Summary
Small conductance Ca2+-activated K+ channels (SK, KCa2.1, KCa2.2, KCa2.3) are expressed at high levels in brain regions critical for learning and memory. The activation of dendritic SK channels limits the induction of synaptic plasticity that may underlie hippocampal and amygdala dependent memory. EBIO facilitates SK channel activation by increasing their sensitivity to calcium. The compound CyPPA selectively activates SK2 and SK3 channels in a similar manner. To date there has been no report of the effects of SK channel activators on memory. Therefore, the present study examined the effects of systemic EBIO on mice in a behavioral task battery. Significant effects of EBIO on memory and motor activity were validated and extended by examining the effects of systemic CyPPA. Systemic EBIO and CyPPA both produced a transient decline in locomotor behavior. Neither SK channel activator affected anxiety. EBIO (17.5 mg/kg) impaired the encoding, but not retrieval, of object memory in a spontaneous object recognition task. A similar impairment of object memory encoding was observed in CyPPA (15 mg/kg)-treated mice. These memory-impairing effects were not due to changes in motivation, attention or movement. Systemic EBIO did not affect contextual or cued fear memory after conditioning with a 3 tone (CS)-footshock (US) pairing protocol or a 1 CS-US pairing protocol. Interestingly, apamin (0.4 mg/kg) enhanced contextual fear memory in mice conditioned with a 1 CS-US pairing protocol. These results suggest that SK channel activation impairs the encoding of non-aversive memory but not memory for aversive events. These data support converging evidence that SK channels regulate cellular mechanisms of memory encoding.
Keywords: memory encoding, object recognition, apamin, mice, fear conditioning, SK channel, EBIO, CyPPA
1. Introduction
Small conductance calcium-activated potassium channels (KCa 2.1–2.3, SK channels, coded for by genes KCNN1, KCNN2 and KCNN3) are voltage insensitive and activated through nanomolar increases in intracellular calcium (Stocker, 2004). All three SK channel subunits (SK1, SK2 and SK3) are expressed ubiquitously in the central nervous system. However compared to SK3, high levels of SK1 and SK2 subunits are found in the hippocampus and amygdala - brain regions critical for explicit memory (Sailer et al., 2002; Sailer et al., 2004; Stocker and Pedarzani, 2000). Contingent upon their subcellular localization, SK channels contribute K+ current to the afterhyperpolarization, regulate synaptic integration, neuronal pacemaking and modulate rapid spiking (for a review see Bond et al., 2005). SK channels colocalize with NMDA-type glutamate receptors on the dendritic spines of pyramidal neurons in the hippocampus and lateral amygdala. Dendritic SK channels shunt NMDA receptor activation, depress glutamatergic excitatory postsynaptic potentials, affect synaptic integration, and limit the induction of synaptic plasticity (Faber et al., 2005; Lin et al., 2008; Ngo-Anh et al., 2005). In light of this SK channel-mediated negative feedback loop, considerable effort has been made to define the influence of SK channels on memory, and their efficacy as targets to treat memory disorders.
Most studies of SK channel behavioral influences have used the peptide apamin, a channel blocker, (Garcia et al., 1991). Apamin facilitates spatial and non-spatial learning in laboratory rodents (Brosh et al., 2007; Deschaux and Bizot, 1997; Deschaux et al., 1997; Fournier et al., 2001; Mpari et al., 2005; Mpari et al., 2008; Stackman et al., 2002). Further, transgenic mice that overexpress the SK2 channel subunit exhibit significant impairments in the encoding of spatial and contextual memory in the Morris water maze and delay fear conditioning paradigm, respectively (Hammond et al., 2006; Stackman et al., 2008).
1-ethyl-2-benzimidazolinone (EBIO) activates SK channels by shifting the Ca2+ sensitivity via stabilization of the interaction between the channel’s α-subunits and Ca2+ bound to the constitutive calmodulin molecule at each C terminus. Thus, EBIO reduces the internal [Ca2+] required for SK channel activation and delays channel deactivation (Pedarzani et al., 2001). EBIO also activates the intermediate conductance Ca2+-activated K+ channel (IK, SK4, KCa3.1,) found in the periphery, but not in the central nervous system (Jensen et al., 1998; Pedersen et al., 1999). Systemic EBIO produces dose-dependent ataxia (Anderson et al., 2006) and disrupts burst firing of substantia nigra dopaminergic neurons (Ji and Shepard, 2006). Chronic intracerebellar EBIO rescues ataxia in the ducky mutant mouse by restoring the intrinsic pacemaking property of cerebellar Purkinje neurons (Walter et al., 2006). Cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA) selectively activates SK2/SK3 subunits over SK1/IK, and is more potent than EBIO (Hougaard et al., 2007; Pedarzani and Stocker, 2008). To date there have been no reports of SK channel activators on learning and memory. Converging neurophysiological and behavioral evidence supports an important role of SK channels in synaptic integration and synaptic plasticity that may be fundamental for memory. Thus, further effort should be placed on defining the potential of SK channels as targets for treatment of learning and memory and motor disorders. Here, we report a broad behavioral characterization of two SK channel activators in male C57BL/6NHsd mice.
2. Methods and Materials
2.1. Animals
All subjects were male 8 to 12-week-old C57BL/6NHsd mice purchased from Harlan Laboratories (Indianapolis, IN). Mice were housed in groups of 4 per standard polycarbonate cage with ad libitum access to food and water. Cages were maintained in a temperature- and humidity-controlled vivarium with a 12 h light/dark cycle (lights on at 0700 h). All behavioral testing took place during the lights on phase of the cycle from 1200–1700 h. The mice were allowed to habituate to the vivarium environment for at least 5 days before the start of any experimental testing. All procedures were conducted in accordance with the guidelines as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Prior to initiating experiments, the Institutional Animal Care and Use Committee at Florida Atlantic University approved all procedures.
2.2. Drugs
1-ethyl-2-benzimidazolinone (EBIO) was purchased from Tocris (Ellisville, MO), dimethyl sulfoxide (DMSO) and Cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA) were purchased from Sigma (St. Louis, MO), apamin was purchased from Calbiochem (La Jolla, CA), and sterile saline (0.9% NaCl) was purchased from AmTech (St. Joseph, MO). Cremophor RH40 was generously contributed by BASF (Ludwigshafen, Germany). A 1% solution of DMSO in sterile saline served as the vehicle in all EBIO (10–25 mg/kg, 10 ml/kg, SC) experiments, except where noted. The vehicle for all studies of CyPPA (15 mg/kg, 10 ml/kg, IP) was a 5% solution of Cremophor RH40 in sterile saline. Solutions of apamin (0.4 mg/kg, 10 ml/kg, IP) were prepared in a 0.9% saline vehicle. All solutions of EBIO, CyPPA and apamin were freshly prepared at the time of administration.
2.3. Behavioral tasks
All mice were naïve at the start of behavioral testing and no mice were re-tested on similar tasks, nor were they tested again after fear conditioning. All mice were acclimated to the laboratory prior to initiating behavioral testing. Mice were removed from the vivarium and transported to the laboratory holding room for at least 1 h per day for one or two days. On the next day, mice were transported to the laboratory holding room where each animal’s tail was marked with a blue Sharpie and body weight was recorded to establish identity.
2.3.1. Open Field
A dose-dependent analysis of the effects of systemic EBIO on locomotor activity was conducted to determine the appropriate EBIO dosage range, the interval between EBIO administration and the initiation of testing, and any side effects that would interfere with the expression of behavior in learning and memory tasks. In an initial pilot study to define a working dose range, we found that mice administered 35 mg/kg EBIO in DMSO vehicle exhibited severe immobility. A 1:1 dilution yielded our mid-range 17.5 mg/kg dose (Vick and Stackman, unpublished data). For the present study, each mouse received an injection of EBIO (10, 17.5 or 25 mg/kg, SC), CyPPA (15 mg/kg, IP) or respective vehicle. Immediately following the injection, the mouse was placed into the center of a white opaque arena (37.5 cm by 37.5 cm by 50.8 cm tall). The floor and walls of the arena were constructed of white ABS acrylic sheet. Before each test day the arena floor and walls were thoroughly cleaned with disposable germicidal wipes (Sani-Cloth PLUS, PDI, Orangeburg NY) and rinsed with deionized water 5 min later. Additionally, the arena floor and walls were washed with a 10% ethanol solution to remove any olfactory cues between each mouse. The locomotor activity trial lasted for 60 min during which behavior was tracked via an overhead video camera interfaced with behavioral tracking software Ethovision XT v5.1 (Noldus Information Technology, Leesburg, VA). The Ethovision software detects the mouse by image contrast (in this case a black mouse on a white background) in each video frame (6.0 fps) and tracks the movement of the digitized pixels that represent the “center point” of the mouse. Locomotor behavior was assessed by four dependent measures. Distance traveled, defined as the sum of recorded movement of the center point of the mouse, in cm over the duration of the trial. Thigmotaxis, defined as the time spent in a central or inner zone of the arena (23 cm by 23 cm) divided by the total time spent in the inner and outer zones of the arena:
A lower thigmotaxis index score indicates less time spent near the center of the arena and more time within 7.25 cm of the walls. Thigmotaxis is an index of anxiety; the more anxious the mouse, the less time that mouse will spend in the inner zone of the arena and the more time it will spend in contact with the walls (Clement and Chapouthier, 1998). Immobility, defined as the amount of time, in seconds, that Ethovision failed to detect any linear or angular movement of the animal. Immobility was determined by measuring the amount of change in pixels from one 3-frame sample to the next; if the total pixel area representing the mouse changed by less than 20%, then the mouse was considered to be immobile. A mouse that reared or was grooming would not be detected as immobile. Total meander, defined as the change in movement direction relative to distance traveled, was analyzed as a measure of movement path shape:
Meander might be useful as an index of motor coordination or gait asymmetry; a low meander score indicates a straight movement path with little curvature while a higher meander score indicates a movement path with high sinuosity. Measures of meander were restricted to periods during which the mouse was determined to be moving but not thigmotaxic.
