Abstract
Objective
Chronic inflammation and cellular senescence are intertwined in the pathogenesis of premature aging, which is considered as an important contributing factor in driving chronic obstructive pulmonary disease (COPD). SIRT1, a NAD+-dependent protein/histone deacetylase, regulates inflammation, senescence/aging, stress resistance, and DNA damage repair via deacetylating intracellular signaling molecules and chromatin histones. The present review describes the mechanism and regulation of SIRT1 by environmental agents/oxidants/reactive aldehydes and pro-inflammatory stimuli in lung inflammation and aging. The role of dietary polyphenols in regulation of SIRT1 in inflammaging is also discussed.
Methods
Analysis of current research findings on the mechanism of inflammation and senescence/aging (i.e., inflammaging) and their regulation by SIRT1 in premature aging of the lung.
Results
COPD is a disease of lung inflammaging, which is associated with the DNA damage response, transcription activation and chromatin modifications. SIRT1 regulates inflammaging via regulating FOXO3, p53, NF-κB, histones and various proteins involved in DNA damage and repair. Polyphenols and its analogs have been shown to activate SIRT1 although they have anti-inflammatory and antioxidant properties.
Conclusions
Targeting lung inflammation and cellular senescence as well as premature lung aging using pharmacological SIRT1 activators or polyphenols would be a promising therapeutic intervention for COPD/emphysema.
Keywords: Inflammaging, COPD, SIRT1, Tobacco smoke, Oxidative stress, NF-κB, DNA damage response, FOXO3, Histone modifications, Polyphenols
Introduction
The word “inflammaging“ is coined by C Franceschi in 2000, which refers to a progressive increase in proinflammatory status, a major characteristic of the aging process. This can be reflected in diseases where underlying chronic abnormal inflammation exist (Franceschi et al., 2000). For example, chronic inflammation is associated with aging and its related diseases, such as diabetes, atherosclerosis, cancer, and chronic obstructive pulmonary disease (COPD). The inflammation and cellular senescence are intertwined in the process of accelerated or premature aging. The causal role of inflammation and aging in certain conditions and diseases remains unknown. COPD is the fourth leading cause of chronic morbidity and mortality in the United States and globally, affecting an estimated 23 million people. It includes airway obstruction/chronic bronchitis and emphysema, which is linked with lung inflammaging and premature aging (accelerated decline in lung function) due to inhaled cigarette smoke-derived oxidants and free radicals, and noxious gases. However, in certain conditions, such as in pulmonary emphysema inflammation is almost absent but the disease/lung destruction progresses.
The NAD+-dependent protein deacetylase, sirtuin1 (SIRT1), has been reported as an important regulator of aging phenomena, such as apoptosis/senescence, stress resistance, and inflammation through the deacetylation of intracellular signaling molecules and chromatin histones (Chung et al., 2010). The level/activity of SIRT1 deacetylase is decreased in chronic lung inflammatory conditions and premature aging where sustained oxidative/carbonyl (due to reactive aldehydes-acrolein and 4-hydroxy-2-nonenal) stress occurs. SIRT1 is oxidatively down-regulated by cigarette smoke/aldehydes, leading to post-translational modifications, inactivation and protein loss via the proteasome (Caito et al., 2010b). However, very little is known whether SIRT1 regulates inflammaging, particularly in the development of COPD. In this review, we describe the mechanism and regulation of SIRT1 by oxidants/aldehydes generated by environmental and pro-inflammatory stimuli in lung inflammaging, particularly in pathogenesis of COPD, a disease of accelerated premature aging and inflammation of the lung. We also discuss the role of dietary polyphenols and pharmacological analogs in regulation of SIRT1 in inflammaging.
Etiology and comorbidities of COPD
COPD is characterized by destruction of the alveolar wall, decline in lung function, and chronic lung inflammatory response. An estimated 1015–17 oxidants/free radicals and ~4,700 different highly reactive chemical compounds/aldehydes are present in per puff of cigarette smoke, which is the major risk factor in the development of COPD. It accounts for ~80%-90% of COPD cases in USA (Sethi and Rochester, 2000). Additionally, noxious environmental gases/particles, such as NO2, SO2, and particulate matters, as well as exposure to second-hand tobacco smoke and smoke derived from burning of biomass fuels can trigger inflammatory response in lungs of a susceptible population. Maternal smoking is another contributing factor in promoting COPD in the offspring during the later stages of life (Beyer et al., 2009). Tobacco smoking has also been associated with cardiovascular disease, skin wrinkling, as well as several types of cancer (e.g. lung) and premature aging of the lungs (accelerated decline in lung function). Chronic inflammation, oxidative/carbonyl stress and protease/antiprotease imbalance resolve very slowly after smoking cessation, the resolution demanding from months to years (Louhelainen et al., 2009; Louhelainen et al., 2010; Nagai et al., 2006). This may explain why smoking cessation alone is not the only “therapy“ to prevent COPD progression. COPD also can develop in non-smokers especially in women, or in those with childhood respiratory problems, asthma, as well as long exposure to smoke-derived from biomass fuel burning and environmental pollutants (Lamprecht et al., 2011; Salvi and Barnes, 2009).
In addition to intra-pulmonary manifestations, comorbidities, such as lung cancer, cardiovascular disease, diabetes, metabolic syndrome, osteoporosis, muscle atrophy, skin wrinkling/aging and depression, are the major causes for mortality in COPD. For example, the skeletal muscle wasting and depression negatively affect the quality of life in COPD patients. Although COPD increases the susceptibility for lung tumorigenesis up to 4.5-fold (Sundar et al., 2011; Yao and Rahman, 2009), the causal pathways that links COPD and other comorbid conditions remains to be studied. It is apparent that besides local inhaled therapies, systemic/oral therapies with minimal side effects are necessary to slow the COPD lung disease and its systemic manifestations.
Inflammaging phenotype in COPD
A variety of cellular processes, such as inflammation, aging/senescence, oxidative stress, apoptosis/proliferation, autophagy, and autoimmunity, are involved in the pathogenesis of COPD/emphysema (Yao and Rahman, 2009, 2011). Hence, the specific molecules that regulate aging/senescence and inflammatory/immune events will provide the possible therapies for intervention in COPD.
Lung function decreases with age along with additional age-related alterations, such as changes of the elastic recoil of the lung, increased alveolar size, and reduced defense mechanisms. The prevalence of COPD increases with aging, and upregulation of pro-inflammatory genes occurs in lungs of COPD patients, suggesting the association of inflammation and aging/senescence in the pathogenesis of COPD/emphysema. Lung cellular senescence is accelerated in COPD, which has been found to be independently associated with lowered antioxidant defense, elevated oxidative stress, protease/antiprotease imbalance, and elastolysis (Ito and Barnes, 2009; MacNee, 2009). The telomere length in circulating lymphocytes is shortened (i.e., replicative senescence) in patients with COPD as compared to non-smokers (Houben et al., 2009; Morla et al., 2006; Mui et al., 2009; Savale et al., 2009). Furthermore, the telomere length was positively correlated with PaO2, SaO2, 6-minute walking distance, and lung function in patients with COPD (Mui et al., 2009; Savale et al., 2009). It is postulated that these senescent immune cells have downregulated humoral and cellular immunity, or lose the ability to self-recognition, thereby leading to impaired host defense and autoantibody generation. Cigarette smoke/oxidants/aldehydes exposure has been shown to induce senescence (i.e., stress-induced premature senescence, SIPS) in both alveolar epithelial cells and fibroblasts, which is independent of telomere shortening (Muller et al., 2006; Nyunoya et al., 2006; Nyunoya et al., 2009; Tsuji et al., 2004, 2006, 2010). In addition to type II epithelial cells, the number of senescent Clara cells is also increased in patients with COPD as compared to nonsmokers (Aoshiba and Nagai, 2009). Both Type II epithelial cells and Clara cells are the progenitors of alveolar and airway epithelial cell for regeneration, respectively. Hence, the senescence of these progenitor cells may dampen the repair of damaged lung tissue, which explains why COPD progresses even after cessation of smoking.
