Abstract
In the classical renin-angiotensin system, circulating ANG II mediates growth stimulatory and hemodynamic effects through the plasma membrane ANG II type I receptor, AT1. ANG II also exists in the intracellular space in some native cells, and tissues and can be upregulated in diseases, including hypertension and diabetes. Moreover, intracellular AT1 receptors can be found associated with endosomes, nuclei, and mitochondria. Intracellular ANG II can function in a canonical fashion through the native receptor and also in a noncanonical fashion through interaction with alternative proteins. Likewise, the receptor and proteolytic fragments of the receptor can function independently of ANG II. Participation of the receptor and ligand in alternative intracellular pathways may serve to amplify events that are initiated at the plasma membrane. We review historical and current literature relevant to ANG II, compared with other intracrines, in tissue culture and transgenic models. In particular, we describe a new transgenic mouse model, which demonstrates that intracellular ANG II is linked to high blood pressure. Appreciation of the diverse, pleiotropic intracellular effects of components of the renin-angiotensin system should lead to alternative disease treatment targets and new therapies.
Keywords: intracrine, mitochondria, nuclear intracrine
the renin-angiotensin system is a phylogenetically ancient hormonal pathway, existing as a circulating system and serving pressor functions, even in primitive vertebrate cyclostomes, such as the river lamprey (127). In recent times, it has become clear that angiotensin can accumulate in various mammalian tissues and organs, including kidney (75, 100, 123), pancreas (22, 23, 86, 95), heart (78, 118, 144), and brain (89, 136, 165) independently of the circulating system and can mediate local effects. In both the circulating and local systems, the canonical effects of ANG II are mediated through the ANG II type 1 (AT1R) and type 2 (AT2R) G protein-coupled receptors. AT1R is the predominant receptor in adult animals and that which mediates contractile, pressor, secretory, and growth effects. AT2R, which shares only about 30% identity with AT1R, in contrast, may play a role in abrogating vasoconstrictor and growth responses to ANG II-mediated AT1R activation (21, 146, 147). AT1R is typically coupled through Gαq/11 to the second messengers 1,2 diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which, in turn, activate PKC and trigger release of intracellular calcium stores. AT1R can also be coupled to Ras, Raf-1, MAPK, phospholipase A, JAK-STAT, and Jun kinase pathways, among others (68, 103, 149, 163, 170). The AT1R is also reciprocally coupled to a number of receptor tyrosine kinases (RTKs), including EGF receptor and insulin-like growth factor-1 (117, 154). AT1R transactivates RTKs, thus stimulating ERK1/2 phosphorylation activation in hepatic cells, vascular smooth muscle cells (VSMC), and breast cancer cells (36, 113, 117, 138). This pathway appears to involve sequential activation of the AT1R, Gαq/11, PLC, and Src. The protein tyrosine kinase Src, in turn, activates a matrix metalloprotease [ADAM17 in the case of VSMCs (116)]. The matrix metalloprotease catalyzes cleavage and shedding of heparin-binding EGF, which activates the EGF receptor (141). Src may also directly bind and activate the receptor. Clearly then, ligand stimulation of AT1R at the plasma membrane influences multiple pathways, the collective outcome depending on cell type and environment.
Functional Intracellular AT1 Receptor
. . . In the cytoplasm.
For the G protein-coupled receptors (GPCRs), binding of agonist sequentially activates multiple G protein assemblies until the receptor desensitizes to agonist. Desensitization generally involves receptor phosphorylation by G protein-coupled receptor kinases, leading to enlistment of the β-arrestin adaptor protein and targeting the receptor for internalization into clathrin-coated pits. Classical GPCR signaling ceases following internalization into clathrin-coated pits. However, signaling can continue through an alternate G protein-independent β-arrestin-dependent pathway. β-arrestin can act as a scaffolding protein to link the AT1R to other signaling pathways, including p38 MAPK, ERK1/2, and JNK3 (c-Jun N-terminal kinase 3) (25, 63, 93, 142). While β-arrestin proteins were traditionally known for reducing receptor signals from the plasma membrane, it is clear that they can also initiate signals in the cytoplasm from the same receptors that they “desensitize” at the plasma membrane (142).
Ligand stimulation of AT1R can activate ERK1/2 directly through Gαq/11 or indirectly through a β-arrestin-mediated signaling pathway. G-protein-mediated AT1R activation of ERK is rapid, transient, and blocked by PKC inhibitors, and results in nuclear translocation of phospho-ERK. In contrast, the β-arrestin pathway is slower to commence and is characterized by persistent activated ERK associated with endocytic vesicles. Clearly, AT1R retains a function, albeit a static function, following internalization into cells. This permits signaling to continue “after receptor internalization” into endosomes. We believe this signaling pathway coincides with the large endosomes that have been described by Hunyady et al. (67) and Innamorati et al. (71) as the Rab11-positive perinuclear recycling compartment. Hunyady et al. (67) describe two pathways by which the AT1R may traffic. One is the Rab4-positive short recycling pathway, which leads to rapid recycling of the receptor to the cell surface. The other is the Rab11-positive long recycling pathway, in which the AT1R collects in endosomes of the perinuclear recycling compartment (PNRC). In a similar fashion, Shenoy and Lefkowitz (142) suggest that GPCRs can be categorized as class A (primarily recycled rapidly, and includes β2-adrenergic, ETA, and the μ-opioid receptor), receptors of which preferentially bind β-arrestin 1 and dissociate rapidly. Class B GPCRs (which include AT1R, neurotensin I, and TRHR) show equal affinity for β-arrestin 1 and β-arrestin 2 (142). The Class B GPCRs form stable complexes with and traffic together with arrestins, and they colocalize in endosomes for extended periods of time. In the absence of evidence to the contrary, we believe the arrestin-AT1R (class B) complexes, which are stable and continue signaling for significant periods of time, reside in the endosomes, described by Hunyady et al. (67) as the long recycling pathway, or PNRC.
We have also found that this compartment shares membranes with the Golgi apparatus (30), suggesting that materials could be retrotransported via the PNRC endosomes.
. . . At the nuclear membrane.
A number of prototypical GPCRs, including the type I lysophosphatidic acid (LPA) GPCR (LPA1), and the β-adrenergic receptor, exist as holoproteins in the nuclear membrane and possess nuclear functions. LPA1 is both constitutive in nucleus and traffics to nucleus in response to LPA treatment, and stimulates phosphorylation of intranuclear proteins, including the Akt kinase protein (52, 164). Moreover, LPA mediates, through the LPA1 receptor, eNOS translocation to perinuclear and nuclear sites. eNOS through nitric oxide, in turn, modulates calcium homeostasis and gene transcription (53). Similarly, β1- and β3-adrenergic receptors are present on nuclei of ventricular cardiomyocytes, bind ligand, and stimulate downstream effects, including adenylyl cyclase activity and MAPK activation (15, 159).
The AT1 receptor and the ANG-(1–7) receptor both localize within the nuclear membrane (30, 62, 111). Because the nuclear double-membrane is continuous with the endoplasmic reticulum (ER), receptors can flow freely between the two compartments (see Fig. 1). The diffusion-retention model for nuclear trafficking predicts that transmembrane or integral membrane proteins in the ER can diffuse laterally in a retrograde direction from the ER through the outer nuclear membrane and then through the lateral channels of the nuclear pore complexes and into the inner nuclear membrane (150, 166). This model predicts that proteins collect in the inner nuclear membrane at higher steady-state levels than in other compartments based on an association with resident proteins, which immobilize the diffusing species. Full-length GPCRs, such as AT1R, therefore, can accumulate in the inner nuclear membrane by retrograde passage from the ER (30). While Golgi modifications are not required for function of many GPCRs, including the AT1R (88), some nuclear membrane-associated receptors do appear to be glycosylated, suggesting that they may traffic to the ER from the Golgi via retrograde COPI vesicles (5) and then accumulate in the nuclear membrane via lateral diffusion from the ER.
Fig. 1.
Illustration of the nuclear envelope cross section. Receptors from the ER can traffic through the outer nuclear membrane, pore membrane domain, and into the inner nuclear membrane. Receptors can be maintained in the inner membrane by attachments to the lamina or chromatin. Figure was reproduced with kind permission from Springer Science+Business Media B.V. from The Local Cardiac Renin-Angiotensin Aldosterone System, 2nd ed., 2009, Chapter 4. “Intracellular Accumulation and Nuclear Trafficking of Angiotensin II and the Angiotensin II Type I Receptor,” Cook and Re, Fig. 4.1a, p. 31. [From Cook and Re (30).]