2.3.2. Elevated Plus Maze
Mice were tested in the elevated plus maze task to further examine the influence of systemic SK channel activation upon anxiety. The elevated plus maze consists of two open and two enclosed horizontal perpendicular arms (5 cm wide, 30.5 cm long) extending from a central platform (5 × 5 cm), which were elevated 61 cm above the floor. Behavior on the plus maze was acquired by the Ethovision XT system, and behavior on the central platform (5 cm wide, 5 cm long) was excluded from analysis. Prior to elevated plus maze testing, each mouse was habituated to an empty clean polycarbonate mouse cage for a 5-min period. On the second day each mouse also received an injection of vehicle (1% DMSO, SC or 5% Cremophor, IP) to further habituate mice to the needle prick and bolus. On the day of testing each mouse received an injection of EBIO (17.5 mg/kg, SC), CyPPA (15 mg/kg, IP) or respective vehicle. Following the injection the mouse was immediately placed in an open holding cage for 20 min after injection of EBIO, or 30 min after injection of CyPPA. These injection-to-test delay intervals were chosen based on the influence of EBIO and CyPPA on mice in the open field (see Fig. 1 & Fig. 2). After the delay, the mouse was placed on the central platform of the elevated plus maze facing an intersection between open and closed arms. From the 5-min exploration trial, Ethovision determined the number of entries into zones representing the open and closed arms, the amount of time spent in the open and closed arms, and the total distance traveled (in cm). The criterion for arm entry was that the tracked center point of the mouse, as detected by Ethovision, had to be inside the zone representing the maze arm. The maze was thoroughly washed with 10% ethanol after each mouse to remove any olfactory cues. The open arm dwell ratio and open arm entry ratio were analyzed as measures of anxiety. Open arm dwell ratio was calculated by dividing the amount of time (in s) spent in the open arms by the total amount of time spent in the open + closed arms, with the result expressed as a percent:
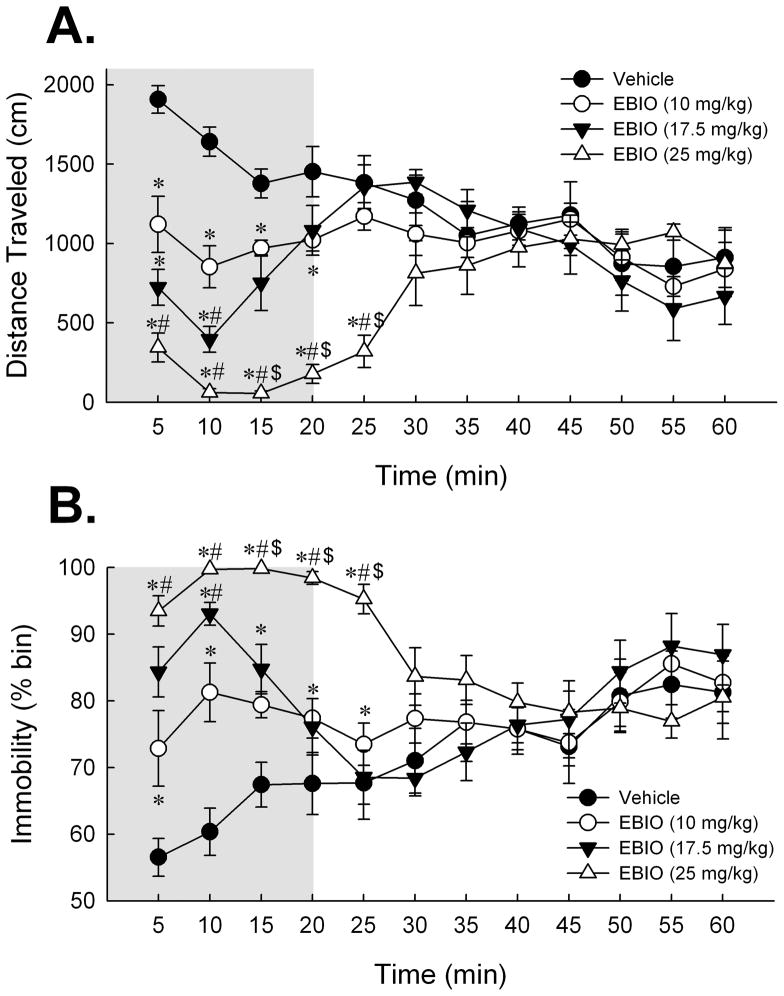
Figure 1.
Activation of SK channels with systemic EBIO depresses locomotor responding in C57BL/6NHsd mice. (A) Dose-dependent effect of EBIO on mean distance traveled in the 60 min period in the open field expressed as cm traveled per 5-min time bin. Post-hoc Tukey HSD tests revealed that mice treated with EBIO exhibited a significant decrease in distance traveled during the first 15 min of the session. Exploratory motor activity recovered to the level of the 1% DMSO vehicle group by 20 min in the 10 and 17.5 mg/kg EBIO-treated mice and by 30 min in the 25 mg/kg EBIO-treated mice. (B) Dose-dependent effect of EBIO on immobility over the 60 min period expressed as percent of time deemed immobile by the Ethovision software per 5-min time bin. Post-hoc Tukey HSD tests revealed that measures of immobility recovered to vehicle levels within 20 min for the 10 and 17.5 mg/kg dose groups and by 30 min for the 25 mg/kg dose group. Shaded region depicts the 20 min post-injection interval over which all doses of EBIO impaired exploratory motor behavior in mice. In light of this effect, a 20 min delay was imposed after EBIO administration and before behavioral testing for all subsequent experiments. Error bars are SEM. *, P < 0.05 vs. vehicle; #, P < 0.05 vs. 10 mg/kg EBIO; and $, P < 0.05 vs. 17.5 mg/kg EBIO. Vehicle, n = 8; 10 mg/kg EBIO, n = 8; 17.5 mg/kg EBIO, n = 8; 25 mg/kg EBIO, n = 7.
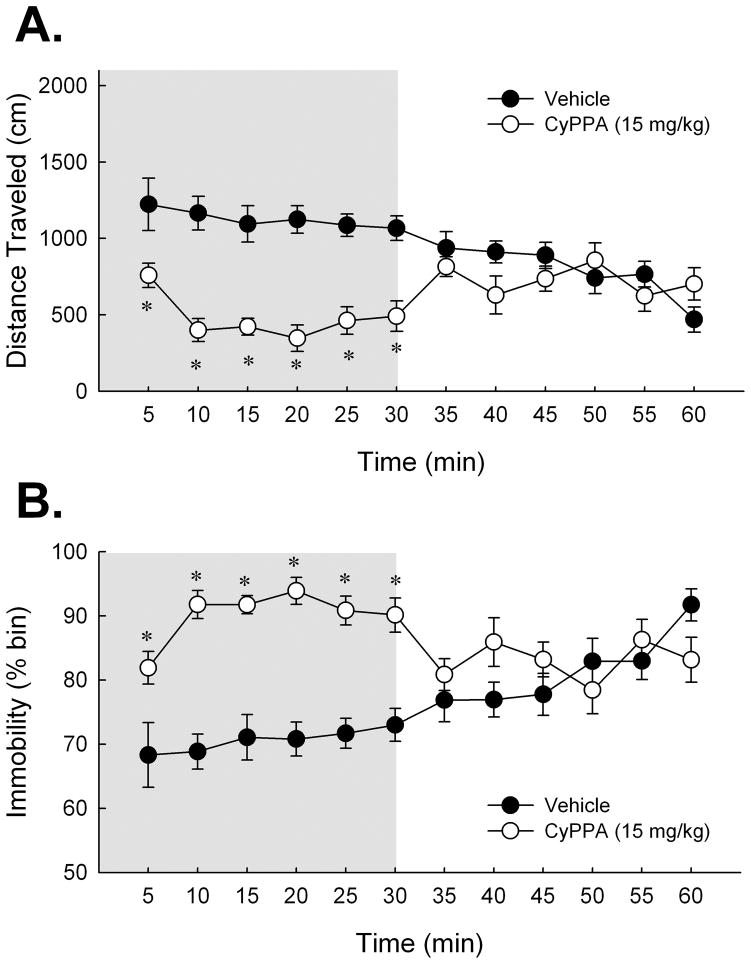
Figure 2.