The role of cellular senescence in COPD/emphysema is further attested by the animal studies. The deficiency of Klotho or senescence marker protein-30 gene leads to emphysematous phenotype in mouse lung (Sato et al., 2006; Suga et al., 2000). Knockout of telomerase, a holoenzyme required for maintaining telomere length, also increases the mean linear intercept of airspace in mouse (Lee et al., 2009). These findings implicate that cellular senescence is an essential process contributing to the development/progression of COPD/emphysema. Nevertheless, further study is required to which phenomenon drives the progression of COPD/emphysema, replicative senescence, SIPS, or both. It is also important to bear in mind that the telomere length is longer in mice than that in long-living mammals due to high levels of telomerase, suggesting caution when extrapolating the data from mouse to human.
Cellular senescence is a permanent state of cell cycle arrest, which prevents the growth of cells exposed to potential oncogenic stimuli. However, the senescent cells are metabolically active, and prone to secrete pro-inflammatory cytokines (i.e., IL-6 and IL-8) and matrix metalloproteinases (i.e., MMP-3 and MMP-9) involved in inflammaging (Freund et al., 2010; Rodier et al., 2009). These pro-inflammatory mediators may in turn initiate and maintain cellular senescence as the deficiency of IL-6, IL-6R, or CXCR2 prevented SIPS (Acosta et al., 2008; Kuilman et al., 2008) (Figure 1). Thus, the low grade of chronic inflammation and cellular senescence form a vicious cycle via an autocrine and paracrine manner, which may render lung progenitor cells unable to repair the damaged lung tissues, leading to aggressive lung destruction. Indeed, the increased percentage of pro-inflammatory senescent type II cells expressing both p16 and phosphorylated NF-κB (i.e., senescent-associated secretory phenotype, SASP) was observed in lungs of patients with COPD as compared to smokers and nonsmokers (Tsuji et al., 2010). Furthermore, cellular senescence is also associated with apoptosis/autophagy (Vicencio et al., 2008). Thus, the cellular senescence is an important cellular status that allows the damaged cells to adapt to stress or undergo programmed cell death in the pathogenesis of COPD/emphysema. The cellular senescence also facilitates bacterial adhesion to the lung cells under the persistent SIPS leading to exacerbations of the disease (Shivshankar et al., 2011; Zhou et al., 2011).
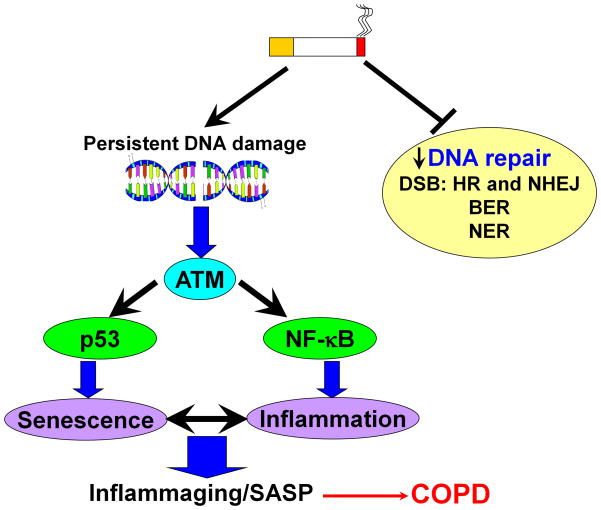
Figure 1. Oxidative stress induces persistent DNA damage leading to cellular senescence and inflammation.
Sustained or persistent DNA damage from oxidative/carbonyl stress recruits checkpoint kinase ataxia telangiectasia mutated (ATM) leading to cellular senescence and inflammatory response through activation of p53 and NF-κB, respectively. Oxidative/carbonyl stress also damages the DNA repair pathways, such as double-strand break (DBS), base excision repair (BER), and nucleotide excision repair (NER), which further cause DNA damage. The cellular senescence and inflammation will form a positive feedback to compromise normal cellular homeostasis.
Mechanism of inflammaging in COPD
A variety of molecules have been shown to regulate the inflammaging or in other word SASP, which includes DNA damage response (DDR), transcription factors, and epigenetic (more specifically epigenomic ) modifications. Thus, the study of these molecules/pathways particularly in response to cigarette smoke will provide an avenue in preventing the progression of COPD/emphysema.
DNA damage response
Cigarette smoke/oxidative stress has been shown to cause DNA damage, which is increased in patients with COPD/emphysema (Caramori et al., 2011). Recent studies have demonstrated that persistent DNA damage causes SIPS with increased pro-inflammatory mediators release (Coppe et al., 2008; Rodier et al., 2009) (Figure 1). It seems that SASP is induced by genotoxic stress, such as oxidants/reactive aldehydes (reactive oxygen species, ROS)/smoking and ionizing radiation, rather than proliferative arrest per se (Coppe et al., 2008; Rodier et al., 2009). The upstream signals of DDR, including ataxia telangiectasia mutated (ATM) and its substrates Nijmegen breakage syndrome 1 (NBS1), Werner syndrome protein as well as checkpoint kinase 2 (CHK2), are required for inflammatory cytokines secretion in senescent cells (Rodier et al., 2009). The mechanism of ATM signal-mediated pro-inflammatory release is associated with NF-κB activation (Elkon et al., 2005; Wu et al., 2006).
The double-strand break (DSB) is the most dramatic form of DNA damage, which can be repaired either by homologous recombination (HR), classic or alternative non-homologous end jointing (NHEJ). The former corrects DSB damage in a precise manner using a way that retrieves information from a homologous and undamaged DNA, whereas the latter is a primary pathway to repair DSB defects in an error-prone manner which does not require a homologous template. Usually, the classic NHEJ has faster kinetics than alternative NHEJ and HR to monitor end jointing (Han and Yu, 2008; Mao et al., 2008a). The HR-based repair usually happens in late S-and G2-phases of cell cycle while the early rapid repair is active throughout the cell cycle (Mao et al., 2008b; Neal and Meek, 2011). The decision that damaged cells choose HR or NHEJ for DSB repair depends on the factors/situations including the cell cycle stage and available undamaged sister chromatid as well as the availability of repairing proteins/enzymes at the time of damage acquisition. Cigarette smoke/oxidative/carbonyl stress has been shown to decrease the NHEJ repair proteins such as Ku70, Ku80 and Ku86 directly or indirectly (Caramori et al., 2011; Song et al., 2003), which may lead to sustained DNA damage and account for SASP observed in patients with COPD.
