Abstract
The hippocampal formation has been implicated in a growing number of disorders, from Alzheimer's disease and cognitive ageing to schizophrenia and depression. How can the hippocampal formation, a complex circuit that spans the temporal lobes, be involved in a range of such phenotypically diverse and mechanistically distinct disorders? Recent neuroimaging findings indicate that these disorders differentially target distinct subregions of the hippocampal circuit. In addition, some disorders are associated with hippocampal hypometabolism, whereas others show evidence of hypermetabolism. Interpreted in the context of the functional and molecular organization of the hippocampal circuit, these observations give rise to a unified pathophysiological framework of hippocampal dysfunction.
Neuroimaging and neuropsychological studies have, among other observations, implicated the hippocampal formation in Alzheimer's disease, temporal lobe epilepsy (TLE), cognitive ageing, post-traumatic stress disorder (PTSD), transient global amnesia, schizophrenia, and depressive and anxiety disorders. Although overlaps exist, these disorders are clearly not phenocopies and, more importantly, are thought to have distinct pathogenic mechanisms. Resolving how the hippocampal formation can be affected by a broad range of disorders is the goal of this Review.
Until recently, most neuroimaging and neuropsychological tests have evaluated the hippocampal formation as a singular structure, but it is in fact a complex circuit made up of functionally and molecularly distinct subregions. Moreover, the complexity of the hippocampal formation extends beyond its internal circuit organization. Most neural information funnels into the circuit through a restricted area, whereas the outflow fans out, monosynaptically connecting with a broad range of cortical and subcortical sites. In the first section of this Review we will briefly summarize the internal circuitry of the hippocampal formation and describe how hippocampal efferents connect with separate brain networks (FIG. 1).
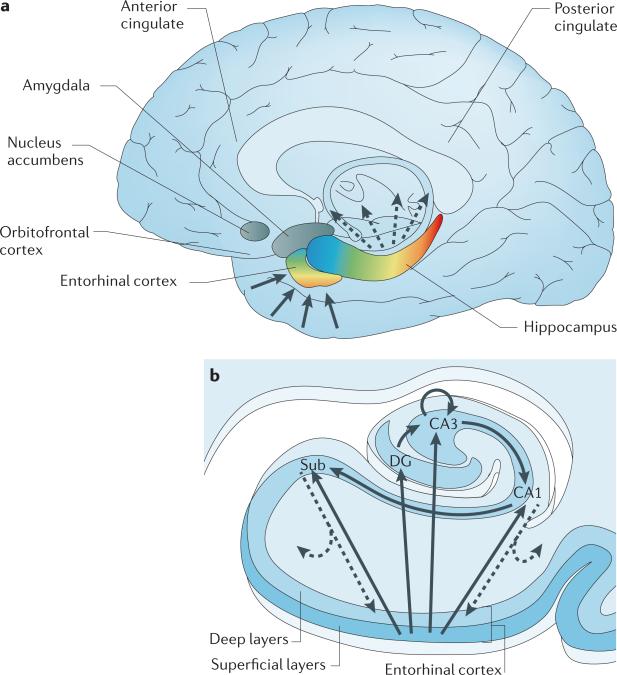
Figure 1. The organization of the hippocampal formation.
a | The hippocampal formation, which is made up of the entorhinal cortex and hippocampus (shown in colour) extends over the anterior-to-posterior axis of the brain. The colour gradients reflect the topological input–output relations between the hippocampal formation and other brain areas, as well as its internal functional and molecular organization. Input to the hippocampus is shown by solid arrows, hippocampal outflow is shown by dashed arrows. Cortical and subcortical information funnels onto superficial layers of the entorhinal cortex, and this input is organized in an anterior–medial to posterior–lateral gradient (shown by the colour gradient in the entorhinal cortex). This anatomical gradient is largely preserved as the entorhinal cortex conveys this information to the hippocampus (shown by the corresponding colour code in the hippocampus). The long-axis gradient is preserved in the output pattern of the hippocampus. As well as reconnecting with the entorhinal cortex, the hippocampus monosynaptically connects with — from anterior to posterior — the orbitofrontal cortex, anterior cingulate, amygdala, nucleus accumbens and posterior cingulate. b | In the hippocampal transverse axis, superficial layers of the entorhinal cortex connect with the dentate gyrus (DG), CA3, CA1 and the subiculum (Sub). The trisynaptic circuit connects the dentate gyrus to CA3, to CA1 and to the subiculum. Through auto-association fibres, CA3 neurons interconnect with other CA3 neurons throughout the long axis. The CA1 and primarily the subiculum provide the main hippocampal outflow (shown by dashed arrows), back to the deep layers of the entorhinal cortex and also to a range of cortical and subcortical sites (as shown in part a).
In the second section of this Review we survey studies that use high-resolution variants of structural and functional MRI (fMRI) that can visualize and assess the integrity of individual hippocampal subregions (BOX 1; FIG. 1). By simultaneously assessing multiple subregions, the hippocampal formation can be interrogated as a circuit, and these imaging approaches are well suited to pinpoint subregions that are differentially affected by, or resistant to, a particular disorder. Over the past few years, these high-resolution imaging methods have been applied to numerous disorders. As will be reviewed, when these studies are examined together, a clear picture emerges in which patterns of regional changes can differentiate disorders that are associated with hippocampal dysfunction.
The concept of regional vulnerability, across the brain and within the hippocampal formation in particular, is not new. For example, the CA1 subfield is known to be the hippocampal subregion differentially vulnerable to vascular disease1,2, whereas the dentate gyrus is known to be differentially vulnerable to the effects of adrenalectomy3. Recent gene-expression studies have established that each hippocampal subregion has a distinct molecular profile4–6, and this ‘molecular anatomy’ provides a partial substrate for regional vulnerability. So, the relatively high expression of certain NMDA receptors7 in CA1 helps to explain its vulnerability to excitotoxicity in the context of hypoxia and ischaemia associated with vascular disease1,2, whereas the high levels of mineralocorticoid receptors in the dentate gyrus confer vulnerability to the effects of reductions in the level of circulating corticosteroids8. As will be discussed, demonstrating and reinforcing the concept of regional vulnerability is important for providing insights into pathogenic mechanisms as well as for explaining phenotypic variability.
In the third section of the Review we show that functional imaging techniques have identified another, perhaps more surprising, factor by which diseases that affect the hippocampal formation can be segregated. Imaging techniques such as positron emission tomography (PET) and some variants of fMRI can identify the metabolic state of the hippocampal formation. In many diseases the hippocampal formation is found to be hypometabolic. Hypometabolism is expected because these disorders are characterized by hippocampal ‘loss of function’, such as memory deficits. In other disorders, however, there is evidence that the hippocampal formation is in a hypermetabolic state. This observation introduces the interesting idea that, as in TLE, some hippocampus-based disorders may cause ‘gain of function’ symptoms by stimulating hippocampal outflow areas. We will review studies that raise the intriguing possibility that hypermetabolism in the anterior hippocampus might mediate a gain of psychotic and affective symptoms.
When the concepts of regional vulnerability and metabolic state are viewed in light of the functional and molecular organization of the hippocampal circuit, a general pathophysiological framework of hippocampal dysfunction begins to emerge. In the final section of this Review, we will elaborate on this framework, showing its utility in explaining and better charact erizing phenotypes that distinguish hippocampal disorders, and demonstrating how it can be used to shed light on pathogenic mechanisms.
Organization of the hippocampal formation
The hippocampal formation spans the posterior-to-anterior extent of the base of the temporal lobes (FIG. 1a). In its transverse axis the hippocampal formation is organized as a mainly unidirectional circuit made up of multiple subregions — the entorhinal cortex, the dentate gyrus, the CA1 and CA3 subfields, and the subiculum9 (FIG. 1b). Notably, in his classic study, Lorente de Nó — who first parsed the hippocampal formation using the Cornu Ammonis nomenclature — also described the CA2 subfield10. Although there has been renewed interest in CA2, it is not commonly imaged in vivo, and will therefore not be featured in this Review.
In the ‘trisynaptic pathway’, layer II of entorhinal cortex connects to the dentate gyrus through the perforant pathway, and the dentate gyrus connects to CA3 through the mossy fibres. CA3 neurons interconnect with other CA3 neurons up and down the hippocampal long axis through ‘auto-associative’ tracts, or with CA1 through the Shaffer collaterals. Finally, CA1 connects to the subiculum. In addition to this pattern of connectivity, layer II of the entorhinal cortex projects to CA3, and layer III of entorhinal cortex can send direct connections to CA1 and the subiculum (FIG. 1b).
The entorhinal cortex serves as the gateway into the hippocampal formation and receives monosynaptic input from numerous regions, including the perirhinal cortex, the parahippocampal cortex, the auditory and olfactory cortices, and the amygdala. The input to the entorhinal cortex is organized in an anterior-to-posterior gradient, and the gradient is largely preserved in the way that the entorhinal cortex connects with the rest of the hippocampus (FIG. 1a). So, for example, input from the amygdala connects with anterior and medial aspects of the entorhinal cortex, which then communicate this input to anterior aspects of the hippocampus. By contrast, visual cortex input is delivered to the hippocampal formation through the perirhinal and parahippocampal cortices, which connect to posterior and lateral aspects of the entorhinal cortex, and from there to posterior aspects of the hippocampus.
The subiculum and, to a lesser extent, CA1 are the hippocampal subregions that provide the dominant out-flow of the hippocampal circuit11. Both CA1 and the subiculum connect to deep layers of the entorhinal cortex (FIG. 1b), which then re-connects — via the parahippocampal gyrus — with neocortical sites that provided the original hippocampal input12. This hippocampal– neocortical network has historically received the most attention for its role in the consolidation of long-term memories13. Anatomical tracing studies have established that the outflow from the subiculum and CA1 can also bypass the entorhinal cortex, monosynaptically connecting with a range of other brain areas, including the amygdala, the medial prefrontal and orbitofrontal cortices, the anterior and posterior cingulate cortex, and the nucleus accumbens14–16 (FIG. 1c). These direct hippocampal efferents show a striking topographical organization, such that the more posterior (dorsal in rodents) part projects to the posterior cingulate cortex, whereas more anterior parts show denser projections to medial prefrontal and orbitofrontal cortices and the amygdala.
