Abstract
The inhibitory kappa B kinases (IKKs) are well recognized as key regulators of the nuclear factor kappa B (NF-κB) cascade and as such represent a point of convergence for many extracellular agents that activate this pathway. The IKKs generally serve to transduce pro-inflammatory and growth stimulating signals that contribute to major cellular processes but also play a key role in the pathogenesis of a number of human diseases. Therefore, the catalytic IKKs represent attractive targets for intervention with small molecule kinase inhibitors. IKK isoforms are assembled as variable multi-subunit IKK complexes that regulate not only NF-κB dimers, but also protein substrates out-with this cascade. Consequently, close consideration of how these individual complexes transduce extracellular signals and more importantly what impact small molecule inhibitors of the IKKs have on functional outcomes are demanded. A number of adenosine triphosphate (ATP)-competitive IKKβ-selective inhibitors have been developed but have demonstrated a lack of activity against IKKα. A number of these chemicals have also exhibited detrimental outcomes such as cellular toxicity and immuno-suppression. The impact of small molecule inhibitors of IKK catalytic activity will therefore be reappraised, examining the advantages and potential disadvantages to this type of intervention strategy in the treatment of diseases such as arthritis, intestinal inflammation and cancer. Furthermore, we will outline some emerging strategies, particularly the disruption of protein–protein interactions within the IKK complex, as an alternative route towards the development of novel pharmacological agents. Whether these alternatives may negate the limitations of ATP-competitive molecules and potentially avoid the issues of toxicity will be discussed.
Keywords: inhibitory kappa B kinase, nuclear factor kappa B, kinase inhibitors, protein–protein interactions, inflammation
Introduction
In the early 1980s, Sen and Baltimore (1986) embarked on a study to identify nuclear factors required to regulate immunoglobulin G (IgG) gene expression in B cells. One of these bound specifically within the promoter of the Ig κ light chain and believing the factor to be B cell specific, called this nuclear factor bound to the κ site of B cells (NF-κB). This initial seminal work, and the subsequent realization of the ubiquitous nature of this transcription factor, has made NF-κB one of the most intensively studied signalling paradigms in the last 25 years. NF-κB has provided a model for the understanding of inducible membrane to nuclear signalling, in particular linking cytokine receptors to interior signalling platforms. In addition, because of its role in a number of disease conditions including arthritis, cancer, cardiovascular disorders and neurodegeneration, the NF-κB pathway has become a key therapeutic target for drug development.
NF-κB comprises the family of Rel proteins of which there are five members: p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2). These transcription factors mediate signalling from the cell surface to regulate key genes involved in inflammation, cell division and immunity. The basic paradigm characterized in the early 1990s indicated that NF-κB isoforms reside in the cytosol, some as larger precursors, bound to a series of inhibitory proteins called the inhibitory kappa Bs (IκBs). Following stimulation, IκBα underwent phosphorylation, ubiquitination and proteolytic degradation to release NF-κB, allowing it to translocate to the nucleus where it bound to specific κB sites to regulate gene transcription (Figure 1). Identifying the enzyme(s) which mediated phosphorylation of the IκBs proved difficult and not achieved until sometime later through the isolation of the inhibitory kappa B kinases (IKKs), the major regulatory kinases within the pathway. This review focuses on the regulation and functions of these kinases, progress in designing drugs to inhibit their activities and the use of these drugs pharmacologically in models of disease.
Figure 1.
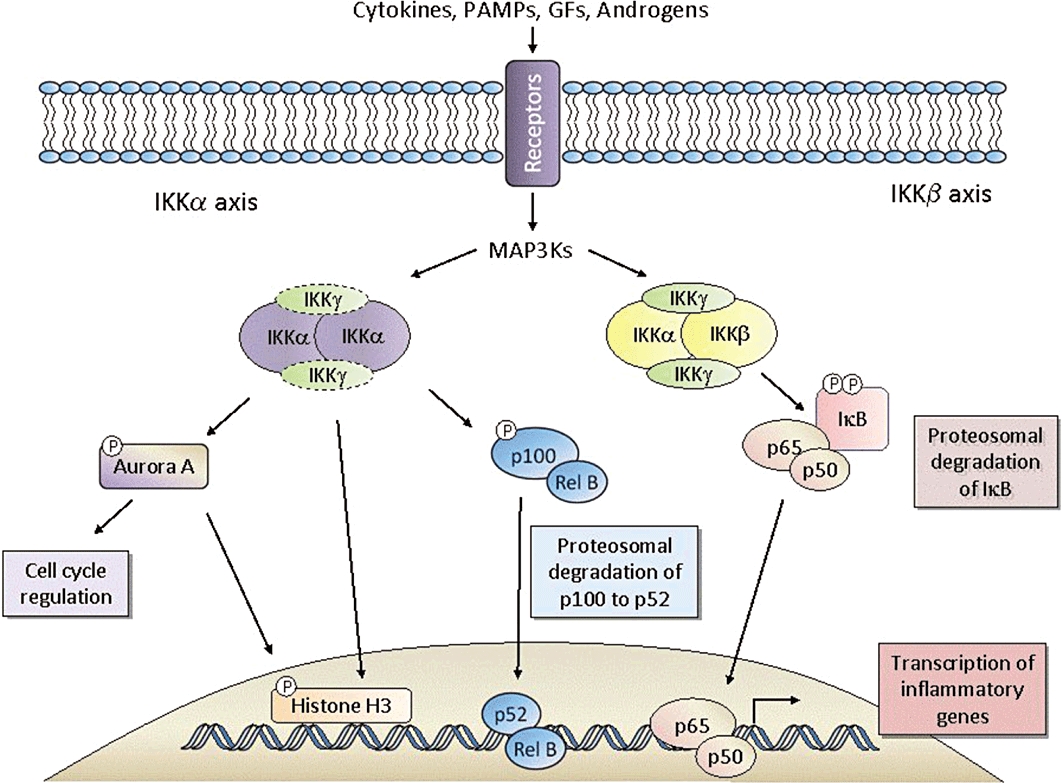
Schematic representation of IKKα and IKKβ-mediated cell signalling encompassing the canonical NF-κB cascade (NEMO dependent —), non-canonical NF-κB cascade (NEMO dependent/independent ---) and substrates outwith these pathways. A variety of extracellular agents; pro-inflammatory cytokines, pathogen-associated molecular profiles (PAMPs), growth factors (GFs) and hormones, in a variety of cell types, display the ability to engage these diverse signalling events leading to gene transcription and chromatin modification that impact on inflammatory responses, cell cycle progression and cell growth/apoptosis.