Activation of SK channels with systemic CyPPA depresses locomotor responding in C57BL/6NHsd mice. (A) Effect of CyPPA (15 mg/kg) on mean distance traveled during the 60 min period in the open field. Student’s t-test analysis determined locomotor depression of CyPPA to return to that of 5% Cremophor vehicle levels by 35 min. (B) Effect of CyPPA on immobility over the 60 min period. Students t-test determined that measures of mobility returned to vehicle levels by 35 min. Shaded region depicts the 30 min post-injection interval over which CyPPA impaired locomotor behavior in mice. In light of this effect, a 30-min delay was imposed after CyPPA administration and before behavioral testing for all subsequent experiments. Error bars are SEM. *, P < 0.05 vs. vehicle. Vehicle, n = 8; 15 mg/kg CyPPA, n = 8.
Open arm entry ratio was calculated by dividing the number of entries into the open arms by the total number of arm entries then expressing the result as a percent:
2.3.3. Spontaneous Object Recognition Task
To determine the effects of the SK channel activators on non-spatial learning and memory naïve mice were tested using a spontaneous novel object recognition paradigm demonstrated to be sensitive to hippocampal inactivation (Hammond et al., 2004) and to SK channel blockade (Stackman et al., 2002). During the sample session each mouse explored two novel toy objects in a familiar white opaque arena (37.5 cm × 37.5 cm × 50.8 cm tall). During the test session presented 24 hr later, one of the objects from the sample session was replaced with a novel object and the mouse was re-introduced into the arena. The time spent exploring the novel object was compared with that of the familiar object. Since rodents have a tendency to explore novel, non-threatening items, the difference in time spent exploring each object is a reliable measure of memory (Ennaceur and Delacour, 1988).
Prior to testing object recognition, each mouse was habituated to an empty clean polycarbonate mouse cage for a 5-min period (1/day for two consecutive days). Next, each mouse received two 10-min habituation sessions (1/day) to acclimate to the white arena. The first cohort of mice was injected with EBIO (17.5 mg/kg, SC) or vehicle 20 min prior to the sample session to investigate the effects of SK channel activation on the encoding of object memory. Vehicle was given to all mice 20 min prior to the test session. The second cohort of mice was injected with EBIO (17.5 mg/kg, SC) or vehicle 20 min prior to the test session to investigate the effects of SK channel activation on the retrieval of object memory. All mice in the second cohort received vehicle injections 20 min prior to the sample session. A third cohort of naïve mice were injected with the selective SK2/SK3 channel activator CyPPA (15 mg/kg, IP) 30 min prior to the sample session to validate and extend the results observed with EBIO on object memory. Immediately after each injection, the mouse was placed into an empty polycarbonate holding cage for 20 or 30 min before being released into the arena.
During the sample session, the mouse was placed into the arena with two identical novel metal objects affixed to opposite corners (NE and SW) of the arena. The objects were positioned approximately 2 cm away from the arena wall. Object exploration was recorded with digital stopwatches for the sample session and with Ethovision’s manual event encoder for the test session. Exploration was defined as time spent with the head oriented towards the object and within 2–3 cm with vibrissae moving. One experimenter (MG), blind to the treatment condition of the mice, coded all of the exploratory behavior. Male C57BL/6NHsd mice demonstrate strong novel object preference 24 hr after a sample session during which the sample objects were explored for 30–38 s (Stackman et al., 2002; Vick and Guidi, unpublished data). In the present study, each mouse was permitted a maximum of 10 min to accumulate 38 s of exploration of either sample object (two identical metal leveling feet) or a total of 30 s of exploration of both sample objects. Mice were immediately removed from the arena after the encoding criterion was met; any mice that failed to reach the encoding criterion within 10 min were removed from the study. Twenty-four hr later, a test session was conducted in the same arena except that one of the familiar objects was replaced with a novel object (plastic toy gorilla). Each mouse was allowed to explore the arena for 5 min during the test session. The absolute position of the novel object was counterbalanced between the NE and SW corners of the arena across mice to reduce the spatial significance of the objects. After each session the arena and objects were cleaned thoroughly with 10% ethanol solution to limit olfactory cues.
Measures of object exploration, the latency to accumulate sample object exploration, and distance traveled were determined from the sample session behavior. Object exploration was defined as time spent with the head oriented towards and within 2–3 cm of the object, and with the vibrissae moving. Latency to accumulate sample object exploration was defined as the amount of time (in s) each mouse took to reach the encoding criterion during the sample session. Distance traveled was defined as the total distance moved (in cm) of the mouse over the entire session. Measures of novel object preference were inferred from the test session behavior. Novel object preference ratio was defined as time spent exploring the novel object divided by the total time spent exploring both objects:
Novel object preference ratio scores above 0.5 indicate novel object preference and suggest a successful recognition of the familiar object. A novel object preference ratio score of 0.5 indicates chance performance or equal exploration of the novel and familiar objects. Discrimination ratio was defined as a second test measure of object memory, and was calculated by subtracting the time spent exploring the familiar object from the time spent exploring novel object and dividing the result by the total time spent exploring both objects:
The discrimination ratio scores range from −1 to 1 with 0 representing equal or chance exploration levels. Discrimination ratio scores above 0 indicate novel object preference and suggest a successful recognition of the familiar object.
2.3.4. Fear Conditioning
The influence of systemic EBIO on learning and memory was further examined using a contextual and cued fear conditioning paradigm. The hippocampus is considered critical for encoding the association between the context and footshock (Kim and Fanselow, 1992; Matus-Amat et al., 2004), while the amygdala is essential for encoding the associations of footshock with both the context and the tone (Phillips and LeDoux, 1992). Delay fear conditioning was conducted using the MED Associates Near-Infrared Video Fear Conditioning system (Georgia, VT) comprising of four rectangular chambers (30.5 cm by 24.1 cm by 21 cm) constructed of brushed aluminum side walls and Plexiglas top, front and back walls. Each chamber contained a speaker through which audible tones were delivered, and was illuminated by an overhead white light. The chamber floor was constructed of parallel stainless steel rods (36 rods, 3.2 mm dia, 7.9 mm apart) designed for mice, and connected to a scrambled shock generator. Each chamber was housed inside a larger noise-attenuating box and a ventilation fan provided background noise. All behavior was acquired through the front wall of the chamber by a near infrared camera mounted 35 cm outside each chamber. The chambers were identical to each other and after each trial they were thoroughly cleaned with 1% LiquiNox (White Plains, New York) to remove olfactory cues.
On the first day, each mouse was allowed to freely explore a conditioning chamber during a 5 min context pre-exposure session. On the following day each mouse was returned to the same chamber for a 3 CS-US or a 1 CS-US conditioning session, depending on the experiment (see below). After a 60 s exploration interval to establish baseline freezing, a tone (90 dB, 5000 Hz, CS) was presented for 30 s that co-terminated with a 1-s, 0.5 mA footshock (US). For mice conditioned with 3 CS-US pairings, the presentation of the CS-US pairing was repeated twice more (120 s ISI). All mice were removed from the chamber and returned to their home cages 60 s after the final CS-US pairing was presented. Twenty-four hr after conditioning, each mouse was returned to the conditioning chamber for a 5 min context test but no footshock or tone stimuli were presented. Freezing, a rodent’s natural response to fear (LeDoux, 1993), was measured by the automated Video Fear software tracking system (MED Associates, Georgia, VT). At least two hours later each mouse was tested for freezing to the conditioning tone in a modified chamber. The modified chamber included a white Plexiglas floor, a black Plexiglas triangular insert, which altered the lighting and geometry of the chamber, and the floors and inserts were cleaned with 70% ethanol prior to animal introduction to alter olfactory cues. Sixty seconds after introduction into the modified chamber, the 30-s tone CS was presented and freezing behavior was measured. Mice that demonstrated robust (greater than 20%) freezing during the first 60 s of the tone test (pre-tone) were excluded from tone analysis due to generalized freezing rather than cued freezing. For conditioning and the tone test sessions, tone-elicited freezing was considered as freezing episodes that occurred during the 30 s of the tone and the 30 s interval following the tone. The total percentage of time spent freezing was determined for the context pre-exposure and context test sessions. Freezing was defined as the lack of all activity, other than respiration, lasting more than 0.6 s (20 au, 30 fps, 18 frames). The automated software program operationally defined freezing episodes as the duration of time, greater than 0.6 s, during which less than 20 pixels of each video frame were detected to have changed. The video capture rate was set at 30 fps which resulted in freezing being recorded computationally after less than 20 pixels of motion per frame over the time course of 18 frames.
