Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is a rare and progressive disease. Several processes are believed to lead to the fatal progressive pulmonary arterial narrowing seen in IPAH including vasoconstriction, cellular proliferation inflammation, vascular remodeling, abnormalities in the lung matrix, and in situ thrombosis. Nitric oxide (NO) produced by NO synthases (NOS) is a potent vasodilator and plays important roles in many other processes including platelet function. Reduced NO levels in patients with IPAH are known to contribute to the development of pulmonary hypertension and its complications. Platelet defects have been implied in IPAH, but original research supporting this hypothesis has been limited. Normal platelets are known to have NOS activity, but little is known about NOS expression and NO production by platelets in patients with IPAH. Here we characterized the phenotype of the platelets in IPAH and show a defect in their ability to be activated in vitro by thrombin receptor activating protein but not adenosine diphosphate. We also show that endothelial NOS (eNOS) levels in these platelets are reduced and demonstrate that NO is an important regulator of platelet function. Thus reduced levels of eNOS in platelets could impact their ability to regulate their own function appropriately.
Keywords: nitric oxide synthases, l-NAME, TRAP
pulmonary arterial hypertension (PAH) is a rare and progressive disease that leads to deterioration in cardiopulmonary function and premature death. It is characterized by increases in pulmonary vascular tone, pulmonary vascular remodeling (28), and mean pulmonary artery pressure and an imbalance in vasoconstrictors and vasodilators, particularly prostacyclin, endothelin-1, and nitric oxide (NO; Ref. 13). Idiopathic pulmonary arterial hypertension (IPAH), in which PAH occurs in the absence of underlying disease, is characterized by complex and abnormal vascular responses and pathology. Several processes are believed to lead to the fatal progressive pulmonary arterial narrowing seen in pulmonary hypertension (PH) including vasoconstriction, cellular proliferation inflammation, vascular remodeling, abnormalities in the lung matrix, and in situ thrombosis (4, 16, 28).
The thrombotic lesions that are often found in pulmonary arteries in IPAH suggest that platelet dysfunction is a potentially important pathophysiologic process in the disease (36). Thrombosis in pulmonary arteries could be initiated by abnormalities in the clotting cascade or platelets. von Willebrand factor, which plays a crucial role in platelet adhesion and aggregation (23), can be increased in patients with IPAH (44) and may be a predictor of long-term prognosis (19). In addition to having a role in clotting, platelets also release many different bioactive substances, which regulate vasoconstriction and thrombosis (15).
NO has been proposed as a major physiologic regulator of blood vessel tone (33, 34). NO in the lung is involved in multiple functions including vascular smooth muscle relaxation, proliferation, and inflammation (5, 6). Interestingly, NO and the reaction products of NO are reduced in patients with IPAH compared with healthy individuals (17) and are related in a quantitative fashion to the degree of PAH. One of the earliest observations of NO action was that upon platelets. While this was believed to impact platelets from the endothelial-derived NO, it is now very clear that NO is also produced by platelets themselves (18, 19). Both endothelial (eNOS) and inducible NO synthase (iNOS) proteins have been found in platelets (2, 3, 40). While eNOS appears to be constitutively present, iNOS protein is found only under inflammatory conditions (40). However, these observations are controversial since other studies fail to detect mRNA or proteins for these enzymes in the platelet (25, 26).
Here we have characterized the phenotype of the platelets in IPAH and have identified that eNOS levels in these platelets are reduced. We also demonstrate that NO is an important regulator of platelet function and this reduced level of eNOS would impact the ability of the platelets to regulate themselves correctly. The physiological consequence of this dysregulation leads to several known pathological features of IPAH.
MATERIALS AND METHODS
Study population.
Platelet counts were evaluated in 154 patients with IPAH (114 female, age; 50.8 ± 13.5 yr) identified based on the World Health Organization criteria as shown in Table 1 (13, 39) and 105 healthy control volunteers (83 female, age; 44.3 ± 13.5 yr; Table 1). Healthy controls were individuals with no history of pulmonary or cardiac disease or symptoms. In a subset of these individuals [22 IPAH (14 female, age 51.9 ± 12.6 yr) and 21 controls (14 female, age 36.5 ± 10.4 yr)], we performed more detailed evaluation of platelet structure and function including cyclooxygenase (COX) and NOS expression (mRNA: n = 13 IPAH, 12 controls; Western blots: n = 9 IPAH, 9 controls).
Table 1.
Demographics and clinical characteristics of IPAH study subjects
| Subjects with IPAH | |
|---|---|
| n | 154 |
| Mean age, yr | 50.8 ± 13.5 |
| Sex (female) | 114 |
| FEV1, 1 | 2.1 ± 0.1 |
| FEV1, %predicted | 70 ± 1.5 |
| FVC, 1 | 2.8 ± 0.1 |
| FEV, %predicted | 76 ± 1.7 |
| MPAP, mmHg | 51.2 ± 2 |
| PVR, Wood units | 10 ± 0.6 |
| CI, l·min−1·m−2 | 2.4 ± 0.1 |
| AST, mg/dl | 28.3 ± 1.5 |
| ALT, mg/dl | 22.5 ± 1.5 |
| Bilirubin, mg/dl | 0.9 ± 0.1 |
| Creatinine, mg/dl | 1.1 ± 0.1 |
| Medications | No. of patients |
| Prostacyclin | 54 |
| Other medications | 47 |
Values are means ± SE. IPAH, idiopathic pulmonary arterial hypertension; FEV1, forced expiratory volume in 1 s; MPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; CI, cardiac index; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The platelet aggregation studies were performed on freshly isolated platelets from blood obtained from three IPAH patients [at the time of a diagnostic right heart catheterization and before initiation of any PH-specific therapies] and three controls (on the same day as the patients).