The role of the IKKs in the regulation of the NF-κB pathway
The IKKs are a series of four enzymes, three of which were initially purified as part of a high-molecular-weight, multi-subunit kinase of approximately 700–900 kDa (Chen et al., 1996). IKKα and IKKβ are the catalytic subunits and share 52% overall sequence homology, with 64% identity across their catalytic domains (Woronicz et al., 1997). IKKγ or NF-κB essential modulator (NEMO) is a 48 kDa non-catalytic protein that plays a scaffolding/regulatory role and is required for kinase function (Rothwarf et al., 1998; Yamaoka et al., 1998). Although IKKβ is the isoform that appears to bind with higher affinity to NEMO, the predominant and most active form of the complex contains one molecule each of IKKα and IKKβ bound to two molecules of IKKγ (Huynh et al., 2000; Miller and Zandi, 2001; May et al., 2002; Rushe et al., 2008). A third IKK known as IKK-i/ε and an IKK-related kinase known as TANK-binding kinase 1 (TBK1)/NF-κB-activating kinase (NAK)/tumour necrosis factor-α (TNF-α) receptor-associated factor 2-associated kinase (T2K) have been isolated but do not function as NF-κB inducing kinases, although have been suggested as potential modulators of p65 transactivation (Buss et al., 2004; Adli and Baldwin, 2006; Wietek et al., 2006). While TBK1 is expressed constitutively and is activated in response to agonist stimulation (Tojima et al., 2000), IKK-i/ε is an inducible enzyme and, once expressed, is constitutively active (Shimada et al., 1999). These enzymes predominantly regulate members of the family of interferon regulatory factors (IRFs) that contribute to specific gene transcription events such as cytokine production and suppression in the context of infection and immunity (Chau et al., 2008). TBK1 has also been implicated in angiogenesis (Korherr et al., 2006; Czabanka et al., 2008), and IKK-i in the constitutive activation of gene transcription in a number of cancer cell lines (Eddy et al., 2005; Adli and Baldwin, 2006).
Activation of the IKK isoforms α and β regulate two divergent NF-κB signalling pathways, the classical canonical pathway and the non-canonical pathway. Each cascade relies on different IKKs for maximal activation, and it is these differences that give rise to the potential for therapeutic intervention and the development of isoform selective inhibitors. The canonical pathway is activated by pro-inflammatory stimuli such as TNF-α, interleukin-1 (IL-1) and lipopolysaccharide (LPS) through the toll-like receptors (TLRs) (Figure 1). This results in the recruitment of a number of well-described adaptor molecules identified as the TNF receptor-associated factors (TRAFs). This facilitates the recruitment of key enzymes such as MAP or ERK kinase kinase 3 (MEKK3) and transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) which specifically activate IKKβ through phosphorylation of amino residues Ser 177 and Ser 181 within the activation loop (Yang et al., 2001; Schmidt et al., 2003). Activated IKKβ then phosphorylates Ser 32 and Ser 36 of IκBα (or Ser 19 and Ser 23 of IκBβ) (Brown et al., 1995; DiDonato et al., 1996), leading to polyubiquitination and subsequent degradation of IκB by the proteasome. As IκB is usually bound to NF-κB, the nuclear localization sequence (NLS) within the Rel homology domain of NF-κB is masked and as such is retained predominantly in the cytoplasm (there is some evidence for shuttling of NF-κB : IκBα complexes) (Malek et al., 2001). The removal of IκB therefore results in the ‘unmasking’ of the NLS to induce nuclear translocation of NF-κB and binding to consensus binding sites within promoter regions of specific genes. IKKβ can also mediate phosphorylation of NF-κB p65 at Ser 536 to initiate transactivation leading to increased transcriptional activation following DNA binding (Sakurai et al., 1999).