Our lab had previously reported that transgenic mice overexpressing SK2 channels (SK2+/T) exhibit significant impairments in contextual and cued fear memory after the same 3 CS-US pairing protocol described above (Hammond et al., 2006; Stackman et al., 2008). To determine whether contextual and cued fear conditioning were sensitive to pharmacological manipulation of SK channels, one cohort of mice received EBIO (17.5 mg/kg, SC) or vehicle (0.4% DMSO, SC) 20 min before conditioning with the 3 CS-US pairing protocol. A second cohort of mice received the SK channel blocker, apamin (0.4 mg/kg, IP) or 0.9% saline, 30 min before conditioning with the 3 CS-US pairing protocol. Intraperitoneal injection of 0.4 mg/kg apamin has been shown previously to facilitate encoding of object and spatial memory in C57BL/6NHsd mice (Stackman et al., 2002). To address the possibility that the 3 CS-US pairing protocol represented overtraining that limited the ability of SK channel drugs to affect fear memory, follow-up experiments were conducted in which a naïve cohort of mice received EBIO (17.5 mg/kg, SC) or vehicle (1% DMSO, SC) 20 min before conditioning with a 1 CS-US pairing protocol. A second cohort of naïve mice received apamin (0.4 mg/kg, IP) or 0.9% saline, 30 min before conditioning with a 1 CS-US pairing protocol. In all experiments, mice received vehicle injections before the context pre-exposure and context test sessions. Since EBIO was found to not affect fear conditioning with either protocol, the effects of CyPPA on fear conditioning were not examined.
2.4. Data analysis
Data are presented as mean ± SEM with significance set at P < 0.05. Independent groups Student’s t-tests were conducted to determine significance between two independent groups or between one group and chance. The Mann-Whitney U test was used to determine significance between two independent groups when equal variance amongst groups was not present. Repeated-measures ANOVAs were used to analyze open field motor responses and measures of freezing during conditioning and the tone test sessions, followed by post hoc two-tailed Student’s t-tests or multiple comparisons Tukey’s HSD tests where appropriate. All statistical tests were conducted using the SPSS v16 (Chicago, IL) software package.
3. Results
3.1. Systemic SK channel activation and locomotor activity
3.1.1 Effect of systemic EBIO on locomotor activity
Systemic EBIO produced a dose-dependent impairment in several measures of locomotor behavior over the 60 min period in the open field (see Figure 1A & B). A repeated measures ANOVA on distance traveled measured in 5-min bins yielded a significant effect of EBIO dose [F(3,27) = 8.42; P < 0.001, see Fig. 1A], a significant effect of time bin [F(11,297) = 5.33; P < 0.001]; and a significant EBIO dose × time bin interaction [F(33,297) = 7.36; P < 0.001]. Post-hoc Tukey HSD tests run to further explore the main effect of EBIO dose revealed significant differences in distance traveled between vehicle-treated mice and those that received 17.5 mg/kg, P < 0.05; and 25 mg/kg, P < 0.001, and between 10 mg/kg and 25 mg/kg-treated mice, P < 0.05. To further investigate the time course of EBIO’s effects on measures of distance traveled, one-way ANOVA’s were run on each 5-min bin. These analyses indicated that all doses of EBIO significantly reduced locomotor activity compared to vehicle-treated mice for the first 15 min of the 60 min period. In agreement with what is depicted in Fig. 1A, post-hoc Tukey multiple comparisons tests indicated that recovery of locomotor activity by EBIO-treated mice to that of vehicle-treated levels was as follows: 10 mg/kg and 17.5 mg/kg EBIO-treated mice recovered by 20 min post-injection; and 25 mg/kg EBIO by 30 min post-injection.
Comparable analyses on immobility measures yielded a significant effect of EBIO dose [F(3,27) = 5.58; P < 0.01, see Fig. 1B], a significant effect of time bin [F(11,297) = 4.47; P < 0.001]; and a significant EBIO dose × time bin interaction [F(33,297) = 6.29; P < 0.001]. Post-hoc Tukey HSD tests run to further explore the main effect of EBIO dose effect revealed a significant difference in immobility over the 60 min session between vehicle-treated mice and those that received 25 mg/kg, P < 0.01. One-way ANOVA’s were run on each 5-min bin to further examine the time course of EBIO’s effect on immobility. Subsequent post-hoc Tukey multiple comparisons tests found that measures of immobility from 10 and 17.5 mg/kg EBIO-treated mice recovered to that of vehicle-treated levels by 20 min post-injection; and 25 mg/kg EBIO by 30 min post-injection. Given that EBIO appeared to produce its most robust influence on locomotor responses during the first 30 min after administration, measures of total meander (reflecting degree of angular direction changes over distance traveled) and thigmotaxis scores were determined for the first 30 min of the motor activity session. One way ANOVA yielded neither a significant effect of EBIO dose on total meander [F(3,27) = 1.08; n.s.] nor on thigmotaxis [F(3,27) < 1; n.s.].
3.1.2. Effect of systemic CyPPA on locomotor activity
To verify that the motor influences observed in EBIO-treated mice reflect activation of SK channels, we also examined the influence of the SK2/SK3 channel activator, CyPPA on locomotor responses in a naïve cohort of mice. Consistent with the effects observed after EBIO, systemic injection of CyPPA also impaired locomotor responses (See Figure 2A & B). A repeated measures ANOVA on distance traveled across 5-min time bins yielded a significant treatment effect [F(1,14) = 16.15; P < 0.01, see Fig. 2A], a significant effect of 5-min time bin [F(11,154) = 3.07; P < 0.01], and a significant treatment × time bin interaction [F(11,154) = 9.68; P < 0.001]. A similar analysis of immobility measures also revealed a significant effect of treatment [F(1,14) = 14.48; P < 0.01, see Fig. 2B], a significant effect of 5-min time bin [F(11,154) = 3.06; P < 0.01], and a significant treatment × time bin interaction [F(11,154) = 10.00; P < 0.001]. Post-hoc multiple comparisons tests indicated that measures of distance traveled and immobility of CyPPA treated mice recovered to those of vehicle-treated mice by 35 min post-injection. Thus, a 30-min delay was imposed between the time of the injection and the commencement of behavioral testing in all subsequent experiments involving 15 mg/kg CyPPA. Analyses of thigmotaxis and meander over the first 30-min post-injection interval yielded non-significant effects of treatment on thigmotaxis [t(14) = 1.89; n.s.] and meander [t(14) < 1; n.s.]; consistent with the locomotor effects observed after EBIO.
3.2. Systemic SK channel activation and the elevated plus maze
The effect of EBIO on anxiety was further examined by testing vehicle (1% DMSO, n=10)- and EBIO (17.5 mg/kg, n=12)-treated mice on the elevated plus maze. There was no significant effect of EBIO on the open arm dwell ratio [t(20) = 1.02; n.s.; vehicle mean = 33.43 ± 8.38; EBIO mean = 22.63 ± 6.66] or open arm entry ratio [t(20) < 1; n.s.; vehicle mean = 42.49 ± 6.29; EBIO mean = 47.11 ± 6.62]. These data indicate that EBIO did not produce a detectable anxiolytic or anxiogenic effect in the mice. There was a significant reduction in total distance moved in the elevated plus maze by EBIO-treated mice compared to vehicle-treated mice [t(20) = 3.30; P < 0.01; vehicle mean = 732.89 ± 47.33; EBIO mean = 436.30 ± 69.06].
In a follow up experiment designed to examine the selective effects of SK2 and SK3 channel activation on anxiety, CyPPA (15 mg/kg, n = 9)- and vehicle (5% Cremophor, n=8)-treated mice were tested on the elevated plus maze. Similar to EBIO, independent t-tests revealed no significant treatment effect on open arm dwell ratio [t(15) = −1.1; n.s.; vehicle mean = 10.99 ± 3.10; CyPPA mean = 15.09 ± 2.16] or open arm entry ratio [Mann-Whitney U(15) = 28; n.s.; vehicle mean = 28.27 ± 5.45; CyPPA mean = 36.98 ± 2.10]. Analyses also revealed no significant treatment effect on total distance traveled in the maze, [t(15) = 1.27; n.s.; vehicle mean = 1066.69 ± 129.55; CyPPA mean = 983.58 ± 46.34]. Together, these data indicate that systemic injection of SK channel activators does not significantly affect anxiety in C57BL/6NHsd mice.