The observed input and outflow gradients suggest that the hippocampal long axis is functionally organized, an interpretation that agrees with data from gene expression profiling4,6, fMRI studies17, and electrophysiological studies that have documented long-axis differences in mechanisms of plasticity18–20 and place field activity21,22. Building on these observations, a range of studies have established that the posterior extents of the hippocampal long axis are more likely to be involved in memory and cognitive processing, whereas the anterior extents of the hippocampal long axis are more involved in other complex behaviours such as stress, emotion, sensory–motor integration and goal-directed activity4,23–26. Notably, and as will be discussed below, the anterior hippocampus and some of its associated outflow areas are key components of networks that are implicated in schizophrenia and depression.
Hippocampal regional vulnerability
Rather than reviewing all disorders in which the hippocampal formation is implicated, we will focus on a number of major ones, with the purpose of establishing the principle of differential vulnerability. The term ‘differential’ (rather than ‘selective’) is deliberately used: because of connections within the hippocampal circuit, a primary lesion or dysfunction in any subregion can, over time, affect neighbouring subregions. By comparing studies that have mapped patterns of anatomical alterations within the hippocampal circuit, patterns that differentiate disorders can be determined, thereby pinpointing differentially vulnerable and resistant subregions.
For disorders in which there is death of primary hippocampal neurons, mapping patterns of cell loss provides a histological indication of regional vulnerability. In vascular disease, CA1 shows the most reliable loss of neurons27,28. In Alzheimer's disease, the pattern of cell death and other histological findings suggests that the entorhinal cortex is prominently affected by the disease29–31, that CA1 and the subiculum are also heavily involved, and that the dentate gyrus and CA3 are relatively preserved29,30,32. Although cell death in TLE33 is extensive — occurring predominantly in the dentate gyrus and CA subfields — two findings differentiate TLE from Alzheimer's disease. First, the subiculum is a subregion with relatively little cell loss in TLE. Second, although entorhinal cortex cell death is observed in both diseases, it is more pronounced in Alzheimer's disease, in which it starts in layer II, whereas in TLE it seems to be confined to layer III neurons34.
High-resolution variants of functional MRI have successfully detected the differential vulnerability of CA1 to vascular disease in living patients2. Furthermore, these technologies have been able to detect Alzheimer's disease-associated alterations in entorhinal cortex, CA1 and subiculum, on a background of relatively resistant dentate gyrus and CA3 (REFS 35–37), and have confirmed that the subiculum is relatively unaffected in TLE38. By showing concordance with histologically detected patterns of vulnerability, these observations validate that neuroimaging can accurately map hippocampal pathology in vivo.
The other disorders considered in this Review are notable for their relative absence of loss of primary neurons and are therefore often referred to as ‘functional’ disorders. Mapping patterns of differential vulnerability for this class of disorders is particularly challenging — not only because of the absence of cell death but also because the ‘functional’ pathology readily spreads over time. It is for these disorders that high-resolution neuro-imaging has proven most useful (BOX 1). Neuroimaging techniques can map not only patterns of hippocampal dysfunction in living subjects but, as we will highlight, can also map spread over time. We will begin by reviewing disorders that typically affect the hippocampal formation in later-life and then review those that occur more commonly in younger patients.
Pinpointing vulnerable subregions in the hippocampal formation in late life
Although ageing is itself not a disease, cognitive decline that occurs during ageing has deleterious consequences, and because it occurs without prominent cell loss it is considered a prime example of a functional disorder39. A great number of imaging studies have investigated the hippocampal formation in ageing individuals and many findings have been reported. Nevertheless, the effect of ageing on hippocampal function is often confounded by diseases2, in particular Alzheimer's disease and vascular disease, which commonly occur in older subjects and can cause hippocampal dysfunction independent of ageing2. When attempting to isolate the hippocampal pattern of dysfunction reflective of ageing per se, it is therefore important to exclude the effect of Alzheimer's disease and vascular disease.
The first clues that ageing manifests with a pattern of hippocampal dysfunction that is distinct from Alzheimer's disease or vascular disease came from studies using a high-resolution variant of fMRI that was explicitly developed to map patterns of basal hippocampal metabolism in humans and animal models40–42. The term ‘basal metabolism’, as historically used in the functional imaging field, refers to the resting-state metabolism in a brain region. This resting-state metabolism can change slowly during ageing or in disease, in contrast with acute changes in metabolism that are evoked by a transient stimulus (BOXES 1,2)). As Alzheimer's disease and vascular disease do not typically occur in non-human primates or rodents, a comparison of fMRI findings across species can isolate a pattern of dysfunction that is differentially linked to ageing. These fMRI variants have been applied to ageing human subjects43 (with or without Alzheimer's disease35 and with or without vascular disease2), ageing rhesus monkeys41, ageing mice and mice that express disease-causing mutations in the amyloid precursor protein35. Besides confirming the differential link of Alzheimer's disease to the entorhinal cortex and vascular disease to CA1, results from these cross-species studies suggested that ageing itself differentially affects the dentate gyrus2,35,41,43.
Informed by these fMRI findings, recent studies have set out to develop a cognitive task that can assess the functional integrity of the dentate gyrus in humans. As first theorized by Marr44, and empirically established in rodent studies45–48, the human dentate gyrus plays a part in ‘pattern separation’49, a cognitive operation that allows similar stimuli flowing through the hippocampal circuit to be represented with distinct neural codes. Accordingly, memory tasks have been developed that can assess pattern separation ability in humans49. Impairments in these tasks have been documented in ageing subjects50,51 and have been interpreted as confirming previous fMRI findings that implicated the dentate gyrus in normal ageing39,40. Although the computational process underlying pattern separation occurs within the dentate gyrus itself, impaired performance might also be expected with upstream lesions in the entorhinal cortex, which provides the main dentate gyrus input (FIG. 1b). Indeed, impairment in pattern separation tasks has been suggested in subjects who might be in the early stages of Alzheimer's disease, in which the entorhinal cortex is affected52. We will return to this point in the last section, in which we discuss how cognitive tests that engage the entorhinal cortex suggest that there are cognitive pheno-types that differentiate Alzheimer's disease from ageing.
Volumetric techniques using structural MRI were originally used to estimate the volume of the entorhinal cortex and the rest of the hippocampus (including the dentate gyrus, CA3, CA1 and the subiculum) as a single region of interest (ROI). Longitudinal studies have shown that although entorhinal cortex shrinkage can be observed at very old ages, the rest of the hippocampus (not the entorhinal cortex) showed a reliable age-related decline across age groups, tracking the time course of age-related memory decline53,54. More recently, techniques have been developed that can estimate the volumes of the entorhinal cortex as well as the other individual hippocampal subregions, and therefore can pinpoint which subregion is driving the observed effect. Because of the difficulty in visualizing the precise boundaries between the dentate gyrus and CA3, in these studies the two subregions are typically lumped into a single ROI. A study that used these high-resolution techniques to compare patients with Alzheimer's disease with age-matched controls observed Alzheimer's disease-associated shrinkage in the entorhinal cortex, CA1 and subiculum, with sparing of the dentate gyrus or CA3 (REF. 36). When evaluating healthy subjects across the age span, the entorhinal cortex was found to be relatively resistant to ageing, with changes isolated in the dentate gyrus and CA3, and in CA1 (REF. 37). The effect in dentate gyrus and CA3 was more pronounced and better tracked with the temporal profile of age-related memory decline. Moreover, the ageing effect in CA1 was linked to white matter hyperintensities (S. Mueller, unpublished observations), a marker of vascular disease. A more recent volumetric study also showed age-related effects in CA1, and dentate gyrus and CA3 (REF. 55), with relative preservation of entorhinal cortex volume. Here, shrinkage of dentate gyrus and CA3 correlated with age-related memory decline, whereas CA1 shrinkage was associated with hypertension and was therefore interpreted as reflecting the differential sensitivity of CA1 to vascular disease2.
fMRI can also be used to map stimulus-induced changes in the blood oxygen level-dependent (BOLD) response, which is thought to reflect evoked neural activity (BOXES 1,2). Recently, a high-resolution variant of BOLD imaging has been developed and has been applied in ageing individuals and patients with Alzheimer's disease. Normal ageing was characterized by an abnormal stimulus-evoked BOLD response that was restricted to the combined dentate gyrus and CA3 ROI, with normal responses observed in the entorhinal cortex and subiculum51. By contrast, subjects with presumptive early Alzheimer's disease showed an abnormal evoked BOLD response in both the entorhinal cortex and in the downstream dentate gyrus or CA3 (REF. 52). Finally, a recent study investigated ageing subjects using a novel variant of diffusion tensor imaging (DTI)56, an MRI-based technique that may detect white matter and dendritic integrity57. The authors interpreted their result as being suggestive of perforant path alterations and, importantly, an age-related decrease in dendritic integrity that was localized selectively to dentate gyrus or CA3, and not observed in entorhinal cortex, CA1 or subiculum58.