In parallel to this paradigm of IKK-mediated NF-κB activation, it is also recognized that NF-κB complexes can be mobilized in an atypical, IKK-independent manner, reliant on alternative mechanisms of regulation. The phosphorylation of IκBα at Tyr42 has been reported in response to a variety of stimuli such as treatment with hydrogen peroxide, the tyrosine-phosphatase inhibitor pervanadate, exposure to nerve growth factor and in response to hypoxia and reperfusion (reviewed in Perkins, 2006; Perkins and Gilmore, 2006). Alternative casein kinase-II (CK2)-mediated phosphorylation of IκBα in its C-terminal PEST domain may also lead to NF-κB activation, for example, exposure of cells to ultraviolet (UV) light, or the expression of the erbB2 oncogene in breast cancer cells (reviewed in Perkins, 2006). Therefore, through differential phosphorylation of IκBs, additional mechanisms of activation of NF-κB subunits can be achieved.
The identification of the IKK complex allowed the role of IKKβ within the canonical pathway to be defined very quickly. Studies using dominant-negative mutants of IKKβ and IKKβ knockout (KO) mice confirmed the requirement for this isoform in nuclear translocation and expression of key NF-κB genes such as those involved in inflammation, apoptosis and cell survival (Li et al., 1999; Tanaka et al., 1999). In fact, the inhibition of IKKβ in a variety of cells has now identified clearly the key role this isoform has in regulating survival of cells and protecting against cellular apoptosis (Mustapha et al., 2000; Wullaert et al., 2011). However, defining the role of IKKα in regulation of NF-κB dependent transcription was more challenging. Although inhibition or gene deletion of IKKα revealed no positive regulatory role in IκBα degradation and subsequent NF-κB translocation, no other clear mechanistic function was apparent. In fact, initial studies implicated IKKα as a potential negative regulator of IKKβ and IKK complex catalytic activity (O'Mahony et al., 2000; Lawrence et al., 2005; Li et al., 2005). Nevertheless, studies showed that IKKα KO mice have a phenotype distinct from those of the IKKβ KO, which indicated a unique cellular function for IKKα (Takeda et al., 1999; Hu et al., 2001).
These KO studies and a closer investigation of NF-κB isoforms have identified IKKα as a key component in the alternative, non-canonical pathway. In this model, albeit demonstrated in very few systems (Matsushima et al., 2001; Dejardin et al., 2002), NF-κB precursors of higher molecular weight (e.g. p100) are processed to generate other lower-molecular-weight NF-κB isoforms (e.g. p52). Another MEKK distinct from MEKK3, namely NFκB-inducing kinase (NIK), first phosphorylates IKKα on Ser 176 (Ling et al., 1998). IKKα then, in turn, phosphorylates p100 at multiple sites (serines 99, 108, 115, 123 and 872) (Xiao et al., 2004) which target it for ubiquitination and proteolytical processing to p52. In support of this model, IKKα−/− B cells show defective processing of p100, while the constitutive cleavage of p105 is unchanged (Senftleben et al., 2001). The nuclear translocation of p52 : RelB heterodimers results in the transcription of genes involved in B cell maturation and lymphoid organogenesis. The composition of NF-κB dimers in this system, which is distinct from p65, ultimately leads to a different pattern of gene transcription which is cell-type specific.
More recently, IKKα has also been shown to participate in the canonical pathway via a novel nuclear mechanism. After TNF-α stimulation, IKKα translocates to the nucleus where it plays a role in modulating gene expression through the phosphorylation of histone H3 (Anest et al., 2003; Yamamoto et al., 2003). In addition, IKKα has been shown to modulate NF-κB gene expression by regulating silencing mediator for retinoic acid and thyroid hormone receptor (SMRT) derepression (Hoberg et al., 2004), influence cell cycle progression via phosphorylation of Aurora A (Prajapati et al., 2006), and regulate mammary gland development (Cao et al., 2001) and angiogenesis (Agarwal et al., 2004). Interestingly, expression of a kinase inactive IKKα mutant in IKKα−/− murine embryonic fibroblasts (MEFs) rescued expression of a subset of NF-κB genes, suggesting that the catalytic activity of IKKα is not always essential (Massa et al., 2005). Further evidence for this has been provided from studies that show IKKα to be essential for keratinocyte differentiation, independent of its kinase activity (Hu et al., 2001; Sil et al., 2004).
Thus, despite also functioning within the canonical NF-κB pathway, IKKα can nevertheless initiate a distinct pattern of gene expression overlapping with, but distinct to, that induced by IKKβ. This again presents IKKα as a potential target for drug development. However, despite the opportunity to develop isoform selective inhibitors, IKKβ has thus far proven to be the more tractable target.
The development of novel small molecule inhibitors of the IKKs
Based on early studies that identified IKKβ as the key driver of classical NF-κB signalling, large pharmaceutical companies have developed diverse, large-scale, high-throughput screening (HTS) programmes encompassing hit-to-lead development and characterization of structure–activity relationships (SAR), all in the absence of resolved crystal structures for IKKα and β. This has led to a number of chemical entities of relatively low molecular weight with drug-like features that commonly function as IKKβ selective inhibitors (see Table 1). The majority of these compounds perform as adenosine triphosphate (ATP)-competitive molecules or alternatively possess allosteric action to limit IKK activities (see Table 1). Furthermore, a number of these molecules have been pursued in vivo in animal models of disease. In short, full characterization of mechanism of action related to potency in vitro, efficacy in cell-based experiments and animal studies have been requisite to their potential movement from preclinical analysis to patient trials, although, to date, use of these inhibitors clinically has yet to be reported.