Such receptors have potential to interact with ligands present in the internuclear membrane space and to signal events in the nucleus through nuclear membrane signal transduction events (51) that may recapitulate plasma membrane events. For nuclear membrane-associated AT1R, the ligand binding site presumably exists in the internuclear membrane space (INMS). ANG II, therefore, must be present in the INMS to activate the receptor. In isolated nuclei studies, ANG II can enter the INMS via disruptions in the outer membrane or in the ER-nuclear membrane interface that are caused by the nuclear isolation technique. The availability of the receptor canonical binding site to ANG II should be dependent on the quality of the nuclei and contiguity of inner and outer nuclear membranes (79, 80). How might ANG II reach its nuclear membrane-associated receptor target in an intact cell? We suggest that ANG II can only gain access to the INMS through intercompartment membrane fusions (since the nuclear membrane-ER is a closed membrane system). A candidate population for vesicular delivery of ANG II is the endosomes of the perinuclear recycling compartment. We have shown (as have others) that AT1R localizes to these vesicles following internalization. We have further shown that these endosomes colocalize with Golgi (30). This fusion would, in theory, permit retrograde trafficking of ANG II to the INMS via COPI vesicles (5). This would also represent a mechanism for trafficking of modified (Golgi-processed) AT1R in a retrograde manner into the inner nuclear membrane.
Several studies have shown directly that the endothelin (ETB but not ETA) receptor and AT1R are present in both nuclear membranes and nucleosol (11, 13, 14, 30) and are directly activated to increase nuclear free calcium, suggesting that they are functional receptors. The fact that the corresponding ligands can be found within the nucleus as well, suggests that ligand-receptor interactions, which recapitulate those found at the plasma membrane, may exist at the nuclear membrane-nucleosol interface. Chappell and colleagues (61, 119, 120) have characterized AT1 nuclear membrane receptors in rat and in sheep kidney. They find both that ANG II upregulates reactive oxygen species in isolated renal nuclei through AT1 receptors and that nuclear AT2 receptors are functionally linked to nitric oxide production. In both fetal and adult sheep, most cortical nuclear and plasma membrane sites are AT2 receptor-like, while most medullary nuclear and plasma membrane sites correspond to AT1 receptors.
Several reports point to the existence and induction of phosphatidylinositol diphosphate (PIP), IP3, DAG, phospholipase C (PLC), and PKC in the nuclear compartment of many cell types, including 293, PC12, and NIH 3T3 (72, 73, 109, 152). For example, cellular treatment of human osteosarcoma cells with IL-1α induces intranuclear PIP and PLC (174), while treatment of 3T3 cells with IGF-1 induces enhanced nuclear DAG levels (106). Moreover, several cell types, including those which possess nuclear AT1, ET1, and neuropeptide Y GPCRs (including heart, hepatic, vascular endothelial, and smooth muscle cells) also possess calcium channels and Na+-H+ exchangers (11, 12), which have been proposed to modulate gene expression, proliferation, and cell remodeling. The data supporting the concept of nuclear signaling cascades that recapitulate plasma membrane cascades are abundant and support the dogma that the nucleus represents a modified “cell within a cell.”
. . . Intracellular receptor fragments.
A number of GPCRs, including the vasopressin V2, β2 adrenergic, and ETB receptors, are reported to undergo regulated limited proteolysis to produce peptides with possible bioactivity (57, 58, 60, 83, 84). For most GPCRs, however, it is unclear whether an intracellular fragment (as compared with an ectodomain fragment) is also generated during proteolysis, generally because the appropriate assays have not yet been performed. In addition, several GPCRs have been identified, associated with cellular nuclei, including those for ACh, ANG II, apelin, dynorphin B, endothelin 1, and prostaglandin E2, and often using multiple different approaches (51, 92). For instance, the AT1 receptor has been localized to nuclei in several different independent studies using techniques that include radioligand binding and chromatin solubilization assays of rat liver nuclei, immunohistochemistry of rat brain, electrophysiology assays of rat cardiac myocytes, ANG II microinjection and calcium assays, immunocytochemistry, and Western blot of rat brain neurons, and immunocytochemistry and Western blot of human VSMCs (28, 51, 129, 131). In these nuclear association studies, assays have not generally been designed to differentiate between cleaved receptor fragments and holoreceptors.
In tissue culture studies, we have specifically addressed the nature of the intranuclear AT1 receptor using a double-fusion protein of AT1R, in which yellow fluorescent protein is fused downstream and cyan fluorescent protein (CFP) is fused upstream. We have shown that the fluors colocalize in vehicle-treated transfected cells, whereas the fluors diverge in ANG II-treated transfected cells (28). The receptor, therefore, is cleaved in a ligand-dependent manner. Cyan fluorescence is lost from the cell surface (seen as a reduction in blue fluorescence at the circumference), while yellow fluorescence accumulates in nuclei. Similar results are obtained when alternate tags (myc downstream and Flag upstream) are substituted for the fluorescent moieties. The cleavage occurs in genetically unmodified protein as well, releasing a stable 6-kDa protein within cells (28, 30, 32). The cleavage fragment (CF or AT1RCF) does possess a nuclear localization signal, and mutation of the consensus reduces, but does not inhibit, nuclear localization (32). Active transport may occur to increase accumulation of the CF, but the size of the cleavage fragment should permit passive diffusion into the nucleus through nuclear pores (76, 77).
For comparison, an intracellular fragment is also produced from the GPCR, D-frizzled 2, a receptor for the ligand, wingless (or Wnt in mammals), which is involved in pattern development in Drosophila and mammals. The intracellular domain of D-frizzled 2 is cleaved from endosomes and translocated to the nucleus where it is involved in transcriptional events that support pattern formation (108). The carboxy terminus of Frizzled-7 is also cleaved, although the fate of the resulting intracellular fragment has not yet been reported (151) Furthermore, an intracellular cleavage fragment is produced from the carboxy terminus of polycystin, a noncanonical GPCR having 11 transmembrane domains (9, 101). Following nuclear localization, the carboxy-terminal fragment inhibits the ability of β-catenin to induce T-cell factor-dependent gene transcription and, thereby, inhibits the Wnt signaling pathway (87). The detailed processes and enzymes responsible for cleavage of most multipass receptors are not clear, although a family of intramembrane proteases referred to as IMPAS/PSH/signal peptide peptidase (or SPP) is implicated (112). Collectively, these studies indicate that cleavage of receptors and other cell surface proteins, as well as accumulation of stable intracellular products, can be regulated processes, which serve, perhaps, to further amplify or enhance effects of ligand-receptor signal transduction events, which initiate at the plasma membrane.
We have recently determined the precise cleavage site within the AT1R by mass spectrometry and Edman sequencing (32). Cleavage occurs between Leu305 and Gly306 at the junction of the 7th transmembrane domain and the intracellular cytoplasmic carboxy-terminal domain. To evaluate the function of the CF distinct from the holoreceptor, we generated a construct encoding the CF as an in-frame yellow fluorescent protein fusion. The CF accumulates in nuclei and induces apoptosis, as determined by nuclear fragmentation and disintegration, phosphotidylserine displacement in the plasma membrane caspase activation, poly(ADP-ribose) polymerase upregulation, and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) labeling and DNA fragmentation. CF-induced apoptosis appears to be a general phenomenon, as it is observed in multiple cell types, including smooth muscle cells and cardiomyoblasts.
Interestingly, many intracellular cleavage products correlate with cell death and apoptosis, consistent with the action of AT1RCF. For example, the receptor for advanced glycation end products (RAGE) has been linked to several chronic diseases thought to result from vascular damage, including atherosclerosis, peripheral vascular disease, Alzheimer's disease, and congestive heart failure. RAGE is targeted by regulated intramembrane proteolysis, producing both an extracellular soluble fragment (sRAGE), as well as an intracellular domain; the intracellular protein is detected in both the cytoplasm and nucleus (50). Transfected human embryonic kidney 293 cells that exhibit accumulation of this product in the nucleus also show nuclear condensation and cell shrinkage. This is accompanied by a 16% and 38% reduction in cell viability at 16 and 40 h posttransfection, respectively, and also in an increase in TUNEL-positive cells at 16 h posttransfection.