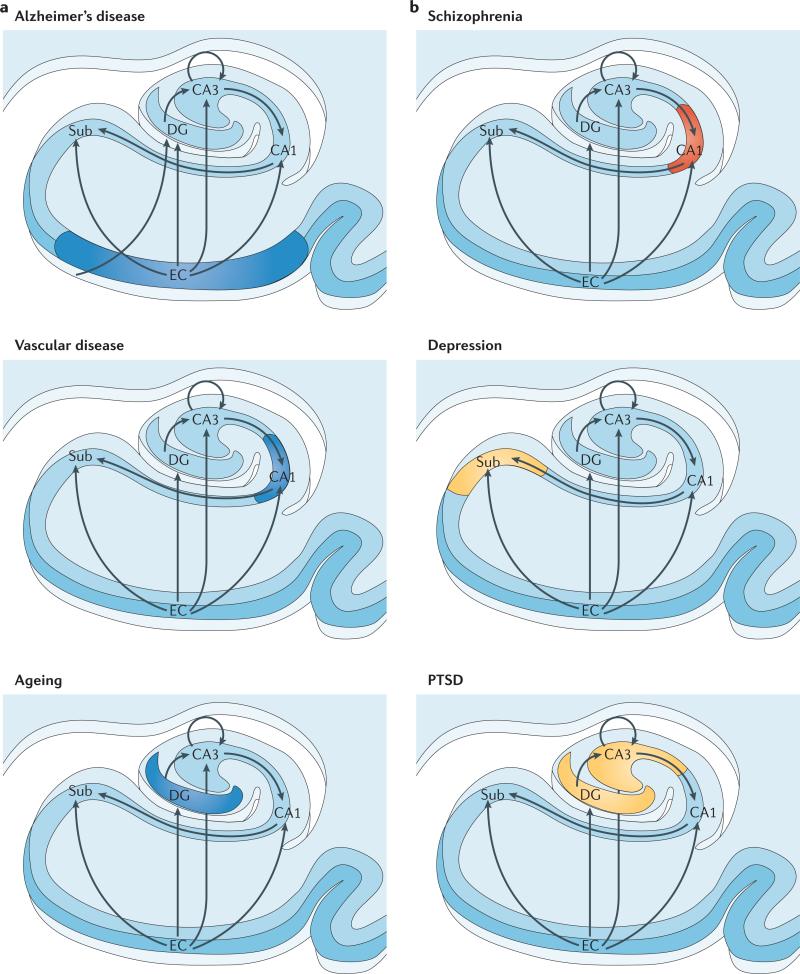
Taken together, the diverse range of imaging studies show that Alzheimer's disease, vascular disease and ageing all contribute to hippocampal alterations in late life, and over time can affect overlapping subregions. Nevertheless, a direct comparison identifies patterns of alterations within the transverse hippocampal circuit that differentiates the three conditions: the entorhinal cortex is differentially associated with Alzheimer's disease, CA1 with vascular disease, and the ageing process per se seems to differentially target the dentate gyrus (FIG. 2a). Over the hippocampal long axis, posterior CA1 is differentially affected by vascular disease59. Although a systematic investigation of age-related dentate gyrus alterations over the hippocampal long axis has not yet been completed, a recent study suggests that age-related pattern separation defects occur in relatively posterior aspects58.
Figure 2. Regional vulnerability and metabolic state differentiate disorders that affect the hippocampal formation.
Although multiple hippocampal subregions can be affected in disorders, by comparing patterns of alterations that are observed by functional and structural MRI it is possible to isolate individual subregions differentially affected by each disorder. Furthermore, functional imaging techniques that are sensitive to metabolic state have suggested that some hippocampus-based disorders are characterized by hypometabolism (shown in blue), whereas others are abnormally hypermetabolic (shown in red). a | Alzheimer's disease, vascular disease and ageing all contribute to hippocampal alterations in late life. A direct comparison suggests that the entorhinal cortex (EC) is differentially associated with Alzheimer's disease and the CA1 with vascular disease, whereas the ageing process per se seems to differentially target the dentate gyrus (DG). Hypometabolism has been localized to the EC in Alzheimer's disease, to CA1 in vascular disease and the DG in ageing. b | Schizophrenia and depression typically begin during adolescence, and post-traumatic stress disorder (PTSD) currently has its highest incidence in young adulthood. A direct comparison suggests that CA1 is differentially associated with schizophrenia and that the subiculum (Sub) may be differentially associated with depression. In the case of PTSD, both CA3 and the DG have been implicated. Evidence for global hippocampal hypermetabolism exists for both schizophrenia and depression. In schizophrenia, this hypermetabolism may be driven by hypermetabolism in CA1, whereas subicular hypermetabolism in depression is a hypothesis that remains untested (shown in yellow). In PTSD the metabolic state of the hippocampal formation is as yet undetermined (shown in yellow).
Pinpointing vulnerable subregions in the hippocampal formation in adolescence and young adulthood
Although they can occur at any age, schizophrenia and depression are diseases that typically begin in adolescence. Triggered by a traumatic event, PTSD can of course also occur at any age. Nevertheless, reflective of current events, the incidence of PTSD is highest in young adults, and we have therefore included a discussion of PTSD in this section.
As well as estimating the volume of individual subregions, mapping alterations in the three-dimensional shape of the hippocampus has emerged as another structural MRI technique that can localize anatomical differences in the hippocampal formation. In patients with schizophrenia, this approach has implicated anterior aspects of the hippocampal long axis and, within the hippocampal circuit, changes were found in the anterior CA1 and subiculum60,61. Interestingly, these studies suggest that the left hippocampus is typically more affected than the right. A subsequent high-resolution fMRI study that mapped basal hippocampal metabolism in patients with schizophrenia confirmed this pattern62. This study also imaged subjects without psychosis who were at risk of developing the disease and, by following the subjects over time, was able to identify patterns of dysfunction in prodromal stages of the disease. The findings suggested that the disease process might start in anterior CA1 and spread, over time, to the anterior subiculum and other brain regions62.
Mapping hippocampal shape has also been used to investigate major depressive illness. As with schizophrenia, the greatest difference between the results from patients with depression and controls was predominantly in anterior aspects of the hippocampal long axis63,64. Within the hippocampal circuit, one study pinpointed shrinkage to the anterior subiculum63 (FIG. 2b). A second study confirmed the finding in the anterior subiculum but also found shrinkage in anterior CA1 (REF. 64). Besides technical issues, a main difference between these two studies is that the latter study included elderly subjects and, as the authors suggest, the involvement of CA1 might in part reflect an interaction with diseases of late life, such as vascular disease.
As with schizophrenia, it would be useful to image subjects who are at risk of developing depression to map the earliest prodromal stages of disease. This has not yet been done in humans, but a recent PET study in rhesus monkeys established that the anterior hippocampus is selectively affected in monkeys with ‘anxious temperament’65. Notably, as a trait, anxious temperament in children is an established risk factor for developing depression and anxiety disorders in adulthood65 (and human studies have suggested a substantial phenotypic and anatomical overlap between anxiety and depressive disorders66).
Numerous volumetric MRI studies have documented a smaller hippocampus in PTSD patients (as first described in REF. 67). So far, there is only one high-resolution MRI study that has mapped volumetric differences across hippocampal subregions in PTSD patients and controls68. This study reported the greatest volume reduction in a single ROI that included both the dentate gyrus and CA3 (FIG. 2b). In the last section, we discuss how, guided by the functional organization of the hippocampal circuit, neuropsychological testing might be able to disambiguate CA3 from dentate gyrus dysfunction, and how cognitive profiles are expected to vary depending on whether dysfunction occurs in the posterior or anterior aspects of the hippocampal long axis.
Metabolic state and hippocampal dysfunction
Most disorders of the brain are associated with regional changes in basal metabolism. Energy metabolism in neurons requires glucose uptake and oxygen consumption. The metabolic state in any brain structure can be determined in vivo by directly measuring glucose uptake or oxygen consumption using PET, or by indirectly measuring correlates of oxygen consumption: cerebral blood flow (CBF) or cerebral blood volume (CBV) with PET, single-photon emission tomography (SPECT) or fMRI.
It is important to note that although the stimulus-evoked BOLD response measured by fMRI is designed to map transient evoked changes in neural activity, studies have established that the BOLD response is not simply coupled to metabolism69,70, and therefore by itself cannot characterize the metabolic state of the brain71. This limitation applies to normal control subjects, in whom haemodynamic coupling varies across the brain72,73, but is even more problematic when imaging ageing individuals and patients suffering from disease. The BOLD response is in fact influenced by neurovascular factors74–76 that act as confounds when trying to interpret between-group differences in the BOLD response (BOX 2). These neurovascular factors are affected in ageing and disease77,78, and unsurprisingly studies have shown that differences in the BOLD response in ageing and disease do not necessarily reflect changes in activity or metabolism77,78. It is important to emphasize that these limitations can be addressed. For example, it is now possible to calibrate the BOLD response against these neurovascular confounds74, thereby deriving a measure of brain metabolism. When changes in the uncalibrated BOLD response are observed in ageing and disease they probably suggest that something is wrong in a particular region, but they do not necessarily inform on abnormalities in neuronal activity, and they certainly cannot determine metabolic abnormalities (BOX 2).
Considering the volume of evidence for hippocampal atrophy and loss of hippocampus-dependent memory function in ageing and disease, it might be expected that functional imaging studies that assess metabolism should document hippocampal hypometabolism in all conditions. This is certainly the case in Alzheimer's disease, in vascular disease and in ageing, in which evidence for hypometabolism has been localized to the entorhinal cortex35,79, CA1 (REF. 2) and the dentate gyrus, respectively41,43 (FIG. 2a). Using global measures of hippocampal metabolism, inconsistent results have been found in PTSD80–82, which perhaps will be clarified in the future with the use of high-resolution technologies.
Notably, however, studies that have imaged hippocampal metabolism in patients with schizophrenia and depression have yielded surprising results. In the case of schizophrenia, a range of functional imaging techniques have clearly established that the hippocampal formation is characterized by an abnormal hypermetabolic state83–87. Most of these studies used techniques that did not possess high spatial resolution, and simply assessed metabolism in the whole hippocampus. In the one published high-resolution fMRI study, anterior CA1 was found to be especially hypermetabolic in people with schizophrenia, together with hypermetabolism in the anterior subiculum and the orbitofrontal cortex62. In the early prodromal stages of the disease, evidence for hypermetabolism was found exclusively in anterior CA1 (REF. 62) (FIG. 2b).
In depression, some cross-sectional studies comparing patients with healthy controls have found evidence of hippocampal hypermetabolism88–90. A more consistent observation emerges, however, from a growing number of studies in which patients were imaged before and after receiving pharmacological or behavioural treatments. These studies have found a decrease in metabolism in the global hippocampal formation in association with treatment efficacy (reviewed in REF. 91). Thus, the hippocampal formation in patients with depression seems to be in a relative state of hypermetabolism. In agreement with this conclusion, a PET study showed that an ‘anxious temperament’ trait in rhesus monkeys65 is selectively associated with hypermetabolism in the anterior hippocampus. So far, high-resolution functional imaging techniques have not yet been used in patients with depression, and hence, whether hippocampal hypermetabolism is localized to the subiculum — as might be suggested — remains unknown.
Interestingly, TLE is a disorder in which the hippocampal formation alternates between basal hypometabolism92 and transient hypermetabolism (reflective of underlying seizures)93. Loss of memory function in patients with TLE is seen during hippocampal hypometabolic states and exists, of course, in a more profound manner during active seizures. Informatively, during active seizures psychotic and affective symptoms, such as hallucinations and delusions and alterations in mood that often phenocopy schizophrenia and depression, can also occur94,95. As seizure activity begins in, and emanates from, the hippocampal formation, abnormal stimulation of hippocampal outflow areas — those that mediate either psychotic or affective behaviour — has been invoked to explain these gain-of-function symptoms in TLE94.