3.3. Systemic SK channel activation and spontaneous novel object recognition
3.3.1. Effect of systemic EBIO on the encoding of object memory
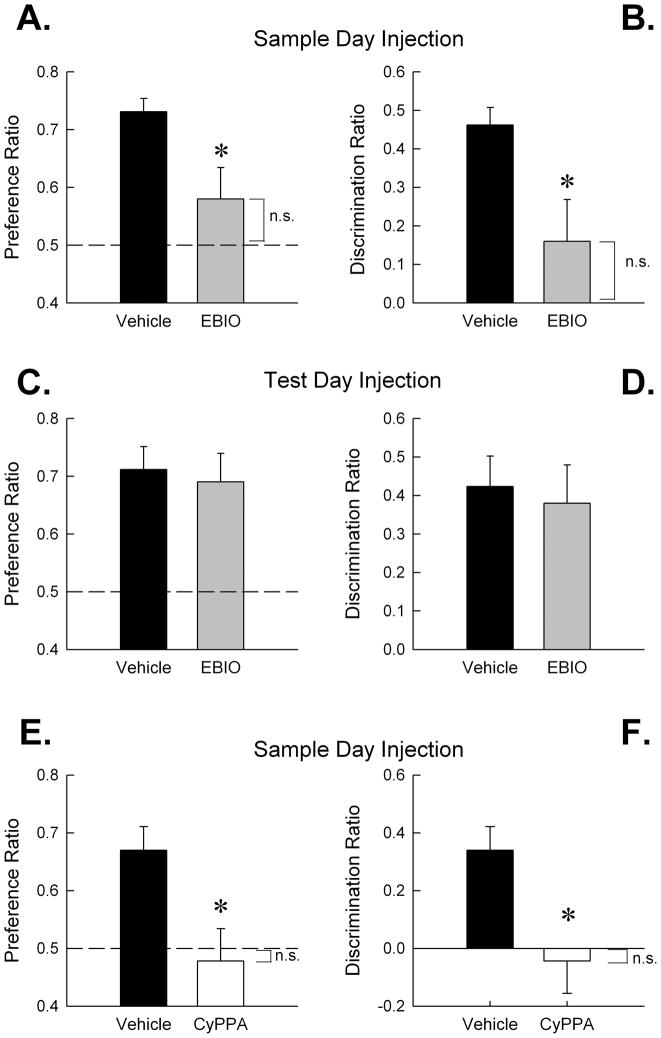
We previously showed that blockade of SK channels with systemic apamin facilitated the encoding of object memory in mice in the spontaneous object recognition task (Stackman et al., 2002). Here, mice received vehicle or EBIO (17.5 mg/kg) and 20 min later were released into the arena to explore the two sample objects. The data for five mice (2 vehicle-treated and 3 EBIO-treated mice) were dropped due to insufficient object exploration or a failure to meet the encoding criterion. There was no effect of EBIO treatment on the latency to accumulate 38 s of sample object exploration [Mann-Whitney U(20) = 38.5; n.s.; vehicle mean = 323.51 ± 17.41 s; EBIO mean = 393.40 ± 41.28 s] and no effect on object exploration behavior [t(20) = 1.72; n.s.]. These results indicate that EBIO did not affect the motivation of the mice to explore the sample objects. There was no effect of EBIO treatment on the distance moved during the sample session [t(20) = 1.15; n.s.]. This result is consistent with the lack of significant effect on locomotor activity in the mice 30–40 min after injection of EBIO (see Fig 1). However, mice that encoded sample object memory under the influence of EBIO exhibited significantly less novel object preference during the test session 24 h later (see Fig. 3A & B). Analyses of novel object preference measures revealed a significant treatment effect on novel object preference ratio (Fig. 3A) [Mann-Whitney U(20) = 30; P < 0.05]; and on the discrimination ratio (Fig. 3B) [Mann-Whitney U(20) = 30; P < 0.05]. As expected, vehicle-treated mice exhibited novel object preference during the test session that was significantly greater than chance (defined as 0.50 novel object preference ratio or 0 discrimination ratio) [both t(11)’s > 10; P < 0.001]. In contrast, EBIO-treated mice exhibited chance level of novel object preference [both t(9) < 1.5; n.s.]. These data indicate that systemic EBIO impairs the encoding of object memory in mice in the spontaneous object recognition task.
Figure 3.
Activation of SK channels with systemic EBIO (17.5 mg/kg) or CyPPA (15 mg/kg) impaired object memory encoding (A) Mice treated with EBIO (n = 10) before the sample session exhibited significantly less novel object preference during the test session 24 h later compared to vehicle-treated mice (n = 12), as measured by the novel object preference ratio; (B) and the discrimination ratio. Both novel object exploration ratios were significantly different from chance for vehicle mice (both P < 0.001), but non-significantly different from chance for EBIO mice (both P > 0.1). (C) A naïve cohort of mice received a vehicle injection before the sample session and then an injection of EBIO (n = 7) or vehicle (n = 8) prior to the test session 24 h later. Vehicle- and EBIO-treated mice exhibited equivalent preference for the novel object as measured by the novel object preference ratio and (D) the discrimination ratio. Both vehicle and EBIO groups demonstrated novel object exploration ratios significantly above chance (all P < 0.01). (E) A final cohort of naïve mice received an injection of CyPPA (n = 8) or 5% Cremophor vehicle (n = 10) before the sample session. CyPPA-treated mice exhibited significantly less novel object preference during the test session as measured by novel object preference ratio; (F) and the discrimination ratio. Both novel object exploration ratios were significantly above chance for the vehicle (both P < 0.01) mice but not significantly different from chance for the CyPPA mice (both P > 0.1). Error bars are SEM. *, P < 0.05, vs. vehicle; n.s., ratio not significant from chance exploration (P > 0.05). Dashed line at 0.5 of A, C and E represents chance performance or a lack of discrimination between the novel and familiar object.
3.3.2. Effect of systemic EBIO on the retrieval of object memory
To further examine the influence of EBIO on object memory processes, a naïve cohort of mice received vehicle injections prior to a sample session and then received EBIO (17.5 mg/kg) or vehicle prior to the test session 24 h later. The data for one EBIO-treated mouse were excluded due to lack of exploration on the test day. Analyses revealed no significant effect of EBIO treatment on novel object preference ratio [t(13) < 1; n.s., Fig. 3C], or discrimination ratio [t(13) < 1; n.s., Fig. 3D]. Importantly, all mice exhibited significantly greater than chance level of novel object preference [all t(6 or 7)’s > 3.5; P < 0.01]. Further, there was no treatment effect on total exploration time [t(13) = 1.83; n.s.; vehicle mean = 83.18 ± 8.03; EBIO mean = 58.96 ± 10.82]. There was a significant treatment effect on distance traveled during the test session [t(13) = 2.95; P < 0.05], with vehicle-treated mice traveling a greater distance during the test session than EBIO-treated mice. These data indicate that although systemic EBIO did affect locomotor responses of the mice during the test session, both vehicle- and EBIO-treated mice exhibited a strong preference for exploring the novel object during the test session.
3.3.3. Effect of systemic CyPPA on the encoding of object memory
To validate the results observed with EBIO on object memory as due to a specific action of the drug on SK channels, we also examined the effects of systemic CyPPA on object memory. Naïve mice were administered CyPPA (15 mg/kg, IP), or vehicle (5% Cremophor, IP) 30 min before the sample session. The data for two mice (one CyPPA and one vehicle) were excluded from analysis due to failure of the mice to meet the object exploration criterion. There was no significant treatment effect on the latency to accumulate 38 s of object exploration during the sample session [t(16) = −1.59; n.s.; vehicle mean = 271.00 ± 27.43 s; CyPPA mean = 340.11 ± 34.30 s] or on the total amount of object exploration exhibited by the mice [t(16) = 1.66; n.s.]. These data indicate that CyPPA did not affect the motivation to explore the objects during the sample session. Furthermore there was no significant effect of treatment on distance traveled during the sample session [t(16) < 1; n.s.] confirming that no motor impairment was present during the exploration of objects.
Complementary to the EBIO-treated mice, mice injected with CyPPA before the sample session exhibited impaired novel object recognition 24 hr later in the test session (see Fig. 3E & F) compared to vehicle and chance. Specifically, mice injected with 15 mg/kg CyPPA before the sample session exhibited significantly lower discrimination and preference ratios than vehicle-treated mice [t(16) = 2.84; P < 0.05]. Vehicle-treated mice exhibited novel object preference during the test session that was significantly greater than chance level [both t’s(9) = 4.17; both P’s < 0.01], while CyPPA-treated mice exhibited chance performance in both ratios [t(7) < 1; n.s.]. These results confirm the effects seen with EBIO, and suggest that the object memory deficit observed after EBIO is a consequence of the activation of SK2 and SK3 subunits specifically.