Isolated purified platelets were also obtained from normal volunteers (n = 3) undergoing plasmapheresis for research donation of blood products from the Cleveland Clinic General Clinical Research Center. The pheresis samples (n = 3) were only used for mRNA and protein expression experiments but not in the functional studies or to determine the platelet counts.
All participants signed a consent form that was approved by the Cleveland Clinic Institutional Review Board before participation in these studies.
Platelet isolation.
Whole blood from healthy volunteers and from the patients with IPAH was collected in citrated tubes (1/7 volume of [acid citrate dextrose (ACD)]) as described previously (21), and platelet counts were calculated as platelets × 109 per liter. Blood was spun for 20 min at 1,000 rpm at room temperature (RT) to obtain platelet rich plasma (PRP). PRP was placed into another tube in the presence of 100 nM prostaglandin E1 (PGE1; Sigma cat no: P-5515) to prevent aggregation then respun for 20 min at 1,500 rpm at RT to get a pellet-containing platelets. The pellet was washed two times with 1× PIPES-saline-glucose buffer containing 5 mM PIPES (Sigma), 145 mM NaCl, 50 mM Na2HPO4, 1 mM MgCl2-6 H2O, and 5.5 mM glucose. Washed platelets were spun 10 min at 1,500 rpm at RT, and then mRNA extraction or protein isolation was performed. Platelets obtained from this source were further purified by being mixed with PGE1 to a final concentration of 100 nM and spun at 1,800 rpm for 20 min. The supernatant was discarded, and the upper layer containing the platelets was carefully removed, without disturbing the red blood cells, and resuspended in Tyrode buffer lacking calcium. These platelets were then respun, and the process was repeated two more times. At this time, no red blood cell contaminates were visible. After the final spin, platelets were resuspended in Tyrode buffer containing 1 mM calcium and used in subsequent experiments. Platelet numbers and purity were confirmed using Abbott Cell-Dyn 3700 hematology analyzer.
Immunostaining.
Isolated platelets were cytospun and then fixed in 4% paraformaldehyde for 7 min at RT followed by permeabilization in 0.5% Triton X-100 for 7 min. Slides were washed with PBS and incubated with primary antibodies for eNOS and CD42b [Biosciences, Pharmingen, and Santa Cruz Biotechnology (Santa Cruz, CA), respectively] in humidified chambers for 90 min at RT. After three washes in PBS, they were stained with fluorescence-conjugated secondary antibodies. Slides were rinsed three times with PBS and once with distilled water followed by mounting on the glass microscope slides using Vectashield mounting medium containing DAPI (Vector Laboratories, CA). Confocal XY images were taken using ×63 objective lens (zoom 2) of a Leica TCS-SP/SP-AOSB laser confocal microscope (Leica-Microsystems, Wetzlar) using Leica confocal software version 2.5. The excitation (Ex)/emission (Em) wavelengths were as follows: DAPI: Ex 351 nm, Em 370–420 nm; Alexa Fluor 488: Ex 488 nm, Em 500–550 nm; and Alexa Fluor 568: Ex 561 nm, Em 575–630 nm.
cDNA synthesis and conventional RT-PCR.
Total RNA was isolated from platelets using Trizol reagent (Invitrogen) according to the manufacturer's protocol. For cDNA synthesis, 0.5 ug of RNA from each sample was digested by DNAseI (Invitrogen) and total RNA was reverse-transcribed using the Moloney murine leukemia virus enzyme (Invitrogen) and Oligo dT (Invitrogen). cDNAs were amplified using Taq polymerase (Invitrogen) with the primer sequences listed in Table 2 and conditions in Table 3. PCR products were separated on 2% agarose gels containing ethidium bromide and visualized under a ultraviolet lamp.
Table 2.
Primers and conditions used for RT-PCR
| Name | Forward Primer | Reverse Primer | Length | Accession No. |
|---|---|---|---|---|
| eNOS | CAGTGTCCAACATGCTGCTGGAAATTG | AAGGTCTTCTTCCTGGTGATGC | 468 | NM_000603 |
| COX1 | TGCCCAGCTCCTGGCCCGCCGCTT | GTGCATCAACACAGGCGCCTCTTC | 304 | AF440204 |
| COX2 | TTCAAATGAGATTGTGGGAAAATTGCT | AGATCATCTCTGCCTGAGTATCTT | 304 | AY462100 |
| GAPDH | ACCACAGTCCATGCCATC | TCCACCACCCTGTTGCTGTA | 451 | BC025925 |
eNOS, endothelial nitric oxide synthase; COX, cyclooxygenase.
Table 3.
RT-PCR conditions for the different primers
| Gene | Cycle Profile |
|---|---|
| eNOS | 95°C/30 s; 58°C/30 s; 72°C/45 s for 40 cycles |
| COX1 | 95°C/30 s; 58°C/30 s; 72°C/1 min for 35 cycles |
| COX2 | 95°C/30 s; 58°C/30 s; 72°C/1 min for 35 cycles |
| GAPDH | 95°C/30 s; 58°C/30 s; 72°C/1 min for 35 cycles |
Western blot for protein determination.