A pathophysiological framework
Based on the wide range of neuroimaging studies reviewed in the previous sections, we propose that any disorder that affects the hippocampal formation does so by differentially targeting specific subregions of the hippocampal circuit, and that this either leads to a decrease or an increase in neuronal metabolism. These two factors — regional vulnerability and metabolic state — provide a framework for how any disorder that affects the hippocampal formation should be characterized (FIG. 3). In this section we will first discuss how, guided by hippocampal functional anatomy, this framework generates hypotheses on how diseases that affect the hippocampal formation might nevertheless manifest with phenotypic variability. Moreover, guided by the molecular anatomy of the hippocampal formation, we will illustrate how this framework provides the basis for understanding mechanisms of disease.
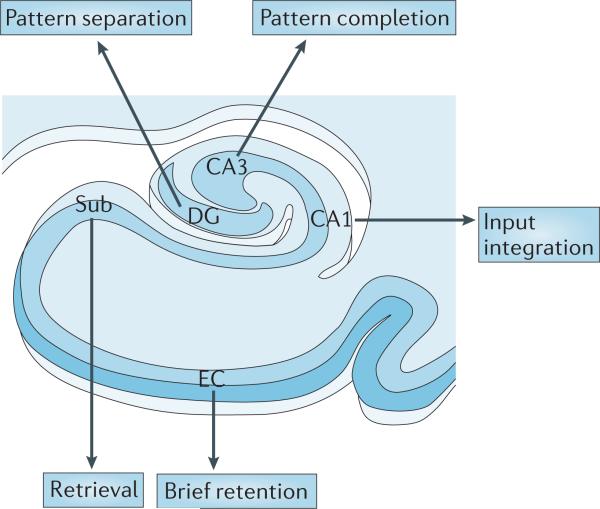
Figure 3. A proposed ‘functional map’ of the hippocampal circuit.
Although multiple subregions are engaged by different tasks, as neural information flows through the hippocampal circuit, each hippocampal subregion is thought to perform a distinct cognitive or computational operation. It is possible to begin to chart a ‘functional map’ of the hippocampal circuit, linking distinct operations to individual hippocampal subregions. Here, CA1 is important in integration of inputs, the dentate gyrus (DG) has a role in pattern separation, CA3 has a role in pattern completion, the subiculum (Sub) is involved in memory retrieval and the entorhinal cortex (EC) is involved in brief retention in hippocampus-dependent memory tasks.
Using regional vulnerability to understand phenotypic diversity
Hippocampus-dependent tests in humans and other mammals were originally developed to assess the hippocampal formation globally, to contrast its function with that of other brain areas or to implicate the hippocampal formation in patients with cognitive impairments96. Recently, however, the cognitive importance of individual hippocampal subregions has come to the fore. When empirical studies are interpreted in the context of computational considerations it is possible to begin charting a ‘functional map’ of the hippocampal formation, whereby specific cognitive or computational operations can be differentially linked to individual subregions (FIG. 3). Again, we emphasize the term differential, because obviously all subregions in the hippocampal circuit participate in multiple operations. Although construction of a functional map has only just begun, once charted it will be possible to develop more nuanced neuropsychological tests to assess the integrity of one subregion over another, thereby improving the ability to phenotype and distinguish disorders. At the same time, applying these tests to disorders for which imaging studies have established patterns of hippocampal vulnerability will help to validate emerging theories about the function of individual subregions. For example, showing that ageing manifests with pattern separation deficits50,51 confirms imaging findings (as reviewed above) — which are after all only indirect measures of function — and validates basic studies that have suggested that the dentate gyrus is differentially involved in this cognitive operation.
As mentioned, because the entorhinal cortex is upstream to the dentate gyrus, cell death in this subregion is also expected to cause pattern separation deficits and defects in cognitive operations that are linked to other downstream subregions. In fact, recent studies have suggested that pattern separation deficits might occur in the early stages of Alzheimer's disease (characterized by entorhinal cortex abnormalities)52. From a clinical perspective, this introduces the important point that hippocampal subregions really serve two functions: they perform a distinct operation as neural information flows through the circuit, but they also act as conduits that deliver information onto downstream subregions. In this regard, as the entorhinal cortex is the most upstream subregion in the hippocampal circuit, it is important to identify cognitive operations that differentially engage this subregion.
Rodent studies suggest that some cells in the entorhinal cortex, particularly those in its medial aspect, show unique grid-cell properties97. A recent human fMRI study described a cognitive task based on predictions extracted from grid-cell properties, and showed that this task activated the entorhinal cortex98. The task, however, was found to also activate numerous other brain regions outside the hippocampal formation. A convergence of other findings suggests that the entorhinal cortex is selectively linked to a cognitive operation performed during brief delay periods of hippocampus-dependent tasks. This link was first suggested by unit recordings in monkeys and rats99,100. Here, sustained activity was observed in the entorhinal cortex during the delay period of matching- or non-matching-to-sample paradigms. In slice preparations, the entorhinal cortex has been found to have electrophysiologically unique ‘persistent-firing’ neurons that show intrinsically-driven, sustained activity lasting minutes101. Although they have not yet been confirmed in vivo, persistent-firing neurons have been incorporated into computational models of hippocampal function in which the entorhinal cortex is proposed to act as a ‘memory buffer’ during brief delays102, and importantly, these neurons have been linked to the emergence of grid cells in the entorhinal cortex103.
These electrophysiological findings have been used to interpret observations in a growing number of human fMRI studies. One study observed sustained activation in a large area that included the entorhinal cortex during a delay in a matching-to-sample task, and sustained activity in this area predicted performance on delayed recognition for visual stimuli104,105. A separate study, using higher spatial resolution, showed sustained activity that was unambiguously localized to the entorhinal cortex during a delay period in a matching-to-sample task of faces106. Although these fMRI studies were interpreted in the context of previous electrophysiological findings, it is notable that stimulus-induced activity was also observed in multiple downstream hippocampal subregions. Two fMRI studies have documented a selective link with the entorhinal cortex. One study found sustained activity in the entorhinal cortex during a memory encoding phase, and the level of sustained activity predicted performance during delayed cue-recall107. Another study used fMRI to map basal metabolism in multiple hippocampal subregions in subjects who received an extensive neuropsychological battery of tests outside the scanner. Basal metabolism in the entorhinal cortex was found to differentially correlate with performance on a derived measure of delayed retention108.
When these electrophysiological, computational and fMRI studies are viewed as a whole, it is possible to conclude that the entorhinal cortex has an important role in ‘delayed retention’ during brief delays (FIG. 3). Although many tasks, such as delayed matching-to-sample, activate the entorhinal cortex, performance on these tasks does not isolate this cognitive operation. In clinical neuropsychological tests, delayed retention is typically isolated by normalizing the amount of information that is recalled after a delay by the amount of information that is recalled immediately. Defects in this derived measure of delayed retention is one of the hallmark cognitive features of Alzheimer's disease109, confirming its link to the entorhinal cortex. Moreover, defects in delayed retention have also been observed in the preclinical stage of the disease, decades before the onset of dementia110. Most importantly, delayed retention is one of the few hippocampus-dependent cognitive abilities that is often found to be relatively resistant to ageing compared to Alzheimer's disease109,111–113. These observations confirm neuroimaging findings that suggest that the entorhinal cortex is relatively resistant to the ageing process, and shows how our pathophysiological framework can predict phenotypic differences between Alzheimer's disease and ageing.
In addition to distinguishing the entorhinal cortex from other hippocampal subregions, it is also important to be able to use cognitive tests to assess the function of other hippocampal subregions. As mentioned, in the case of PTSD neuroimaging studies have so far been unable to anatomically dissociate the dentate gyrus from CA3. Therefore, in addition to pattern separation tasks, which engage the dentate gyrus, it would be useful to have neuropsychological tests that differentially assess the functional integrity of CA3. In fact, the CA3 subregion has been linked to a cognitive operation termed pattern completion — the ability to reactivate a complete hippocampus-dependent memory when presented with only a fragment of the memory (FIG. 3). As first described by Lorente de Nó10, CA3 is unique in that it contains an ‘association tract’ that connects CA3 neurons up and down the hippocampal long axis (FIG. 1) and, as computationally suggested by Marr44, this auto-association system is an anatomical feature that is ideally suited for pattern completion. A mouse fMRI study114 and in vivo recording studies115,116 have provided empirical confirmation of the importance of CA3 for pattern completion. Interestingly, studies suggest that performance on cognitive tasks that are sensitive to pattern completion are relatively preserved in ageing50, supporting the idea that compared with the dentate gyrus, CA3 is relatively preserved.
Based on the functional organization of the hippocampal long axis, reviewed above, we propose that the anterior dentate gyrus and CA3 are involved in pattern separation and pattern completion, respectively, of emotionally charged stimuli, whereas the posterior subregions are predicted to be engaged by stimuli that are emotionally neutral. Although future studies are required to test these specific predictions, BOLD fMRI studies support this long-axis organization117, showing, for example, that emotionally charged faces activate the anterior hippocampus26, whereas activation of the posterior hippocampus is more typically found when emotionally neutral faces are used17. Indeed, the mnemonic defects that are observed in PTSD generally involve memories of emotionally charged stimuli118.
Reflecting the dual input that the CA1 subregion receives from layer III entorhinal cortex and from CA3 (FIG. 1b), CA1 neurons are well positioned to play a part in integrating or comparing information from these converging sources of input. Computational and electro-physiological studies have confirmed that CA1 has a role as an integrator or comparator of converging entorhinal cortex and CA3 input119–123. This role has been invoked to explain a human fMRI study that showed that posterior CA1 is differentially activated when subjects encode allocentric spatial information124. A recent study has observed selective lesions to CA1 in patients with transient global amnesia, and showed that this CA1-selective lesion was associated with defects in learning in a virtual water maze125.