3.4. Systemic SK channel manipulation and contextual and cued fear conditioning
3.4.1. Systemic EBIO or apamin prior to conditioning with 3 CS-US pairing protocol
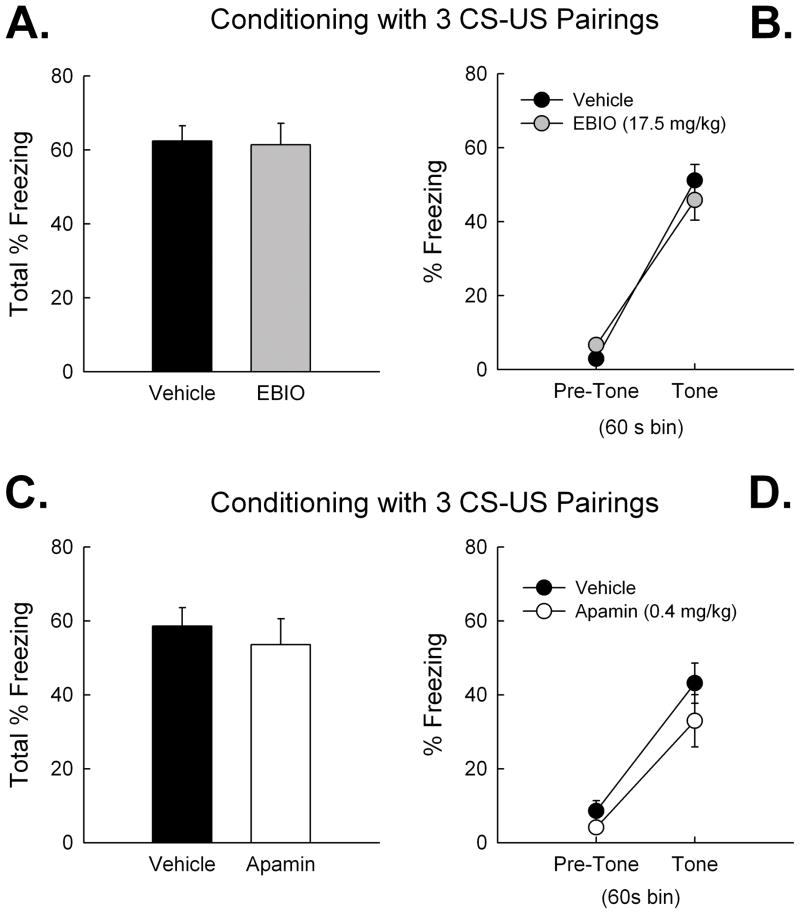
We previously reported that SK2 overexpression impairs the encoding of contextual and cued fear memories after a 3 CS-US conditioning protocol (Hammond et al., 2006; Stackman et al., 2008). To date there have been no reports of the effects of systemic SK channel drugs on delay fear conditioning in laboratory rodents. Therefore, we tested the influence of systemic EBIO (17.5 mg/kg) and apamin (0.4 mg/kg) on contextual and cued fear conditioning with a 3 CS-US pairing protocol in separate cohorts of naïve mice. All mice of the first cohort received a 5-min context pre-exposure session under the influence of the vehicle. Twenty-four hr later, mice received EBIO or vehicle and 20 min later were returned to the same chamber and received 3 CS-US pairings. A two-factor ANOVA on % freezing data, with treatment as a between-subjects variable and CS-US pairing as a repeated measures variable revealed a significant effect of CS-US pairing, [F(3,132) = 93.83; P < 0.001], a significant main effect of treatment, F(1,44) = 9.64; P < 0.01], and a non-significant treatment × CS-US pairing interaction, F(3,132) = 0.98; n.s.]. Overall, these results indicate that there was a significant difference in freezing during the conditioning session between the vehicle- and EBIO-treated mice. However, both groups of mice exhibited a significant increase in freezing over the course of the 3 CS-US conditioning session. All mice displayed conditioned fear to the context when tested 24 hr after conditioning, expressed as an increase in % freezing relative to the pre-exposure session (Fig. 4A). There were no differences in freezing between EBIO and vehicle-treated mice during the context test [Mann-Whitney U(44) = 255.0; n.s.]. There were also no differences in tone-elicited freezing during the tone test (Fig. 4B). Repeated measures ANOVA revealed no treatment effect on tone test freezing [F(1,44) < 1; n.s.] and no interaction of treatment × time bin [F(1,44) < 1; n.s.]. These findings indicate that EBIO did not affect conditioned contextual or cued fear memory after a 3 CS-US pairing protocol.
Figure 4.
Activation of SK channels with EBIO (17.5 mg/kg), or blockade of SK channels with apamin (0.4 mg/kg) did not affect the encoding of contextual or cued fear memory during conditioning with a 3 CS-US pairing protocol. Mice that received EBIO (n = 23) or vehicle (n = 23) before conditioning exhibited equivalent freezing during (A) the context test 24 h later, and during (B) the tone test. Mice that received apamin (0.4 mg/kg, n = 10) or saline (n = 10) before conditioning with 3 CS-US pairs also exhibited equivalent freezing during (C) the context test 24 h later, and during (D) the tone test. Error bars are SEM.
All mice of the second cohort received a 0.9% saline injection (10 ml/kg, IP) and 30 min later were placed into a conditioning chamber for a 5-min context pre-exposure session. Twenty-four hr later mice received apamin (0.4 mg/kg, IP) or 0.9% saline vehicle and 30 min later were returned to the same chamber and received 3 CS-US pairings. A two-factor ANOVA on % freezing data revealed a significant effect of CS-US pairing, [F(3,54) = 38.43; P < 0.001], and a significant treatment × CS-US pairing interaction effect, [F(3,54) =5.28; P < 0.01], but no significant main effect of apamin treatment, [F(1,18) = 1.26; n.s]. The higher freezing observed of apamin treated mice prior the first CS-US pair may have contributed to the interaction effect. All mice exhibited conditioned fear to the context when tested 24 hr after conditioning, expressed as an increase in % freezing relative to the pre-exposure session (Fig. 4C). However, there were no differences in freezing between apamin- and vehicle-treated mice during the context test [t(18) < 1; n.s.]. There were also no differences in tone-elicited freezing during the tone test (Fig. 4D). Repeated measures ANOVA revealed a significant effect of time bin (pre-tone vs tone) on freezing [F(1,13) = 45.21; P < 0.001], but no effect of treatment [F(1,13) = 2.26; n.s.] or treatment × time bin interaction [F(1,13) < 1; n.s.]. These findings indicate that apamin administered before the 3 CS-US pairing conditioning session did not affect retention of conditioned contextual and cued fear memory, compared to vehicle.
3.4.2 Systemic EBIO or apamin prior to conditioning with 1 CS-US pairing protocol
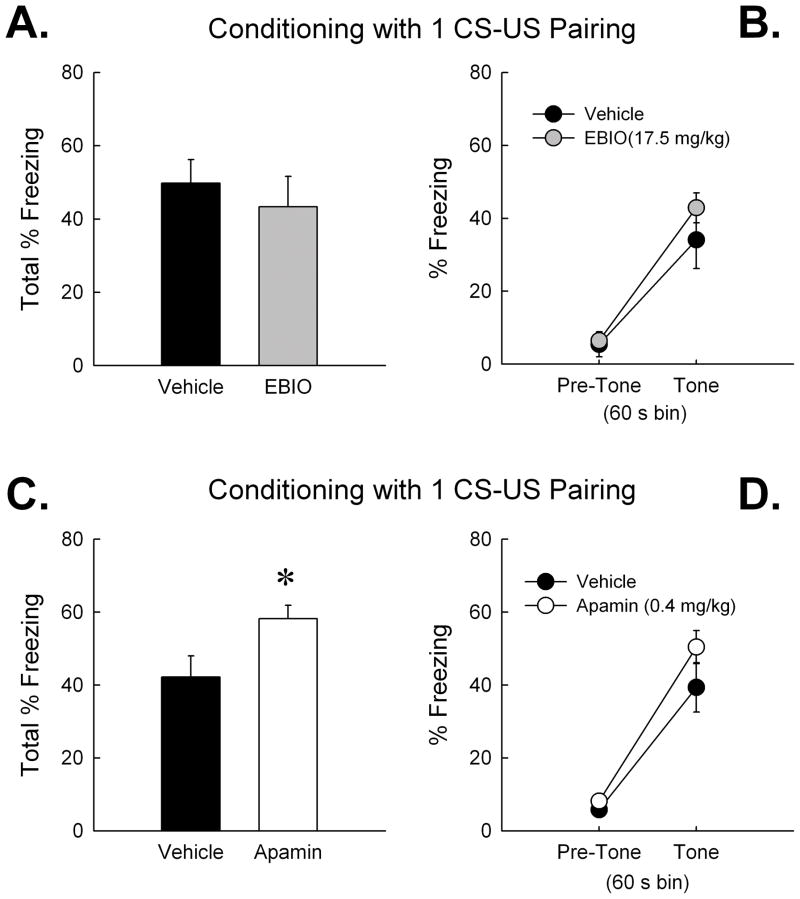
Our laboratory has previously shown that SK channel manipulation affects the rate of learning or memory encoding. For instance, systemic apamin (0.4 mg/kg) facilitates object memory in mice permitted limited, but not extensive, time to explore sample objects (Stackman et al., 2002). This result and others suggest that SK channels may influence an early stage of learning called memory encoding, but not affect memory retention or retrieval. Given the lack of contribution of the SK channel activator or blocker on conditioned fear memories encoded during a 3 CS-US pairing protocol, we next examined the influence of EBIO and apamin in two cohorts of mice receiving a more limited, 1 CS-US pairing, conditioning protocol. The first cohort received a vehicle injection and then a 5-min context pre-exposure session on Day 1. Twenty-four hr later, mice received either EBIO or vehicle and 20 min later were returned to the same chamber and received 1 CS-US pairing. Analyses revealed a non-significant effect of treatment on freezing, [F(1,15) < 1; n.s.], and a non-significant treatment × CS-US pairing interaction, [F(1,15) < 1; n.s.]. There were no differences in freezing between EBIO and vehicle-treated mice during the context test 24 h after conditioning (Fig. 5A) [t(15) = 1.18; n.s.]. Repeated measures ANOVA revealed no effect of treatment on freezing during the tone test (Fig. 5B) [F(1,15) < 1; n.s.]. These findings indicate that EBIO did not affect conditioned contextual and cued fear memory after the more limited 1 CS-US pairing protocol. The effects of CyPPA on fear conditioning were not tested since EBIO failed to influence conditioned fear memories.