Table 1.
Chemical scaffolds, potency and selectivity of IKK inhibitor molecules characterized in cell-based studies and animal models in vivo
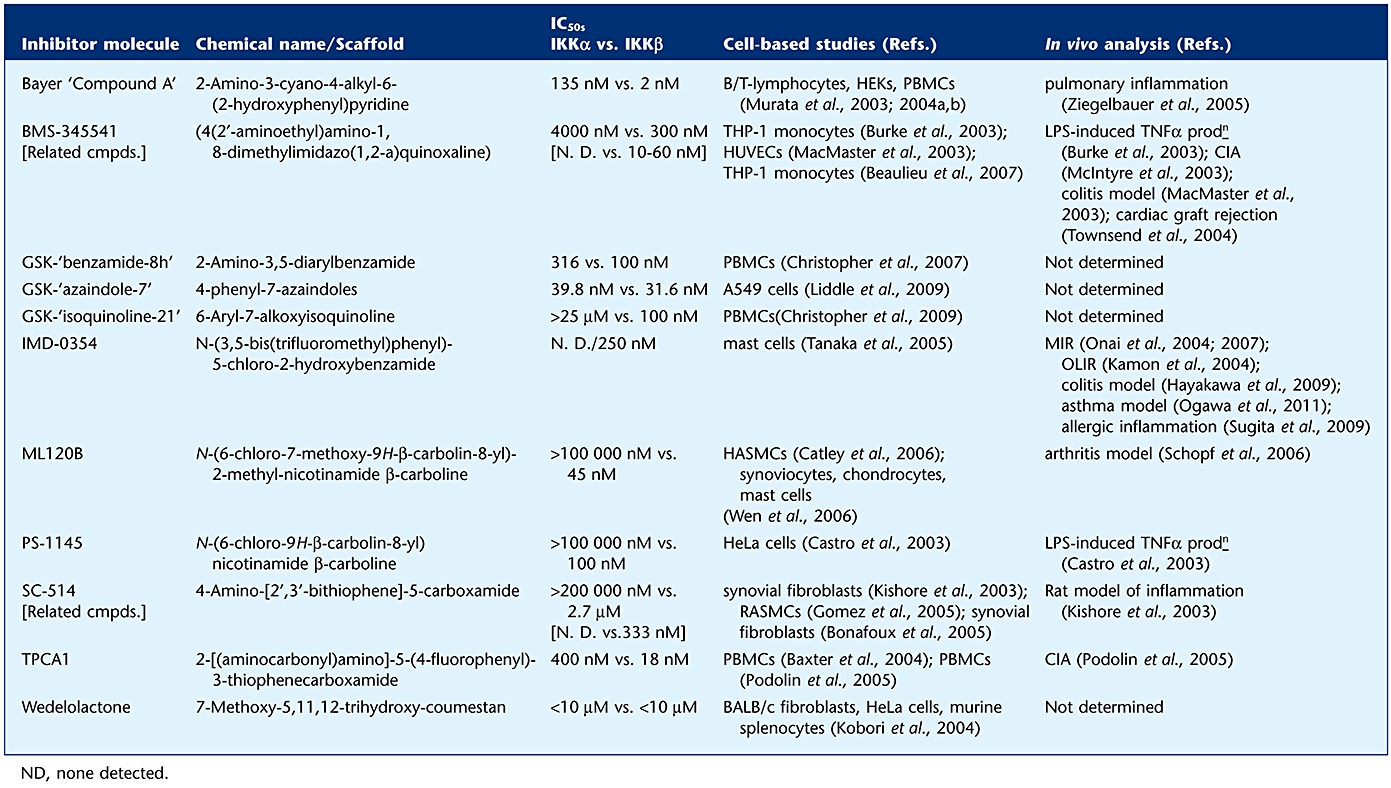 |
Well-recognized and thoroughly studied IKK inhibitors, depicted in Figure 2, include Bayer ‘Compound A’ (1), PS-1145 (2) and related ML120B (3) based on a β-carboline scaffold, thiophene carboximides such as SC-514 (4) and TPCA1 (5), diarylbenzamides (6), hydroxybenzamide compounds [e.g. IMD-03; (7)] and the more recently developed GlaxoSmithKline (GSK) classes of alkoxyisoquinoline (8) and azaindoles (9). Interestingly, both Bayer ‘Compound A’ and TPCA1 also display activity against IKKα albeit with 100-fold and 10-fold selectivity, respectively, toward IKKβ (see Table 1). The Bristol Myers Squibb (BMS) compound BMS-345541 (10) also displays action against both IKKα and IKKβ, displaying 10-fold selectivity towards IKKβ (see Table 1). Interestingly however, it possesses very different pharmacology. BMS-345541 acts as an allosteric inhibitor of both IKKα and IKKβ (see Table 1), binding to IKKβ is in a non-mutually exclusive manner with respect to adenosine diphosphate (ADP), while binding to IKKα has a secondary influence on the active site and therefore effects ATP binding (Burke et al., 2003).
Figure 2.
