the presence of normal levels of Ca ATPase and Na/Ca exchanger (2). In support of this hypothesis is the recent observation that acute administration of anthracyclines causes a severe reduction in SR Ca releases most likely through an interaction with Casq2 and oxidation of RyR2 (13, 30).
The tricyclic antidepressant amitriptyline binds to Casq2 at low micromolar concentrations and causes increased spontaneous Ca releases and consequent depletion of the SR Ca content. However, the contribution of drug binding to Casq2 is probably minimal, since the RyR2 hyperactivity results from direct interaction with the RyR2 channel and can be reproduced in myocytes lacking Casq2 (16, 110). Whether amitriptyline binding to Casq2 contributes to cardiotoxicity during long-term exposure is yet to be determined.
Conclusions and Future Developments
The last decade brought a huge progress in our understanding of Casq2-linked CPVT. Basic and translational research on CPVT mouse models have provided information about the intracellular Ca handling and have identified potential drugs for CPVT treatment that have recently been tested in patients with promising results. However, further investigations are needed to clarify the complex interactions among SR proteins that play a central role not only in inherited but also in acquired arrhythmogenic conditions. Future studies will also focus on how cardiac cells subtypes (Purkinje cells and ventricular myocytes in particular) are affected by impaired Ca handling and new treatments developed that specifically target the cell type responsible for CPVT initiation. Finally, the contribution of Casq2 alterations (e.g., altered protein expression levels, genetic variants, and haploinsufficiency in heterozygous carriers of Casq2-null mutations) to arrhythmia risk in ischemic heart disease and/or heart failure will be explored.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants H-L88635 and HL-71670 (to B. C. Knollmann), American Heart Association Established Investigator Award 0840071N (to B. C. Knollmann), and the Heart Rhythm Society Fellowship Award (to M. Faggioni).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, LeMarec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8: 1308–1339, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Arai M, Tomaru K, Takizawa T, Sekiguchi K, Yokoyama T, Suzuki T, Nagai R. Sarcoplasmic reticulum genes are selectively downregulated in cardiomyopathy produced by doxorubicin in rabbits. J Mol Cell Cardiol 30: 243–254, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Baher AA, Uy M, Xie F, Garfinkel A, Qu Z, Weiss JN. Bidirectional ventricular tachycardia: ping pong in the His-Purkinje system. Heart Rhythm 8: 599–605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bal NC, Jena N, Sopariwala D, Balaraju T, Shaikh S, Bal C, Sharon A, Gyorke S, Periasamy M. Probing cationic selectivity of cardiac calsequestrin and its CPVT mutants. Biochem J 435: 391–399, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Bal NC, Sharon A, Gupta SC, Jena N, Shaikh S, Gyorke S, Periasamy M. The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization. J Biol Chem 285: 17188–17196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Gyorke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res 90: 493–502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37: 417–429, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res 93: 487–490, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Berte B, Eyskens B, Meyfroidt G, Willems R. Bidirectional ventricular tachycardia in fulminant myocarditis. Europace 10: 767–768, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Campbell KP, MacLennan DH, Jorgensen AO, Mintzer MC. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem 258: 1197–1204, 1983 [PubMed] [Google Scholar]
- 12. Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O'Connell R, Berenfeld O, Anumonwo J, Pandit SV, Vikstrom K, Napolitano C, Priori SG, Jalife J. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res 101: 1039–1048, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charlier HA, Jr, Olson RD, Thornock CM, Mercer WK, Olson DR, Broyles TS, Muhlestein DJ, Larson CL, Cusack BJ, Shadle SE. Investigations of calsequestrin as a target for anthracyclines: comparison of functional effects of daunorubicin, daunorubicinol, and trifluoperazine. Mol Pharmacol 67: 1505–1512, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res 101: 617–626, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Chopra N, Knollmann BC. Cardiac calsequestrin: the new kid on the block in arrhythmias. J Cardiovasc Electrophysiol 20: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Chopra N, Laver D, Davies SS, Knollmann BC. Amitriptyline activates cardiac ryanodine channels and causes spontaneous sarcoplasmic reticulum calcium release. Mol Pharmacol 75: 183–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, Jones LR, Pessah IN, Allen PD, Franzini-Armstrong C, Knollmann BC. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci USA 106: 7636–7641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM, Wilkinson JD, Orav EJ, Cuniberti L, Salbert BA, Lipshultz SE. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J 155: 998–1005, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cozens B, Reithmeier RA. Size and shape of rabbit skeletal muscle calsequestrin. J Biol Chem 259: 6248–6252, 1984 [PubMed] [Google Scholar]