Figure 5.
Activation of SK channels with EBIO (17.5 mg/kg) did not affect the encoding of contextual or cued fear memory after conditioning with a 1 CS-US pairing protocol. However blockade of SK channels with apamin (0.4 mg/kg) did enhance the encoding of contextual fear memory after the 1 CS-US pairing protocol. Mice that received EBIO (n = 9) or vehicle (n = 8) before conditioning exhibited equivalent freezing during (A) the context test 24 h later, and during (B) the tone test. Mice that received apamin (0.4 mg/kg, n = 11) before conditioning exhibited significantly greater freezing during (C) the context test 24 h later compared to vehicle-treated mice (n = 9). There were no treatment effects on tone-elicited freezing during (D) the tone test. Error bars are SEM. *, P < 0.05 vs. vehicle.
The final cohort of mice received a 0.9% saline vehicle injection and then a 5-min context pre-exposure session on Day 1. Twenty-four hr later, mice received apamin (0.4 mg/kg) or vehicle and 30 min later were returned to the same chamber and received 1 CS-US pairing. Analyses of freezing measures from the conditioning session revealed a significant effect of time bin [F(1,18) = 22.99; P < 0.001], but non-significant effects of treatment [F(1,18) = 1.05; n.s.], and treatment × time bin interaction [F(1,18) < 1; n.s.]. These data suggest that both groups of mice displayed similar freezing patterns during the 1 CS-US pairing conditioning session. However, mice that received apamin before conditioning, exhibited a significantly greater degree of freezing during the context test 24 hr after conditioning (see Fig 5C) [t(18) = −2.42; P < 0.05]. Repeated measures ANOVA on tone test freezing measures revealed a significant effect of time bin (pre-tone vs. tone) (see Fig. 5D) [F(1,13) = 100.81; P < 0.001], but non-significant effects of treatment or treatment x time bin interaction [both F(1,13) < 2.3; both P > 0.15, n.s.]. These findings suggest that systemic apamin facilitated the encoding of contextual fear memory during the limited 1 CS-US pairing protocol. Mice treated with the SK channel blocker exhibited significantly greater freezing during the context test than did the saline-treated control mice.
4. Discussion
The present results indicate that the SK channel activators EBIO and CyPPA impaired motor and cognitive behavior in male C57BL/6NHsd mice. To our knowledge, this is the first published report of the in vivo effects of CyPPA and the first report of the influence of SK channel activators on learning and memory. Systemic EBIO and CyPPA transiently decreased the total distance traveled in an open field. Neither systemic EBIO nor CyPPA affected the anxiety of mice as measured by thigmotaxis in the open field or arm entries in the elevated plus maze. Systemic EBIO (17.5 mg/kg) and CyPPA (15 mg/kg) both impaired the encoding of object memory in mice during a spontaneous object recognition task. Injection of systemic EBIO before the test session did not affect the retrieval of object memory. These results are consistent with previous reports that SK channel blockade facilitated object memory encoding but not retrieval (Deschaux et al., 1997; Stackman et al., 2002). The behavioral effects observed after EBIO are likely due to the drug’s ability to activate SK channels. However, EBIO has also been shown to activate peripheral IK channels and to increase the non-SK mediated slow component of the afterhyperpolarization (Pedarzani et al., 2001). Our findings that CyPPA, an activator of SK2 and SK3 channels, impaired object memory similar to that after EBIO suggests that the amnestic effects of EBIO are due to its activation of SK2 and SK3 channels. However, given that CyPPA is a relatively new compound of which there is limited in vivo data, we cannot rule out the possibility that non-SK channel influences of these two compounds contribute to the observed behavioral effects.
The dose of EBIO that impaired object memory encoding, failed to significantly affect the encoding of contextual or cued fear memory during distinct conditioning paradigms that lead to weak and strong fear memory retention in vehicle-treated mice, respectively. Interestingly, systemic apamin improved contextual fear memory after a 1 CS-US pairing protocol; mice administered apamin prior to conditioning with a 1 CS-US pairing exhibited context test freezing levels comparable to those of vehicle-treated mice conditioned with 3 CS-US pairings. These data imply that fear conditioning may be sensitive to pharmacological blockade but not activation of SK channels. Furthermore, these data suggest that the influence of EBIO on memory may be task-specific. The overexpression of SK2 channels impairs contextual and cued fear memory (Hammond et al., 2006; Stackman et al., 2008). Each of the three distinct SK channel subtypes exhibits equivalent sensitivity to activation by EBIO (Pedarzani et al., 2001). Thus, it appears that the pan-SK channel activating property of EBIO is unlikely to produce all of the behavioral effects observed in mice that selectively overexpress SK2 channels. Taken together, these results indicate that activation of SK channels by systemic administration of EBIO impairs motor behavior and object memory, but spares fear memory and anxiety. Apamin-induced blockade of SK channels enhanced contextual fear memory encoded during a limited 1 CS-US conditioning session, but had no effect on fear memories encoded during a more extensive 3 CS-US pairing conditioning session. The effects of the SK channel activators or the SK channel blocker on object and fear memory, respectively, were not the result of changes in motor responses or motivational influences.
4.1. EBIO influence upon motor activity
Anderson et al. (2006) reported that systemic EBIO (> 20 mg/kg) produced motor ataxia in NMRI mice on the rotarod. However, doses of EBIO up to 40 mg/kg did not affect exploratory motor activity, as assessed by open field photobeam breaks averaged over a 1 h session (Anderson et al. 2006). We found that EBIO (> 10 mg/kg) depressed exploratory motor responses, which recovered to vehicle levels within 20–30 min (Fig 1A). It is likely that differences in mouse strain, vehicle, and the analysis method may have contributed to the disparity between our findings and those of Anderson et al. (2006).
The precise site(s) of action in the brain responsible for the motor-impairing effects of EBIO are not known. Chronic intracerebellar microinfusion of EBIO rescues ataxic behavior in ducky and tottering mutant mouse lines by restoring the intrinsic pacemaking property of cerebellar Purkinje neurons (Walter et al., 2006). Although it is possible that the motor deficits induced by systemic EBIO reflect the activation of SK channels within the cerebellum, chronic intracerebellar EBIO did not affect locomotor responses of C57BL/6J mice on the rotarod or heterozygous ducky mutant mice in the open field (Walter et al., 2006). Thus, the disruptive effects of EBIO on motor responses reported by Andersen et al. (2006) and here may not reflect the activation of cerebellar SK channels.
SK channels, predominantly SK3, are highly expressed within the mouse substantia nigra (Sailer et al., 2004). In vivo recordings of individual dopaminergic nigral neurons reveal that systemic EBIO decreased burst firing patterns, but not the average firing rate, within 5–10 min of administration (Ji and Shepard, 2006). The rate of onset of EBIO’s effects on firing patterns of midbrain dopamine neurons approximates the rate of onset of EBIO-induced motor deficits we observed in the open field. Furthermore, the microinfusion of apamin into the A10 dopaminergic region of midbrain potentiated the exploratory locomotor activity of rats in the open field (Steketee and Kalivas, 1990). Therefore, it is likely that systemic EBIO impairs motor function by transiently suppressing activity within the nigrostriatal dopaminergic and/or the mesolimbic/mesocortical pathways that are critical for motor behavior.