Regulated intramembrane proteolysis is also involved in the pathogenesis of Alzheimer's disease through a pathway distinct from RAGE. The transmembrane amyloid precursor protein (APP) gives rise to the Aβ peptide cleavage product, which is found in plaque fibrils and tangles (4). The APP also gives rise to an APP intracellular domain (AICD), which translocates to the nucleus and appears to contribute to the pathogenesis of Alzheimer's disease, perhaps by regulating nuclear signaling (160). Recent studies have shown that overexpression of the AICD in neurons induces cell death, as determined by TUNEL assays and DNA laddering (115), possibly in collaboration with Fe65 and p53. Another example of cleavage fragment-induced apoptosis occurs in a family of receptors that are involved both in internalization of ligands and also in signal transduction and neurotransmission. Cleavage of both the low-density lipoprotein receptor-related protein (LRP), as well as the related LRP1 contributes to apoptosis. LRP undergoes regulated intramembrane proteolysis in response to ischemia in neurons with nuclear translocation of the intracellular domain. The latter induces caspase-3 cleavage, TUNEL positivity, and significant cell death (124).
Clearly then, other receptor cleavage fragments, like the AT1RCF, have been associated with nuclear transport and apoptosis. An underlying homology in the sequences of the cleaved peptides, however, is not readily apparent. Nor is there any unambiguous reason why regulated proteolysis of these particular diverse receptors might be linked to cell death. Further investigation of the caspase pathways activated by the AT1RCF may be helpful in formulating a thesis.
Functional Intracellular Angiotensin
Studies have been reported since the 1970s that support the existence and functionality of intracellular or intracrine angiotensin II (iANG II). These studies originate in many laboratories and include a broad spectrum of models and techniques that can be collected into distinct bodies of work that support the following ideas: 1) exogenous ANG II associates with cellular nuclei and other subcellular organelles from rat heart and liver (3, 18, 66, 130, 132–134, 136–138, 159, 160), 2) endogenous iANG II exists in distinct cells and tissues, such as rat juxtaglomerular cells, proximal tubule cells, and brain [including anterior pituitary, subfornical organ, hypothalamus, and third ventricle (2, 45, 70, 110, 135, 153, 161, 172, 173)], 3) ANG II can be detected in nuclei of native cells in rat cerebellar cortex, human endocardial endothelial cells, rat renal cortex, and porcine kidney and adrenals (45, 49, 74, 99, 158), 4) introduced (microinjected or genetically expressed) ANG II possesses intracellular function in, among other tissues, rat hepatoma and VSMCs, hamster ventricular cardiac myocytes, rabbit proximal tubule cells, and transfected cells, including CHO-K1 and COS-7 cells (26, 27, 29, 31–33, 37, 39, 40, 173), and 5) ANG II stimulation can cause AT1R translocation to nucleus in human and rat VSMCs, CHO-K1, and COS-7 cells, and rat brain neurons (14, 27–30, 102).
. . . Intracellular ANG II from intracellular angiotensinogen.
To investigate the potential for (and subsequent effect of) ANG II generation within cells, we mutated an angiotensinogen (AGT) cDNA to remove the signal peptide required for secretion through the secretory pathway. We ligated it into an expression plasmid to produce a nonsecreted form of AGT in transfected cells and confirmed that ANG II is, indeed, expressed and retained in cells following transfection. ANG II produced from this construct is generated through an intracrine mechanism. We investigated the effect of this plasmid, following transient and stable transfections and in the presence of various effectors, upon cellular proliferation (33). Proliferation of rat hepatoma cell lines was increased an average of 39%, growth that was not affected by the AT1R blocker candesartan (which acts exogenously) but was blocked by renin antisense phosphorothioate oligomers, suggesting that growth is stimulated through a renin-dependent pathway. This study showed that ANG II can be processed from AGT, which is retained within cells. Therefore, assuming that AGT can be internalized and accumulate in native cells and tissues or assuming that a nonsecreted form of AGT might be generated and retained in cells, there is potential for ANG II intracellular generation in native tissues (30).
A number of other hormones and growth factors, including prolactin, insulin, nerve growth factor, epidermal growth factor, somatostatin, neuropeptide Y, and platelet-derived growth factor have been found to have biologically significant intracellular activities (17, 41, 46, 54, 85, 126, 128, 130). Clearly, where growth factors have dual extracellular and intracellular roles, they may be 1) secreted locally and internalized or 2) processed from a precursor (where applicable) and retained with the cell. Hepatoma-derived growth factor (HDGF), a heparin-binding growth factor, similar to the fibroblast growth factors, functions through the latter mechanism. It is a secreted growth factor ubiquitously expressed and mitogenic for fibroblasts and endothelial cells (46, 114). However, HDGF is also present in nuclei (its sequence contains a putative nuclear localization element) of a variety of cell types and is involved in proliferation during development and disease (46, 114). Following transfection of hemagglutinin-tagged HDGF, the tag is observed in scattered isolated nuclei, not in cell clusters, suggesting that it directly translocates to the nucleus and is not locally secreted, internalized, and subsequently translocated to the nucleus. HDGF can reside in the cytoplasm or nucleus, or be secreted, depending on the cell type and cell-cycle phase, and nuclear HDGF has been associated with hepatocellular carcinoma, pancreatic cancer, colorectal stromal tumors, and nasopharyngeal carcinoma (65, 66, 94, 155, 162).
Consistent with evidence that elevation of intracellular HDGF may directly reflect the disease state of a cell, iANG II in myocardium (myocytes and endothelial cells) of diabetic rats and diabetic hypertensive patients may similarly reflect disease severity. In rat models, high glucose stimulates elevation of intracellular ANG II, oxidative stress, and fibrosis (145). In human diabetic patients, intracellular ANG II levels in hearts are increased 3.4- and 3.1-fold, respectively, in myocytes and endothelial cells over levels present in nondiabetic patients. Intracellular ANG II levels appear to be increased an additional twofold in diabetic hypertensive patients compared with diabetic nonhypertensive patients (48). The authors suggest that local elevations of ANG II, which accompany diabetes and hypertensive diabetes, in turn, enhance oxidative damage and activate cardiac cell apoptosis and necrosis. Elevated iANG II may, therefore, be both a marker of disease and a contributor to disease progression.
In some cases, internal production or internalization of a peptide or protein has been proven to mediate very different effects from those resulting from interaction with cell surface receptors. For example, parathyroid hormone-related protein (PTHrP) acting within nuclei of vascular smooth muscle cells stimulates cell division and contributes to growth of breast and colon cancers, whereas extracellular PTHrP inhibits proliferation (10, 85, 107). The PDGF family of proteins provides an additional example. Binding of the v-sis or PDGF B proteins to the (underglycosylated) 160-kDa PDGF receptor within the secretory compartment elicits a transforming signal that is not reproduced by v-sis or PDGF B binding to the mature 180-kDa receptor on the cell surface (7, 8, 18). Ligand-receptor binding within the secretory compartment is both necessary and sufficient for v-sis-mediated transformation. Neither v-sis nor PDGF-B (which possesses 94% amino acid homology to v-sis) can transform immortalized cell lines by binding surface PDGF receptors. In addition to the well-characterized PDGF external autocrine loop and internal autocrine (intracrine) loop involving receptor activation within the processing compartments, PDGF may have a third autocrine function. v-Sis has been localized to the nucleus (as well as polyribosomes, endoplasmic reticulum, and Golgi apparatus) of simian sarcoma virus-transformed fibroblasts (122, 171). Indeed, prominent immunoreactive proteins have been found in association with nuclear chromatin in intact cells and in isolated nuclei. Nuclear localization signals have also been mapped to v-sis, PDGF B, and PDGF A protein products (91, 105), suggesting that PDGF might be transported to the nucleus following binding and internalization. PDGF is an example of a growth factor that appears to function at multiple sites and through multiple pathways to achieve its regulatory effects. We believe that ANG II in a similar fashion may act at the cell surface or within the nucleus or mitochondria and that it may be internalized or generated internally, depending upon cell type, cell age, genetic background, and cellular environment (or culture conditions).
In conclusion, these studies support the hypothesis that angiotensin and a growing list of additional peptides, growth factors, and cytokines can generate biologically relevant effects by acting at intracellular sites. At least some of these events follow retention and action of the intracrine in the cell of synthesis.
. . . Internalized extracellular ANG II.
The existence of an intracellular renin-angiotensin system (iRAS) implies that components of the RAS are made locally and result in biologically functional intracellular angiotensins, renin, and/or receptor. Studies show that measurable levels of ANG II exist within some cells and that ANG II may be released from certain cell types (e.g., cardiac myocytes and mesangial cells) following mechanical stimuli, such as stretching (6, 38, 64, 69, 134, 139, 157). From where does this intracellular ANG II originate? Existing intracellular ANG II may be internalized from the circulation or extracellular fluid, or alternatively, produced intracellularly.
