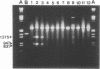
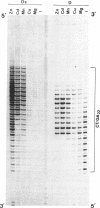
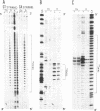
Abstract
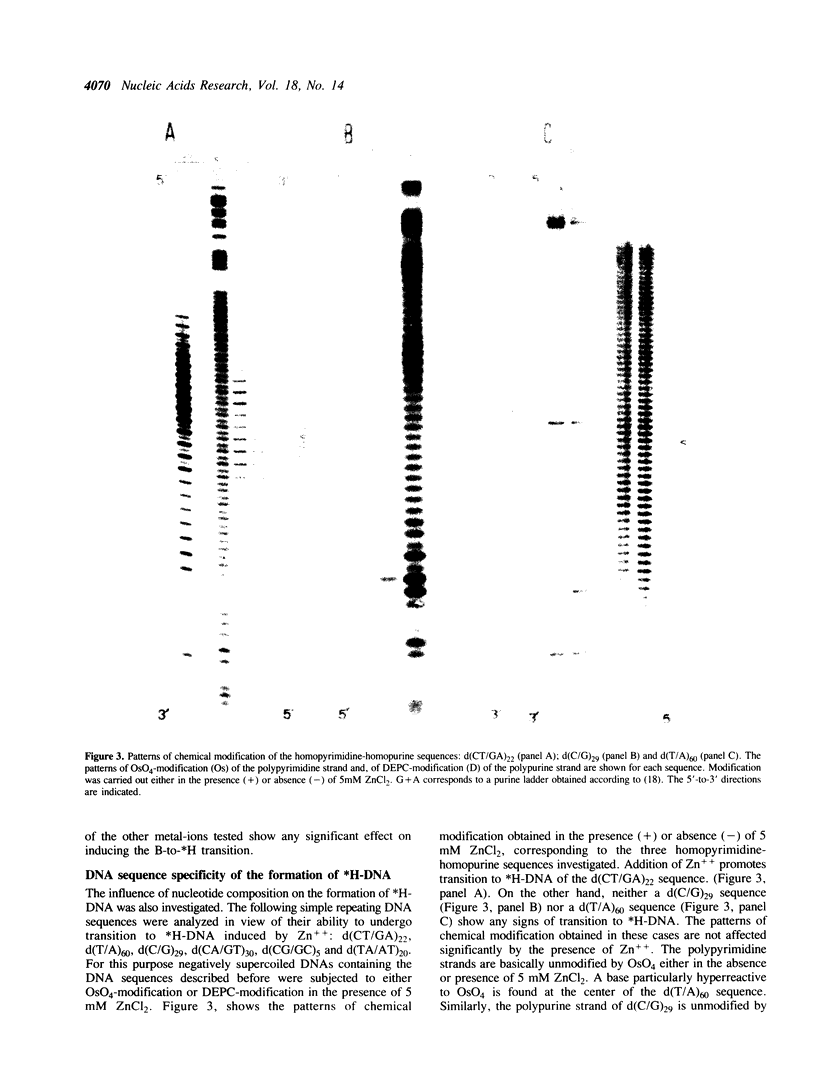
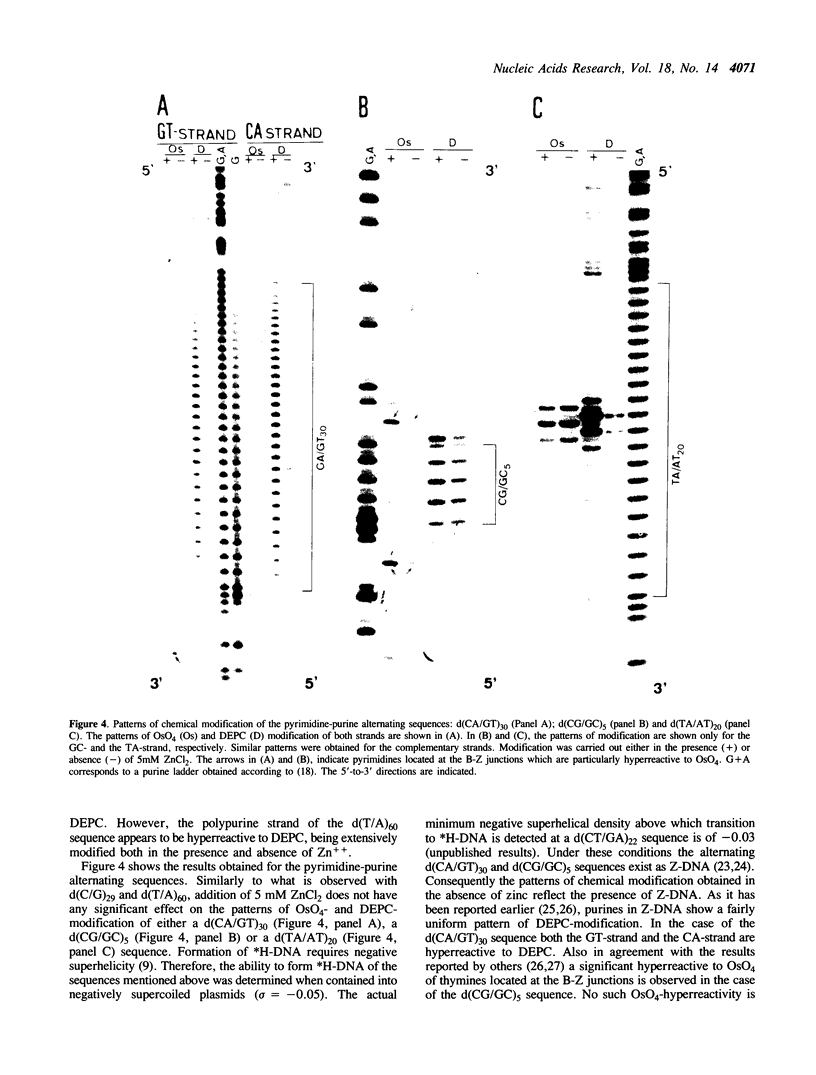
The homopyrimidine-homopurine sequence d(CT/GA)22 undergoes, in the presence of zinc ions, transition to an altered DNA conformation (*H-DNA) which is neither H-DNA nor B-DNA. *H-DNA is characterized by a peculiar chemical reactivity pattern in which most of the polypyrimidine strand is hyperreactive to osmium tetroxide and the central part of the polypurine strand is sensitive to diethylpyrocarbonate. Formation of *H-DNA is specific of metal-ion. *H-DNA is detected in the presence of Zn++, Cd++ and Mn++. The efficiency on promoting the transition is in the order of Zn++ greater than Cd++ much greater than Mn++. Formation of *H-DNA is also specific of nucleotide sequence. From all the different homopolymeric sequences tested only the d(CT/GA)22 sequence showed the zinc-induced transition to *H-DNA. These results suggest that stabilization of *H-DNA involves the formation of a specific complex between the metal-ion and the nucleotide sequence. The biological relevance of these results is discussed in view of the important role that zinc ions play on many nucleic acids processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antao V. P., Gray D. M., Ratliff R. L. CD of six different conformational rearrangements of poly[d(A-G).d(C-T)] induced by low pH. Nucleic Acids Res. 1988 Jan 25;16(2):719–738. doi: 10.1093/nar/16.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymami J., Coll M., Frederick C. A., Wang A. H., Rich A. The propeller DNA conformation of poly(dA).poly(dT). Nucleic Acids Res. 1989 Apr 25;17(8):3229–3245. doi: 10.1093/nar/17.8.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernués J., Beltrán R., Casasnovas J. M., Azorín F. Structural polymorphism of homopurine--homopyrimidine sequences: the secondary DNA structure adopted by a d(GA.CT)22 sequence in the presence of zinc ions. EMBO J. 1989 Jul;8(7):2087–2094. doi: 10.1002/j.1460-2075.1989.tb03617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casasnovas J. M., Ellison M. J., Rodriguez-Campos A., Azorin F. The obtention of simian virus 40 recombinants carrying d(CG.GC)n, d(CA.GT)n and d(CT.GA)n sequences. Stability of the inserted simple repeating sequences. Eur J Biochem. 1987 Sep 15;167(3):489–492. doi: 10.1111/j.1432-1033.1987.tb13363.x. [DOI] [PubMed] [Google Scholar]