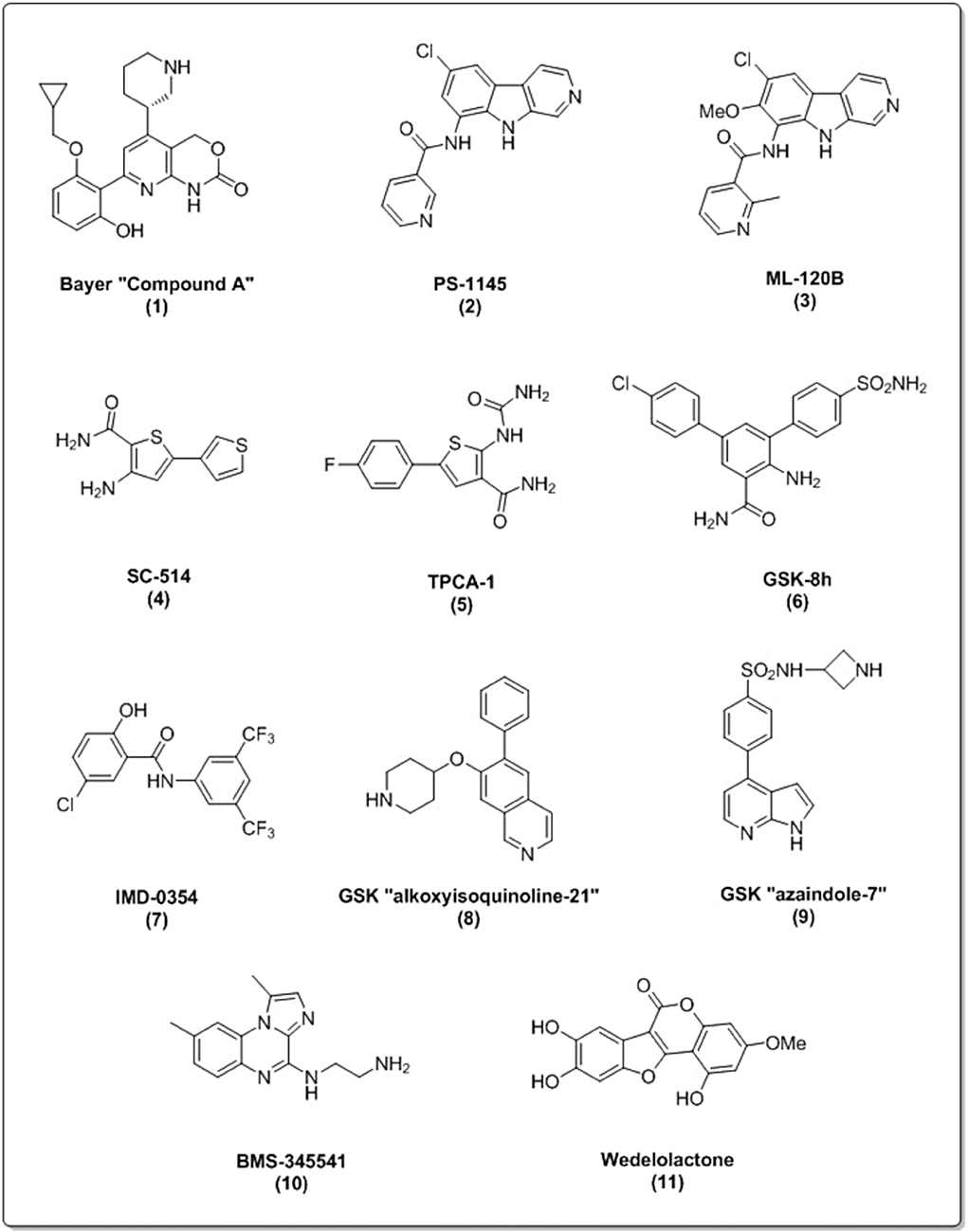
Chemical structures of IKK inhibitors.
Collectively, these inhibitors, perhaps expectedly, block numerous agonist-stimulated gene transcription events linked to IKKβ-NF-κB activation. This includes regulated upon activation, normal T cell expressed and secreted (RANTES) protein and monocyte chemoattractant proteins (MCPs) in synoviocytes and matrix metalloproteinases (MMPs) in chondrocytes which are related to cellular changes and disease onset in arthritis/joint destruction (see Section Arthritis), the production of cytokines such as TNF-α and ILs 1, 6 and 8 in monocytic cells which are linked to inflammation (see Section Intestinal Inflammation) and the modulation of cell cycle regulators in tumour development (see Section Cancer). However, only a subset of these inhibitors has been utilized in studies in vivo, although the introduction of these molecules into ever-diversifying models is ongoing (see Table 2). Of particular note is that molecules such as BMS-345541, ML120B and TPCA1 have been efficacious in rodent models of arthritis. Both BMS-345541 and ML120B have been reported to display desirable pharmacokinetics in mice and rats with good oral bioavailability; oral administration dose dependently inhibited both cellular inflammation and associated disease incidence and severity (McIntyre et al., 2003). In contrast TPCA1 was administered intra-peritoneally due to poor oral bioavailability, but also resulted in a reduction in disease severity (Podolin et al., 2005). SC-514 also displayed poor oral bioavailability (Kishore et al., 2003), yet upon intra-peritoneal administration was able to reduce LPS-stimulated TNF-α production.