One model for the accumulation of iANG II relies on the knowledge that ANG II, within ligand-receptor complexes, is internalized via clathrin-coated pit-mediated endocytosis. Because it is well known that contents of endosomes can leak into the cytoplasm (19, 20, 24), ANG II may directly discharge into the cytosol and could access binding sites within the cytosol and nucleosol (the latter via nuclear pores). Several studies show that megalin may play a role in internalization of AGT and ANG II in the proximal nephron. Megalin is a multiligand scavenger receptor involved in protein endocytosis. Studies suggest that in several cell types, including yolk sac epithelium and proximal tubule (brush border membrane vesicles), megalin can bind to and internalize ANG II and ANG 1–7 (55, 56). In more recent studies, megalin was also found to internalize AGT in the early proximal tubule (123). The favored model is that AT1R-mediated ANG II uptake favors iANG II accumulation, whereas megalin-mediated ANG II uptake favors iANGII degradation, but megalin could, in theory, mediate ANG II transport to intracellular sites.
To our collective knowledge, few studies have been designed to address ANG II transport to the internuclear membrane space. We have proposed and have conducted some preliminary studies to support the idea that ANG II may reach the intranuclear membrane space by way of recycling endosomes (30) (see subsection . . . In the cytoplasm).
Transgenic Mouse Models
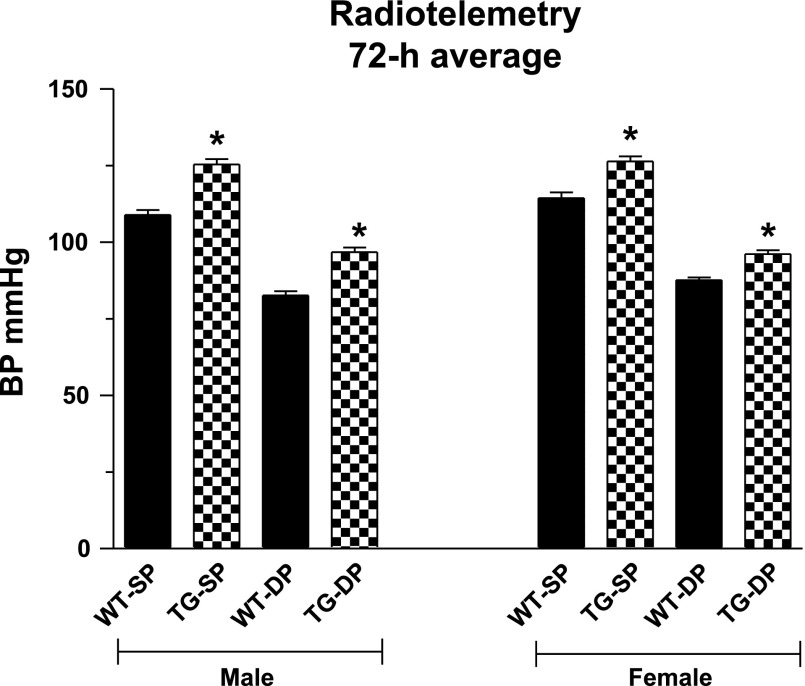
The functions of renin, angiotensin, ACE, and RAS receptors has been extensively studied in transgenic models (47, 59, 82, 96, 98, 135). Moreover, the role of intracellular renin has also be evaluated in several transgenic studies (90, 97, 121, 167, 168). While intracellular angiotensin has been broadly studied in cell culture and in vivo following adenoviral delivery (3, 97), we recently reported the first study describing a transgenic mouse model, which directly expresses intracellular ANG II, independent of secreted AGT or secreted ANG II (133). Our approach was based on results from our earlier in vitro studies of a protein fusion of angiotensin, in which ANG II is fused downstream and in-frame with enhanced cyan fluorescent protein (ECFP/ANG II) (with a 10-amino acid spacer arm between the two moieties). Following transfection, ANG II in the context of this fusion is detectable within cells using anti-ANG II antibodies, by Western blot, and by enzyme immunoassay. ECFP/ANG II is shown to remain intact and not degraded by Western blot analysis. In tissue culture studies, we found iANG II to alter the steady-state distribution of fluorescently labeled AT1R with receptor translocating to the nucleus of COS-7, CHO-K1, and rat vascular smooth muscle cells. We also found ANG II to increase proliferation and activate CREB in all of these cell types. Moreover, we confirmed that ECFP/ANG II is maintained within cells (not released) and activates some signal transduction pathways uniquely different from exogenous ANG II signaling. These studies prompted us to generate a transgenic mouse line overexpressing ECFP/ANG II (133). We selected the metallothionein promoter to drive expression to a wide array of tissues with the potential for metal regulation of transgene expression. While transgene is expressed (RNA and protein) in all major tissues in two independent mouse lines, kidney is the only tissue in which we detect a phenotypic change. As early as 2 mo of age, these mice demonstrate elevated systolic and diastolic blood pressures, as determined by radiotelemetry (Fig. 2) (133). Some mice also display kidney thrombotic microangiopathy (TMA) and microthrombosis in the glomerular capillaries and small vessels. TMA has been observed in other transgenic models that overexpress components of the RAS (104, 140). iANG II-mediated changes in blood pressure (BP) are observed as early as 2 mo of age. TMA, which follows at 4–6 mo of age (and ranges from very mild to severe), is not, therefore, responsible for elevated BP in this model. We observe no sex-specific effects of ECFP/ANG II on BP or TMA; males and females are affected to the same extent by the transgene. Circulating ANG II can, in contrast, cause sex-specific effects. Elevation of circulating ANG II, as occurs in rodent infusion of ANG II, can cause sexually dimorphic changes in BP that have been linked to gonadal hormones, baroreflex control of HR/BP, and sympathetic nerve activity (169). We are not aware of any reports in which intracellular ANG II or receptor show sex-specific differences in distribution or function. However, because iANG II can act at a number of divergent locations in the cell and through a number of distinct mechanisms, some of the intracellular effects could potentially be sex-specific. Studies have shown that ANG II infusion into mice stimulates NAD(P)H oxidase and reactive oxygen species (ROS) species formation in a sex-specific fashion (44, 156). There also exists evidence that ANG II treatment of isolated nuclei increases NAD(P)H oxidase activity and ROS formation through an AT1R pathway (120), suggesting that some nuclear iANG events might also be sex-specific.
Fig. 2.
Enhanced cyan fluorescent protein (ECFP)/ANG II (line A mice) transgenic mice exhibit increased average (day and night) blood pressures compared with wild-type littermates by radiotelemetry. Bars represent averages over a 72-h data collection period (30 s every 30 min). WT, wild-type; TG, transgenic; SP, systolic pressure; DP, diastolic pressure. Values are expressed as means ± SE; n = 9 for all groups. *P < 0.001 for TG vs. corresponding WT (unpaired t-test).
Analysis of isolated mouse embryonic fibroblasts (MEFs) from transgenic mice shows the presence of ECFP/ANG II in the cytoplasm and in some (30–40%) nuclei. The cytoplasmic fluorescence is often punctate and significantly colocalizes with mitochondrial markers, suggesting a mitochondrial function (31, 132). Our studies and those from other laboratories have demonstrated ANG II and ANG II binding sites associated with mitochondria of MEFs, kidney, adrenals, and brain (45, 132, 148, 158), and mitochondria have been implicated in RAS-mediated disease, including vascular endothelial dysfunction, hypertension, and diabetes (31, 34, 35, 42, 43, 132). Unquestionably, mitochondria play a role in RAS-mediated disease. It remains to be determined whether and to what extent ANG II mediates its mitochondrial effects through canonical (membrane-associated receptor) vs. noncanonical means. Early studies from our laboratory indicate a direct interaction between iANG II and mitochondrial electron transport chain components, as well as effects on ATP generation and the formation of ROS. Direct binding of iANG II to mitochondrial proteins would preclude binding to the binding pocket of integral membrane AT1R. These data, therefore, suggest a noncanonical action of ANG II at mitochondria (31, 132). The fact that intracellular ANG II also increases blood pressure in our transgenic model suggests that noncanonical ANG II action at mitochondria plays a role in ROS generation and hypertension.
Abadir et al. (1) have recently presented compelling evidence for the existence of functional intramitochondrial angiotensin receptor. They evaluated several cell types (including mouse cardiomyocytes and renal tubule, vascular endothelial, and neuronal cells) and showed the presence of AT2R in all of these using Immunogold staining. AT1R was typically present only very rarely in 5-mo-old mice but increased in prevalence in aged mice (greater than 1 yr of age). For example, in proximal tubules, this paper reports an average of two occurrences of AT1R per mitochondrion, (increasing to 12 molecules in aged mice) and ∼25 copies of AT2R per mitochondria, that number falling to 10 in aged mice. Moreover, they show mitochondrial AT2R to be coupled to increased mitochondrial nitric oxide production, suggesting a link between AT2R and mitochondrial aging in disease. These data support a canonical role for angiotensin receptors in mitochondria.