Finally, we come to the last subregion in the hippocampal circuit — the subiculum. A growing number of human fMRI studies have observed that the subiculum shows a differential BOLD response during the retrieval (compared to the encoding) of new hippocampus-dependent memories106,126–129, probably reflecting the fact that the subiculum receives input from CA1 and the entorhinal cortex130.
As highlighted, a definitive functional map of the hippocampal formation has not yet been completed. Nevertheless, the studies that are reviewed above establish the principle that such a map exists (FIG. 3), and illustrate the general approach by which this map may be charted with greater certainty. Informed by computational and experimental observations, human neuroimaging — in particular, high-resolution BOLD131 — will be needed to link specific cognitive operations to distinct subregions, and are likely to show how these operations vary over the hippocampal long axis. For clinical purposes, such a functional map will hopefully guide the development of a new wave of standardized neuropsychological tests (FIG. 3) designed to assess the function of multiple subregions in a single battery. As emphasized, because the hippocampal subregions are interconnected to form a circuit, a ‘lesion’ in one subregion might cause secondary dysfunction in other subregions, and thus impaired performance might be observed in multiple tests. However, by administering an extensive battery, it should be possible to identify the test in which the greatest impairment occurs, thereby pinpointing the anatomical source of hippocampal dysfunction.
Using metabolic state to understand phenotypic diversity
Patients who have extensive bilateral resections of the medial temporal lobes (such as the famous patient H.M.), including of the hippocampal formation, develop profound cognitive defects but are notably free of changes in personality or affect132. As a hippocampectomy can be considered an extreme version of hippocampal loss of function, this confined cognitive phenotype supports the idea that disorders that manifest with hypometabolism are characterized primarily by cognitive symptoms.
Of course, as shown in TLE, hypermetabolism within the hippocampal formation will also interfere with its mnemonic function, although by its very nature hypermetabolism might not be linked to specific cognitive operations. More interestingly, when hippocampal hypermetabolism is observed in TLE, it can (in addition to a loss of memory function) be associated with a ‘gain’ in psychotic and affective symptoms, thus phenocopying the symptoms of schizophrenia or depression95.
This raises the intriguing possibility that the hippocampal hypermetabolism that is observed in schizophrenia and depression may, by stimulating specific hippocampal outflow areas, actually drive the psychotic and affective symptoms that are characteristic of these diseases (FIG. 4). If this is true, then selectively deactivating the hippocampal formation pharmacologically or by other means133 should ameliorate symptoms. As far as we know, this has never been formally tested as most experimental manipulations do not exclusively target the hippocampal formation. Vagal nerve stimulation reduces hippocampal metabolism and has proven to be an effective treatment for depression, but it reduces metabolism in other brain areas as well134. It is also possible that hippocampal hypermetabolism in schizophrenia and depression might, in the long term, entrain hypermetabolism in hippocampal outflow areas by altering their metabolic set-point. In this scenario, hypermetabolism in these areas becomes hippocampus-independent over time, and hippocampus-selective interventions would therefore be effective only if administered during the earliest stages of the disease.
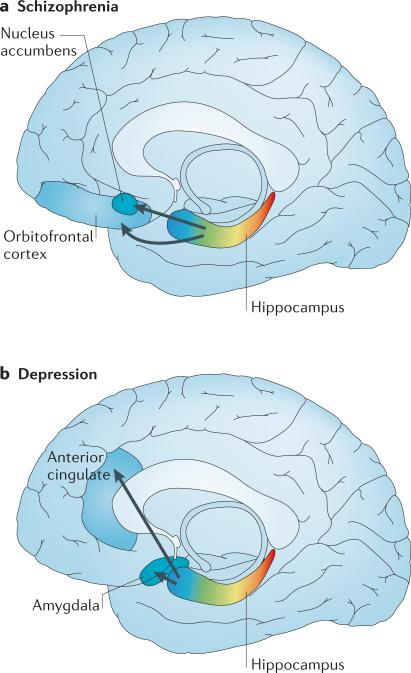
Figure 4. Proposed hippocampus-based networks in schizophrenia and depression.
a | In schizophrenia, hypermetabolism in the anterior hippocampus occurs early in the disease process, and through monosynaptic connections this can be linked to orbitofrontal hypermetabolism that emerges later in the disease course. In animal models, hyperactivity in the anterior hippocampus stimulates the nucleus accumbens, leading to increased striatal dopamine release. b | Studies in patients with depression suggest that hypermetabolism in the anterior hippocampus occurs early in the disease process, and via monosynaptic connections this may be linked to hypermetabolism observed in the amygdala and the anterior cingulate.
Currently, only correlative observations and animal studies provide evidence for the idea that the hippocampal formation acts as an anatomical driver of gain-of-function symptoms in schizophrenia and depression (FIG. 4). In the case of schizophrenia (FIG. 4a), one study found that hippocampal hypermetabolism occurs first, and that orbitofrontal hypermetabolism is observed only later in the disease course62. Furthermore, CA1 hyper-metabolism was found to be associated with measures of delusions and hallucinations. As the hippocampus monosynaptically connects with the orbitofrontal cortex14, it is possible that hippocampal hypermetabolism acts to drive this later extra-hippocampal effect. The hippocampus also monosynaptically connects with the nucleus accumbens, and animal studies have established a mechanism by which hippocampal hypermetabolism links to increased dopamine release in the ventral striatum. Specifically, increased activity in the ventral hippocampus (equivalent to the anterior hippocampus in humans) stimulates the nucleus accumbens through monosynaptic connections, leading to increased striatal dopamine release135 (FIG. 4a). Not only is increased striatal dopamine release observed in schizophrenia136,137 but elevations in dopamine are a neurochemical cause of psychotic symptoms137.
In depression, hypermetabolism occurs, in addition to the hippocampal formation, in multiple regions of the frontal lobe — in particular, the subgenual anterior cingulate and in subregions of the amygdala, caudate, occipital lobe, cerebellum and thalamus91. A brain network linked to depression has been suggested based on the connectivity among some of these areas138. An important feature of this network is the connection between the hippocampus and the subgenual anterior cingulate (FIG. 4b), a connection that was first suggested by tracer studies in non-human primates14 and confirmed more recently by in vivo imaging in humans139. This connection is particularly interesting because selectively reducing hypermetabolism in the subgenual anterior cingulate with deep brain stimulation ameliorates affective symptoms in some patients with depression and anxiety disorders66.
Future studies are needed to establish whether there is a causal link between the hippocampal hypermetabolism that is observed in schizophrenia and depression, and their respective symptoms. If this link is established, the pathophysiological framework generates a number of predictions. Although there are some overlaps between the symptoms of schizophrenia and depression, we predict that differences in symptoms might be explained by differential regional hippocampal hypermetabolism. As reviewed, there is already some evidence that these disorders target different hippocampal subregions, with schizophrenia differentially targeting CA1 and depression targeting the subiculum. We predict that the hippocampal long axis will also show this regional vulnerability. As well as explaining phenotypic differences between schizophrenia and major depressive illness, the framework might also explain phenotypic variability within the two diagnostic categories. Specifically, it might explain why some patients with depression have accompanying psychotic symptoms, and why there is variation in the degree of affective symptomatology of patients diagnosed with schizophrenia and related psychotic disorders.
Using the framework to understand pathogenic mechanisms
Clearly, for nearly all of the disorders reviewed here, the hippocampal formation is not the only brain area that is affected. However, by mapping patterns of vulnerability within the hippocampal formation and determining whether vulnerable subregions are hypo- or hypermetabolic, a basic assumption of the framework is that this information can be exploited to clarify pathogenesis. Showing that two diseases target different subregions would provide compelling evidence that they are mediated by separate processes. For example, there has been an ongoing debate about whether Alzheimer's disease and ageing overlap mechanistically. The observed anatomical dissociation — with Alzheimer's disease differentially targeting the entorhinal cortex and ageing differentially targeting the dentate gyrus with relative sparing of the entorhinal cortex — suggests that Alzheimer's disease and ageing have distinct aetiologies.
More importantly, if a pattern of vulnerability is established, it can be used experimentally to isolate cellular and molecular mechanisms of disease. For example, using gene-expression profiling, a study relied on the differential pattern of hippocampal dysfunction in Alzheimer's disease to isolate deficiencies in molecules related to membrane sorting and trafficking in those subregions that are more affected in Alzheimer's disease140. Studies have confirmed that these intracellular pathways contribute to the pathogenesis of the disease141, leading to a general cell biological hypothesis of late-onset Alzheimer's disease142.
These studies in Alzheimer's disease show the framework's utility. As long as post-mortem samples can be accessed for a given disease, molecular correlates of regional vulnerability and resistance can be identified. Even if post-mortem samples are unavailable, pinpointing a hippocampal subregion that is differentially vulnerable to a given disorder can provide clues regarding pathogenesis. For example, the molecular map of the hippocampal formation shows differential expression of glucocorticoid receptors in the CA3 (REFS 143,144), which may link this subregion to PTSD. Indeed, previous rodent studies have found that both acute and chronic stress differentially target the CA3 subregion143–149. Interestingly, recent studies in rodents suggest that the effects of stress-induced glucocorticoid release is greatest in the anterior (or ventral) hippocampus150. Similarly, when the dentate gyrus is implicated in a disorder, a potential link of the disease to adult neurogenesis — a feature that is unique to this subregion151,152 — must be considered. One straightforward possibility is that a reduction in neurogenesis underlies hippocampal dysfunction. Indeed, age-related reductions in neurogenesis have been observed in rodents153, and recent studies have shown that blocking neurogenesis in mice causes defects in pattern separation48, whereas increasing neurogenesis enhances pattern separation154. Alternatively, neurogenesis during development — which persists longest in the dentate gyrus compared to other subregions — can ‘imprint’ the expression of molecules that mediate transcriptional regulation. This form of gene-expression imprinting has been documented155, and some studies suggest that the dentate gyrus is differentially engaged in histone modification and other epigenetic mechanisms important for the regulation of transcription.
Interestingly, a range of high-resolution fMRI studies in humans, non-human primates and rodents have shown that high levels of blood glucose causes metabolic defects selectively in the dentate gyrus2. An increase in post-prandial blood glucose (owing to age-related insulin resistance) is observed in ageing humans and other mammals156,157. Thus, an age-related increase in blood glucose might account for why dentate gyrus dysfunction is observed with ageing across species (as reviewed above). These observations suggest that manipulations that improve glucose homeostasis might reduce or prevent age-related memory decline. Indeed, physical exercise is one manipulation that improves glucose homeostasis158, differentially increases dentate gyrus basal metabolism159 and brain-derived neurotrophic factor (BDNF) expression160, and ameliorates age-related atrophy measured in the whole hippocampus161.