- Casasnovas J. M., Ellison M. J., Rodriguez-Campos A., Martinez-Balbas A., Azorin F. In vivo assessment of the Z-DNA-forming potential of d(CA.GT)n and d(CG.GC)n sequences cloned into SV40 minichromosomes. J Mol Biol. 1989 Aug 20;208(4):537–549. doi: 10.1016/0022-2836(89)90146-0. [DOI] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté J., Renaud J., Ruiz-Carrillo A. Recognition of (dG)n.(dC)n sequences by endonuclease G. Characterization of the calf thymus nuclease. J Biol Chem. 1989 Feb 25;264(6):3301–3310. [PubMed] [Google Scholar]
- Duckett D. R., Murchie A. I., Lilley D. M. The role of metal ions in the conformation of the four-way DNA junction. EMBO J. 1990 Feb;9(2):583–590. doi: 10.1002/j.1460-2075.1990.tb08146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley G. V. Zinc Z-DNA. Nucleic Acids Res. 1984 Apr 25;12(8):3643–3648. doi: 10.1093/nar/12.8.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong J. C., Lilley D. M. Highly selective chemical modification of cruciform loops by diethyl pyrocarbonate. Nucleic Acids Res. 1986 May 27;14(10):3995–4007. doi: 10.1093/nar/14.10.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazka G., Palecek E., Wells R. D., Klysik J. Site-specific OsO4 modification of the B-Z junctions formed at the (dA-dC)32 region in supercoiled DNA. J Biol Chem. 1986 May 25;261(15):7093–7098. [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C. Homocopolymer sequences in the spacer of a sea urchin histone gene repeat are sensitive to S1 nuclease. Nature. 1982 Feb 25;295(5851):714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- Herr W. Diethyl pyrocarbonate: a chemical probe for secondary structure in negatively supercoiled DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8009–8013. doi: 10.1073/pnas.82.23.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H., Dahlberg J. E. Single strands, triple strands, and kinks in H-DNA. Science. 1988 Sep 30;241(4874):1791–1796. doi: 10.1126/science.3175620. [DOI] [PubMed] [Google Scholar]
- Htun H., Lund E., Dahlberg J. E. Human U1 RNA genes contain an unusually sensitive nuclease S1 cleavage site within the conserved 3' flanking region. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7288–7292. doi: 10.1073/pnas.81.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H., Lund E., Westin G., Pettersson U., Dahlberg J. E. Nuclease S1-sensitive sites in multigene families: human U2 small nuclear RNA genes. EMBO J. 1985 Jul;4(7):1839–1845. doi: 10.1002/j.1460-2075.1985.tb03858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Kohwi Y. Cationic metal-specific structures adopted by the poly(dG) region and the direct repeats in the chicken adult beta A globin gene promoter. Nucleic Acids Res. 1989 Jun 26;17(12):4493–4502. doi: 10.1093/nar/17.12.