Perspectives and Significance
Collectively, the published reports suggest that ANG II may be internalized or generated through an intracellular system and that it may alter cellular properties both through cytoplasmic protein interactions and through nuclear translocation, receptor binding, and transcriptional regulation of gene expression (Fig. 3). iANG II that is both internalized through receptor-mediated endocytosis and potentially processed from intracellular AGT may have noncanonical functions in the cytoplasm or nucleosol or may function in a canonical fashion through nuclear membrane-associated receptor. Moreover, ANG II may act at the level of the mitochondria through canonical (mitochondrial membrane-associated receptor) or noncanonical mechanisms. Most importantly, iANG II alters cellular proliferation and signal transduction and elevates blood pressure through a renal mechanism in transgenic mice. Furthermore, the AT1 receptor can function independently of ANG II, participating in a cytoplasmic β-arrestin-mediated scaffold for signaling events. Moreover, cleavage fragments of the receptor can traffic to the nucleus and induce apoptosis.
Fig. 3.
Model of pathways through which the iRAS may mediate intracellular effects. [1] Endosomes containing embedded AT1R can be internalized and AT1R can continue to signal through the β-arrestin:AT1R complex which serves as a scaffold for assembly of ERK and JNK signaling components (142). [2] Acidification of endosomes (20, 24) permits release of ANG II into the cytoplasm, where it may traffic to nucleus [3] or mitochondria [4], either free or bound to other proteins. Cytosolic ANG II can mediate signaling effects by modifying protein complex activity, or it can be transported into the nucleus as a complex or as a free peptide. In the nucleus, association of ANG II with other proteins, including, potentially, a nuclear form of ANG II receptor may permit modification of gene expression or DNA replication. In addition, a cleaved fragment of the AT1R (COOH terminus) (28) [5, 6] may associate with a protein complex that includes ANG II [7] and traffic to nucleus [8]. AT1R can accumulate in nuclear membrane (11, 30) presumably by retrograde membrane diffusion from the endoplasmic reticulum (ER)/Golgi apparatus. Receptor associated with the inner nuclear membrane [by movement from the outer nuclear membrane around the nuclear pore complex (137, 166)] is positioned such that the COOH terminus is within the nucleosol [9] and available for signaling through nuclear second messenger signaling pathways (11, 12, 16, 81, 125). Presumably, nuclear signaling through nuclear membrane-associated AT1R is ligand mediated. We believe that ANG II may gain access to the ER lumen and, subsequently, to the intranuclear membrane space, via trafficking through the slow recycling endosome pathway (30, 67). [10] In addition, ANG II may be generated within cells from intracellular AGT (30, 143).
Clearly, the RAS is proving to be far more complex than could have been predicted a mere decade ago and contributes to the principle that biological systems are inherently efficient, often showing signal amplification in successive steps of a given pathway, with reutilization and minimal waste. Although we know that signal transduction contributes to the specificity of ligand-mediated responses, it more importantly contributes to amplification of the response. We argue that the continued signaling that occurs in intracrine systems and through nonclassical intracellular receptor functions represents an extension of the amplification principle, permitting increased magnitude and/or duration of response.
The development of new drugs directed to noncanonical functions of intracrines and atypical intracellular receptors and receptor fragments represents a new pharmaceutical industry interest. Most GPCR-modulating drugs on the market were not initially targeted to a specific protein but were developed on the basis of a functional activity assay. The observation that a candidate drug activated or inhibited a GPCR, in most cases, was only later discovered. GPCR-targeted drugs typically inhibit cell surface receptors and are often not specifically designed to be efficiently internalized into cells. Moreover, they are not designed to target noncanonical intracrine functions. Effective targeting of nonclassical functions of GPCRs and targeting of cleaved fragments or intracellular domains generated from plasma membrane proteins will, in most cases, require novel strategies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.L.C. interpreted results of experiments; J.L.C. prepared figures; J.L.C. drafted manuscript; J.L.C. and R.N.R. edited and revised manuscript; J.L.C. and R.N.R. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by the Ochsner Clinic Foundation and National Institutes of Health/National Heart, Lung, and Blood Institute Grant HL-072795.
REFERENCES
- 1. Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amedeo Modesti P, Zecchi-Orlandini S, Vanni S, Polidori G, Bertolozzi I, Perna AM, Formigli L, Cecioni I, Coppo M, Boddi M, Serneri GG. Release of preformed Ang II from myocytes mediates angiotensinogen and ET-1 gene overexpression in vivo via AT1 receptor. J Mol Cell Cardiol 34: 1491–1500, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept 120: 5–13, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Barten DM, Albright CF. Therapeutic strategies for Alzheimer's disease. Mol Neurobiol 37: 171–186, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Beck R, Rawet M, Wieland FT, Cassel D. The COPI system: molecular mechanisms and function. FEBS Lett 583: 2701–2709, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Becker BN, Yasuda T, Kondo S, Vaikunth S, Homma T, Harris RC. Mechanical stretch/relaxation stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Exp Nephrol 6: 57–66, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Bejcek BE, Hoffman RM, Lipps D, Li DY, Mitchell CA, Majerus PW, Deuel TF. The v-sis oncogene product but not platelet-derived growth factor (PDGF) A homodimers activate PDGF alpha and beta receptors intracellularly and initiate cellular transformation. J Biol Chem 267: 3289–3293, 1992 [PubMed] [Google Scholar]
- 8. Bejcek BE, Li DY, Deuel TF. Transformation by v-sis occurs by an internal autoactivation mechanism. Science 245: 1496–1499, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Bertuccio CA, Chapin HC, Cai Y, Mistry K, Chauvet V, Somlo S, Caplan MJ. Polycystin-1 C-terminal cleavage is modulated by polycystin-2 expression. J Biol Chem 284: 21011–21026, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhatia V, Saini MK, Falzon M. Nuclear PTHrP targeting regulates PTHrP secretion and enhances LoVo cell growth and survival. Regul Pept 158: 149–155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bkaily G, Avedanian L, Jacques D. Nuclear membrane receptors and channels as targets for drug development in cardiovascular diseases. Can J Physiol Pharmacol 87: 108–119, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Bkaily G, Nader M, Avedanian L, Choufani S, Jacques D, D'Orleans-Juste P, Gobeil F, Chemtob S, Al-Khoury J. G-protein-coupled receptors, channels, and Na+-H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can J Physiol Pharmacol 84: 431–441, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Bkaily G, Pothier P, D'Orleans-Juste P, Simaan M, Jacques D, Jaalouk D, Belzile F, Hassan G, Boutin C, Haddad G, Neugebauer W. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol Cell Biochem 172: 171–194, 1997 [PubMed] [Google Scholar]
- 14. Bkaily G, Sleiman S, Stephan J, Asselin C, Choufani S, Kamal M, Jacques D, Gobeil F, Jr, D'Orleans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol 81: 274–287, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci 122: 2337–2350, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Bouche G, Gas N, Prats H, Baldin V, Tauber JP, Teissie J, Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0–G1 transition. Proc Natl Acad Sci USA 84: 6770–6774, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Browder TM, Dunbar CE, Nienhuis AW. Private and public autocrine loops in neoplastic cells. Cancer Cells 1: 9–17, 1989 [PubMed] [Google Scholar]
- 19. Brown MS, Anderson RG, Basu SK, Goldstein JL. Recycling of cell-surface receptors: observations from the LDL receptor system. Cold Spring Harb Symp Quant Biol 46: 713–721, 1982 [DOI] [PubMed] [Google Scholar]