Table 2.
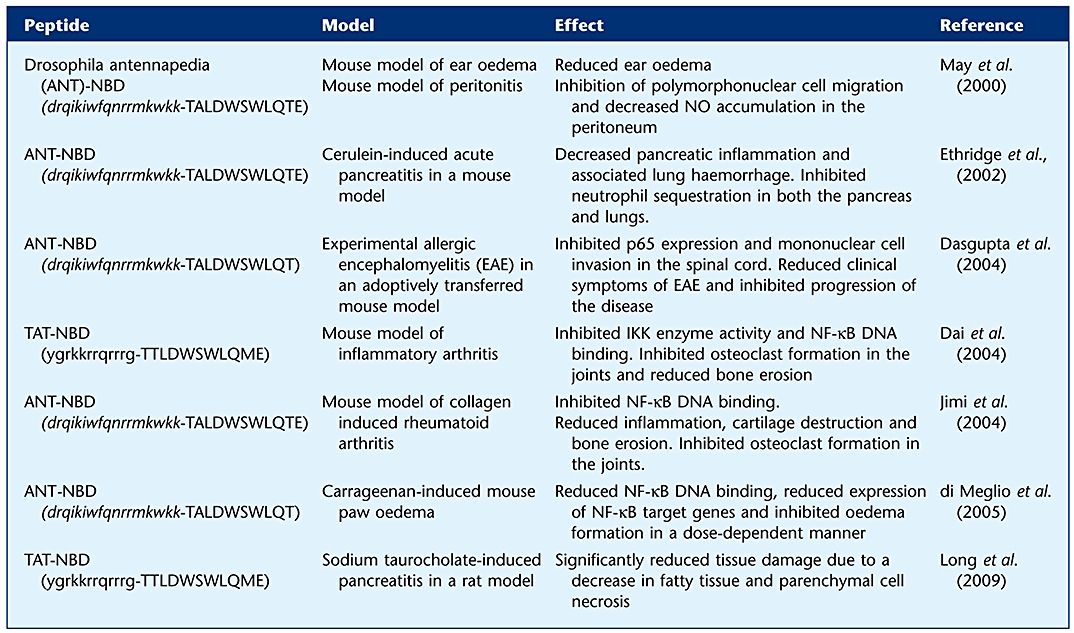
In vivo effect of cell-permeable peptides targeting the NBD
 |
Aside from HTS strategies with synthetic small molecules, it is also well recognized that natural products may represent a route towards novel pharmacological agents that target the IKKs. For example, wedelolactone [Figure 2, (11)] has been suggested to act as an irreversible inhibitor of both IKKα and IKKβ (Kobori et al., 2003). Another compound, noraristeromycin, has been shown to be a selective inhibitor of IKKαin vitro (Asamitsu et al., 2008); however, the assay conditions employed in the study did not exclude an additional effect upon IKKβ. Indeed, apart from a few key examples, the characterization of natural products as IKK inhibitors has been limited, tending to stop short of a proper elucidation of the mechanism of inhibition within the IKK/NF-κB pathway. Nevertheless, the diversity of compounds, both synthetic or natural, and the constituent chemical scaffolds that have been reported to inhibit components of the NF-κB pathway, including but not limited to the IKKs, continue to grow (Bremner and Heinrich, 2002; Gupta et al., 2010a,b; Luqman and Pezzuto, 2010). The Gilmore laboratory (Boston University, MA, USA) has compiled extensive listings of similar and related molecules that intervene in IKK-NF-κB signalling/transcription (see http://www.nf-kb.org) as a resource to aid in the further development of IKK inhibitors.
Targeting IKK inhibition in disease
The well-recognized role of NF-κB in underpinning cellular inflammation has implicated the IKKs as important intermediates involved in a number of disease conditions including asthma, atherosclerosis, neurodegeneration, rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) (Grivennikov et al., 2010). Ideally, the inhibition of NF-κB would therefore be a worthwhile therapeutic strategy; unfortunately, however, there are caveats. NF-κB proteins play a pivotal role in normal physiological functions such as innate immunity and cell survival. Inhibition of NF-κB could therefore have a catastrophic effect upon susceptibility to infection and normal development. Hence, targeting the pathway in some conditions is feasible, but not without problems. In the succeeding discussion, we focus on RA and IBD, as inflammatory conditions in which drug development has the potential to make the greatest immediate impact, and also on cancer, a multifaceted condition in which the IKKs may be targetable.
Arthritis
In RA, up-regulation of IKK activity, predominantly IKKβ, is well recognized (Han et al., 1998). Studies in cultured synovial cells, demonstrating enhanced IKK and NF-κB (Bannai et al., 1996; Roshak et al., 1996; Yamasaki et al., 2001), correlate with studies in human arthritic joints (Danning et al., 2000; Carlsen et al., 2002; Benito et al., 2004) and in animals with experimentally induced arthritis (Tsao et al., 1997; Han et al., 1998). Increased NF-κB is associated with many aspects of disease progression; autoreactive T-cells, B-cell antibody production and macrophage activation may all be increased. Correspondingly, genetic deletion of key IKK-regulated NF-κB subunits, for example p50, can prevent many of these features and consequently stop the development of either collagen- or BSA-induced arthritis (Campbell et al., 2000). Increased IKK/NF-κB activity is also associated with enhanced adhesion molecule expression in the joint synovium, recruitment of lymphocytes, production of inflammatory mediators such as IL-1β and TNF-α, cartilage destruction and pannus formation (Han et al., 1998). Infection with catalytically inactive, dominant-negative variants of IKKβ profoundly inhibited these parameters in vitro (Andreakos et al., 2003) and ameliorated adjuvant-induced arthritis in vivo (Tak et al., 1999).
