Abstract
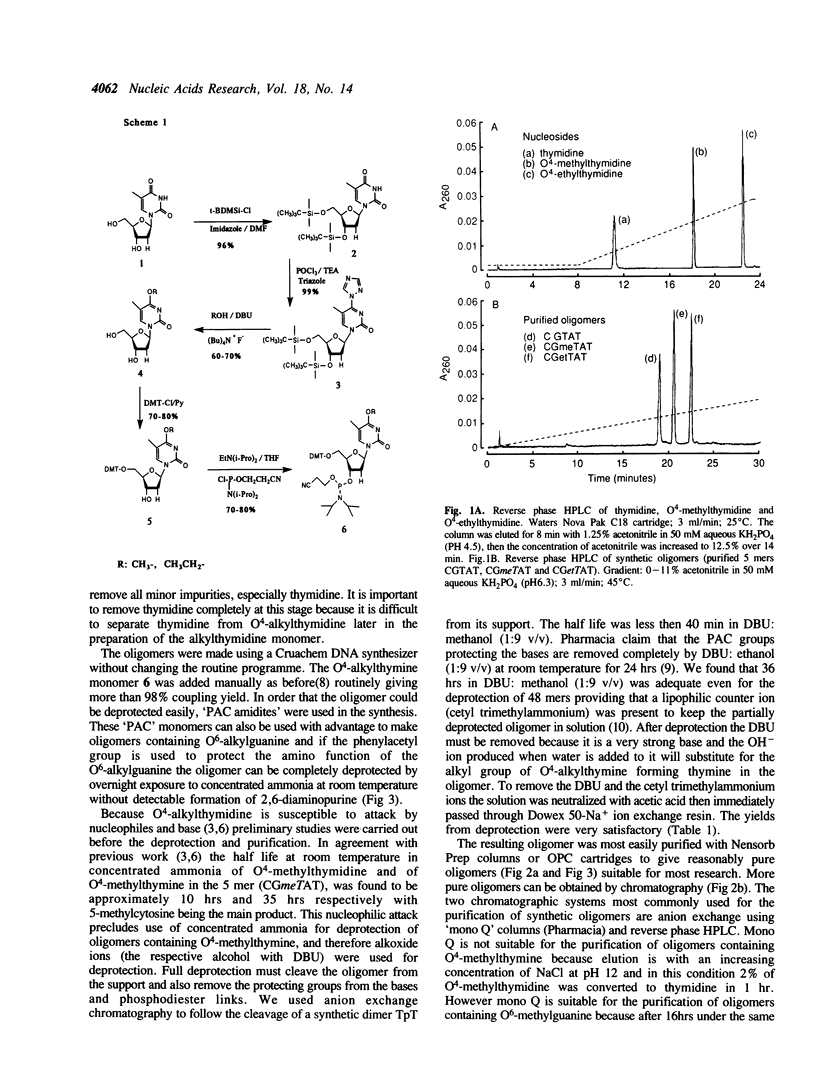
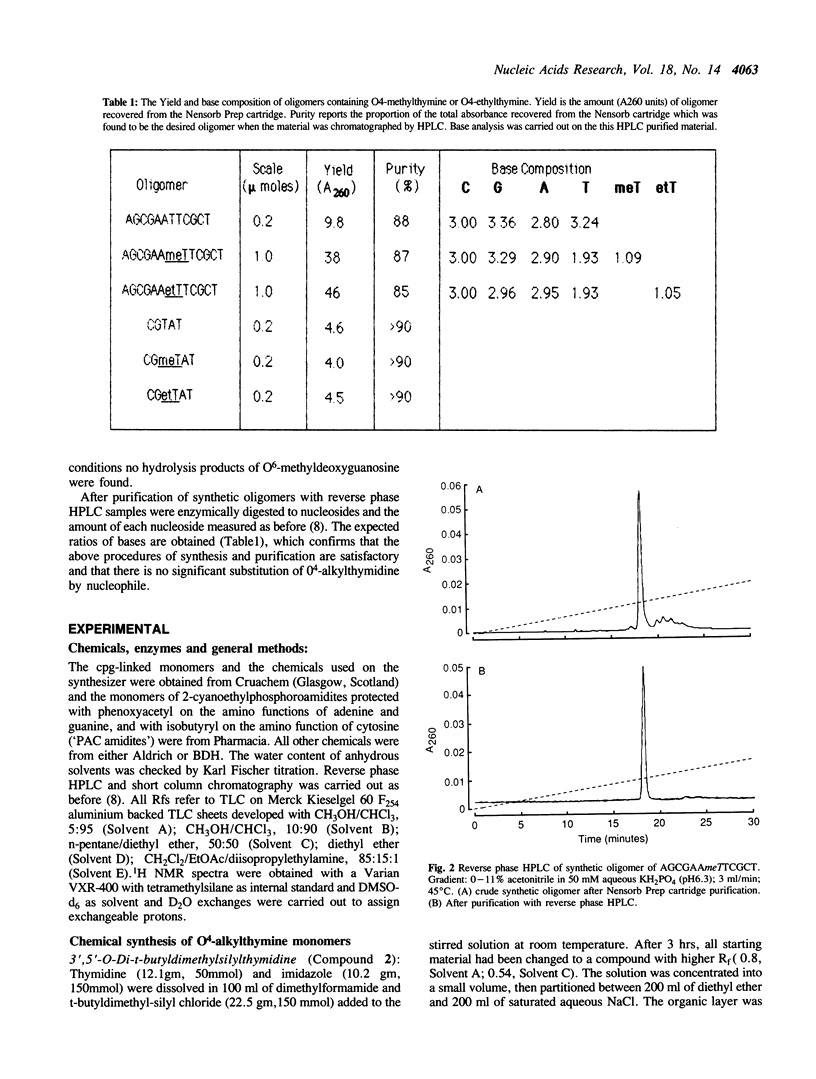
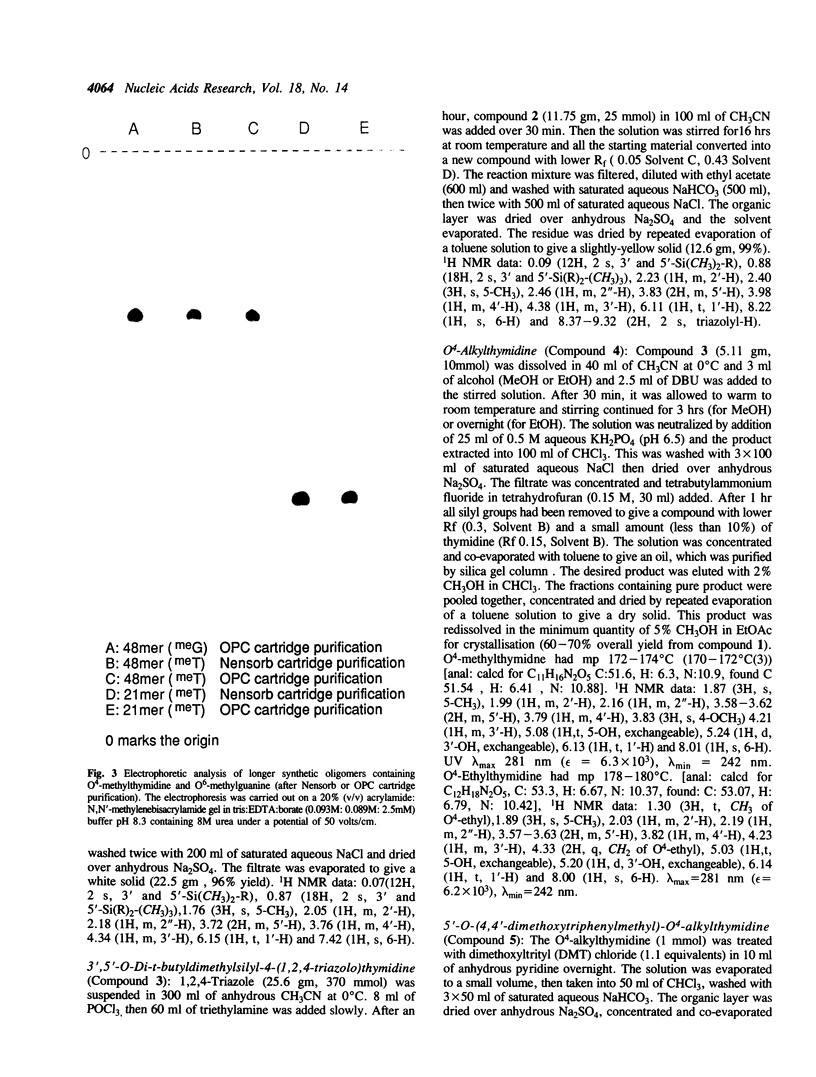
A route to prepare the cyanoethyl-phosphoramidite monomer of O4-alkylthymine and a method for the routine solid-phase synthesis of oligodeoxynucleotides containing O4-alkylthymine are described. This method has been used to make DNA sequences up to 48 bases in length. The amino function of the adenine and guanine in the sequence were protected with the phenoxyacetyl group, and that of cytosine with the isobutyryl group. The phosphodiesters were protected with the cyanoethyl group. This allowed complete deprotection of the oligomer with alkoxide ions (methanol/1,8- diazabicyclo[5.4.0]undec-7-ene (DBU) for the oligomers containing O4-methylthymine, or ethanol/DBU for those containing O4-ethylthymine) thus avoiding the use of ammonia which is known to attack the O4-alkylthymine to form 5-methylcytosine. There was no detectable loss of the alkyl group to form thymine.