- 20. Brown MS, Anderson RG, Goldstein JL. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell 32: 663–667, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 35: 155–163, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Chan WP, Fung ML, Nobiling R, Leung PS. Activation of local renin-angiotensin system by chronic hypoxia in rat pancreas. Mol Cell Endocrinol 160: 107–114, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Chappell MC, Millsted A, Diz DI, Brosnihan KB, Ferrario CM. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens 9: 751–759, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol 55: 721–734, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Cook JL. G protein-coupled receptors as disease targets: emerging paradigms. Ochsner J 10: 2–7, 2010 [PMC free article] [PubMed] [Google Scholar]
- 26. Cook JL, Giardina JF, Zhang Z, Re RN. Intracellular angiotensin II increases the long isoform of PDGF mRNA in rat hepatoma cells. J Mol Cell Cardiol 34: 1525–1537, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol 40: 696–707, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Cook JL, Mills SJ, Naquin RT, Alam J, Re RN. Cleavage of the angiotensin II type 1 receptor and nuclear accumulation of the cytoplasmic carboxy-terminal fragment. Am J Physiol Cell Physiol 292: C1313–C1322, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol 36: 75–90, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Cook JL, Re RN. Intracellular accumulation and nuclear trafficking of angiotensin II and the angiotensin II type 1 receptor. In: The Local Cardiac Renin-Angiotensin Aldosterone System (2nd ed), edited by Frohlich ED, Re RN. New York: Springer, 2009, chapt.4, p. 29–41 [Google Scholar]
- 31. Cook JL, Singh A, Aguiluz RN, Re RN, Alam J. Intracellular angiotensin II binds to mitochondrial proteins of the NADH dehydrogenase complex and modifies oxidative phosphorylation, P387LB. Hypertension 56: e135, 2010 [Google Scholar]
- 32. Cook JL, Singh A, Deharo D, Alam J, Re RN. Expression of a naturally occurring angiotensin AT1 receptor cleavage fragment elicits caspase-activation and apoptosis. Am J Physiol Cell Physiol 301: C1175–C1185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res 89: 1138–1146, 2001 [DOI] [PubMed] [Google Scholar]
- 34. de Cavanagh EM, Ferder L, Toblli JE, Piotrkowski B, Stella I, Fraga CG, Inserra F. Renal mitochondrial impairment is attenuated by AT1 blockade in experimental Type I diabetes. Am J Physiol Heart Circ Physiol 294: H456–H465, 2008 [DOI] [PubMed] [Google Scholar]
- 35. de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol 27: 545–553, 2007 [DOI] [PubMed] [Google Scholar]
- 36. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 37. De Mello WC. Intracrine action of angiotensin II in the intact ventricle of the failing heart: angiotensin II changes cardiac excitability from within. Mol Cell Biochem 358: 309–315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Mello WC. Is an intracellular renin-angiotensin system involved in control of cell communication in heart? J Cardiovasc Pharmacol 23: 640–646, 1994 [DOI] [PubMed] [Google Scholar]
- 39. De Mello WC, Gerena Y. Eplerenone inhibits the intracrine and extracellular actions of angiotensin II on the inward calcium current in the failing heart. On the presence of an intracrine renin angiotensin aldosterone system. Regul Pept 151: 54–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Mello WC, Gerena Y. Further studies on the effects of intracrine and extracellular angiotensin II on the regulation of heart cell volume. On the influence of aldosterone and spironolactone. Regul Pept 165: 200–205, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Delrieu I. The high molecular weight isoforms of basic fibroblast growth factor (FGF-2): an insight into an intracrine mechanism. FEBS Lett 468: 6–10, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens 25: 1263–1271, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Erdmann B, Fuxe K, Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension 28: 818–824, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Everett AD, Lobe DR, Matsumura ME, Nakamura H, McNamara CA. Hepatoma-derived growth factor stimulates smooth muscle cell growth and is expressed in vascular development. J Clin Invest 105: 567–575, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 106: 373–382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Fu ML, Schulze W, Wallukat G, Elies R, Eftekhari P, Hjalmarson A, Hoebeke J. Immunohistochemical localization of angiotensin II receptors (AT1) in the heart with anti-peptide antibodies showing a positive chronotropic effect. Receptors Channels 6: 99–111, 1998 [PubMed] [Google Scholar]
- 50. Galichet A, Weibel M, Heizmann CW. Calcium-regulated intramembrane proteolysis of the RAGE receptor. Biochem Biophys Res Commun 370: 1–5, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol 84: 287–297, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Gobeil F, Jr, SG, Vazquez-Tello A, Brault S, Beauchamp MH, Quiniou C, Marrache AM, Checchin D, Sennlaub F, Hou X, Nader M, Bkaily G, Ribeiro-da-Silva A, Goetzl EJ, Chemtob S. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J Biol Chem 278: 38875–38883, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Gobeil F, Jr, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, D'Orleans-Juste P, Goetzl EJ, Chemtob S. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem 281: 16058–16067, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Goldfine ID, Smith GJ, Wong KY, Jones AL. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci USA 74: 1368–1372, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gonzalez-Villalobos R, Klassen RB, Allen PL, Johanson K, Baker CB, Kobori H, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin-(1–7). Am J Physiol Renal Physiol 290: F1270–F1275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol 288: F420–F427, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Grantcharova E, Furkert J, Reusch HP, Krell HW, Papsdorf G, Beyermann M, Schulein R, Rosenthal W, Oksche A. The extracellular N terminus of the endothelin B (ETB) receptor is cleaved by a metalloprotease in an agonist-dependent process. J Biol Chem 277: 43933–43941, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Grantcharova E, Reusch HP, Grossmann S, Eichhorst J, Krell HW, Beyermann M, Rosenthal W, Oksche A. N-terminal proteolysis of the endothelin B receptor abolishes its ability to induce EGF receptor transactivation and contractile protein expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 26: 1288–1296, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grossmann S, Higashiyama S, Oksche A, Schaefer M, Tannert A. Localisation of endothelin B receptor variants to plasma membrane microdomains and its effects on downstream signalling. Mol Membr Biol 26: 279–292, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296: F1484–F1493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 299: F983–F990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 91: 672–680, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Hermann K, Ring J. Association between the renin angiotensin system and anaphylaxis. Adv Exp Med Biol 377: 299–309, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Hu TH, Huang CC, Liu LF, Lin PR, Liu SY, Chang HW, Changchien CS, Lee CM, Chuang JH, Tai MH. Expression of hepatoma-derived growth factor in hepatocellular carcinoma. Cancer 98: 1444–1456, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Hu TH, Lin JW, Chen HH, Liu LF, Chuah SK, Tai MH. The expression and prognostic role of hepatoma-derived growth factor in colorectal stromal tumors. Dis Colon Rectum 52: 319–326, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 157: 1211–1222, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Imig JD, Navar GL, Zou LX, O'Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT(1A) receptors. Am J Physiol Renal Physiol 277: F303–F311, 1999 [DOI] [PubMed] [Google Scholar]
- 70. Inagami T, Mizuno K, Higashimori K. Juxtaglomerular cells as a source of intrarenal angiotensin II production. Kidney Int Suppl 32: S20–S22, 1991 [PubMed] [Google Scholar]
- 71. Innamorati G, Le Gouill C, Balamotis M, Birnbaumer M. The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J Biol Chem 276: 13096–13103, 2001 [DOI] [PubMed] [Google Scholar]
- 72. Irvine RF. Inositol lipids in cell signalling. Curr Opin Cell Biol 4: 212–219, 1992 [DOI] [PubMed] [Google Scholar]
- 73. Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol 4: 349–360, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Jacques D, Abdel Malak NA, Sader S, Perreault C. Angiotensin II and its receptors in human endocardial endothelial cells: role in modulating intracellular calcium. Can J Physiol Pharmacol 81: 259–266, 2003 [DOI] [PubMed] [Google Scholar]