- 20. de la Fuente S, Van Langen IM, Postma AV, Bikker H, Meijer A. A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin Electrophysiol 31: 916–919, 2008 [DOI] [PubMed] [Google Scholar]
- 21. di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation 114: 1012–1019, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Di Maio A, Ter Keurs HE, Franzini-Armstrong C. T-tubule profiles in Purkinje fibres of mammalian myocardium. J Muscle Res Cell Motil 28: 115–121, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Dirksen WP, Lacombe VA, Chi M, Kalyanasundaram A, Viatchenko-Karpinski S, Terentyev D, Zhou Z, Vedamoorthyrao S, Li N, Chiamvimonvat N, Carnes CA, Franzini-Armstrong C, Gyorke S, Periasamy M. A mutation in calsequestrin, CASQ2D307H, impairs sarcoplasmic reticulum Ca2+ handling and causes complex ventricular arrhythmias in mice. Cardiovasc Res 75: 69–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franzini-Armstrong C, Kenney LJ,. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol 105: 49–56, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galimberti ES, Knollmann BC. Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca(2+) waves in permeabilized myocytes lacking calsequestrin. J Mol Cell Cardiol 51: 760–768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gergs U, Berndt T, Buskase J, Jones LR, Kirchhefer U, Muller FU, Schluter KD, Schmitz W, Neumann J. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am J Physiol Heart Circ Physiol 293: H728–H734, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Grimard C, De Labriolle A, Charbonnier B, Babuty D. Bidirectional ventricular tachycardia resulting from digoxin toxicity. J Cardiovasc Electrophysiol 16: 807–808, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86: 2121–2128, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han P, Cai W, Wang Y, Lam CK, Arvanitis DA, Singh V, Chen S, Zhang H, Zhang R, Cheng H, Kranias EG. Catecholaminergic induced arrhythmias in failing cardiomyocytes associated with human HRCS96A variant overexpression. Am J Physiol Heart Circ Physiol 301: H1588–H1595, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanna AD, Janczura M, Cho E, Dulhunty AF, Beard NA. Multiple actions of the anthracycline daunorubicin on cardiac ryanodine receptors. Mol Pharmacol 80: 538–549, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Hanninen SL, Ronkainen JJ, Leskinen H, Tavi P. Mitochondrial uncoupling downregulates calsequestrin expression and reduces SR Ca2+ stores in cardiomyocytes. Cardiovasc Res 88: 75–82, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 119: 2426–2434, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Hirose M, Stuyvers B, Dun W, Ter Keurs H, Boyden PA. Wide long lasting perinuclear Ca2+ release events generated by an interaction between ryanodine and IP3 receptors in canine Purkinje cells. J Mol Cell Cardiol 45: 176–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Houle TD, Ram ML, Cala SE. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc Res 64: 227–233, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Hwang H, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, Yin H, Knollmann BC. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 4: 128–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iyer V, Hajjar RJ, Armoundas AA. Mechanisms of abnormal calcium homeostasis in mutations responsible for catecholaminergic polymorphic ventricular tachycardia. Circ Res 100: e22–31, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Jones LR, Cala SE. Biochemical evidence for functional heterogeneity of cardiac sarcoplasmic reticulum vesicles. J Biol Chem 256: 11809–11818, 1981 [PubMed] [Google Scholar]
- 38. Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest 101: 1385–1393, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalyanasundaram A, Bal NC, Franzini-Armstrong C, Knollmann BC, Periasamy M. The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering. J Biol Chem 285: 3076–3083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalyanasundaram A, Viatchenko-Karpinski S, Belevych A, Lacombe VA, Hwang HS, Knollmann BC, Gyorke S, Periasamy M. Functional consequences of stably expressing a mutant calsequestrin (Casq2d307h) in the Casq2 null background. Am J Physiol Heart Circ Physiol 302: H253–H261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kang C, Nissen MS, Sanchez EJ, Lam KS, Milting H. Potential adverse interaction of human cardiac calsequestrin. Eur J Pharmacol 646: 12–21, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Kang G, Giovannone SF, Liu N, Liu FY, Zhang J, Priori SG, Fishman GI. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res 107: 512–519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kashimura TBS, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the RyR2(R4496C) mouse model of CPVT, β-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res 107: 1483–1489, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Kiarash A, Kelly CE, Phinney BS, Valdivia HH, Abrams J, Cala SE. Defective glycosylation of calsequestrin in heart failure. Cardiovasc Res 63: 264–272, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Kim E, Youn B, Kemper L, Campbell C, Milting H, Varsanyi M, Kang C. Characterization of human cardiac calsequestrin and its deleterious mutants. J Mol Biol 373: 1047–1057, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Kirchhefer U, Wehrmeister D, Postma AV, Pohlentz G, Mormann M, Kucerova D, Muller FU, Schmitz W, Schulze-Bahr E, Wilde AA, Neumann J. The human CASQ2 mutation K206N is associated with hyperglycosylation and altered cellular calcium handling. J Mol Cell Cardiol 49: 95–105, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol 587: 3081–3087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knollmann BC. Power and pitfalls of using transgenic mice to optimize therapy for CPVT: a need for prospective placebo-controlled clinical trials in genetic arrhythmia disorders. Heart Rhythm 7: 1683–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knollmann BC. A “rough” journey to the sarcoplasmic reticulum–implications of altered calsequestrin trafficking for cardiac arrhythmia. J Mol Cell Cardiol 49: 554–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116: 2510–2520, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kornyeyev D, Petrosky AD, Zepeda B, Ferreiro M, Knollmann B, Escobar AL. Calsequestrin 2 deletion shortens the refractoriness of Ca(2+) release and reduces rate-dependent Ca(2+)-alternans in intact mouse hearts. J Mol Cell Cardiol 52: 21–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kornyeyev D, Reyes M, Escobar AL. Luminal Ca2+ content regulates intracellular Ca2+ release in subepicardial myocytes of intact beating mouse hearts: effect of exogenous buffers. Am J Physiol Heart Circ Physiol 298: H2138–H2153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lahat H, Eldar M. Autosomal recessive catecholamine-induced polymorphic ventricular tachycardia. Isr Med Assoc J 4: 1095–1096, 2002 [PubMed] [Google Scholar]
- 54. Lahat H, Eldar M, Levy-Nissenbaum E, Bahan T, Friedman E, Khoury A, Lorber A, Kastner DL, Goldman B, Pras E. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13–21. Circulation 103: 2822–2827, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 69: 1378–1384, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 91: 1512–1519, 1995 [DOI] [PubMed] [Google Scholar]
- 57. Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118: 2230–2245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 109: 3208–3214, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Liu N, Denegri M, Ruan Y, Avelino-Cruz JE, Perissi A, Negri S, Napolitano C, Coetzee WA, Boyden PA, Priori SG. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res 109: 291–295, 2011 [DOI] [PubMed] [Google Scholar]
- 60. Liu OZ, Lederer WJ, Sobie EA. Does the Goldilocks Principle apply to calcium release restitution in heart cells? J Mol Cell Cardiol, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu QQ, Oberti C, Zhang XQ, Ke T, Zhang T, Scheinman M, Hu DY, Wang QK. [A novel mutation of F189L in CASQ2 in families with catecholaminergic polymorphic ventricular tachycardia]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 25: 334–337, 2008 [PubMed] [Google Scholar]
- 62. Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Dynamic regulation of sarcoplasmic reticulum Ca(2+) content and release by luminal Ca(2+)-sensitive leak in rat ventricular myocytes. Biophys J 81: 785–798, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci USA 68: 1231–1235, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McFarland TP, Milstein ML, Cala SE. Rough endoplasmic reticulum to junctional sarcoplasmic reticulum trafficking of calsequestrin in adult cardiomyocytes. J Mol Cell Cardiol 49: 556–564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mohamed U, Gollob MH, Gow RM, Krahn AD. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm 3: 1486–1489, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Murphy RM, Mollica JP, Beard NA, Knollmann BC, Lamb GD. Quantification of calsequestrin 2 (CSQ2) in sheep cardiac muscle and Ca2+-binding protein changes in CSQ2 knockout mice. Am J Physiol Heart Circ Physiol 300: H595–H604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 4: 675–678, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Novak A, Binah O, Itskovitz-Eldor J, Barad L, Zeevi-Levin N. Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation. J Cell Mol Med 2011. November 2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem 279: 18026–18033, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Park H, Wu S, Dunker AK, Kang C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J Biol Chem 278: 16176–16182, 2003 [DOI] [PubMed] [Google Scholar]
- 71. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394, 1999 [DOI] [PubMed] [Google Scholar]
- 72. Pertille A, de Carvalho CL, Matsumura CY, Neto HS, Marques MJ. Calcium-binding proteins in skeletal muscles of the mdx mice: potential role in the pathogenesis of Duchenne muscular dystrophy. Int J Exp Pathol 91: 63–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pizzale S, Gollob MH, Gow R, Birnie DH. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 19: 1319–1321, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res 91: e21–e26, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet 42: 863–870, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 106: 69–74, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103: 196–200, 2001 [DOI] [PubMed] [Google Scholar]
