Abstract
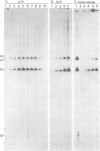
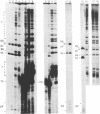
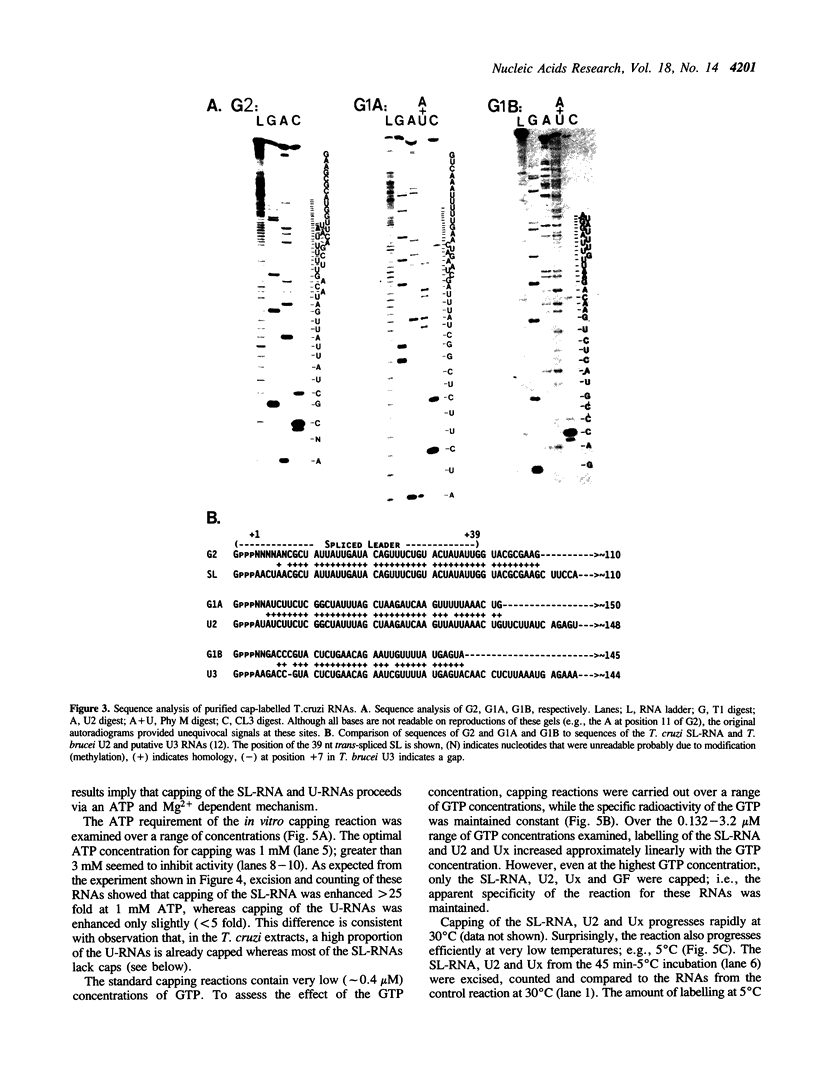
Messenger RNA maturation in trypanosomes requires a trans-splicing event in which a capped 39 nucleotide leader sequence, the spliced leader (SL), from the 5' terminus of a small RNA (SL-RNA) is joined to the 5' termini of protein coding gene transcripts. We have developed nuclear extracts from Trypanosoma cruzi that label three small endogenous RNAs in the presence of [alpha-32P]GTP. Herein, we have characterized this labelling as 5' capping and shown that the capping activity exhibits an unusual ATP dependence. Moreover, partial sequence analysis identified the three cap-labelled RNAs as the T. cruzi SL-RNA, and two U-RNAs previously uncharacterized in T. cruzi, U2 and Ux. Finally, the capping reaction in the T. cruzi extracts showed apparent specificity for these RNAs--other endogenous or exogenous transcripts were not capped. The apparent specificity of this in vitro capping activity closely reflects the in vivo requirements; i.e., only the SL- and U-RNAs need to be capped since mature mRNAs are capped via trans-splicing. These observations are consistent with the hypothesis that one of the functions of trans-splicing is to supply 5' caps to mature trypanosome mRNAs.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindereif A., Green M. R. An ordered pathway of snRNP binding during mammalian pre-mRNA splicing complex assembly. EMBO J. 1987 Aug;6(8):2415–2424. doi: 10.1002/j.1460-2075.1987.tb02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Hieter P. A., Levy C. C. Identification of a cytidine-specific ribonuclease from chicken liver. J Biol Chem. 1980 Mar 10;255(5):2160–2163. [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Bunick D., Zandomeni R., Ackerman S., Weinmann R. Mechanism of RNA polymerase II--specific initiation of transcription in vitro: ATP requirement and uncapped runoff transcripts. Cell. 1982 Jul;29(3):877–886. doi: 10.1016/0092-8674(82)90449-4. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Cheng S. C., Abelson J. Spliceosome assembly in yeast. Genes Dev. 1987 Nov;1(9):1014–1027. doi: 10.1101/gad.1.9.1014. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Field A. S., Luse D. S. Promoter-proximal pausing by RNA polymerase II in vitro: transcripts shorter than 20 nucleotides are not capped. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1251–1255. doi: 10.1073/pnas.80.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Freistadt M. S., Cross G. A., Branch A. D., Robertson H. D. Direct analysis of the mini-exon donor RNA of Trypanosoma brucei: detection of a novel cap structure also present in messenger RNA. Nucleic Acids Res. 1987 Dec 10;15(23):9861–9879. doi: 10.1093/nar/15.23.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Cross G. A., Robertson H. D. Discontinuously synthesized mRNA from Trypanosoma brucei contains the highly methylated 5' cap structure, m7GpppA*A*C(2'-O)mU*A. J Biol Chem. 1988 Oct 15;263(29):15071–15075. [PubMed] [Google Scholar]
- Jove R., Manley J. L. In vitro transcription from the adenovirus 2 major late promoter utilizing templates truncated at promoter-proximal sites. J Biol Chem. 1984 Jul 10;259(13):8513–8521. [PubMed] [Google Scholar]
- Jove R., Manley J. L. Transcription initiation by RNA polymerase II is inhibited by S-adenosylhomocysteine. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5842–5846. doi: 10.1073/pnas.79.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Association of U2, U4, U5, and U6 small nuclear ribonucleoproteins in a spliceosome-type complex in absence of precursor RNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5459–5462. doi: 10.1073/pnas.85.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986 Sep 12;46(6):845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., Kooter J. M., Loosbroek N., Borst P. Mature mRNAs of Trypanosoma brucei possess a 5' cap acquired by discontinuous RNA synthesis. Nucleic Acids Res. 1985 Jun 25;13(12):4253–4266. doi: 10.1093/nar/13.