- 75. Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol 298: F125–F132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jans DA, Hassan G. Nuclear targeting by growth factors, cytokines, and their receptors: a role in signaling? Bioessays 20: 400–411, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Jans DA, Hubner S. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol Rev 76: 651–685, 1996 [DOI] [PubMed] [Google Scholar]
- 78. Kajstura J, Bolli R, Sonnenblick EH, Anversa P, Leri A. Cause of death: suicide. J Mol Cell Cardiol 40: 425–437, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Kihlmark MaH E. Preparation of Nuclei and Nuclear Envelopes. San Diego, CA: Academic, 1998 [Google Scholar]
- 80. Kirschner RH, Rusli M, Martin TE. Characterization of the nuclear envelope, pore complexes, and dense lamina of mouse liver nuclei by high resolution scanning electron microscopy. J Cell Biol 72: 118–132, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Klein C, Malviya AN. Mechanism of nuclear calcium signaling by inositol 1,4,5-trisphosphate produced in the nucleus, nuclear located protein kinase C and cyclic AMP-dependent protein kinase. Front Biosci 13: 1206–1226, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938–F945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kojro E, Fahrenholz F. Ligand-induced cleavage of the V2 vasopressin receptor by a plasma membrane metalloproteinase. J Biol Chem 270: 6476–6481, 1995 [DOI] [PubMed] [Google Scholar]
- 84. Kojro E, Postina R, Gilbert S, Bender F, Krause G, Fahrenholz F. Structural requirements for V2 vasopressin receptor proteolytic cleavage. Eur J Biochem 266: 538–548, 1999 [DOI] [PubMed] [Google Scholar]
- 85. Kumari R, Robertson JF, Watson SA. Nuclear targeting of a midregion PTHrP fragment is necessary for stimulating growth in breast cancer cells. Int J Cancer 119: 49–59, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Lai PB. Local renin-angiotensin system in the pancreas: the significance in acute pancreatitis. JOP 2: 13–15, 2001 [PubMed] [Google Scholar]
- 87. Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 17: 3105–3117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lanctot PM, Leclerc PC, Escher E, Leduc R, Guillemette G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry 38: 8621–8627, 1999 [DOI] [PubMed] [Google Scholar]
- 89. Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension 43: 1116–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 90. Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension 47: 461–466, 2006 [DOI] [PubMed] [Google Scholar]
- 91. Lee BA, Maher DW, Hannink M, Donoghue DJ. Identification of a signal for nuclear targeting in platelet-derived-growth-factor-related molecules. Mol Cell Biol 7: 3527–3537, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem 279: 7901–7908, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science 308: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Lepourcelet M, Tou L, Cai L, Sawada J, Lazar AJ, Glickman JN, Williamson JA, Everett AD, Redston M, Fox EA, Nakatani Y, Shivdasani RA. Insights into developmental mechanisms and cancers in the mammalian intestine derived from serial analysis of gene expression and study of the hepatoma-derived growth factor (HDGF). Development 132: 415–427, 2005 [DOI] [PubMed] [Google Scholar]
- 95. Leung PS. Local renin-angiotensin system in the pancreas: the significance of changes by chronic hypoxia and acute pancreatitis. JOP 2: 3–8, 2001 [PubMed] [Google Scholar]
- 96. Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD. Renal proximal tubule angiotensin AT1a receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol 301: R1067–R1077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol 300: F1076–F1088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 293: F586–F593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung 89: 427–438, 2002 [DOI] [PubMed] [Google Scholar]
- 100. Liebau MC, Lang D, Bohm J, Endlich N, Bek MJ, Witherden I, Mathieson PW, Saleem MA, Pavenstadt H, Fischer KG. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol 290: F710–F719, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell 10: 57–69, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology 139: 365–375, 1998 [DOI] [PubMed] [Google Scholar]
- 103. Luchtefeld M, Bandlow N, Tietge UJ, Grote K, Pfeilschifter J, Kaszkin M, Beck S, Drexler H, Schieffer B. Angiotensin II type 1-receptor antagonism prevents type IIA secretory phospholipase A2-dependent lipid peroxidation. Atherosclerosis 194: 62–70, 2007 [DOI] [PubMed] [Google Scholar]
- 104. Luft FC, Mervaala E, Muller DN, Gross V, Schmidt F, Park JK, Schmitz C, Lippoldt A, Breu V, Dechend R, Dragun D, Schneider W, Ganten D, Haller H. Hypertension-induced end-organ damage : A new transgenic approach to an old problem. Hypertension 33: 212–218, 1999 [DOI] [PubMed] [Google Scholar]
- 105. Maher DW, Lee BA, Donoghue DJ. The alternatively spliced exon of the platelet-derived growth factor A chain encodes a nuclear targeting signal. Mol Cell Biol 9: 2251–2253, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Martelli AM, Tabellini G, Bortul R, Manzoli L, Bareggi R, Baldini G, Grill V, Zweyer M, Narducci P, Cocco L. Enhanced nuclear diacylglycerol kinase activity in response to a mitogenic stimulation of quiescent Swiss 3T3 cells with insulin-like growth factor I. Cancer Res 60: 815–821, 2000 [PubMed] [Google Scholar]
- 107. Massfelder T, Dann P, Wu TL, Vasavada R, Helwig JJ, Stewart AF. Opposing mitogenic and anti-mitogenic actions of parathyroid hormone-related protein in vascular smooth muscle cells: a critical role for nuclear targeting. Proc Natl Acad Sci USA 94: 13630–13635, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310: 1344–1347, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mazzotti G, Zini N, Rizzi E, Rizzoli R, Galanzi A, Ognibene A, Santi S, Matteucci A, Martelli AM, Maraldi NM. Immunocytochemical detection of phosphatidylinositol 4,5-bisphosphate localization sites within the nucleus. J Histochem Cytochem 43: 181–191, 1995 [DOI] [PubMed] [Google Scholar]
- 110. Mercure C, Ramla D, Garcia R, Thibault G, Deschepper CF, Reudelhuber TL. Evidence for intracellular generation of angiotensin II in rat juxtaglomerular cells. FEBS Lett 422: 395–399, 1998 [DOI] [PubMed] [Google Scholar]
- 111. Merjan AJ, Kanashiro CA, Krieger JE, Han SW, Paiva AC. Ligand-induced endocytosis and nuclear localization of angiotensin II receptors expressed in CHO cells. Braz J Med Biol Res 34: 1175–1183, 2001 [DOI] [PubMed] [Google Scholar]
- 112. Moliaka YK, Grigorenko A, Madera D, Rogaev EI. Impas 1 possesses endoproteolytic activity against multipass membrane protein substrate cleaving the presenilin 1 holoprotein. FEBS Lett 557: 185–192, 2004 [DOI] [PubMed] [Google Scholar]
- 113. Muscella A, Greco S, Elia MG, Storelli C, Marsigliante S. PKC-zeta is required for angiotensin II-induced activation of ERK and synthesis of c-Fos in MCF-7 cells. J Cell Physiol 197: 61–68, 2003 [DOI] [PubMed] [Google Scholar]
- 114. Nakamura H, Kambe H, Egawa T, Kimura Y, Ito H, Hayashi E, Yamamoto H, Sato J, Kishimoto S. Partial purification and characterization of human hepatoma-derived growth factor. Clin Chim Acta 183: 273–284, 1989 [DOI] [PubMed] [Google Scholar]
- 115. Nakayama K, Ohkawara T, Hiratochi M, Koh CS, Nagase H. The intracellular domain of amyloid precursor protein induces neuron-specific apoptosis. Neurosci Lett 444: 127–131, 2008 [DOI] [PubMed] [Google Scholar]
- 116. Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol 26: e133–e137, 2006 [DOI] [PubMed] [Google Scholar]
- 117. Olivares-Reyes JA, Shah BH, Hernandez-Aranda J, Garcia-Caballero A, Farshori MP, Garcia-Sainz JA, Catt KJ. Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors. Mol Pharmacol 68: 356–364, 2005 [DOI] [PubMed] [Google Scholar]
- 118. Oliveira EM, Sasaki MS, Cerencio M, Barauna VG, Krieger JE. Local renin-angiotensin system regulates left ventricular hypertrophy induced by swimming training independent of circulating renin: a pharmacological study. J Renin Angiotensin Aldosterone Syst 10: 15–23, 2009 [DOI] [PubMed] [Google Scholar]
- 119. Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Peters J, Wanka H, Peters B, Hoffmann S. A renin transcript lacking exon 1 encodes for a non-secretory intracellular renin that increases aldosterone production in transgenic rats. J Cell Mol Med 12: 1229–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pierce GF, Shawver LK, Milner PG, Yeh HJ, Thomason A, Deuel TF. Identification and purification of PDGF/sis-like proteins from nuclei of simian sarcoma virus-transformed fibroblasts. Oncogene Res 2: 235–244, 1988 [PubMed] [Google Scholar]
- 123. Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Polavarapu R, An J, Zhang C, Yepes M. Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol 172: 1355–1362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Raben DM, Tu-Sekine B. Nuclear diacylglycerol kinases: regulation and roles. Front Biosci 13: 590–597, 2008 [DOI] [PubMed] [Google Scholar]