- 79. Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol 131: 325–334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori SG. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res 103: 298–306, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Rosso R, Kalman JM, Rogowski O, Diamant S, Birger A, Biner S, Belhassen B, Viskin S. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 4: 1149–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Sato Y, Ferguson DG, Sako H, Dorn GW, Kadambi VJ, II, Yatani A, Hoit BD, Walsh RA, Kranias EG. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem 273: 28470–28477, 1998 [DOI] [PubMed] [Google Scholar]
- 83. Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, Mosca F, Nespoli L, Rimini A, Rosati E, Salice P, Spazzolini C. Prevalence of the congenital long-QT syndrome. Circulation 120: 1761–1767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J 79: 2682–2691, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, Gronau P, Maier LS, Vos MA, Lai FA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res 87: 50–59, 2010 [DOI] [PubMed] [Google Scholar]
- 86. Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002 [DOI] [PubMed] [Google Scholar]
- 87. Slupsky JR, Ohnishi M, Carpenter MR, Reithmeier RA. Characterization of cardiac calsequestrin. Biochemistry 26: 6539–6544, 1987 [DOI] [PubMed] [Google Scholar]
- 88. Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 117: 1814–1823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sonmez O, Gul EE, Duman C, Duzenli MA, Tokac M, Cooper J. Type II bidirectional ventricular tachycardia in a patient with myocardial infarction. J Electrocardiol 42: 631–632, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP. Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord 18: 371–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stevens SC, Terentyev D, Kalyanasundaram A, Periasamy M, Gyorke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol 587: 4863–4872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Swan H, Laitinen P, Kontula K, Toivonen L. Calcium channel antagonism reduces exercise-induced ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia patients with RyR2 mutations. J Cardiovasc Electrophysiol 16: 162–166, 2005 [DOI] [PubMed] [Google Scholar]
- 93. Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol 34: 2035–2042, 1999 [DOI] [PubMed] [Google Scholar]
- 94. Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res 98: 1151–1158, 2006 [DOI] [PubMed] [Google Scholar]
- 95. Terentyev D, Viatchenko-Karpinski S, Vedamoorthyrao S, Oduru S, Gyorke I, Williams SC, Gyorke S. Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J Physiol 583: 71–80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Townsend D, Yasuda S, McNally E, Metzger JM. Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex. FASEB J 25: 3106–3114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, Willems AR, van der Veen MJ, Watanabe H, Laborderie J, Haissaguerre M, Knollmann BC, Wilde AA. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol 57: 2244–2254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vassalle M, Lin CI. Calcium overload and cardiac function. J Biomed Sci 11: 542–565, 2004 [DOI] [PubMed] [Google Scholar]
- 99. Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility–consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol 58: 1309–1324, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol 5: 476–483, 1998 [DOI] [PubMed] [Google Scholar]
- 101. Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med 15: 380–383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA 103: 511–518, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Werdich AA, Baudenbacher F, Dzhura I, Jeyakumar LH, Kannankeril PJ, Fleischer S, LeGrone A, Milatovic D, Aschner M, Strauss AW, Anderson ME, Exil VJ. Polymorphic ventricular tachycardia and abnormal Ca2+ handling in very-long-chain acyl-CoA dehydrogenase null mice. Am J Physiol Heart Circ Physiol 292: H2202–H2211, 2007 [DOI] [PubMed] [Google Scholar]
- 104. Wilson LD, Rosenbaum DS. Mechanisms of arrythmogenic cardiac alternans. Europace 9, Suppl 6: vi77–82, 2007 [DOI] [PubMed] [Google Scholar]
- 105. Wong CH, Koo SH, She GQ, Chui P, Lee EJ. Genetic variability of RyR2 and CASQ2 genes in an Asian population. Forensic Sci Int 192: 53–55, 2009 [DOI] [PubMed] [Google Scholar]
- 106. Yu L, Shi E, Gu T. Single-stage repair of interrupted aortic arch with simultaneous coronary artery bypass grafting without cardiopulmonary bypass in an adult. Ann Thorac Surg 92: 1110–1113, 2011 [DOI] [PubMed] [Google Scholar]
- 107. Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, Hahn HH, Zhao W, Waggoner JR, Jones LR, Jones WK, Bers DM, Dorn GW, Wang HS, II, Valdivia HH, Chu G, Kranias EG. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation 115: 300–309, 2007 [DOI] [PubMed] [Google Scholar]
- 108. Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272: 23389–23397, 1997 [DOI] [PubMed] [Google Scholar]
- 109. Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca(2+) release. Nat Med 17: 1003–1009, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zima AV, Qin J, Fill M, Blatter LA. Tricyclic antidepressant amitriptyline alters sarcoplasmic reticulum calcium handling in ventricular myocytes. Am J Physiol Heart Circ Physiol 295: H2008–H2016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]