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Dorfman D. M., Donelson J. E. The spliced leader sequence of Trypanosoma brucei has a potential role as a cap donor structure. Mol Cell Biol. 1985 Sep;5(9):2487–2490. doi: 10.1128/mcb.5.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- McCarthy-Burke C., Taylor Z. A., Buck G. A. Characterization of the spliced leader genes and transcripts in Trypanosoma cruzi. Gene. 1989 Oct 15;82(1):177–189. doi: 10.1016/0378-1119(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kaziro Y. Messenger RNA capping enzymes from eukaryotic cells. Prog Nucleic Acid Res Mol Biol. 1987;34:1–28. doi: 10.1016/s0079-6603(08)60491-2. [DOI] [PubMed] [Google Scholar]
- Morel C., Chiari E., Camargo E. P., Mattei D. M., Romanha A. J., Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram J., Perry K. L., Lizardi P. M., Lührmann R., Agabian N., Nelson R. G. Isolation and sequence of four small nuclear U RNA genes of Trypanosoma brucei subsp. brucei: identification of the U2, U4, and U6 RNA analogs. Mol Cell Biol. 1989 Mar;9(3):1212–1223. doi: 10.1128/mcb.9.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Siliciano P. G., Guthrie C. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell. 1987 Apr 24;49(2):229–239. doi: 10.1016/0092-8674(87)90564-2. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Watkins K. P., Agabian N. Trypanosome mRNAs have unusual "cap 4" structures acquired by addition of a spliced leader. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8190–8194. doi: 10.1073/pnas.84.23.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Busch H. Small nuclear RNAs and RNA processing. Prog Nucleic Acid Res Mol Biol. 1983;30:127–162. doi: 10.1016/s0079-6603(08)60685-6. [DOI] [PubMed] [Google Scholar]
- Reed R., Griffith J., Maniatis T. Purification and visualization of native spliceosomes. Cell. 1988 Jun 17;53(6):949–961. doi: 10.1016/s0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F. Translation in vitro of vesicular stomatitis virus mRNA lacking 5'-terminal 7-methylguanosine. Nature. 1976 Jul 1;262(5563):32–37. doi: 10.1038/262032a0. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Zamore P. D., Green M. R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988 Jan 29;52(2):207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Schaufele F., Gilmartin G. M., Bannwarth W., Birnstiel M. L. Compensatory mutations suggest that base-pairing with a small nuclear RNA is required to form the 3' end of H3 messenger RNA. 1986 Oct 30-Nov 5Nature. 323(6091):777–781. doi: 10.1038/323777a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shea C., Lee M. G., Van der Ploeg L. H. VSG gene 118 is transcribed from a cotransposed pol I-like promoter. Cell. 1987 Aug 14;50(4):603–612. doi: 10.1016/0092-8674(87)90033-x. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Singh R., Gupta S., Reddy R. Capping of mammalian U6 small nuclear RNA in vitro is directed by a conserved stem-loop and AUAUAC sequence: conversion of a noncapped RNA into a capped RNA. Mol Cell Biol. 1990 Mar;10(3):939–946. doi: 10.1128/mcb.10.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Pelletier J. Poliovirus translation: a paradigm for a novel initiation mechanism. Bioessays. 1989 Nov;11(5):128–132. doi: 10.1002/bies.950110504. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. The cap of both miniexon-derived RNA and mRNA of trypanosomes is 7-methylguanosine. Mol Cell Biol. 1988 Jan;8(1):494–496. doi: 10.1128/mcb.8.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Richards F. F., Ullu E. The U2 RNA analogue of Trypanosoma brucei gambiense: implications for a splicing mechanism in trypanosomes. Nucleic Acids Res. 1986 Nov 25;14(22):8893–8903. doi: 10.1093/nar/14.22.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Zwierzynski T. A., Widmer G., Buck G. A. In vitro 3' end processing and poly(A) tailing of RNA in Trypanosoma cruzi. Nucleic Acids Res. 1989 Jun 26;17(12):4647–4660. doi: 10.1093/nar/17.12.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]