The occurrence of hippocampal hypermetabolism offers a separate set of clues about underlying mechanisms. For example, studies have suggested that in schizophrenia there might be an abnormal elevation in synaptic glutamate levels162,163, and therefore tonic stimulation of AMPA receptors might act as a source of hyper-metabolism. The loss of CA3 GABAergic interneurons in patients with schizophrenia164 could cause hyperactivity in conjunction with increased synaptic glutamate release and, based on hippocampal circuit properties, this effect has been proposed to occur most prominently in the CA1 subregion164. Of course, many molecules regulate synaptic glutamate and some, like glutaminase, have been genetically165,166 and functionally167 implicated in schizophrenia. However, future studies are required to establish whether molecules that regulate glutamate metabolism are differentially altered in CA1 of patients with schizophrenia.
In the case of depression, mechanistic insight is provided by the fact that, when effective, anti-depressants suppress hippocampal hypermetabolism91. Serotonin reuptake inhibitors (SSRIs) can, by increasing synaptic serotonin levels and thereby stimulating specific serotonin receptors, suppress hippocampal synaptic activity, including in the subiculum168, and has recently been shown to suppress an fMRI correlate of hippocampal metabolism169. Moreover, in vivo radioligand and postmortem studies have suggested that in depression there might be a deficiency in hippocampal serotonin receptors170, which could contribute to hypermetabolism. An alternative mechanism by which SSRIs might suppress hippocampal hypermetabolism is suggested by an intriguing finding showing that a reduction in dentate gyrus neurogenesis causes an increase in spontaneous synchronized activity171. Newly born neurons in the den-tate gyrus seem, therefore, to have an inhibitory effect on the hippocampal circuit. Although recent studies suggest that increasing neurogenesis per se does not have antidepressant effects154, studies nevertheless show that the effect of SSRIs are mediated in part by enhancing neurogenesis172. Finally, very low doses of the NMDA receptor antagonist ketamine can be rapidly effective in depression, and a recent study showed that, among its many effects, low-dose ketamine reduces spontaneous hippocampal activity173.
Conclusions
The proposed pathophysiological framework of hippocampal dysfunction explains, on the one hand, how alterations in single brain structure can lead to phenotypic diversity, and on the other hand, how hippocampal dysfunction can be caused by a range of mechanistically distinct disorders. As its core feature, the framework is guided by the awareness that the hippocampal formation is not a singular structure; the hippocampal formation is a complex circuit that is functionally and molecularly segregated over its longitudinal and transverse axes.
The functional anatomy of the hippocampal circuit can explain how diseases manifest non-overlapping symptoms by differentially targeting specific sites within the circuit. Moreover, the framework informs recent theories about the computational operations performed by individual hippocampal subregions. It supports the view that the function historically assigned to the hippocampal circuit should be broadened, from an exclusive focus on memory to include affect, personality and other complex behaviours. As reviewed, the molecular anatomy of the hippocampal circuit and the unique cellular features of individual subregions provide a general principle for why the hippocampal formation is vulnerable to a broad range of mechanistically distinct disorders.
As we have emphasized, many specific questions about phenotypic features that distinguish each disorder, and the manner in which hippocampal dysfunction is causally linked to these phenotypes, remain undetermined. Moreover, although in some cases clues have emerged, the full complement of pathogenic mechanisms underlying hippocampal dysfunction have not yet been isolated. Just as advances in functional and structural MRI have generated findings that form the basis of the framework, newer technological innovation promises to address these outstanding issues. With higher field strength scanners, it will be possible, for example, to visualize divisions within the entorhinal cortex174 and other hippocampal subregions, and magnetic resonance spectroscopy (MRS) could be used to pinpoint putative glutamate abnormalities175 in CA1 or neurogenesis defects in the dentate gyrus176. Higher resolution and better signal-to-noise ratios will accelerate the development of ‘molecular imaging’, with the promise of visualizing receptors and other molecular abnormalities that are thought to be pathogenic in specific diseases. Additionally, a new crop of technologies that are designed to map patterns of connectivity — including DTI, resting-state functional connectivity MRI177 and manganese-enhanced MRI178 — are already available. These technologies are well suited to further clarify patterns of connectivity within the hippocampal circuit, to map how the hippocampal long axis differentially interconnects with other brain areas, and to understand how disease and ageing affect the hippocampal circuit and the larger brain networks to which the hippocampal circuit belongs. Lastly, all of these imaging technologies, both old and new, can be used to longitudinally follow large number of subjects, tracking how ageing and disease spreads over the hippocampal circuit.
By providing a basis for better understanding hippocampal dysfunction in ageing and disease, the framework proposed in this Review will help to guide future studies, with the ultimate goal of identifying pathogenic mechanisms and of developing effective interventions for some of the most common disorders of the brain.
Box 1 | fMRI and the hippocampal circuit.
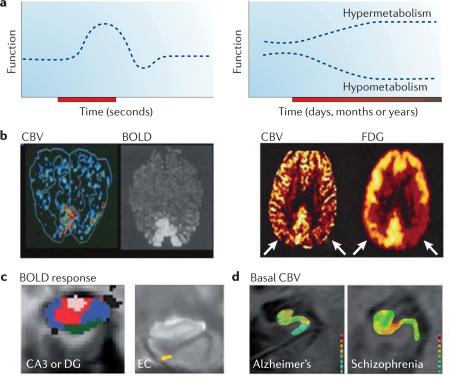
A long history of positron emission tomography (PET) and other functional imaging studies have established that cerebral blood flow (CBF) and cerebral blood volume (CBV) are correlates of brain function and metabolism. Informed by these observations, the first human functional MRI (fMRI) study used a technique that measured CBV179, and shortly thereafter, studies established that fMRI can also measure CBF180 and blood oxygen level-dependent (BOLD)181 responses to map brain function (see BOX 2 for a detailed discussion of the BOLD response).
When imaging brain function, it is important to consider that, neurophysiologically, function can change acutely and transiently, such as when changes in neural activity are evoked by an external and transient stimulus (see the figure; part a, left panel; part b, left panel; and part c. Changes can also occur slowly and chronically, affecting a region's basal metabolic state (see the figure; part a, right panel; Part b, right panel; and part d). Examples of chronic stimuli that change basal metabolism include the dendritic remodelling that underlies long-term memory, neuronal dysfunction that occurs during ageing and in many diseases, and the effects of therapeutic interventions. fMRI measures of CBV, CBF or BOLD responses can map acute changes in brain function (see the figure, part b, left panel, in which visual stimulation is shown to activate visual cortex using either CBV or BOLD measurements). As fMRI measures of CBV and CBF are quantitative, they are better suited to mapping chronic changes in basal metabolism that are associated with ageing and disease (see the figure, part b, right panel, in which CBV defects (as detected with fMRI) match fluorodeoxyglucose (FDG) uptake defects detected with PET) (see BOX 2 for a discussion of the limitations of BOLD in mapping metabolism in ageing and disease).
Different approaches have been used to assess group data in fMRI studies to generate functional maps of the hippocampal formation using CBV, CBF or BOLD. The challenge in evaluating the hippocampal circuit is inherent in the fact that the subregions are very small structures, a few millimetres in dimension. Accordingly, most fMRI studies that evaluate the hippocampal circuit try to generate maps with higher than usual spatial resolution. Because of image acquisition protocols, CBF and BOLD maps are typically generated with an in-plane resolution that nears 1 millimetre. CBV is currently the only fMRI measure that can generate functional maps with sub-millimetre resolution182.
Another challenge is the large degree of subject-to-subject variability in the anatomy of hippocampal subregions, particularly in diseased or ageing populations that often have hippocampal atrophy. Different analytic approaches are now commonly used to overcome this challenge131. One is to evaluate functional maps on a subject-by-subject basis. By drawing regions of interest (ROIs) within hippocampal subregions, group data analysis is performed on functional information that is acquired from each subject (see the figure, part d, which shows individual CBV maps of a patient with Alzheimer's disease35 and of a patient with schizophrenia62. Warmer colours indicate greater CBV).
With the development of sophisticated co-registration techniques, it is now possible to co-register the hippocampal formation across subjects and to perform voxel-based statistical analyses (reviewed in REF. 131). These approaches have been used to show a BOLD response in the dentate gyrus (DG) or CA3 area using a pattern separation task49 (see the figure, part c; pink colour in the left image) and a BOLD response in the entorhinal cortex (EC) during a brief delay in a match-to-sample task106 (see the figure, part c; yellow colour in the right image). By using cortical unfolding techniques, it is also possible to perform voxel-based analyses on ‘flat maps’ of the hippocampal formation126. Figure part c, left image is reproduced, with permission, from REF. 49 © (2008) American Association for the Advancement of Science. Figure part c, right image is reproduced, with permission, from REF. 106 © (2009) Society for Neuroscience. Figure part d, left image is reproduced, with permission, from REF. 35 © (2007) American Medical Association. Figure part d, right image is reproduced, with permission, from REF. 62 © (2009) American Medical Association.
Box 2 | The complexity of interpreting the BOLD response in ageing and disease.
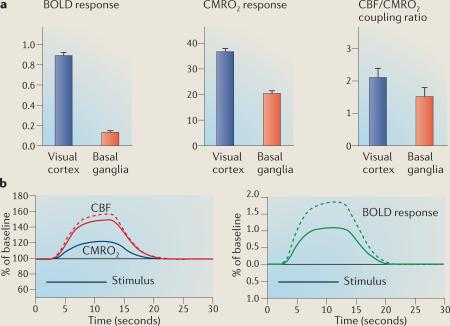
The blood oxygen level-dependent (BOLD) response is a sensitive indicator of where neural activity has acutely changed in response to a transient stimulus. The interpretation of altered responses in disease populations, in ageing, or even in healthy controls on medication75, is intrinsically ambiguous. BOLD is sensitive to changes in deoxyhaemoglobin levels183, which are reduced during acute activation because cerebral blood flow (CBF) increases much more than the cerebral metabolic rate of oxygen (CMRO2) consumption. The BOLD response, however, turns out to be strongly modulated by two additional confounding factors74 that act independently of neural activity: baseline levels of deoxyhaemoglobin and the precise ratio of the fractional changes in CBF and CMRO2.