Transcription regulation
NF-κB is a key transcription factor responsible for pro-inflammatory genes transcription, which is significantly increased in patients with COPD (Caramori et al., 2003; Di Stefano et al., 2002). Interestingly, it also regulates cellular senescence, DNA repair as well as genome stability via an oxidative stress-related mechanism (Acosta et al., 2008; Bernard et al., 2004; Kawahara et al., 2009; Wang et al., 2009). This is important for preventing further DNA damage in the earlier phase of smokers/COPD, although DNA damage was not changed between RelA/p65 deficient and wild-type mouse embryonic fibroblasts (Wang et al., 2009). However, the deletion of RelA/p65 decreases the production of CXCR2 ligands (i.e., IL-8 and GROγ) during SIPS. Similarly, transcription factor C/EBPβ is also involved in SASP (Acosta et al., 2008). Hence, the sustained activation of NF-κB triggered by cigarette smoke/oxidants/aldehydes will dampen the re-epithelialization of damaged airways and alveolar walls due to epithelium senescence, as well as will result in chronic inflammatory response via increasing expression of SASP factors in lungs.
Chromatin/histone modifications
Chromatin remodeling, including DNA methylation and histone modifications (histone acetylation/acetylation and methylation/demethylation), has been shown to regulate cellular senescence (Dimauro and David, 2009). Cigarette smoke and oxidants cause histone acetylation (e.g., H3K9, H3K14, H4K5, H4K8, H4K12, H4K16) and increased expression of NF-κB-dependent pro-inflammatory cytokines in rodent lungs (Yang et al., 2008; Yao et al., 2010). Similarly, increased histone acetylation (specifically histone H4) occurs in lungs of smokers, and in bronchial biopsy/peripheral lung tissue (acetylation of histone H4 on pro-inflammatory gene promoter but no change in global increase in HAT activity) obtained from patients with COPD (Ito et al., 2005). Histone acetylation is dynamic reversible and is regulated by both histone acetyltransferases (HATs) and deacetylases (HDACs). Furthermore, histone modifications, such as H3K9 acetylation and H4K20 methylation, are associated with aging (Michishita et al., 2008; Sarg et al., 2002). The enzymes responsible for histone acetylation/deacetylation (e.g., p300, CBP, SIRT1, and SIRT6) and methylation/demethylation (e.g., SUV39h1, KMD, and EZH2) have been shown to regulate cellular senescence and aging (Bandyopadhyay et al., 2002; Braig et al., 2005; Longo and Kennedy, 2006; Michishita et al., 2008; Mostoslavsky et al., 2006). However, no information is available regarding the regulation of histone modifications in SIPS/SASP particularly in response to cigarette smoke/oxidants/aldehydes exposure in lung cells.
SIRT1 as a target for inflammaging
Unlike class I and II histone deacetylases (HDACs), which require a water molecule for deacetylation, type III SIRTs require NAD+ as a cofactor and are not inhibited by trichostatin A. The deacetylase activity of sirtuins is inhibited by the reaction product, nicotinamide. SIRTs have five homologues in yeast (ySir2 and Hst1–4) and seven in humans (SIRT1–7). The best characterized and well-studied among the human sirtuins is SIRT1, a nuclear protein reported to regulate critical metabolic and physiological processes (Alcendor et al., 2007; Finkel et al., 2009; Lavu et al., 2008; Michan and Sinclair, 2007; Yang and Sauve, 2006). SIRT1 removes the acetyl moieties from the ε-acetamido groups of lysine residues of histones and other signaling proteins, thus facilitating chromatin condensation and silencing of gene transcription. Activation or overexpression of SIRT1 (sir2) has been shown to increase the lifespan of Drosophila, S. cerevisiae and C. elegans up to 70% although resveratrol used in these studies is non-specific activator of SIRT1. Furthermore, the role of resveratrol (via SIRT1) in prolonging lifespan in mammals remains unclear (Bordone et al., 2007; Wood et al., 2004). SIRT1 regulates numerous processes, including inflammation and cellular senescence/aging, due to its ability to deacetylate NF-κB, forkhead box class O (FOXO)3, p53, Werner syndrome protein, Klotho, β-catenin/Wnt, Notch, endothelial nitric oxide synthase (eNOS), and histones (Finkel et al., 2009; Lavu et al., 2008; Michan and Sinclair, 2007) (Figure 2).
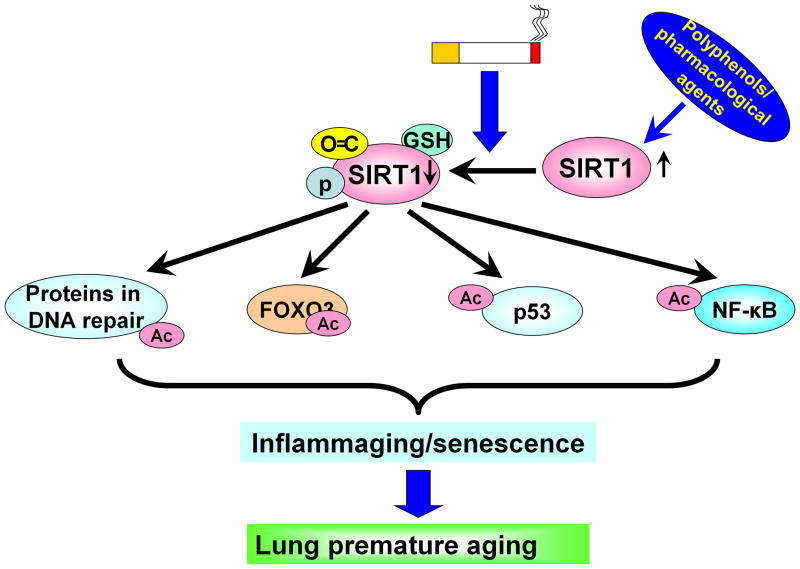
Figure 2. SIRT1 reduction caused by cigarette smoke results in deacetylation of proteins in DNA repair, FOXO3, p53 and NF-κB leading to premature lung aging.
SIRT1 is subjected to posttranslational modifications in response to oxidative/carbonyl stress, which causes the acetylation of various substrates, including ku70, Werner syndrome protein, FOXO3, p53 and NF-κB. These molecules play an important role in initiating and causing inflammation, cellular senescence and DNA damage, which is a major characteristic of lung premature aging. Activation of SIRT1 by polyphenols and its analogs (pharmacological activators) may attenuate lung inflammaging.
Recent studies have shown five single nucleotide polymorphisms of SIRT1 in a population-based study, suggesting SIRT1 variability in the human population (Flachsbart et al., 2006; Zillikens et al., 2009). However, it is unknown whether SIRT1 (SNPs or allelic variants) is a susceptibility factor for development of COPD in smokers. It would be interesting in future study to determine SIRT1 polymorphisms in COPD and link to susceptibility to the disease.