Western blot analysis was utilized to determine eNOS, neuronal NOS (nNOS), and iNOS proteins in isolated platelets from control and IPAH patients. Isolated platelets were lysed with lysis buffer containing 50 mM Tris pH 7,4, 100 mM NaCl, 1 mM EDTA, Nonidet P-40, 10% glycerol, and proteinase inhibitors (200 uM NaO, 20 ug/ml aprotinin, 5 ug/ml leupeptin, 10 ug/ml pepestatin, 1 mM PMSF, and 1 mM DTT) and incubated on ice for 30 min. The protein concentrations in the supernatants were measured after the lysed platelets were centrifuged for 30 min at 14,000 rpm at 4°C, and then proteins were subjected to electrophoresis in SDS-PAGE after solubilization in SDS-PAGE sample buffer with 10% β-mercaptoethanol. The proteins were transferred to nitrocellulose membrane, and Western blot analysis was accomplished by using antibodies for eNOS (Biosciences, Pharmingen), nNOS (Biosciences, Pharmingen), and iNOS (Millipore, Temecula, CA) and secondary antibodies (NA931V; Amersham Biosciences). At the end, Western blots were developed using enhanced chemiluminescence (Amersham).
For detection of CD63, total protein (15 ug) was lysed and subsequently separated on a gradient 4–15% polyacrylamide gel (Bio-Rad) under nonreducing conditions. Protein detection was performed with a primary antibody against CD63 (1:200; sc-5275; Santa Cruz Biotechnology) and GAPDH (1:1000; 14C10; Cell Signaling, Danvers, MA). For detection of protease-activated receptor-1 (PAR1), total protein (15 ug) was lysed and subsequently separated on a gradient 4–15% polyacrylamide gel (Bio-Rad) under reducing conditions. Protein detection was performed with a primary antibody against PAR1 (1:100; ab32611; Abcam, Cambridge, MA) and GAPDH (1:10,000; AM4300; Ambion, Foster City, CA). Blots were incubated with differentially labeled species-specific secondary antibodies after primary antibody incubation [anti-rabbit IRDye 800CW (green) and anti-mouse IRDye 680 (red) 926–32211 and 926–32220; LI-COR Biosciences, Lincoln, NE]. Membranes were scanned using the ODYSSEY infrared imaging system (LI-COR Biosciences) and quantitated using ImageJ (NIH, Bethesda, MD).
Platelet aggregation assay.
Platelets in Tyrode buffer with calcium were incubated at RT in the presence or absence of NO inhibitors for ≥30 min before the aggregation assay. NG-nitro-l- arginine methyl ester (l-NAME), which shows selectivity to eNOS and nNOS, was used at 0, 1, 2, and 5 mM. Aminoguanadine, which shows selective inhibition to iNOS, was used at 2 mM. At the end of the timed incubations, platelets were subjected to aggregometry. Aggregometry was performed using 2 × 108 platelets/ml in a final reaction volume of 500 μl. Aggregation was stimulated using thrombin receptor-activating protein (TRAP) peptide at a final concentration of 5 μM. After all the samples were run, an assay was performed on control platelets to confirm that the platelets were still capable of aggregation with TRAP peptide to the same degree as at the start of the experiments. All assays were performed using a Chrono-log whole blood aggregometer and data was recorded using AGROLINK software.
Whenever possible, platelets were diluted to 2 × 108 platelets/ml with their PRP. However, when platelet numbers from IPAH patients were <2 × 108, the control PRP was diluted to the same numbers as that from the undiluted IPAH patients. In all studies, control PRP was done at the same time as the IPAH PRP. All aggregation assays were performed using a Chrono-log whole blood aggregometer, and data were recorded using AGROLINK software.
Statistical analysis.
All statistical analysis was performed using Jump JMP version 5.0.1.2 for Windows. Continuous variables were compared with the independent two tailed t-test. P ≤ 0.05 was considered as significant.
RESULTS
Low platelet counts in patients with IPAH compared with controls.
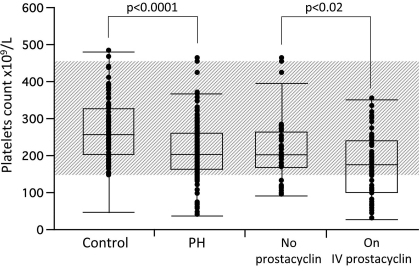
There was a significant difference in platelet counts between IPAH patients and controls [(platelet count × 109/l ± SE) 212 ± 6 for IPAH group and 266 ± 8 for control group; P < 0.0001; Fig. 1]. Although, the patients with IPAH before and after therapy consistently had significantly lower platelet counts than controls there was no significant difference between the platelet count for the patients with IPAH before and after therapy [(plateletscount × 109/l ± SE) 231 ± 6 for IPAH before therapy and 215 ± 7 for IPAH after therapy; P = 0.08]. The patients on intravenous prostacyclin therapy, however, had significantly lower platelet counts compared with patients on other PH-specific medications [(platelets countx109/l ± SE) 173 ± 11.2 for the patients in the prostacyclin group and 215 ± 12.2 for control group; P < 0.02; Fig. 1].
Fig. 1.
Platelet counts (platelets × 109/ml) were determined in 154 patients with idiopathic pulmonary arterial hypertension (IPAH) and 105 healthy controls. Gray area shows the normal range of platelets counts in our clinical laboratory.
Control and IPAH platelets are mature.