4.2. Influence of SK channel activators on learning and memory
A number of reports indicate that blocking SK channels with apamin enhances learning and memory in laboratory rodents (for a review see Tzounopoulos and Stackman, 2003). The SK1 and SK2 subunits are expressed in very high levels in brain regions important for learning and memory including the neocortex, hippocampus, and amygdala (Sailer et al., 2004). Further, SK channels are colocalized with NMDA receptors in dendritic spines of hippocampal and amygdalar pyramidal neurons. Blocking dendritic SK channels increases the amplitude of glutamatergic EPSPs and facilitates the induction of NMDA receptor-mediated synaptic plasticity (Faber et al., 2005; Lin et al., 2008; Ngo-Anh et al., 2005). Despite numerous studies showing the influence of apamin on rodent learning and memory, there have been no reports to date of the effects of SK channel activators on learning and memory. Taken together with evidence that blocking SK channels facilitates memory, the present findings likely reflect the ability of SK channel activators to impair the induction of synaptic plasticity. The present data support the view that SK channels play an important role in regulating cellular mechanisms of explicit memory.
4.2.2. Novel object recognition results
Systemic activation of SK channels during the sample session significantly reduced the amount of time mice explored a novel object presented 24 h later. EBIO-treated mice did not exhibit above chance degree of novel object preference and displayed a significant reduction in preference ratios when compared to vehicle-treated control mice. CyPPA-treated mice also exhibited impaired or chance level performance compared to the vehicle-treated mice. Although both SK channel activators impaired object memory, CyPPA appeared to produce a stronger deficit. These data support a modulatory role of SK channel activation upon object memory and suggests that the impairment produced by EBIO is more likely due to its activation of SK2 and SK3, than SK1 channels. Furthermore, these results indicate that the impairment in novel object preference of EBIO-treated mice was unlikely to be due to the drug activating peripheral IK channels. Consistent with an encoding specific effect, EBIO did not affect novel object preference when it was administered before the test session. Taken together, these results represent the first demonstration of SK channel activators impairing memory. It is interesting to note that activation of SK3 (and SK2) channels impairs memory in the object recognition task while the genetic deletion of the SK3 channels appears to not affect object memory (Jacobsen et al., 2009). It is possible that SK3 knockout may facilitate object memory encoding although this was not explicitly tested in Jacobsen et al. (2009).
In summary, our data provide further support for the view that SK channels regulate the encoding, but not the retrieval, of object memory. The specificity of the influence of SK channels on encoding is consistent with our previous finding that systemic apamin facilitated object memory encoding, but did not affect object memory retention (Stackman et al., 2002). The hippocampus is a critical neural substrate for object memory as assessed with the spontaneous novel object recognition task (Clark et al., 2000; Hammond et al., 2004) as is the perirhinal cortex (Brown and Aggleton, 2001; Bussey et al., 2000). Therefore, it is likely that SK channels, which are expressed at high levels in both neural regions, modulate the encoding of novel object recognition within either or both of these systems.
4.2.3. Delay fear conditioning results
Mice that over-express SK2 subunits exhibit a deficit in conditioned contextual and cued fear memory compared to wild type littermates. The deficit in contextual fear memory in SK2+/T mice was rescued by increasing the duration of context pre-exposure, indicating that SK2 overexpression impairs memory encoding (Stackman et al., 2008). In the present study, no differences were found in freezing between EBIO and vehicle or between apamin and vehicle-treated mice during the tests of contextual or cued fear memory 24 hr after conditioning with 3 CS-US pairings. Wiltgen et al. (2006) found that hippocampal lesions disrupted contextual fear memory after conditioning with one footshock, but spared context-related freezing after conditioning with three footshocks. Systemic EBIO also failed to affect conditioned fear memory after a 1 CS-US pairing protocol. However, mice treated with systemic apamin prior to 1 CS-US conditioning exhibited a significantly greater degree of freezing during the context test 24 h after conditioning compared to saline-treated controls. In fact, apamin-treated mice conditioned with 1 CS-US pairing froze, during the context test, at a level comparable to that of saline-treated mice conditioned with 3 CS-US pairings. These results suggest that blockade of SK channels facilitated the encoding of contextual fear memory during a conditioning protocol that results in considerably weaker freezing scores in vehicle-treated mice. This result is consistent with previous reports demonstrating that apamin facilitates memory encoding (Tzounopoulos and Stackman, 2003).
In summary, systemic EBIO (17.5 mg/kg) and CyPPA (15 mg/kg) impaired the encoding of object memory in C57BL/6NHsd mice. However, EBIO did not affect the encoding of contextual and cued fear memory. The present data support the view that SK channels play an important role in regulating cellular mechanisms of explicit memory. It will be of interest to delineate the CNS sites of action for the present findings using discrete intracranial microinfusion of SK channel activating compounds.
Acknowledgments
A subset of these data was presented in preliminary form at the 37th annual Society for Neuroscience meeting in San Diego, CA (October 2007). Dr. Yidan Lan of BASF generously provided Cremophor RH40. A special thanks is given to Dr. Palle Christophersen for insightful discussions and recommendations for determining the CyPPA dosage. This research was funded in part with an award from the National Science Foundation (Grant # IBN 0630522) to R.W.S.
References
- Anderson NJ, Slough S, Watson WP. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. Eur J Pharmacol. 2006;546:48–53. doi: 10.1016/j.ejphar.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brosh I, Rosenblum K, Barkai E. Learning-induced modulation of SK channels-mediated effect on synaptic transmission. Eur J Neurosci. 2007;26:3253–3260. doi: 10.1111/j.1460-9568.2007.05936.x. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement Y, Chapouthier G. Biological bases of anxiety. Neurosci Biobehav Rev. 1998;22:623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Bizot JC. Effect of apamin, a selective blocker of Ca2+-activated K+-channel, on habituation and passive avoidance responses in rats. Neurosci Lett. 1997;227:57–60. doi: 10.1016/s0304-3940(97)00301-7. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Bizot JC, Goyffon M. Apamin improves learning in an object recognition task in rats. Neurosci Lett. 1997;222:159–162. doi: 10.1016/s0304-3940(97)13367-5. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nature Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Fournier C, Kourrich S, Soumireu-Mourat B, Mourre C. Apamin improves reference memory but not procedural memory in rats by blocking small conductance Ca2+-activated K+ channels in an olfactory discrimination task. Behav Brain Res. 2001;121:81–93. doi: 10.1016/s0166-4328(00)00387-9. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Galvez A, Garcia-Calvo M, King VF, Vazquez J, Kaczorowski GJ. Use of toxins to study potassium channels. J Bioenerg Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, Strobaek D, Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol. 2007;151:655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Redrobe JP, Hansen HH, Petersen S, Bond CT, Adelman JP, Mikkelsen JD, Mirza NR. Selective cognitive deficits and reduced hippocampal brain-derived neurotrophic factor mRNA expression in small-conductance calcium-activated K+ channel deficient mice. Neuroscience. 2009;163:73–81. doi: 10.1016/j.neuroscience.2009.05.062. [DOI] [PubMed] [Google Scholar]
- Jensen BS, Strobaek D, Christophersen P, Jorgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol. 1998;275:C848–856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- Ji H, Shepard PD. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience. 2006;140:623–633. doi: 10.1016/j.neuroscience.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpari B, Regaya I, Escoffier G, Mourre C. Differential effects of two blockers of small conductance Ca2+-activated K+ channels, apamin and lei-Dab7, on learning and memory in rats. J Integr Neurosci. 2005;4:381–396. doi: 10.1142/s0219635205000884. [DOI] [PubMed] [Google Scholar]
- Mpari B, Sreng L, Regaya I, Mourre C. Small-conductance Ca2+-activated K+ channels: Heterogeneous affinity in rat brain structures and cognitive modulation by specific blockers. Eur J Pharmacol. 2008;589:140–148. doi: 10.1016/j.ejphar.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Stocker M. Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KA, Schroder RL, Skaaning-Jensen B, Strobaek D, Olesen SP, Christophersen P. Activation of the human intermediate-conductance Ca2+-activated K+ channel by 1-ethyl-2-benzimidazolinone is strongly Ca2+-dependent. Biochim Biophys Acta. 1999;1420:231–240. doi: 10.1016/s0005-2736(99)00110-8. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Jr, Bond CT, Adelman JP. Contextual memory deficits observed in mice overexpressing small conductance Ca2+-activated K+ type 2 (KCa2.2, SK2) channels are caused by an encoding deficit. Learn Mem. 2008;15:208–213. doi: 10.1101/lm.906808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Effect of microinjections of apamin into the A10 dopamine region of rats: a behavioral and neurochemical analysis. J Pharmacol Exp Ther. 1990;254:711–719. [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Stackman RW. Enhancing synaptic plasticity and memory: a role for small conductance Ca2+-activated K+ channels. Neuroscientist. 2003;9:434–439. doi: 10.1177/1073858403259282. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]