- 126. Rakowicz-Szulczynska EM, Rodeck U, Herlyn M, Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci USA 83: 3728–3732, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rankin JC, Watanabe TX, Nakajima K, Broadhead C, Takei Y. Identification of angiotensin I in a cyclostome, Lampetra fluviatilis. Zoolog Sci 21: 173–179, 2004 [DOI] [PubMed] [Google Scholar]
- 128. Re R. The nature of intracrine peptide hormone action. Hypertension 34: 534–538, 1999 [DOI] [PubMed] [Google Scholar]
- 129. Re RN. Implications of intracrine hormone action for physiology and medicine. Am J Physiol Heart Circ Physiol 284: H751–H757, 2003 [DOI] [PubMed] [Google Scholar]
- 130. Re RN. On the biological actions of intracellular angiotensin. Hypertension 35: 1189–1190, 2000 [DOI] [PubMed] [Google Scholar]
- 131. Re RN, Cook JL. The intracrine hypothesis: an update. Regul Pept 133: 1–9, 2006 [DOI] [PubMed] [Google Scholar]
- 132. Re RN, Cook JL. The mitochondrial component of intracrine action. Am J Physiol Heart Circ Physiol 299: H577–H583, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol 298: H1807–H1818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75: 977–984, 1993 [DOI] [PubMed] [Google Scholar]
- 135. Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sakai K, Sigmund CD. Molecular evidence of tissue renin-angiotensin systems: a focus on the brain. Curr Hypertens Rep 7: 135–140, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Saksena S, Shao Y, Braunagel SC, Summers MD, Johnson AE. Cotranslational integration and initial sorting at the endoplasmic reticulum translocon of proteins destined for the inner nuclear membrane. Proc Natl Acad Sci USA 101: 12537–12542, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sandoval YH, Li Y, Anand-Srivastava MB. Transactivation of epidermal growth factor receptor by enhanced levels of endogenous angiotensin II contributes to the overexpression of Gialpha proteins in vascular smooth muscle cells from SHR. Cell Signal 23: 1716–1726, 2011 [DOI] [PubMed] [Google Scholar]
- 139. Schunkert H, Sadoshima J, Cornelius T, Kagaya Y, Weinberg EO, Izumo S, Riegger G, Lorell BH. Angiotensin II-induced growth responses in isolated adult rat hearts. Evidence for load-independent induction of cardiac protein synthesis by angiotensin II. Circ Res 76: 489–497, 1995 [DOI] [PubMed] [Google Scholar]
- 140. Sethi S, Iida S, Sigmund CD, Heistad DD. Renal thrombotic microangiopathy in a genetic model of hypertension in mice. Exp Biol Med (Maywood) 231: 196–203, 2006 [DOI] [PubMed] [Google Scholar]
- 141. Shah BH, Yesilkaya A, Olivares-Reyes JA, Chen HD, Hunyady L, Catt KJ. Differential pathways of angiotensin II-induced extracellularly regulated kinase 1/2 phosphorylation in specific cell types: role of heparin-binding epidermal growth factor. Mol Endocrinol 18: 2035–2048, 2004 [DOI] [PubMed] [Google Scholar]
- 142. Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J 375: 503–515, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol 288: R539–R546, 2005 [DOI] [PubMed] [Google Scholar]
- 144. Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H939–H948, 2007 [DOI] [PubMed] [Google Scholar]
- 145. Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57: 3297–3306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Siragy HM. AT1 and AT2 receptors in the kidney: role in disease and treatment. Am J Kidney Dis 36: S4–S9, 2000 [DOI] [PubMed] [Google Scholar]
- 147. Siragy HM, Carey RM. Angiotensin type 2 receptors: potential importance in the regulation of blood pressure. Curr Opin Nephrol Hypertens 10: 99–103, 2001 [DOI] [PubMed] [Google Scholar]
- 148. Sirett NE, McLean AS, Bray JJ, Hubbard JI. Distribution of angiotensin II receptors in rat brain. Brain Res 122: 299–312, 1977 [DOI] [PubMed] [Google Scholar]
- 149. Smith RD, Baukal AJ, Dent P, Catt KJ. Raf-1 kinase activation by angiotensin II in adrenal glomerulosa cells: roles of Gi, phosphatidylinositol 3-kinase, and Ca2+ influx. Endocrinology 140: 1385–1391, 1999 [DOI] [PubMed] [Google Scholar]
- 150. Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol 130: 15–27, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Struewing IT, Barnett CD, Zhang W, Yadav S, Mao CD. Frizzled-7 turnover at the plasma membrane is regulated by cell density and the Ca2+-dependent protease calpain-1. Exp Cell Res 313: 3526–3541, 2007 [DOI] [PubMed] [Google Scholar]
- 152. Tanaka K, Horiguchi K, Yoshida T, Takeda M, Fujisawa H, Takeuchi K, Umeda M, Kato S, Ihara S, Nagata S, Fukui Y. Evidence that a phosphatidylinositol 3,4,5-trisphosphate-binding protein can function in nucleus. J Biol Chem 274: 3919–3922, 1999 [DOI] [PubMed] [Google Scholar]
- 153. Thomas MA, Fleissner G, Hauptfleisch S, Lemmer B. Subcellular identification of angiotensin I/II- and angiotensin II (AT1)-receptor-immunoreactivity in the central nervous system of rats. Brain Res 962: 92–104, 2003 [DOI] [PubMed] [Google Scholar]
- 154. Ullian ME, Webb JG, Chen R, Paul RV, Morinelli TA. Mechanisms of vascular angiotensin II surface receptor regulation by epidermal growth factor. J Cell Physiol 200: 451–457, 2004 [DOI] [PubMed] [Google Scholar]
- 155. Uyama H, Tomita Y, Nakamura H, Nakamori S, Zhang B, Hoshida Y, Enomoto H, Okuda Y, Sakon M, Aozasa K, Kawase I, Hayashi N, Monden M. Hepatoma-derived growth factor is a novel prognostic factor for patients with pancreatic cancer. Clin Cancer Res 12: 6043–6048, 2006 [DOI] [PubMed] [Google Scholar]
- 156. van den Meiracker AH, de Ronde W. Gender differences in vascular disease: not a simple explanation. J Hypertens 25: 1193–1194, 2007 [DOI] [PubMed] [Google Scholar]
- 157. van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension 30: 42–49, 1997 [DOI] [PubMed] [Google Scholar]
- 158. van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens 19: 583–589, 2001 [DOI] [PubMed] [Google Scholar]
- 159. Vaniotis G, Del Duca D, Trieu P, Rohlicek CV, Hebert TE, Allen BG. Nuclear beta-adrenergic receptors modulate gene expression in adult rat heart. Cell Signal 23: 89–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Venugopal C, Pappolla MA, Sambamurti K. Insulysin cleaves the APP cytoplasmic fragment at multiple sites. Neurochem Res 32: 2225–2234, 2007 [DOI] [PubMed] [Google Scholar]
- 161. Vila-Porcile E, Corvol P. Angiotensinogen, prorenin, and renin are colocalized in the secretory granules of all glandular cells of the rat anterior pituitary: an immunoultrastructural study. J Histochem Cytochem 46: 301–311, 1998 [DOI] [PubMed] [Google Scholar]
- 162. Wang S, Fang W. Increased expression of hepatoma-derived growth factor correlates with poor prognosis in human nasopharyngeal carcinoma. Histopathology 58: 217–224, 2011 [DOI] [PubMed] [Google Scholar]
- 163. Wang TL, Yang YH, Chang H, Hung CR. Angiotensin II signals mechanical stretch-induced cardiac matrix metalloproteinase expression via JAK-STAT pathway. J Mol Cell Cardiol 37: 785–794, 2004 [DOI] [PubMed] [Google Scholar]
- 164. Waters CM, Saatian B, Moughal NA, Zhao Y, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. Biochem J 398: 55–62, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wright JW, Harding JW. Brain renin-angiotensin-A new look at an old system. Prog Neurobiol 95: 49–67, 2011 [DOI] [PubMed] [Google Scholar]
- 166. Wu W, Lin F, Worman HJ. Intracellular trafficking of MAN1, an integral protein of the nuclear envelope inner membrane. J Cell Sci 115: 1361–1371, 2002 [DOI] [PubMed] [Google Scholar]
- 167. Xu D, Borges GR, Davis DR, Agassandian K, Sequeira Lopez ML, Gomez RA, Cassell MD, Grobe JL, Sigmund CD. Neuron- or glial-specific ablation of secreted renin does not affect renal renin, baseline arterial pressure, or metabolism. Physiol Genomics 43: 286–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension 54: 1240–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005 [DOI] [PubMed] [Google Scholar]
- 170. Yaghini FA, Li F, Malik KU. Expression and mechanism of spleen tyrosine kinase activation by angiotensin II and its implication in protein synthesis in rat vascular smooth muscle cells. J Biol Chem 282: 16878–16890, 2007 [DOI] [PubMed] [Google Scholar]
- 171. Yeh HJ, Pierce GF, Deuel TF. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci USA 84: 2317–2321, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 173. Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zini N, Sabatelli P, Faenza I, Ognibene A, Maraldi NM. Interleukin-1 alpha induces variations of the intranuclear amount of phosphatidylinositol 4,5-bisphosphate and phospholipase C beta 1 in human osteosarcoma Saos-2 cells. Histochem J 28: 495–504, 1996 [DOI] [PubMed] [Google Scholar]