SIRT1 modification by oxidative/carbonyl stress
In addition to NAD+ and nicotinamide, SIRT1 protein level and activity can be affected by posttranslational modifications. There are five known phosphorylation sites in SIRT1 on serine residues S14, S16, S26, S27, and S47. SIRT1 can be phosphorylated or dephosphorylated by PKC, CK2 or PI3K-and ser/thr phosphatase-dependent mechanisms, and possibly by other members of the MAP kinase family. Oxidants/aldehydes derived from tobacco smoke caused SIRT1 phosphorylation in macrophages, epithelial cells as well as in mouse lungs (Caito et al., 2010b). Proteasome inhibitors inhibited SIRT1 phosphorylation, suggesting that phosphorylation in addition to covalent carbonyl/oxidative/nitrosative modifications of SIRT1 cause its irreversible modifications and subsequent proteasomal degradation (Arunachalam et al., 2010;
Caito et al., 2010a; Caito et al., 2010b). Furthermore, SIRT1 is also subject to S-glutathionylation and its enzymatic activity is modulated by intracellular redox GSH status, and is reversed by glutaredoxin1, an enzyme that reverses glutathionylated proteins (Caito et al., 2010b; Zee et al., 2010) (Figure 2). Thus, SIRT1 posttranslational modifications can modify its activity, and cause its nucleocytoplasmic shuttling and subsequent ubiquitination-proteasomal degradation as well as inhibition of ser-thr phosphatases particularly in response to CS exposure. These findings advance the emerging field of research on SIRT1 regulation by suggesting that a simple activation of SIRT1 by pharmacological agents may not be effective since SIRT1 is covalently modified in carbonyl/oxidative/inflammatory conditions. Indeed, increasing cellular NAD+ by PARP-1 inhibitor or NAD+ precursor was unable to restore SIRT1 activity, particularly in response to genotoxic stress caused by cigarette smoke/oxidants/reactive aldehydes (Caito et al., 2010a). Therefore, the reversal of oxidative post-translationally modified SIRT1 may be an avenue before effective therapeutic strategies can be designed for chronic inflammatory diseases.
SIRT1 and regulation of NF-κB
SIRT1 protein directly interacts with RelA/p65 subunit of NF-κB, and deacetylates lys310 residue of RelA/p65, a site that is critical for NF-κB transcriptional activity (Chen et al., 2002; Yeung et al., 2004). We have shown that cigarette smoke-mediated pro-inflammatory cytokine release is regulated by SIRT1 via its interaction with NF-κB in monocytes and mouse lung, as well as in smokers and patients with COPD (Rajendrasozhan et al., 2008; Yang et al., 2007). Both sirtinol (an inhibitor of SIRT1) and SIRT1 knockdown augmented, whereas SRT1720 (a potent SIRT1 activator) inhibited cigarette smoke-mediated pro-inflammatory cytokine release (Nakamaru et al., 2009; Rajendrasozhan et al., 2008; Yang et al., 2007). Furthermore, the level and activity of SIRT1 were decreased in lung of patients with COPD (Nakamaru et al., 2009; Rajendrasozhan et al., 2008). Hence, downregulation of SIRT1 would lead to increased lung inflammatory via regulating NF-κB. Recent studies have shown that SIRT1 also deacetylates and suppresses the transcription activity of activator protein-1 leading to down-regulation of cyclooxygenase-2 gene expression (Gao and Ye, 2008; Zhang et al., 2009; Zhang et al., 2010a), suggesting the multiple targets of SIRT1 in regulating inflammation.
SIRT1 and cell senescence/aging
McBurney et al have shown that mice lacking SIRT1 were smaller and aged faster than their wild-type littermates (McBurney et al., 2003). Senescent mouse embryonic fibroblasts and human endothelial cells have decreased levels of SIRT1 (Orimo et al., 2009; Sasaki et al., 2006). SIRT1 is therefore considered a novel anti-aging protein involved in regulation of cell senescence and proliferation due to its ability to deacetylate FOXO3 and p53 proteins, which is involved in a variety of cellular responses, such as cell cycle arrest, cellular senescence, proliferation, and resistance to oxidative stress and apoptosis (Brunet et al., 2004; Kops et al., 2002; Kyoung Kim et al., 2005; Willcox et al., 2008).
The mechanism of SIRT1/FOXO3 or SIRT1/p53 in cellular proliferation and senescence is associated with altered transcription of downstream cell cycle inhibitors (e.g. p16, p21, and p27) (Furukawa et al., 2007; Ota et al., 2007). Recently, it has been shown that FOXO3 and p53 are acetylated when SIRT1 is reduced in mouse lung exposed oxidants/aldehydes/cigarette smoke, as well as in lungs of patients with COPD (Caito et al., 2010a; Caito et al., 2010b; Hwang et al., 2011). Furthermore, the level of FOXO3 was significantly decreased in lungs of patients with COPD (Hwang et al., 2011; MacNee and Tuder, 2009). Genetic ablation of FOXO3 leads to emphysema and exaggerated inflammation by NF-κB activation and downregulation of antioxidant defense (Hwang et al., 2011). It can be speculated that FOXOs maintenance would offer an alternative insight for SIRT1 s function in COPD (inflammaging/SIPS), but so far this kind of manipulation has not been published in human diseases. Therefore, the study of SIRT1-FOXO3/p53 pathway will provide more insight into the imbalance of cellular proliferation/senescence and apoptosis in response to oxidative stress, and whether polyphenols have any affect on this pathway, since polyphenols (e.g. resveratrol) have been shown to regulate cellular senescence (Stefani et al., 2007; Zamin et al., 2009). It is also possible that SIRT1 regulates the function of p21 by posttranslational modification since p21 itself can be subject to acetylation/deacetylation (Chen et al., 2004). However, it remains to be seen whether SIRT1 is also involved in p21-mediated regulation of cell proliferation/senescence, although p21 is a sensor of cellular stress to trigger lung inflammation and injury (Tuder et al., 2008; Yao et al., 2008).
The functional link between emphysema and aging implied by the findings on regulation of Klotho and senescence marker protein-30 which are implicated in both of these processes (Afanas’ev, 2010; Koike et al., 2010; Sato et al., 2006; Suga et al., 2000). It remains to be known whether SIRT1 regulates these genes and proteins in response to CS exposure. Several other SIRT1 protein substrates involved in cell stress response signaling and cellular senescence have been identified, including ku70, Wnt/β-catenin, Notch, and Werner syndrome protein (Guarani et al., 2011; Holloway et al., 2010; Li et al., 2008; Uhl et al., 2010; Vaitiekunaite et al., 2007) (Table 1). Hence, the study on these molecules in condition of oxidative stress/cigarette smoke will further enhance the understanding of inflammaging in pathogenesis of COPD.
Table 1.