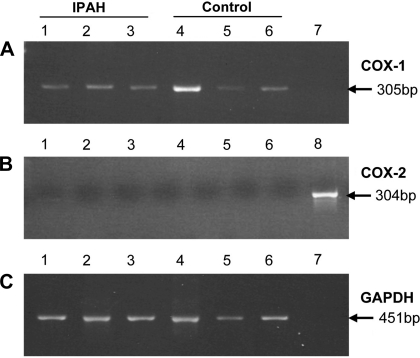
To investigate if the platelets in IPAH patients were younger and therefore suggesting higher turnover, we measured the expression of COX-2, which is present in less mature platelets (10) and COX-1, which is present in more mature platelets. mRNA expression levels of COX-1 and COX-2 were measured by RT-PCR. COX-1 was present in both IPAH and control platelets (Fig. 2A), but COX-2 was not expressed in either group (Fig. 2B). GAPDH was used for normalization (Fig. 2C).
Fig. 2.
Cyclooxygenase-1 (COX-1) expression (A) is similar in both IPAH (3 representative samples from a total n = 13; lanes 1, 2, and 3) and control (3 representative samples from a total n = 12; lanes 3, 4, and 5) platelets, and COX-2 mRNA expression was not detected in either (B). The housekeeping gene GAPDH was used to normalize (C).
Low eNOS protein expression in IPAH platelets compared with controls.
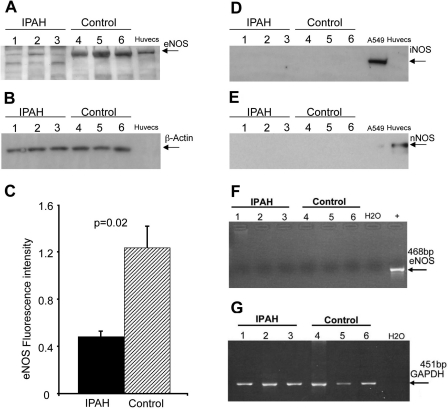
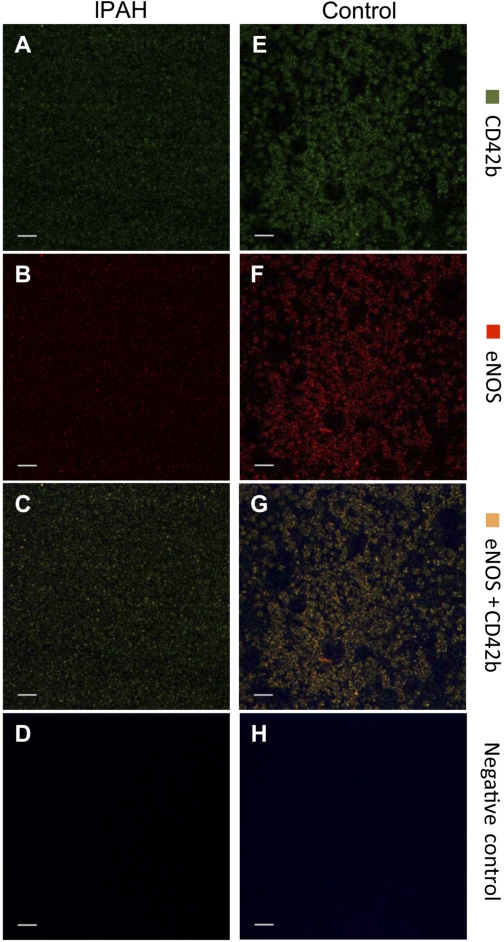
Expressions of eNOS, nNOS, iNOS, and GAPDH in platelets from patients with IPAH and controls were evaluated by Western blot analysis. eNOS protein was present in both but at much lower levels in IPAH compared with controls (Fig. 3A). β-Actin was used to normalize the Western blot (Fig. 3B). Figure 3C shows the ratios of the optical densities for eNOS/β-actin protein (IPAH 0.48 ± 0.05 vs. controls 1.23 ± 0.2; P = 0.02). However, iNOS and nNOS proteins could not be detected in either IPAH or control (Fig. 3, D and E, respectively) platelets. eNOS mRNA expression analysis was done by RT-PCR. The list of the primers and their conditions are shown in Tables 2 and 3, respectively. eNOS mRNA expression was not detected in platelets from either IPAH or control individuals (Fig. 3F) despite strong mRNA expression of GAPDH (Fig. 3G). We also used immunohistochemistry to determine the source of NOS. Platelets from Controls and patients with IPAH were stained for the platelet-specific marker CD42b and eNOS and are shown side by side in Fig. 4, with A–D representing IPAH and E-H representing controls (A and E: CD42b; B and F: eNOS; C and G: colocalization of the 2; and D and H: secondary antibody staining only). eNOS and CD42b were detected both in IPAH (Fig. 4, A and B, respectively) and control (Fig. 4, E and F, respectively). Colocalization of CD42b and eNOS were shown in Fig. 4G for control and in Fig. 4C for IPAH platelets. Figure 4, D and H, shows negative controls of the same sections stained by the secondary antibody only. A signal for eNOS protein is present that co-localizes with the platelet-specific marker CD42b (green color in Fig. 4, C and G), clearly demonstrating that platelets contain eNOS protein. Consistent with the results obtained from the Western blot data, IPAH patients have much lower levels of eNOS protein (Fig. 4B) compared with controls (Fig. 4F). The negative controls, stained with the secondary antibody only, showed only slight background of autofluorescence (Fig. 4, D and H).