Although mathematical modelling of the BOLD response is complex184, the dependence of the BOLD response (ΔS/S0) on CBF changes and on these two confounding factors185 can be approximated as:
The first term, ΔCBF/CBF(base), represents the CBF change. The second term, A, is proportional to baseline deoxyhaemoglobin, which is affected by cerebral blood volume (CBV) and oxygen extraction fraction. The third term, n, is the CBF/CMRO2 coupling ratio, defined as the per cent change in CBF divided by the percent change in CMRO2 (the numerical constant b has a value of about 1.2).
If both baseline deoxyhaemoglobin (A) and the CBF/CMRO2 coupling ratio (n) were invariant, interpreting differences in the BOLD response would be straightforward. Both of these factors, however, are highly variable across different brain regions and in ageing and disease. Therefore, when differences in the BOLD response are observed they do not necessarily reflect differences in neural or metabolic activity. So, for example, subjects at risk of Alzheimer's disease were found to have a reduced BOLD response in the hippocampal formation evoked by a memory task compared to controls78. However, this effect was found to be related to differences in baseline CBF, which probably affects baseline deoxyhaemoglobin and confounds the interpretation of the BOLD response. Similarly, another study showed that age-related changes in baseline deoxyhaemoglobin levels can cause misleading differences in the BOLD response when comparing young and older subjects72.
High variability in the CBF/CMRO2 coupling ratio further contributes to the difficulty in interpreting the BOLD response. For example, a visual-evoked BOLD response in visual cortex was sevenfold larger than a motor-evoked BOLD response in the basal ganglia72 (see the figure, part a, left panel). However, only a twofold difference was observed in the metabolic response between the two regions (see the figure, part a, middle panel). The dramatic mismatch between the BOLD and metabolic responses was due to a modest difference in the CBF/CMRO2 coupling ratio (see the figure, part a, right panel). Using the standard BOLD model it is possible to show how, if a disease subtly increases the CBF/CMRO2 coupling ratio (see the figure, part b; shown by solid and dashed red curves), the same evoked metabolic response (see the figure, part b; shown by the blue curve) can lead to dramatic differences in the BOLD response (see the figure, part b; shown by the green curves). In principle, the opposite can also occur if the disease causes a decrease in coupling.
The confound factors A and n can interact with each other, further adding to the complexity of interpreting the BOLD response. Indeed, this interaction can lead to scenarios in which no differences in the BOLD response are observed, despite underlying metabolic differences. This possibility was recently demonstrated in a study that examined the effects of caffeine intake on the BOLD response186, and which showed that caffeine had countervailing effects on A and n. Notably, in the basal state (that is, without acutely altering metabolism with a transient stimulus) the relationship between CBF, CBV and CMRO2 is typically invariant and tightly coupled69,187, and so fMRI measures of basal CBF or CBV often provide an accurate map of metabolic state. Data in the figure, part a, from REF. 72.
Acknowledgements
S.A.Sm. is supported by US National Institute on Aging (NIA) grants AG025161, AG034618, AG034618 and AG035015, the US National Institute of Mental Health (NIMH) grant MH093398, and the James S. McDonnell Foundation. M.P.W. is supported by the Kavli Foundation and center of excellence grant from the Norwegian Research Council (Nr 145,993). C.A.B. is supported by the McKnight Brain Research Foundation and NIA grant AG003376. S.A.Sc. is supported by NIMH grant K23MH09056. We thank C. Stark, H. Eichenbaum, and I. Asllani for comments on earlier versions of this manuscript.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu W, et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann. Neurol. 2008;64:698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloviter RS, Sollas AL, Dean E, Neubort S. Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: characterization of an in vivo model of controlled neuronal death. J. Comp. Neurol. 1993;330:324–336. doi: 10.1002/cne.903300304. [DOI] [PubMed] [Google Scholar]
- 4.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, et al. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J. Comp. Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CL, et al. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Coultrap SJ, Nixon KM, Alvestad RM, Valenzuela CF, Browning MD. Differential expression of NMDA receptor subunits and splice variants among the CA1, CA3 and dentate gyrus of the adult rat. Brain Res. Mol. Brain Res. 2005;135:104–111. doi: 10.1016/j.molbrainres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Sloviter RS, et al. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- 9.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 10.Lorente de Nó R. Studies on the structure of the cerebral cortex II. Continuation of the study of the ammonic system. J. Psychol. Neurol. 1934;46:113–117. [Google Scholar]
- 11.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nature Rev. Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 12.Lavenex P, Amaral DG. Hippocampal–neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 14.Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J. Comp. Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J. Comp. Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- 17.Small S, Nava A, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nature Neurosci. 2001;4:442–449. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- 18.Papatheodoropoulos C, Kostopoulos G. Dorsalventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neurosci. Lett. 2000;286:57–60. doi: 10.1016/s0304-3940(00)01084-3. [DOI] [PubMed] [Google Scholar]
- 19.Papatheodoropoulos C, Kostopoulos G. Decreased ability of rat temporal hippocampal CA1 region to produce long-term potentiation. Neurosci. Lett. 2000;279:177–180. doi: 10.1016/s0304-3940(99)01002-2. [DOI] [PubMed] [Google Scholar]
- 20.Izaki Y, Takita M, Nomura M. Comparative induction of long-term depression between dorsal and ventral hippocampal CA1 in the anesthetized rat. Neurosci. Lett. 2000;294:171–174. doi: 10.1016/s0304-3940(00)01570-6. [DOI] [PubMed] [Google Scholar]
- 21.Kjelstrup KB, et al. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- 22.Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bast T, Wilson IA, Witter MP, Morris RG. From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol. 2009;7:e1000089. doi: 10.1371/journal.pbio.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Prog. Neurobiol. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 25.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Lau JY, et al. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53:952–961. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J. Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zola SM, et al. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [Using careful measures of neuronal loss, this is one of the first studies to show that the dentate gyrus is relatively resistant to Alzheimer's disease.] [DOI] [PubMed] [Google Scholar]
- 33.Schwarcz R, Witter MP. Memory impairment in temporal lobe epilepsy: the role of entorhinal lesions. Epilepsy Res. 2002;50:161–177. doi: 10.1016/s0920-1211(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 34.Du F, et al. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- 35.Moreno H, et al. Imaging the Aβ-related neurotoxicity of Alzheimer disease. Arch. Neurol. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- 36.Mueller SG, et al. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum. Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller SG, et al. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-resolution MRI at 4 Tesla: preliminary results. Epilepsia. 2009;50:1474–1483. doi: 10.1111/j.1528-1167.2009.02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp. Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 40.Small SA. Measuring correlates of brain metabolism with high-resolution MRI: a promising approach for diagnosing Alzheimer disease and mapping its course. Alzheimer Dis. Assoc. Disord. 2003;17:154–161. doi: 10.1097/00002093-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc. Natl Acad. Sci. USA. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [This study applied a high-resolution variant of fMRI in ageing rhesus monkeys to show that the dentate gyrus is differentially affected by normal ageing, and that the entorhinal cortex is relatively preserved.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno H, Hua F, Brown T, Small S. Longitudinal mapping of mouse cerebral blood volume with MRI. NMR Biomed. 2006;19:535–543. doi: 10.1002/nbm.1022. [DOI] [PubMed] [Google Scholar]
- 43.Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann. Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 44.Marr D. Simple Memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 46.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 47.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 48.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn. Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- 51.Yassa MA, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 May 20; doi: 10.1002/hipo.20808. (doi:10.1002/hipo.20808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yassa MA, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raz N, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 55.Shing YL, et al. Hippocampal subfield volumes: age, vascular risk, and correlation with associative memory. Front. Aging Neurosci. 2011;3:2. doi: 10.3389/fnagi.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc. Natl Acad. Sci. USA. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jespersen SN, Kroenke CD, Ostergaard L, Ackerman JJ, Yablonskiy DA. Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage. 2007;34:1473–1486. doi: 10.1016/j.neuroimage.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 58.Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl Acad. Sci. USA. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashton D, Van Reempts J, Haseldonckx M, Willems R. Dorsal-ventral gradient in vulnerability of CA1 hippocampus to ischemia: a combined histological and electrophysiological study. Brain Res. 1989;487:368–372. doi: 10.1016/0006-8993(89)90842-1. [DOI] [PubMed] [Google Scholar]
- 60.Casanova MF, Rothberg B. Shape distortion of the hippocampus: a possible explanation of the pyramidal cell disarray reported in schizophrenia. Schizophr. Res. 2002;55:19–24. doi: 10.1016/s0920-9964(01)00201-8. [DOI] [PubMed] [Google Scholar]
- 61.Narr KL, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [This study used a structural MRI variant to show selective volume reductions in CA1 in first-episode schizophrenia.] [DOI] [PubMed] [Google Scholar]
- 62.Schobel SA, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [This study used an fMRI variant to show that CA1 is affected even in prodromal stages of schizophrenia, and that the abnormality is suggestive of abnormal hypermetabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Posener JA, et al. High-dimensional mapping of the hippocampus in depression. Am. J. Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [This study used a structural MRI variant to show selective volume reductions in the subiculum in depression.] [DOI] [PubMed] [Google Scholar]
- 64.Ballmaier M, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am. J. Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oler JA, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bremner JD, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [This study used a structural MRI variant to show selective volume reductions in the dentate gyrus (or CA3) in PTSD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl Acad. Sci. USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donahue MJ, et al. Hemodynamic changes after visual stimulation and breath holding provide evidence for an uncoupling of cerebral blood flow and volume from oxygen metabolism. J. Cereb. Blood Flow Metab. 2009;29:176–185. doi: 10.1038/jcbfm.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front. Neuroenergetics. 2010;2:8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ances BM, et al. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage. 2008;39:1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiarelli PA, et al. Flow-metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn. Reson. Med. 2007;57:538–547. doi: 10.1002/mrm.21171. [DOI] [PubMed] [Google Scholar]
- 74.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl Acad. Sci. USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [This is a landmark study that established that, if uncalibrated, BOLD cannot reliably map brain metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown GG, et al. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J. Cereb. Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- 76.Chiarelli PA, Bulte DP, Piechnik S, Jezzard P. Sources of systematic bias in hypercapnia-calibrated functional MRI estimation of oxygen metabolism. Neuroimage. 2007;34:35–43. doi: 10.1016/j.neuroimage.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 77.Ances BM, et al. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum. Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleisher AS, et al. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol. Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Leon MJ, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F] fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc. Natl Acad. Sci. USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin LM, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch. Gen. Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molina ME, Isoardi R, Prado MN, Bentolila S. Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World J. Biol. Psychiatry. 2007:1–9. doi: 10.3109/15622970701472094. [DOI] [PubMed] [Google Scholar]