SIRT1-regulated proteins and their involvements in cellular senescence and DNA damage repair process
| Proteins | Cellular senescence | DNA damage repair | Alteration in response to oxidative stress |
|---|---|---|---|
| FOXO3 | Inhibits cellular senescence | Promotes DNA damage repair via interacting with ATM | Acetylated and degraded |
| p53 | Induces cellular senescence | Causes DNA damage | Acetylated and increased |
| NF-κB | Induces SASP after sustained activation | Protects DNA damage at earlier phase | Acetylated and activated |
| eNOS | Delays endothelial cell senescence | - | Acetylated and deactivated |
| Notch | Induces cellular senescence | Protects DNA damage | Acetylated and destabilization |
| Werner syndrome protein | Protects cellular senescence | Stimulates repair of DNA damage | Degraded |
| Klotho | Suppresses cellular senescence | Reduces DNA damage | - |
| SMP-30 | Protects cellular senescence | Reduces DNA damage | Decreased with aging |
| Wnt/β-catenin | Promotes cellular senescence | - | No significant change in |
| Ku70 | Attenuates cellular senescence | Involved in NHEJ pathway | lungs of COPD patients |
| Ku86 | Suppresses cellular senescence | Involved in NHEJ pathway | Decreased |
SIRT1 and regulation of DNA damage and repair
Accumulating evidence suggests that SIRT1 relocalizes to the sites of DNA damage and promotes DNA damage repair via deacetylating DSB repair and recombination proteins, such as ku70, NBS, Werner helicase, and nibrin (Gorospe and de Cabo, 2008; Jeong et al., 2007; Uhl et al., 2010; Yuan and Seto, 2007; Yuan et al., 2007). Interestingly, inhibition of type I and II HDACs did not repress HR, but they function in the DDR to promote DNA NHEJ (Miller et al., 2010; Uhl et al., 2010), suggesting the differentiated role of HDACs (HDAC1 and HDAC2) in DNA damage and repair. SIRT1 also deacetylates histone H3K56 thereby regulating DNA damage and genomic instability (Vempati et al., 2010; Yuan et al., 2009). Histone methylation (e.g., H3K9me3) can also be regulated by SIRT1 via increasing/deacetylating Suv39h1, or enabling histone H3 toward Suv39h1 (Vaquero et al., 2007). Further study is required to investigate which histone residue(s) are modified and how these modifications affect genomic stability, DNA damage repair, and subsequent cellular senescence.
SIRT1 and lung stem cells: regenerative processes
SIRT1 levels have been found to be significantly higher in embryonic stem cells than in differentiated tissues (Saunders et al., 2010). However, SIRT1 deficiency in the embryonic cells leads to embryonic and developmental abnormalities including defects in the formation of the primitive vascular network (Mantel and Broxmeyer, 2008; Ou et al., 2011). COPD represents a disease with premature ageing and cellular senescence in Clara cells and type II epithelial cells, the progenitor cells of airway and alveolar epithelium. Therefore, the regulation of SIRT1 in these progenitor cells would be a promising therapeutic avenue in treatment of COPD.
SIRT1 and cardiovascular homeostasis
SIRT1 interacts eNOS-nitric oxide generating system thereby regulating vascular senescence and dysfunction as well as atherosclerosis (Arunachalam et al., 2010; Ota et al., 2010). SIRT1 s protection on aging and premature senescence in cardiomyocytes and endothelial cells is due to antioxidant property through FOXO3 (Alcendor et al., 2007; Tanno et al., 2010). Overexpression of SIRT1 in mouse heart regulates aging and resistance to oxidative stress by inhibition of apoptosis and expression of senescence markers (Alcendor et al., 2007). A recent study has shown that resveratrol and other dietary polyphenols attenuate mitochondrial oxidative stress in endothelial cells via activation of SIRT1 (Ungvari et al., 2009). Activation of SIRT1 by resveratrol led to lysine deacetylation in ischemic preconditioning and protects cardiac ischemic-reperfusion (Nadtochiy et al., 2011). Therefore, SIRT1 exhibits a beneficial role in cardiovascular function, which is damaged in patients with COPD.
Clinical implications of SIRT1 activation in inflammaging
Dietary polyphenols
Naturally occurring dietary polyphenols, such as resveratrol, curcumin, quercetin, and catechins, have been shown to activate SIRT1 directly or indirectly in addition to their antioxidant and anti-inflammatory properties. Resveratrol is beneficial in many other cellular dysfunctions that are known to be associated with COPD including diabetes as SIRT1 is antihyperglycemic and protects against diabetic nephropathy (Sharma et al., 2011). Resveratrol prevents ATP decline, lowers the Km of SIRT1 for NAD+, HIF-1α expression and autophagy providing an additional insight into the role of SIRT1/resveratrol in systemic inflammation and metabolic homeostasis (Zhang et al., 2010b). Resveratrol also indirectly regulates SIRT1 via activating nicotinamide phosphoribosyltransferase and AMP-activated kinase (AMPK) leading to increased in increasing intracellular level of NAD+ (Canto et al., 2009; Fulco et al., 2008; Hou et al., 2008; Mukherjee et al., 2009; Suchankova et al., 2009; Um et al., 2010). Furthermore, activation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) increased the SIRT1 protein in skeletal muscle and attenuated LPS-induced lung inflammation (Suwa et al., 2011; Zhao et al., 2008). Recent studies demonstrated that resveratrol is not a specific activator of SIRT1, and treatment with resveratrol reduces SIRT1 levels as well as induces DNA damage (Beher et al., 2009; Pacholec et al., 2010; Pizarro et al., 2011; Tyagi et al., 2011), suggesting the controversial beneficial role of resveratrol in inflammaging.
There are some scanty reports available that quercetin and catechins also activate mammalian SIRT1 or yeast Sir2 albeit to a lesser extent as compared to resveratrol (Davis et al., 2009; de Boer et al., 2006; Howitz et al., 2003). Therefore, the activation of SIRT1 by polyphenols may be beneficial for regulation of inflammaging (Celik and Arinc, 2010). However, the separate studies show that polyphenols, such as epigallocatechin gallate (EGCG) and quercetin did not exhibit any ability to activate SIRT1 in cellular system (Choi et al., 2009; de Boer et al., 2006; Feng et al., 2009). On the contrary, these polyphenols inhibit SIRT1 activity (de Boer et al., 2006). This is due to their instability leading to formation of oxidized form to produce ROS in the medium in particular with reaction with aldehydes/quinones (i.e. resveratrol, EGCG) or SIRT1-inhibitory metabolites (i.e. quercetin and its metabolites) (de Boer et al., 2006). Understanding the role and mechanisms of polyphenols in SIRT1 regulation and cellular functions will help in identification of pharmacological agents for their possible use as nutraceuticals in prevention/management of inflammaging.
Synthetic pharmacological SIRT1 activators
Multiple SIRT1 activators have been developed, and these molecules increase SIRT1 activity by lowering the Km of SIRT1 for the substrates (Milne et al., 2007; Smith et al., 2009). SRT1720 has been reported to be 800–1000-fold more effective than resveratrol in activating SIRT1 (Milne et al., 2007). As compared to resveratrol, SRT2172 is more effective in inhibiting MMP-9 production in monocytes (Nakamaru et al., 2009). The efficiency of the SIRT1 activating compounds is strongly dependent on structural features of the peptide and probably on allosteric regulation (Dai et al., 2010; Pacholec et al., 2010). These molecules such as SRT2104, SRT2172, SRT2183 and SRT2379 are undergoing clinical testing in several metabolic diseases including diabetes, obesity and metabolic syndrome (where increased body mass versus lower oxygen consumption plays an important role in metabolic disorders and hence abnormal aging), and appear to be safe in human healthy volunteers and to be promising against these diseases. Further studies are urgently needed to investigate the efficacy of these molecules in COPD/emphysema and normal aging. However, recent studies showed that SRT1720, SRT2183, and SRT1460 are non-specific for SIRT1 activation (Pacholec et al., 2010). Therefore, development of a specific pharmacological SIRT1 activator is crucial in understanding the role of SIRT1 in cellular function and potentially clinical application of SIRT1 activators in diseases associated with inflammaging.