Fig. 3.
Western analysis of endothelial nitric oxide synthase (eNOS) protein from the platelets of the patients with IPAH (3 representative samples from a total n = 9; A, lanes 1, 2, and 3) and control (3 representative samples from a total n = 9; A, lanes 4, 5, and 6). β-actin was used to normalize (B). Quantitative analysis showed the significant difference of eNOS protein between IPAH and control (C). Inducible NOS (iNOS; D) and neuronal NOS (nNOS; E) proteins could not be detected in either IPAH or control platelets protein lysates. eNOS mRNA expression was not detected in platelets in either IPAH or control platelets (F). Three IPAH patients (lanes 1, 2, and 3) and 3 controls (lanes 4, 5, and 6) are shown. The PCR product size of eNOS mRNA is 468 bp, and human umbilical vein endothelial cell (HUVEC) lines were used as positive control (F). The housekeeping gene GAPDH was used to normalize (G).
Fig. 4.
Isolated platelets from the patients with IPAH and control were stained for eNOS protein (red) and platelet marker, CD42b, (green). eNOS and CD42b were detected both in IPAH (A and B, respectively) and control (E and F, respectively). Colocalization of CD42b and eNOS is shown in G for control and in C for IPAH platelets. D and H: negative controls of the same sections stained by the secondary antibody only.
NO plays a role in platelets aggregation.
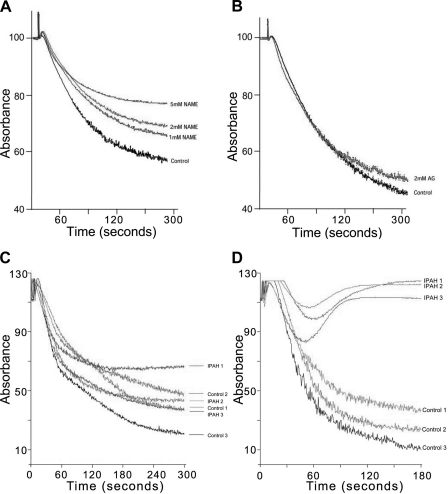
We investigated the effect of NO on platelets using NOS inhibitors. Aggregometry using NOS-specific inhibitors clearly demonstrates that NO is important for platelet aggregation. Consistent with our finding that eNOS is present in the platelets, l-NAME, an eNOS/nNOS-selective inhibitor, prevented platelet aggregation in a dose-dependent fashion (Fig. 5A) but aminoguanidine, an iNOS-selective inhibitor, had no effect (Fig. 5B).
Fig. 5.
Platelets were incubated with various concentrations of NG-nitro-l- arginine methyl ester (l-NAME; A) or aminoguanadine (AG; B) at the end of a 1 h incubation they were tested for aggregation using 5 μM receptor-activating protein (TRAP) activation as described in the text. Platelet rich plasma was isolated from IPAH patients and from controls. Platelet were then counted and diluted to give the same concentration between patient and control. Dose-response curve using 0–10 mM ADP and 0–30 μM TRAP was carried out. Typical responses to TRAP (C) or ADP (D) are demonstrated on 3 patients and same day controls are shown (n = 3 IPAH; n = 3 controls).
Defective platelet aggregation in IPAH patients.
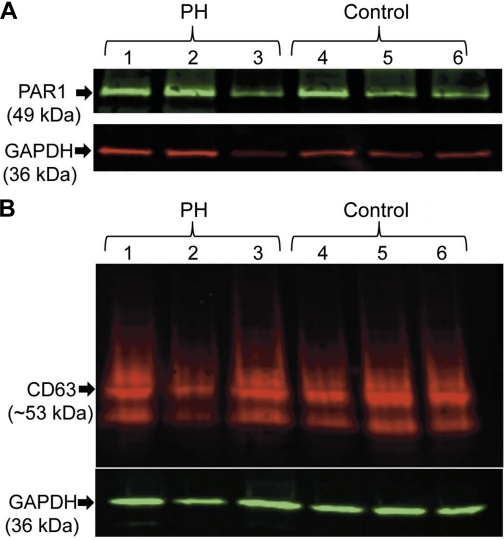
IPAH patients have increased in situ thrombosis formation and reduced NO levels. We therefore investigated if platelet aggregation was defective in these patients. We carried out aggregation studies using the activators TRAP or adenosine diphosphate (ADP). Titrating different doses of ADP or TRAP, we determined the level required to induce aggregation in control and IPAH patients. Interestingly, the dose required to activate platelets using ADP was not much different between IPAH and Control platelets (Fig. 5C). The effect of TRAP activation, however, was markedly different (Fig. 5D). In some IPAH patients, even 10 times the concentration of TRAP could not elicit the same response as the control despite similar ADP responses (Fig. 5C). Since TRAP activation appeared to be defective in IPAH platelets, we investigated the levels of the TRAP receptor (PAR1) in these platelets. We saw no PAR1 difference in the levels of this protein between IPAH and control platelets (Fig. 6A). There was also no difference in CD63 expression, which is marker for the dense granules that contain ADP (Fig. 6B).
Fig. 6.
Expression levels of CD63 protein in platelet. CD63 expression was measured by Western blot in 3 healthy individuals and 3 IPAH patients (A). Expression levels of PAR1 protein in platelet. PAR1 expression was measured by Western blot in 3 healthy individuals and three IPAH patients (B; representative sample from a total n = 9 IPAH; n = 9 controls).