Full text
PDF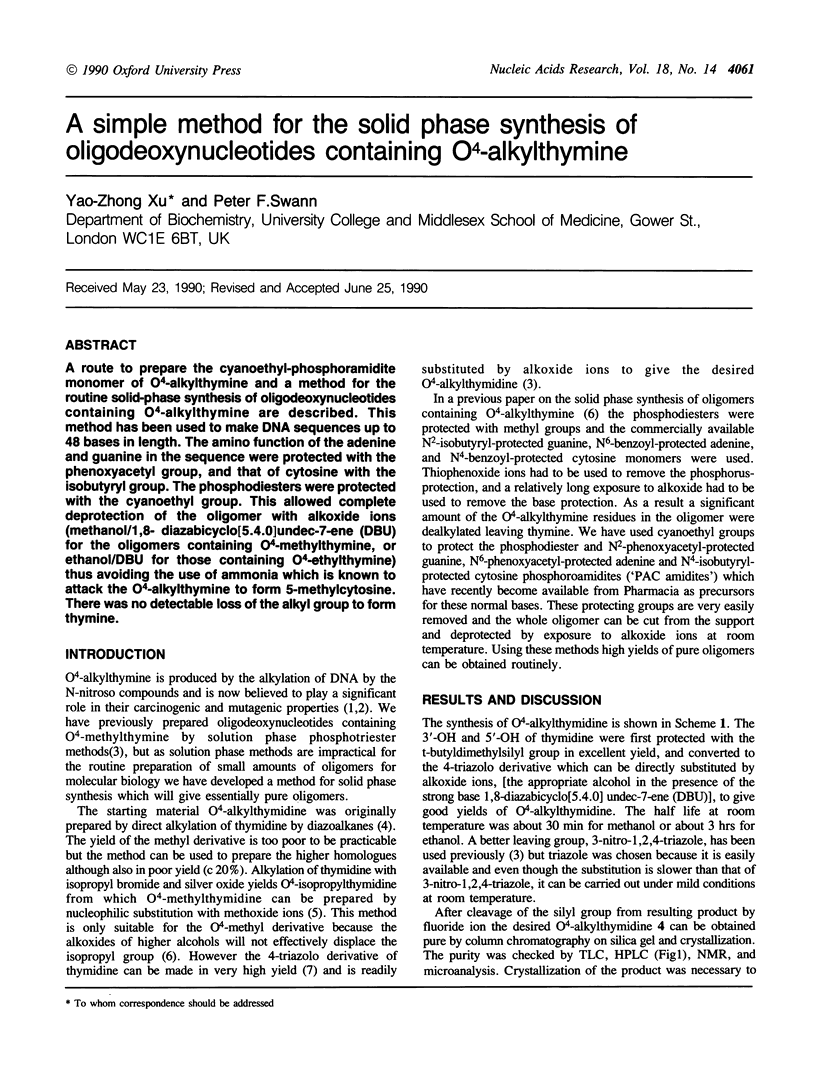

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borowy-Borowski H., Chambers R. W. Solid-phase synthesis and side reactions of oligonucleotides containing O-alkylthymine residues. Biochemistry. 1989 Feb 21;28(4):1471–1477. doi: 10.1021/bi00430a007. [DOI] [PubMed] [Google Scholar]
- Farmer P. B., Foster A. B., Jarman M., Tisdale M. J. The alkylation of 2'-deoxyguanosine and of thymidine with diazoalkanes. Some observations on o-alkylation. Biochem J. 1973 Sep;135(1):203–213. doi: 10.1042/bj1350203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Shah S. A., Farmer P. B., Jarman M. Reaction products from N-methyl-N-nitrosourea and deoxyribonucleic acid containing thymidine residues. Synthesis and identification of a new methylation product, O4-methylthymidine. Biochem J. 1973 Sep;135(1):193–201. doi: 10.1042/bj1350193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. F., Reese C. B., Swann P. F. Synthesis and characterization of oligodeoxynucleotides containing 4-O-methylthymine. Biochemistry. 1987 Feb 24;26(4):1086–1093. doi: 10.1021/bi00378a015. [DOI] [PubMed] [Google Scholar]
- Rappaport H. P. The 6-thioguanine/5-methyl-2-pyrimidinone base pair. Nucleic Acids Res. 1988 Aug 11;16(15):7253–7267. doi: 10.1093/nar/16.15.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. O-alkyl pyrimidines in mutagenesis and carcinogenesis: occurrence and significance. Cancer Res. 1986 Oct;46(10):4879–4885. [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Xu Y. Z., Swann P. F. Solid-phase synthesis of oligodeoxynucleotides containing O6-alkylguanine. Carcinogenesis. 1990 May;11(5):811–816. doi: 10.1093/carcin/11.5.811. [DOI] [PubMed] [Google Scholar]