Other modulators of SIRT1 activity
NAD+ and its dependent deacetylases (e.g., SIRT1) have been linked to lifespan. Due to the wide presence of NAD+ degrading enzymes, the bioavailability of exogenously given NAD+ is low and on the other hand may lead to harmful effects when given systemically. A vitamin B3 component represents a NAD+ precursor, which has low in vivo toxicity. However, it has dual effects of SIRT1: either activation or inhibition (Denu, 2005). Administration of niconamide monucleotide may present potential alternative since it does not activate NAD+ consuming pathways or lead to SIRT1 inhibition. PARP-1 activation drains cellular NAD+ thereby compromising SIRT1 activity. Hence, inhibition of PARP-1 maintains intracellular NAD+ pool with elevated SIRT1 activity though controversial results have also been obtained (Caito et al., 2010a; Hwang et al., 2010). Interestingly, an old drug theophylline prevents NAD+ depletion (Moonen et al., 2005). The NAD+ maintaining compounds have multiple other functions, which can be independent as well, but there is a potential in developing compounds that activate SIRT1 by promoting NAD+ synthesis, though their clinical testing and possible toxicities have not been yet evaluated.
Metformin is a widely used drug especially for adult onset type II diabetes. Recent study has shown that the mechanisms associated with metformin include SIRT1 activation, which is associated with reduced TORC2 protein (Caton et al., 2010). It appears that at least in muscle cells the inhibitory effects of metformin on fatty acid metabolism occur via phosphorylation of AMPK-α (Bogachus and Turcotte, 2010; Caton et al., 2010). Several studies are ongoing on metformin and its effects especially during COPD exacerbations with diabetes.
Conclusions and future directions
Cigarette smoke is the primary cause of COPD characterized by accelerated decline in lung function, abnormal inflammation and premature lung aging. Targeting lung inflammation and cellular senescence as well as premature lung aging would be a promising therapeutic intervention for COPD/emphysema. SIRT1 plays a pivotal role in protecting inflammatory response and cell senescence, which is significantly decreased in lungs of patients with COPD. It is interesting to note that mouse sir2 homolog SIRT6 is also involved in the genomic stability, DNA damage response, inflammation, cellular senescence and aging (Kawahara et al., 2009; Lombard et al., 2008; Michishita et al., 2008; Mostoslavsky et al., 2006; Yang et al., 2009). We have recently shown that SIRT6 in fact promotes DNA repair by activating PARP-1 via lys521 residue, thereby stimulating poly-ADP-ribosylase activity and enhancing DSB repair under oxidative stress (Mao et al., 2011). Hence, the regulation of SIRT1 or SIRT6 activity by dietary polyphenols, specific activators (e.g., SRT2172 and SRT1720), or AMP-activated protein kinase activators (e.g., AICAR), and/or other specific activators of SIRT6 is a promising therapeutic strategy against many chronic inflammatory diseases including the diseases which are associated with inflammaging (e.g., COPD and its comorbidities) (Nakamaru et al., 2009; Tang et al., 2011; Wang et al., 2011). However, the most polyphenols are poorly absorbed, rapidly metabolized and oxidized, and undergo sulfation and glucuronidation, and also lead to formation of their own oxidation products. The biochemical mode of action of dietary polyphenols on activation of SIRT1 is an important area for further research. Further studies are required to validate the involvement of SIRT1 in inflammaging of lung, and whether pharmacological activation of SIRT1 protects lungs against inflammaging triggered by environmental pollutants, cigarette smoke and other inhaled aldehydes/oxidants.
Research Highlights.
Chronic inflammation and cellular senescence occur in premature lung aging.
Cigarette smoke/oxidative stress causes stress-induced premature senescence.
Shortening of telomere and alteration in telomerase occur in patients with COPD.
SIRT1 regulates senescence and inflammaging via telomere, FOXO3, p53, and histones.
Pharmacological or polyphenol activation of SIRT1 would halt lung inflammaging.
Acknowledgments
Supported by the NIH 1R01HL085613 (I.R.), 1R01HL097751 (I.R.), 1R01HL092842 (I.R.) and NIH-NIEHS Environmental Health Science Center grant P30-ES01247. V.L.K is partly supported by the governmental subsidy for health science research (EVO) of the Helsinki University Central Hospital and Finnish Antituberculosis Association Foundation.
Abbreviations
- AICAR
5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- AMPK
AMP-activated kinase
- ATM
ataxia telangiectasia mutated
- CHK2
checkpoint kinase 2
- COPD
chronic obstructive pulmonary disease
- DDR
DNA damage response
- DSB
double-strand break
- EGCG
epigallocatechin gallate
- eNOS
endothelial nitric oxide synthase
- FOXO3
forkhead box class O 3
- HATs
histone acetyltransferases
- HDACs
histone deacetylases
- HR
homologous recombination
- NBS1
Nijmegen breakage syndrome 1
- NHEJ
non-homologous end jointing
- SASP
senescent-associated secretory phenotype
- SIPS
stress-induced premature senescence
- SIRT1
sirtuin1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Afanas’ev I. Reactive oxygen species and age-related genes p66shc, Sirtuin, FOX03 and Klotho in senescence. Oxid Med Cell Longev. 2010;3:77–85. doi: 10.4161/oxim.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:596–601. doi: 10.1513/pats.200904-017RM. [DOI] [PubMed] [Google Scholar]
- Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant-and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay D, Okan NA, Bales E, Nascimento L, Cole PA, Medrano EE. Down-regulation of p300/CBP histone acetyltransferase activates a senescence checkpoint in human melanocytes. Cancer Res. 2002;62:6231–6239. [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Bernard D, Gosselin K, Monte D, Vercamer C, Bouali F, Pourtier A, Vandenbunder B, Abbadie C. Involvement of Rel/nuclear factor-kappaB transcription factors in keratinocyte senescence. Cancer Res. 2004;64:472–481. doi: 10.1158/0008-5472.can-03-0005. [DOI] [PubMed] [Google Scholar]
- Beyer D, Mitfessel H, Gillissen A. Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. Eur J Med Res. 2009;14(Suppl 4):27–31. doi: 10.1186/2047-783X-14-S4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogachus LD, Turcotte LP. Genetic downregulation of AMPK-alpha isoforms uncovers the mechanism by which metformin decreases FA uptake and oxidation in skeletal muscle cells. Am J Physiol Cell Physiol. 2010;299:C1549–1561. doi: 10.1152/ajpcell.00279.2010. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Caito S, Hwang JW, Chung S, Yao H, Sundar IK, Rahman I. PARP-1 inhibition does not restore oxidant-mediated reduction in SIRT1 activity. Biochem Biophys Res Commun. 2010a;392:264–270. doi: 10.1016/j.bbrc.2009.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010b;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, Barnes PJ, Papi A. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66:521–527. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- Caramori G, Romagnoli M, Casolari P, Bellettato C, Casoni G, Boschetto P, Chung KF, Barnes PJ, Adcock IM, Ciaccia A, Fabbri LM, Papi A. Nuclear localisation of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbations. Thorax. 2003;58:348–351. doi: 10.1136/thorax.58.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205:97–106. doi: 10.1677/JOE-09-0345. [DOI] [PubMed] [Google Scholar]