Given the potential of NF-κB and IKKβ as therapeutic targets in inflammatory and autoimmune disorders, synthetic strategies have focussed on the development of highly selective, potent inhibitors of IKKβ. As outlined above, BMS-345541, TPCA1 and ML120B have shown some success in reducing NF-κB activation as well as joint destruction in different animal models (Table 1). In a murine collagen-induced arthritis (CIA) model, the administration of BMS-345541, when initiated in parallel with collagen immunization, reduced arthritic severity and the expression of IL-1β in the joint (McIntyre et al., 2003). When tissue sections from the joints of these rodents were examined histologically, the efficacy of IKKβ inhibitors in this setting was illustrated with a significant reduction in inflammation and cartilage destruction. In the CIA model, TPCA1 has also reduced disease progression in a similar manner to BMS-345541. This inhibitor delayed both onsets and reduced the severity of disease, with an associated decrease in NF-κB p65 nuclear localization and DNA binding. Additionally, administration of TPCA1 resulted in a reduction in the expression levels of NF-κB-regulated cytokines, highlighting the role of IKKβ in NF-κB activation (Podolin et al., 2005). In a related study, oral administration of ML120B to rats in an adjuvant-induced arthritis model resulted in significantly reduced inflammation and bone destruction (Schopf et al., 2006). The use of peptide disruptors of the IKKβ–NEMO interaction have also been an alternative treatment for arthritis (see Section Alternative strategies to target the IKK complex: protein–protein interactions and their disruption & Table 2). These peptides resulted in a reduction in the inflammation of the arthritic joints of rodents, observed as reduced local bone erosion, and the blockade of osteoclastogenesis (Dai et al., 2004; Jimi et al., 2004).
Current therapeutic approaches to RA have been influenced by the intractable nature of the disease. This has necessitated the use of a wide variety of drugs including non-steroidal anti-inflammatory drugs (NSAIDs), steroids, disease-modifying anti-rheumatic drugs (DMARDs) and biologics. While no IKKβ inhibitors have thus far reached the clinic, many of the currently used pharmacological interventions may ascribe their actions at least in part to IKK inhibition. Kopp and colleagues discovered that the cyclo-oxygenase (COX) inhibitors aspirin and certain salicylates blocked NF-κB activation by inhibiting IKKβ activity (Kopp and Ghosh, 1994). These drugs were shown to function by blocking the ATP binding site (Yin et al., 1998). Further studies examining sulindac, ibuprofen and flurbiprofen demonstrated that they display anti-inflammatory and anti-proliferative effects independently of COX activity and prostaglandin E (PGE) synthesis and, at high doses, inhibit NF-κB by decreasing IKKβ kinase activity (Tegeder et al., 2001). The DMARD sulphasalazine, has been shown to inhibit NF-κB activation induced by TNF-α, LPS or phorbol myristoyl acetate (PMA) (Wahl et al., 1998). This inhibition was associated with suppression of IκBα degradation and phosphorylation, suggesting inhibition of IKKβ. The NSAID mesalamine, which is an aminosalycilate derivative, displayed anti-inflammatory properties and prevented IL-1-mediated stimulation of p65 phosphorylation without inhibiting IκBα degradation (Egan et al., 1999). Steroids, while not inhibiting IKK directly, act at least in part to increase expression of IκBα, thereby retaining NF-κB in the cytoplasm (Auphan et al., 1995; De Bosscher et al., 1997; Thiele et al., 1999). Furthermore, in the murine CIA model, it was found that while the ‘gold standard’ treatment for RA, methotrexate, was not effective after therapeutic administration, the IKKβ inhibitor BMS-345541 displayed similar anti-inflammatory efficacy to glucocorticoids (McIntyre et al., 2003), suggesting that specific IKKβ inhibitors may be a useful alternative to currently used therapeutics.
Intestinal inflammation
Several studies have also described excessive or inappropriate NF-κB activation in intestinal inflammation, in both Crohn's disease and ulcerative colitis and in animal models of IBD (Neurath et al., 1996; Rogler et al., 1998; Schreiber et al., 1998). Both colonic mucosal biopsies and lamina propria mononuclear cells from patients with IBD have been shown to exhibit increased levels of p65 in the nucleus as compared to healthy controls (Ellis et al., 1998; Schreiber et al., 1998). Initial studies using animal models demonstrated that administration of antisense oligonucleotides directed against p65 were able to abrogate established trinitrobenzene sulphonic acid (TNBS)-induced colitis in mice (Neurath et al., 1996). This was further confirmed by studies using curcumin to inhibit NF-κB in various mouse models of colitis (Jobin et al., 1999; Sugimoto et al., 2002). At the level of IKKβ, treatment of mice with BMS-345541, in a dextran sulphate sodium (DSS)-induced animal model of colitis, countered weight loss and changes in tissue characteristics of the colon, particularly thickening and shortening (MacMaster et al., 2003), which may be representative of IBD-related clinical outcomes. Additionally, an inhibitory peptide directed against the NEMO-binding domain (NBD) of IKKβ was shown to reduce inflammation in TNBS- and DSS-induced colitis and in spontaneous colitis developed in IL-10 deficient mice (Dave et al., 2007; Shibata et al., 2007).