DISCUSSION
Our data demonstrate that platelet aggregation is defective in IPAH, which may, at least in part, be due to abnormalities in NO levels and NOS expression. We have also conclusively demonstrated that all platelets do contain eNOS protein and that IPAH platelets have lower eNOS protein levels than controls. This important finding suggests that the known NO deficiency state in IPAH encompasses the platelets as well. Our data also confirmed prior reports that platelet counts are decreased in IPAH individuals compared with controls especially in patients receiving IV prostacyclin therapy. Interestingly, our new findings also demonstrate that circulating platelets from IPAH patients are mature, suggesting that the low platelet levels in IPAH are not explained by increased platelet consumption.
NO produced by NOS is a potent vasodilator that plays a major role in lung physiology and is known to be low in patients with IPAH. One of the earliest observations of NO function was the effect on platelets. NO can be derived from the endothelium or generated by the platelets. A number of studies (25, 32, 38) have found NO generated from platelets, but the identity of the platelet NOS isoform remains controversial. The majority of the evidence (1, 8) suggests that platelets contain eNOS; however, one study (32) suggested that platelets contain no eNOS message or eNOS protein. Another possible source of NO could be iNOS since its mRNA has been identified in megakaryocytes (the platelet precursor cells) and in porcine platelets (29). This possibility was corroborated by a study (27) that found that platelets from iNOS-knockout mice have significantly decreased NO production and aggregation time compared with controls. However, other studies (1, 12) have found no iNOS protein in platelets. Therefore, we analyzed for the three major isoform of NOS by RT/PCR, Western blotting, and immunochemistry. Western blotting clearly indicates that eNOS protein is present in the platelet preparations but not nNOS or iNOS. Interestingly, the level of eNOS protein is diminished in platelets obtained from PH patients compared with controls. We next looked for eNOS mRNA in platelets. Our results show that both PH and control platelets contain no mRNA for eNOS. Platelets do contain mRNA that is present from the platelet precursor megakaryocyte stage, but new mRNA synthesis does not occur as these cells lack a nucleus. Since eNOS mRNA half-life is ∼14 h (20), and the life span of a platelet is ∼1 wk (14, 43), it is unlikely that we would find eNOS mRNA in circulating platelets. This finding is consistent with the data from COX-1 absence that indicate that the platelets used in this experiment are mature and thus older than 14 h. This finding also diminishes the possibility that the eNOS protein detected by Western blotting was derived from other cell types since these cell types would contribute to the isolated mRNA and so would have given a positive mRNA eNOS signal. To further address the issue of location of eNOS protein, we carried out immunocytochemistry using an antibody from a different source than that used in the Western blots. It is clear that cells that are positive for eNOS protein are ones that were small, anuclear, and positive for the platelets marker CD42b. Thus we have conclusively demonstrated that eNOS is present in platelets and that platelets from IPAH patients have much lower levels of eNOS compared with controls platelets.
The biological effects of NO are complex since it can have direct effects or can react with other species to generate other reactive agents. The inhibitory pathway of NO involving direct actions on soluble guanylyl cyclase (sGC) leading to the generation of cyclic GMP is well characterized. This s messenger can then mediate inhibition of platelet aggregation by activating specific protein kinases that modify actin and myosin. In the presence of NO and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxal, an inhibitor of sGC, not all affects of NO are lost, suggesting that there must also be sGC-independent effects for NO on platelets (26).
Our novel findings suggest new potential mechanisms of how NO regulates platelet function. In low NO states (simulated by Inhibition of NO synthesis), increased platelet activation was expected, as the inhibitory blockade caused by cGMP is removed. We, however, saw the reverse and platelet aggregation was inhibited. This suggests that NO is also required for platelet activation, which is consistent with an observed burst of NO upon platelet activation (9, 32). Taken together our data demonstrate that levels of NO are critical for platelet function. Too low levels of NO (presented here and seen in IPAH patients) as well as too high levels (previously published; Refs. 30, 35, 37, 41, 42) alter platelet aggregation.
TRAP activation mimics thrombin activation of PAR1. Activation using TRAP is clearly impaired in PH patients, but the ADP response is not. While the initial TRAP response seems normal, i.e., the shape change (increased absorbance upon TRAP addition) and start of platelet aggregation, this aggregation appears to be reversible and not sustained. This triggers several pathways coupled to G-protein subunits. These G proteins cause the release of calcium, the Rho pathway that results in the rearrangement of the actin cytoskeleton, and granule release. This granule release is required to amplify the initial platelet activation signal by releasing ADP and other agonists. From the aggregation traces of PH patients, the secondary phase seems lacking. The ability of the platelets to aggregate is not dysfunctional, as ADP elicits a response similar to controls. Since Levels of PAR1 and CD63 are similar to controls, this suggests that activation and granule formation are normal but granule content maybe aberrant.