- Celik H, Arinc E. Evaluation of the protective effects of quercetin, rutin, naringenin, resveratrol and trolox against idarubicin-induced DNA damage. J Pharm Pharm Sci. 2010;13:231–241. [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chi Y, Bloecher A, Aebersold R, Clurman BE, Roberts JM. N-acetylation and ubiquitin-independent proteasomal degradation of p21(Cip1) Mol Cell. 2004;16:839–847. doi: 10.1016/j.molcel.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, Lee JM, Yoon HG. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69:583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- de Boer VC, de Goffau MC, Arts IC, Hollman PC, Keijer J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech Ageing Dev. 2006;127:618–627. doi: 10.1016/j.mad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Denu JM. Vitamin B3 and sirtuin function. Trends Biochem Sci. 2005;30:479–483. doi: 10.1016/j.tibs.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, Adcock IM. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. [DOI] [PubMed] [Google Scholar]
- Dimauro T, David G. Chromatin modifications: the driving force of senescence and aging? Aging (Albany NY) 2009;1:182–190. doi: 10.18632/aging.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon R, Rashi-Elkeles S, Lerenthal Y, Linhart C, Tenne T, Amariglio N, Rechavi G, Shamir R, Shiloh Y. Dissection of a DNA-damage-induced transcriptional network using a combination of microarrays, RNA interference and computational promoter analysis. Genome Biol. 2005;6:R43. doi: 10.1186/gb-2005-6-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Wu J, Chen L, Luo C, Shen X, Chen K, Jiang H, Liu D. A fluorometric assay of SIRT1 deacetylation activity through quantification of nicotinamide adenine dinucleotide. Anal Biochem. 2009;395:205–210. doi: 10.1016/j.ab.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F, Croucher PJ, Nikolaus S, Hampe J, Cordes C, Schreiber S, Nebel A. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41:98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ye J. Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem Biophys Res Commun. 2008;376:793–796. doi: 10.1016/j.bbrc.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe M, de Cabo R. AsSIRTing the DNA damage response. Trends Cell Biol. 2008;18:77–83. doi: 10.1016/j.tcb.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarani V, Deflorian G, Franco CA, Kruger M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R, Schmidt MH, Zimmermann B, Brandes RP, Mione M, Westphal CH, Braun T, Zeiher AM, Gerhardt H, Dimmeler S, Potente M. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature. 2011;473:234–238. doi: 10.1038/nature09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Yu K. Altered kinetics of nonhomologous end joining and class switch recombination in ligase IV-deficient B cells. J Exp Med. 2008;205:2745–2753. doi: 10.1084/jem.20081623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway KR, Calhoun TN, Saxena M, Metoyer CF, Kandler EF, Rivera CA, Pruitt K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc Natl Acad Sci U S A. 2010;107:9216–9221. doi: 10.1073/pnas.0911325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP–activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben JM, Mercken EM, Ketelslegers HB, Bast A, Wouters EF, Hageman GJ, Schols AM. Telomere shortening in chronic obstructive pulmonary disease. Respir Med. 2009;103:230–236. doi: 10.1016/j.rmed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hwang J, Rajendrasozhan S, Yao H, Chung S, Sundar IK, LHH, Pryhuber GS, Kinnula VL, Rahman I. FoxO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol. 2011 doi: 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys. 2010;500:203–209. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM, Cho MH, Park GH, Lee KH. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39:8–13. doi: 10.1038/emm.2007.2. [DOI] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, Goto S, Takahashi K, Maruyama N, Seyama K, Ishigami A. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L784–792. doi: 10.1152/ajplung.00256.2009. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Kyoung Kim H, Kyoung Kim Y, Song IH, Baek SH, Lee SR, Hye Kim J, Kim JR. Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J Gerontol A Biol Sci Med Sci. 2005;60:4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, Studnicka M, Bateman E, Anto JM, Burney P, Mannino DM, Buist SA. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, Driscoll B. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L57–70. doi: 10.1152/ajplung.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Casta A, Wang R, Lozada E, Fan W, Kane S, Ge Q, Gu W, Orren D, Luo J. Regulation of WRN protein cellular localization and enzymatic activities by SIRT1-mediated deacetylation. J Biol Chem. 2008;283:7590–7598. doi: 10.1074/jbc.M709707200. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Schwer B, Alt FW, Mostoslavsky R. SIRT6 in DNA repair, metabolism and ageing. J Intern Med. 2008;263:128–141. doi: 10.1111/j.1365-2796.2007.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Louhelainen N, Rytila P, Haahtela T, Kinnula VL, Djukanovic R. Persistence of oxidant and protease burden in the airways after smoking cessation. BMC Pulm Med. 2009;9:25. doi: 10.1186/1471-2466-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louhelainen N, Stark H, Mazur W, Rytila P, Djukanovic R, Kinnula VL. Elevation of sputum matrix metalloproteinase-9 persists up to 6 months after smoking cessation: a research study. BMC Pulm Med. 2010;10:13. doi: 10.1186/1471-2466-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD) Biochem Soc Trans. 2009;37:819–823. doi: 10.1042/BST0370819. [DOI] [PubMed] [Google Scholar]
- MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc. 2009;6:527–531. doi: 10.1513/pats.200905-027DS. [DOI] [PubMed] [Google Scholar]
- Mantel C, Broxmeyer HE. Sirtuin 1, stem cells, aging, and stem cell aging. Curr Opin Hematol. 2008;15:326–331. doi: 10.1097/MOH.0b013e3283043819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 2008a;7:1765–1771. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008b;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end–joining. Nat Struct Mol Biol. 2010;17:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen HJ, Geraets L, Vaarhorst A, Bast A, Wouters EF, Hageman GJ. Theophylline prevents NAD+ depletion via PARP-1 inhibition in human pulmonary epithelial cells. Biochem Biophys Res Commun. 2005;338:1805–1810. doi: 10.1016/j.bbrc.2005.10.159. [DOI] [PubMed] [Google Scholar]
- Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27:525–528. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Mui TS, Man JM, McElhaney JE, Sandford AJ, Coxson HO, Birmingham CL, Li Y, Man SF, Sin DD. Telomere length and chronic obstructive pulmonary disease: evidence of accelerated aging. J Am Geriatr Soc. 2009;57:2372–2374. doi: 10.1111/j.1532-5415.2009.02589.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Muller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, Krug N, Nakashima M, Branscheid D, Magnussen H, Jorres RA, Holz O. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7:32. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Betsuyaku T, Kondo T, Nasuhara Y, Nishimura M. Long term smoking with age builds up excessive oxidative stress in bronchoalveolar lavage fluid. Thorax. 2006;61:496–502. doi: 10.1136/thx.2005.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- Neal JA, Meek K. Choosing the right path: Does DNA-PK help make the decision? Mutat Res. 2011;711:73–86. doi: 10.1016/j.mrfmmm.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35:681–688. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunoya T, Monick MM, Klingelhutz AL, Glaser H, Cagley JR, Brown CO, Matsumoto E, Aykin-Burns N, Spitz DR, Oshima J, Hunninghake GW. Cigarette smoke induces cellular senescence via Werner’s syndrome protein down-regulation. Am J Respir Crit Care Med. 2009;179:279–287. doi: 10.1164/rccm.200802-320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, Komuro I. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–894. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. 2010;17:431–435. doi: 10.5551/jat.3525. [DOI] [PubMed] [Google Scholar]