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M., Palecek E. The supercoil-stabilised cruciform of ColE1 is hyper-reactive to osmium tetroxide. EMBO J. 1984 May;3(5):1187–1192. doi: 10.1002/j.1460-2075.1984.tb01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D. Structure of (dG)n.(dC)n under superhelical stress and acid pH. J Biomol Struct Dyn. 1987 Oct;5(2):275–282. doi: 10.1080/07391102.1987.10506393. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D. Structures of homopurine-homopyrimidine tract in superhelical DNA. J Biomol Struct Dyn. 1986 Feb;3(4):667–669. doi: 10.1080/07391102.1986.10508454. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nejedlý K., Kwinkowski M., Gałazka G., Kłysik J., Palecek E. Recognition of the structural distortions at the junctions between B and Z segments in negatively supercoiled DNA by osmium tetroxide. J Biomol Struct Dyn. 1985 Dec;3(3):467–478. doi: 10.1080/07391102.1985.10508435. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Scholten P. M., Nordheim A. Diethyl pyrocarbonate: a chemical probe for DNA cruciforms. Nucleic Acids Res. 1986 May 27;14(10):3981–3993. doi: 10.1093/nar/14.10.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E., Thomas G. H., Bond U. M., Elgin S. C. Characterization of a supercoil-dependent S1 sensitive site 5' to the Drosophila melanogaster hsp 26 gene. Nucleic Acids Res. 1986 Dec 9;14(23):9425–9444. doi: 10.1093/nar/14.23.9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Gaillard C., Prunell A. Helical periodicity of DNA, Poly(dA) . poly(dT) and poly(dA-dT). poly(dA-dT) in solution. Eur J Biochem. 1981 Aug;118(2):215–222. doi: 10.1111/j.1432-1033.1981.tb06389.x. [DOI] [PubMed] [Google Scholar]
- Struhl K. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem Sci. 1989 Apr;14(4):137–140. doi: 10.1016/0968-0004(89)90145-X. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Swaminathan V., Sundaralingam M. The crystal structures of metal complexes of nucleic acids and their constituents. CRC Crit Rev Biochem. 1979;6(3):245–336. doi: 10.3109/10409237909102565. [DOI] [PubMed] [Google Scholar]
- Vojtisková M., Palecek E. Unusual protonated structure in the homopurine.homopyrimidine tract of supercoiled and linearized plasmids recognized by chemical probes. J Biomol Struct Dyn. 1987 Oct;5(2):283–296. doi: 10.1080/07391102.1987.10506394. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]