Reduced levels of eNOS may explain these observations in IPAH platelets. Since platelets have to very precisely balance activation and inhibition, levels of NO need to fall within a narrow range. If too much NO is present, then platelet activation would be difficult as the sGC inhibitory pathway would predominate. However, too little NO (like we see in patients with IPAH) would lead to reduced thresholds for platelet activation by reducing the sGC inhibitory pathway but also by reducing the threshold of granule release by N-ethylmaleimide-sensitive factor. A consequence of reduced NO would be that platelets would be slowly leaching granular contents. This phenomenon may explain the observation of increased plasma levels of serotonin, a constituent of dense granules, in IPAH patients (24). Also, since these platelets are more easily activated, due to the reduced GC pathway inhibition, minor activators could lead to thrombus formation, which can be found in >50% of IPAH patients (11). The idea that the platelets exist in a preactivated state is further supported by a study (7) of PAH patients that found elevated levels of fibrinopeptide, a marker of fibrin breakdown and production, in 100% of IPAH patients. Other studies (22) found elevated von Willebrand factor antigen levels in IPAH and PH associated with congenital heart disease, elevated levels of soluble P-selectin in IPAH patients, and increased von Willebrand factor levels at baseline and follow-up are associated with worse survival in patients with PAH. Based on our findings, when levels of eNOS are very low, then only partial activation of platelets occurs, with activation impaired since NO is also required for a full response. This concept is also supported by the increased presence of p-selectin in eNOS−/− mice in circulating platelets (31) and PH patients having circulating and activated platelets.
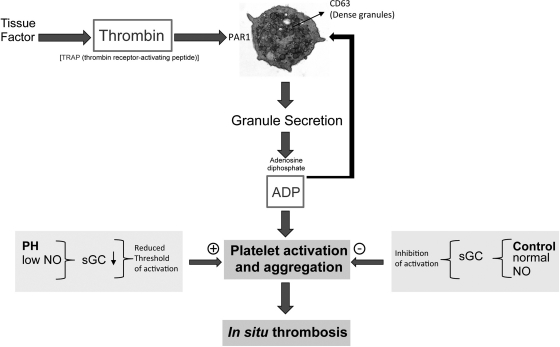
A model based on our findings and what is known about platelet function is depicted in Fig. 7. Activation of thrombin leads to binding and activation of the PAR-1 receptor on platelets. This causes platelet degranulation and release of factors such as ADP, which potentiates the signal causing a strong aggregation response. Under normal conditions, NO causes activation of sGC, which prevents premature activation of platelets. However, in IPAH the level of NO is reduced, which reduces the threshold of platelet activation. In healthy controls, NO is increased upon platelet activation leading to full activation. This step is dysregulated in IPAH patients due to the low level of NO in these patients. Thus platelet dysfunction in IPAH is related to the known global NO deficiency in this disease that is now recognized to encompass the platelets. The central defect in eNOS in IPAH platelets could also have profound effects on other pathobiological features of the disease including (but not limited to) in situ thrombosis.
Fig. 7.
Activation of platelets by thrombin leads to binding and activation of the protease-activated receptor-1 (PAR1) receptor on platelets. This causes platelet degranulation and release of factors such as ADP which potentiates the signal causing a strong aggregation response. Normally NO causes activation of soluble guanylyl cyclase (sGC), which prevents premature activation of platelets. However, in pulmonary hypertension (PH) the level of NO is reduced thus reducing the threshold of platelet activation.
GRANTS
Support for this study was provided by National Institutes of Health Grants HL-081064, HL-107147, HL-095181, and RR026231 and a BRCP 08-049 Third Frontier Program Grant from the Ohio Department of Development (to R. A. Dweik). This work was also supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio and by American Heart Association Grant 0826095H (to M. Aytekin).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A., K.S.A., O.A.M., and R.A.D. conception and design of research; M.A., K.S.A., S.H., R.C., and J.C. performed experiments; M.A. and R.A.D. analyzed data; M.A., K.S.A., and R.A.D. interpreted results of experiments; M.A., K.S.A., and R.A.D. prepared figures; M.A., O.A.M., and R.A.D. drafted manuscript; M.A., K.S.A., and R.A.D. edited and revised manuscript; M.A., K.S.A., and R.A.D. approved final version of manuscript.
REFERENCES
- 1. Berkels R, Bertsch A, Zuther T, Dhein S, Stockklauser K, Rosen P, Rosen R. Evidence for a NO synthase in porcine platelets which is stimulated during activation/aggregation. Eur J Haematol 58: 307–313, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Chen LY, Mehta JL. Further evidence of the presence of constitutive and inducible nitric oxide synthase isoforms in human platelets. J Cardiovasc Pharmacol 27: 154–158, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Chen LY, Mehta JL. Variable effects of l-arginine analogs on l-arginine-nitric oxide pathway in human neutrophils and platelets may relate to different nitric oxide synthase isoforms. J Pharmacol Exp Ther 276: 253–257, 1996 [PubMed] [Google Scholar]
- 4. De Marco T. Pulmonary arterial hypertension and women. Cardiol Rev 14: 312–318, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Dweik RA. Nitric oxide production in the lung and its regulation by oxygen. In: Disease Markers in Exhaled Breath: Basic Mechanisms and Clinical Applications (NATO Science Series), edited by Marczin N, Yacoub MH. Amsterdam, The Netherlands: IOS, 2002, p. 11–17 [Google Scholar]