- Ou X, Chae HD, Wang RH, Shelley WC, Cooper S, Taylor T, Kim YJ, Deng CX, Yoder MC, Broxmeyer HE. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117:440–450. doi: 10.1182/blood-2010-03-273011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro JG, Verdaguer E, Ancrenaz V, Junyent F, Sureda F, Pallas M, Folch J, Camins A. Resveratrol inhibits proliferation and promotes apoptosis of neuroblastoma cells: role of sirtuin 1. Neurochem Res. 2011;36:187–194. doi: 10.1007/s11064-010-0296-y. [DOI] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J Biol Chem. 2002;277:39195–39201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5:413–422. doi: 10.1111/j.1474-9726.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- Sato T, Seyama K, Sato Y, Mori H, Souma S, Akiyoshi T, Kodama Y, Mori T, Goto S, Takahashi K, Fukuchi Y, Maruyama N, Ishigami A. Senescence marker protein-30 protects mice lungs from oxidative stress, aging, and smoking. Am J Respir Crit Care Med. 2006;174:530–537. doi: 10.1164/rccm.200511-1816OC. [DOI] [PubMed] [Google Scholar]
- Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2:415–431. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, Le Corvoisier P, Rideau D, Boczkowski J, Dubois-Rande JL, Chouaid C, Adnot S. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:566–571. doi: 10.1164/rccm.200809-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi JM, Rochester CL. Smoking and chronic obstructive pulmonary disease. Clin Chest Med. 2000;21:67–86. viii. doi: 10.1016/s0272-5231(05)70008-3. [DOI] [PubMed] [Google Scholar]
- Sharma S, Misra CS, Arumugam S, Roy S, Shah V, Davis JA, Shirumalla RK, Ray A. Antidiabetic activity of resveratrol, a known SIRT1 activator in a genetic model for type-2 diabetes. Phytother Res. 2011;25:67–73. doi: 10.1002/ptr.3221. [DOI] [PubMed] [Google Scholar]
- Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Lim JW, Kim H, Morio T, Kim KH. Oxidative stress induces nuclear loss of DNA repair proteins Ku70 and Ku80 and apoptosis in pancreatic acinar AR42J cells. J Biol Chem. 2003;278:36676–36687. doi: 10.1074/jbc.M303692200. [DOI] [PubMed] [Google Scholar]
- Stefani M, Markus MA, Lin RC, Pinese M, Dawes IW, Morris BJ. The effect of resveratrol on a cell model of human aging. Ann N Y Acad Sci. 2007;1114:407–418. doi: 10.1196/annals.1396.001. [DOI] [PubMed] [Google Scholar]
- Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun. 2009;378:836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro OM, Nabeshima Y, Nagai R. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol. 2000;22:26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- Sundar IK, Mullapudi N, Yao H, Spivack SD, Rahman I. Lung cancer and its association with chronic obstructive pulmonary disease: update on nexus of epigenetics. Curr Opin Pulm Med. 2011;17:279–285. doi: 10.1097/MCP.0b013e3283477533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Radak Z, Kumagai S. Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism. 2011;60:394–403. doi: 10.1016/j.metabol.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Tang GJ, Wang HY, Wang JY, Lee CC, Tseng HW, Wu YL, Shyue SK, Lee TS, Kou YR. Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic Biol Med. 2011;50:1492–1502. doi: 10.1016/j.freeradbiomed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80:59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Yun JH, Graham BB. Cigarette smoke triggers code red: p21CIP1/WAF1/SDI1 switches on danger responses in the lung. Am J Respir Cell Mol Biol. 2008;39:1–6. doi: 10.1165/rcmb.2008-0117TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi A, Gu M, Takahata T, Frederick BA, Agarwal C, Siriwardana S, Agarwal R, Sclafani RA. Resveratrol selectively induces DNA damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl M, Csernok A, Aydin S, Kreienberg R, Wiesmuller L, Gatz SA. Role of SIRT1 in homologous recombination. DNA Repair (Amst) 2010;9:383–393. doi: 10.1016/j.dnarep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari ZI, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitiekunaite R, Butkiewicz D, Krzesniak M, Przybylek M, Gryc A, Snietura M, Benedyk M, Harris CC, Rusin M. Expression and localization of Werner syndrome protein is modulated by SIRT1 and PML. Mech Ageing Dev. 2007;128:650–661. doi: 10.1016/j.mad.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- Vempati RK, Jayani RS, Notani D, Sengupta A, Galande S, Haldar D. p300-mediated acetylation of histone H3 lysine 56 functions in DNA damage response in mammals. J Biol Chem. 2010;285:28553–28564. doi: 10.1074/jbc.M110.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, Morselli E, Ben Younes A, Maiuri MC, Lavandero S, Kroemer G. Senescence, apoptosis or autophagy? When a damaged cell must decide its path--a mini-review. Gerontology. 2008;54:92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- Wang J, Jacob NK, Ladner KJ, Beg A, Perko JD, Tanner SM, Liyanarachchi S, Fishel R, Guttridge DC. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO Rep. 2009;10:1272–1278. doi: 10.1038/embor.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585:986–994. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- Yang B, Zwaans BM, Eckersdorff M, Lombard DB. The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle. 2009;8:2662–2663. doi: 10.4161/cc.8.16.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol. 2008;38:689–698. doi: 10.1165/rcmb.2007-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8:E632–643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Hwang JW, Moscat J, Diaz-Meco MT, Leitges M, Kishore N, Li X, Rahman I. Protein kinase C zeta mediates cigarette smoke/aldehyde-and lipopolysaccharide-induced lung inflammation and histone modifications. J Biol Chem. 2010;285:5405–5416. doi: 10.1074/jbc.M109.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–383. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011 doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O’Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Respir Cell Mol Biol. 2008;39:7–18. doi: 10.1165/rcmb.2007-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Pu M, Zhang Z, Lou Z. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle. 2009;8:1747–1753. doi: 10.4161/cc.8.11.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Seto E. A functional link between SIRT1 deacetylase and NBS1 in DNA damage response. Cell Cycle. 2007;6:2869–2871. doi: 10.4161/cc.6.23.5026. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol Cell. 2007;27:149–162. doi: 10.1016/j.molcel.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P, Horn F, Salbego C, Lenz G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009;100:1655–1662. doi: 10.1111/j.1349-7006.2009.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee RS, Yoo CB, Pimentel DR, Perlman DH, Burgoyne JR, Hou X, McComb ME, Costello CE, Cohen RA, Bachschmid MM. Redox regulation of sirtuin-1 by S-glutathiolation. Antioxid Redox Signal. 2010;13:1023–1032. doi: 10.1089/ars.2010.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, Divekar R, McBurney MW, Braley-Mullen H, Zaghouani H, Fang D. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, Yang RF, Zhang Y, Xu J, Wei YS, Liu DP, Liang CC. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem. 2010a;285:7097–7110. doi: 10.1074/jbc.M109.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lowry SF, Guarente L, Haimovich B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. J Biol Chem. 2010b;285:41391–41401. doi: 10.1074/jbc.M110.174482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Onizawa S, Nagai A, Aoshiba K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res. 2011;12:78. doi: 10.1186/1465-9921-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillikens MC, van Meurs JB, Sijbrands EJ, Rivadeneira F, Dehghan A, van Leeuwen JP, Hofman A, van Duijn CM, Witteman JC, Uitterlinden AG, Pols HA. SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin. Free Radic Biol Med. 2009;46:836–841. doi: 10.1016/j.freeradbiomed.2008.12.022. [DOI] [PubMed] [Google Scholar]