- 82.Yehuda R, et al. Changes in relative glucose metabolic rate following cortisol administration in aging veterans with posttraumatic stress disorder: an FDG-PET neuroimaging study. J. Neuropsychiatry Clin. Neurosci. 2009;21:132–143. doi: 10.1176/jnp.2009.21.2.132. [DOI] [PubMed] [Google Scholar]
- 83.Malaspina D, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol. Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawasaki Y, et al. Regional cerebral blood flow in patients with schizophrenia. A preliminary report. Eur. Arch. Psychiatry Clin. Neurosci. 1992;241:195–200. doi: 10.1007/BF02190252. [This is the first in a series of studies that established that the hippocampal formation in schizophrenia is characterized by a hypermetabolic state.] [DOI] [PubMed] [Google Scholar]
- 85.Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET study. Brain. 1992;115:367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- 86.Heckers S, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 87.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 88.Videbech P, et al. The Danish PET/depression project: PET findings in patients with major depression. Psychol. Med. 2001;31:1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- 89.Videbech P, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr. Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- 90.Lui S, et al. Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–484. doi: 10.1148/radiol.2512081548. [DOI] [PubMed] [Google Scholar]
- 91.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [This meta-analysis shows that when antidepressants are effective they typically cause a reduction in hippocampal metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolf RL, et al. Detection of mesial temporal lobe hypoperfusion in patients with temporal lobe epilepsy by use of arterial spin labeled perfusion MR imaging. AJNR Am. J. Neuroradiol. 2001;22:1334–1341. [PMC free article] [PubMed] [Google Scholar]
- 93.Wu RH, et al. MR measurement of regional relative cerebral blood volume in epilepsy. J. Magn. Reson. Imaging. 1999;9:435–440. doi: 10.1002/(sici)1522-2586(199903)9:3<435::aid-jmri11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 94.Elliott B, Joyce E, Shorvon S. Delusions, illusions and hallucinations in epilepsy: 2. Complex phenomena and psychosis. Epilepsy Res. 2009;85:172–186. doi: 10.1016/j.eplepsyres.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Elliott B, Joyce E, Shorvon S. Delusions, illusions and hallucinations in epilepsy: 1. Elementary phenomena. Epilepsy Res. 2009;85:162–171. doi: 10.1016/j.eplepsyres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 96.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 97.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu. Rev. Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 98.Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463:657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J. Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 101.Tahvildari B, Fransen E, Alonso AA, Hasselmo ME. Switching between “On” and “Off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17:257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- 102.Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 103.Hasselmo ME. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18:1213–1229. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schon K, et al. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J. Neurosci. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J. Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olsen RK, et al. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J. Neurosci. 2009;29:11880–11890. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fernandez G, Brewer JB, Zhao Z, Glover GH, Gabrieli JD. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 108.Brickman AM, Stern Y, Small SA. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2010 Sep 7; doi: 10.1002/hipo.20840. (doi:10.1002/hipo.20840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc. Natl Acad. Sci. USA. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elias MF, et al. The preclinical phase of alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch. Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 111.Albert MS. The ageing brain: normal and abnormal memory. Phil. Trans. R. Soc. Lond. B. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petersen RC, Smith G, Kokmen E, Ivnik RJ, Tangalos EG. Memory function in normal aging. Neurology. 1992;42:396–401. doi: 10.1212/wnl.42.2.396. [This is one in a series of papers that shows that performance on delayed retention is relatively unaffected in normal ageing.] [DOI] [PubMed] [Google Scholar]
- 113.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res. Brain Res. Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 114.Kent K, Hess K, Tonegawa S, Small SA. CA3 NMDA receptors are required for experience-dependent shifts in hippocampal activity. Hippocampus. 2007;17:1003–1011. doi: 10.1002/hipo.20332. [DOI] [PubMed] [Google Scholar]
- 115.Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 116.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 117.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 118.Brewin CR. The nature and significance of memory disturbance in posttraumatic stress disorder. Annu. Rev. Clin. Psychol. 2011;7:203–227. doi: 10.1146/annurev-clinpsy-032210-104544. [DOI] [PubMed] [Google Scholar]
- 119.Blum KI, Abbott LF. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 1996;8:85–93. doi: 10.1162/neco.1996.8.1.85. [DOI] [PubMed] [Google Scholar]
- 120.Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 121.Magee JC. Dendritic integration of excitatory synaptic input. Nature Rev. Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- 122.Nolan MF, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 123.Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- 124.Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. J. Neurosci. 2009;29:10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bartsch T, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328:1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- 126.Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- 127.Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 128.Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J. Neurosci. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Viskontas IV, Carr VA, Engel SA, Knowlton BJ. The neural correlates of recollection: hippocampal activation declines as episodic memory fades. Hippocampus. 2009;19:265–272. doi: 10.1002/hipo.20503. [DOI] [PubMed] [Google Scholar]
- 130.O'Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav. Brain Res. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 131.Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65:298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scoville W, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mikell CB, et al. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact. Funct. Neurosurg. 2009;87:256–265. doi: 10.1159/000225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zobel A, et al. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res. 2005;139:165–179. doi: 10.1016/j.pscychresns.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 135.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [This paper shows that hyperactivity in the anterior or ventral hippocampus can lead to increased striatal dopamine release.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laruelle M, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl Acad. Sci. USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abi-Dargham A, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am. J. Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 138.Seminowicz DA, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 139.Fonteijn HM, Norris DG, Verstraten FA. Exploring the anatomical basis of effective connectivity models with DTI-based fiber tractography. Int. J. Biomed. Imaging. 2008;2008:423192. doi: 10.1155/2008/423192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Small SA, et al. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann. Neurol. 2005;58:909–919. doi: 10.1002/ana.20667. [This paper relied on the pattern of hippocampal dysfunction observed in Alzheimer's disease to identify a novel defect in intracellular sorting and trafficking, illustrating the utility of the pathophysiological framework.] [DOI] [PubMed] [Google Scholar]
- 141.Small SA. Retromer sorting: a pathogenic pathway in late-onset Alzheimer disease. Arch. Neurol. 2008;65:323–328. doi: 10.1001/archneurol.2007.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McEwen BS. Corticosteroids and hippocampal plasticity. Ann. N. Y Acad. Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. [DOI] [PubMed] [Google Scholar]
- 144.McEwen BS. The brain is the central organ of stress and adaptation. Neuroimage. 2009;47:911–913. doi: 10.1016/j.neuroimage.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 146.McKittrick CR, et al. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 147.Stewart MG, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 148.Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons-a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- 149.Sandi C, et al. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur. J. Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 150.Christian KM, Miracle AD, Wellman CL, Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gage FH. Neurogenesis in the adult brain. J. Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nature Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 153.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zapala MA, et al. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc. Natl Acad. Sci. USA. 2005;102:10357–10362. doi: 10.1073/pnas.0503357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shimokata H, et al. Age as independent determinant of glucose tolerance. Diabetes. 1991;40:44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 157.Gresl TA, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am. J. Physiol. Endocrinol. Metab. 2001;281:E757–765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 158.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu. Rev. Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 159.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J. Neurosci. Res. 2004;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 161.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am. J. Psychiatry. 2004;161:1116–1118. doi: 10.1176/appi.ajp.161.6.1116. [DOI] [PubMed] [Google Scholar]
- 163.Theberge J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am. J. Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 164.Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol. Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [This landmark paper identified a loss in hippocampal interneurons that is predicted to cause selective hippocampal hyperactivity.] [DOI] [PubMed] [Google Scholar]
- 165.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 166.Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr. Res. 2010;122:38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gaisler-Salomon I, et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia. Neuropsychopharmacology. 2009;34:2305–2322. doi: 10.1038/npp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boeijinga PH, Boddeke HW. Serotonergic modulation of neurotransmission in the rat subicular cortex in vitro: a role for 5-HT1B receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:553–557. doi: 10.1007/BF00167229. [DOI] [PubMed] [Google Scholar]
- 169.Mueggler T, et al. Mapping of CBV changes in 5-HT(1A) terminal fields by functional MRI in the mouse brain. Eur. Neuropsychopharmacol. 2011;21:344–353. doi: 10.1016/j.euroneuro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 170.Drevets WC, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl. Med. Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2010 Sep 29; doi: 10.1002/hipo.20860. (doi:10.1002/hipo.20860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 173.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Augustinack JC, et al. Detection of entorhinal layer II using 7Tesla [corrected] magnetic resonance imaging. Ann. Neurol. 2005;57:489–494. doi: 10.1002/ana.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van Elst LT, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol. Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 176.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Van Dijk KR, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pautler RG. In vivo, trans-synaptic tract-tracing utilizing manganese-enhanced magnetic resonance imaging (MEMRI). NMR Biomed. 2004;17:595–601. doi: 10.1002/nbm.942. [DOI] [PubMed] [Google Scholar]
- 179.Belliveau JW, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 180.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc. Natl Acad. Sci. USA. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kwong KK, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl Acad. Sci. USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lin W, Celik A, Paczynski RP. Regional cerebral blood volume: a comparison of the dynamic imaging and the steady state methods. J. Magn. Reson. Imaging. 1999;9:44–52. doi: 10.1002/(sici)1522-2586(199901)9:1<44::aid-jmri6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 183.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 184.Griffeth VE, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage. 2011;58:198–212. doi: 10.1016/j.neuroimage.2011.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. Neuroimage. 2007;36:1110–1122. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Griffeth VEM, Perthen JE, Buxton RB. Prospects for quantitative fMRI: investigating the effects of caffeine on baseline oxygen metabolism and the response to a visual stimulus in humans. Neuroimage. 2011;57:809–816. doi: 10.1016/j.neuroimage.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leenders KL, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]