- 6. Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 98: 2622–2627, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisenberg PR, Lucore C, Kaufman L, Sobel BE, Jaffe AS, Rich S. Fibrinopeptide A levels indicative of pulmonary vascular thrombosis in patients with primary pulmonary hypertension. Circulation 82: 841–847, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Fleming I, Schulz C, Fichtlscherer B, Kemp BE, Fisslthaler B, Busse R. AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. Thromb Haemost 90: 863–871, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest 100: 350–356, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fries S, Grosser T. The cardiovascular pharmacology of COX-2 inhibition. Hematology Am Soc Hematol Educ Program 445–451, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 70: 580–587, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Gambaryan S, Kobsar A, Hartmann S, Birschmann I, Kuhlencordt PJ, Muller-Esterl W, Lohmann SM, Walter U. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost 6: 1376–1384, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Ghamra ZW, Dweik RA. Primary pulmonary hypertension: an overview of epidemiology and pathogenesis. Cleve Clin J Med 70, Suppl 1: S2–8, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Harker LA, Roskos LK, Marzec UM, Carter RA, Cherry JK, Sundell B, Cheung EN, Terry D, Sheridan W. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood 95: 2514–2522, 2000 [PubMed] [Google Scholar]
- 15. Herve P, Humbert M, Sitbon O, Parent F, Nunes H, Legal C, Garcia G, Simonneau G. Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin Chest Med 22: 451–458, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 158: 917–923, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Kaul S, Waack BJ, Padgett RC, Brooks RM, Heistad DD. Interaction of human platelets and leukocytes in modulation of vascular tone. Am J Physiol Heart Circ Physiol 266: H1706–H1714, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Kawut SM, Horn EM, Berekashvili KK, Widlitz AC, Rosenzweig EB, Barst RJ. von Willebrand factor independently predicts long-term survival in patients with pulmonary arterial hypertension. Chest 128: 2355–2362, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Laufs U, Liao JK. Posttranscriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 273: 24266–24271, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Zhang G, Le Breton GC, Gao X, Malik AB, Du X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J Biol Chem 278: 30725–30731, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lopes AA, Maeda NY. Abnormal degradation of von Willebrand factor main subunit in pulmonary hypertension. Eur Respir J 8: 530–536, 1995 [PubMed] [Google Scholar]
- 23. Lopes AA, Maeda NY, Almeida A, Jaeger R, Ebaid M, Chamone DF. Circulating platelet aggregates indicative of in vivo platelet activation in pulmonary hypertension. Angiology 44: 701–706, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Maclean MR, Dempsie Y. The serotonin hypothesis of pulmonary hypertension revisited. Adv Exp Med Biol 661: 309–322, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Malinski T, Radomski MW, Taha Z, Moncada S. Direct electrochemical measurement of nitric oxide released from human platelets. Biochem Biophys Res Commun 194: 960–965, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Marcondes S, Cardoso MH, Morganti RP, Thomazzi SM, Lilla S, Murad F, De Nucci G, Antunes E. Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: a role for alpha-actinin nitration. Proc Natl Acad Sci USA 103: 3434–3439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marjanovic JA, Stojanovic A, Brovkovych VM, Skidgel RA, Du X. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J Biol Chem 283: 28827–28834, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 114: 1417–1431, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Mehta JL, Chen LY, Kone BC, Mehta P, Turner P. Identification of constitutive and inducible forms of nitric oxide synthase in human platelets. J Lab Clin Med 125: 370–377, 1995 [PubMed] [Google Scholar]
- 30. Mellion BT, Ignarro LJ, Ohlstein EH, Pontecorvo EG, Hyman AL, Kadowitz PJ. Evidence for the inhibitory role of guanosine 3′,5′-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood 57: 946–955, 1981 [PubMed] [Google Scholar]
- 31. Morrell CN, Matsushita K, Chiles K, Scharpf RB, Yamakuchi M, Mason RJ, Bergmeier W, Mankowski JL, Baldwin WM, 3rd, Faraday N, Lowenstein CJ. Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci USA 102: 3782–3787, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naseem KM, Riba R. Unresolved roles of platelet nitric oxide synthase. J Thromb Haemost 6: 10–19, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell 78: 915–918, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Pena E, Padro T, Molins B, Vilahur G, Badimon L. Proteomic signature of thrombin-activated platelets after in vivo nitric oxide-donor treatment: coordinated inhibition of signaling (phosphatidylinositol 3-kinase-gamma, 14–3-3zeta, and growth factor receptor-bound protein 2) and cytoskeleton protein translocation. Arterioscler Thromb Vasc Biol 31: 2560–2569, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Pietra G. The Pathology of Pulmonary Hypertension. New York: Marcel Dekker, 1997 [Google Scholar]
- 37. Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol 92: 181–187, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radomski MW, Palmer RM, Moncada S. An l-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA 87: 5193–5197, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. Primary pulmonary hypertension A national prospective study. Ann Intern Med 107: 216–223, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Sase K, Michel T. Expression of constitutive endothelial nitric oxide synthase in human blood platelets. Life Sci 57: 2049–2055, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Saxon A. Letter: Inhibition of platelet function by nitroprusside. N Engl J Med 295: 281–282, 1976 [DOI] [PubMed] [Google Scholar]
- 42. Sogo N, Magid KS, Shaw CA, Webb DJ, Megson IL. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem Biophys Res Commun 279: 412–419, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Stuart MJ, Murphy S, Oski FA. A simple nonradioisotope technic for the determination of platelet life-span. N Engl J Med 292: 1310–1313, 1975 [DOI] [PubMed] [Google Scholar]
- 44. Veyradier A, Nishikubo T, Humbert M, Wolf M, Sitbon O, Simonneau G, Girma JP, Meyer D. Improvement of von Willebrand factor proteolysis after prostacyclin infusion in severe pulmonary arterial hypertension. Circulation 102: 2460–2462, 2000 [DOI] [PubMed] [Google Scholar]