In the clinical setting, targeting intestinal inflammation has generally taken the same approach as that described for arthritis, with the use of DMARDs and steroids. In an intestinal epithelial cell model, sufasalazine and lefunomide have been shown to inhibit NF-κB activation by preventing the phosphorylation of IκBα (Wahl et al., 1998), and by blocking functionally important post-translational modifications of the p65 subunit (Egan et al., 1999), suggesting the potential for inhibition of IKK to be a feature of their mode of action. Steroids have also been proven to be highly effective in the treatment of active IBD, an effect presumed to be mediated, at least in part, by preventing migration of activated NF-κB into the cell nucleus and subsequent binding to DNA (Auphan et al., 1995; Scheinman et al., 1995). Other treatments for IBD (and arthritis) have centred on the use of anti-cytokine biological agents such as infliximab (chimeric anti-human TNF-α monoclonal antibody), adalimumab (recombinant anti-human TNF-α monoclonal antibody) and anakinra (recombinant form of human IL-1 receptor antagonist), which target cytokines whose expression is regulated by NF-κB but which can also themselves activate the NF-κB pathway (Wahl et al., 1998; Podolsky, 2002). Thus, taken together, these studies would suggest that IKK inhibitors could be excellent new therapies for the treatment of both arthritis and IBD, equally as efficacious as current therapeutics (McIntyre et al., 2003; Podolin et al., 2005), without the damaging side effects associated with some of these compounds (Auphan et al., 1995; Scheinman et al., 1995), more specific in their mode of action than the DMARDs and less likely to encounter resistance in some patient cohorts. However, there are potential problems with cellular and systemic toxicity which need to be addressed. In targeting IKKβ particularly, there may be the potential for significant cell death/apoptosis of normal epithelial and cardiac cells respectively (Mustapha et al., 2000; Wullaert et al., 2011). Effective treatment of inflammatory disorders will require maintaining a delicate balance between suppressing inflammation and interfering with normal cellular functions. Treatments aimed at inhibiting components of the NF-κB pathway in a tissue- or cell-specific manner may have better therapeutic efficacy and reduce systemic toxicity.
Cancer
The early observation that NF-κB could be activated by cellular oncoproteins such as Ras, as well as by a number of viral oncoproteins, suggested that the NF-κB cascade could contribute to the cellular transformation that underpins tumour development (Gilmore, 1999; Valentine et al., 2010). Indeed, a large body of work has identified two features of cellular IKK activation that relates to a potential role in cancer; expression of NF-κB-dependent cell survival genes which play an important anti-apoptotic role and, secondly, a NF-κB-independent role in cell cycle progression. More recently, an additional aspect has emerged, with relevance to NF-κB; a chronic inflammatory state may be of significance in increasing the propensity for tumourigenesis in vivo (Tysnes, 2010). This feature is relevant in colorectal, pancreatic and lung cancers (Charalambous et al., 2003; Katoh, 2007; Yang et al., 2007).
Outwith the context of chronic inflammatory state, hyperactivation of the NF-κB pathway has been reported in multiple tumour cell lines and tissue samples. For example, nuclear p65 phosphorylation has been shown to contribute to the malignant phenotype of head and neck cancer, regulating epithelial to mesenchymal transition (Arun et al., 2009) and leading to less favourable clinical outcomes (Chung et al., 2006). Similarly, nuclear NF-κB in histological sections of prostate adenocarciomas was found to be a prognostic marker for disease relapse (Domingo-Domenech et al., 2005). NF-κB-associated genes are also over-expressed in basal breast cancer cells, a highly proliferative hormone-insensitive cell type associated with poor disease prognosis (Bertucci et al., 2009). In tissue samples from patients with pancreatic cancer, components of the non-canonical pathway, p52 and RelB, have been reported to co-localize in the nuclear compartment. Associated cellular studies have suggested that this may be a consequence of upstream NIK stabilization which leads to constitutive p100 phosphorylation (Wharry et al., 2009).
The constitutive activation of the IKKs themselves have also been observed in various tumour-derived cell lines (Romieu-Mourez et al., 2001; Gasparian et al., 2002; Charalambous et al., 2003; Olsen et al., 2004). To date, however, there is very little evidence to indicate that directly inheritable or somatic mutations in IKK genes underpin their overactivity. In multiple myeloma, mutations in genes encoding regulatory components of the non-canonical cascade, but not the IKKs, have been identified (Keats et al., 2007), indicating that the activity of the IKKs is enhanced indirectly (Romieu-Mourez et al., 2001). Irrespective of this fact, increased cellular IKKs, in particular IKKβ, seem to be a key to enhanced survival. The IKKβ/NF-κB axis encodes numerous survival genes such as superoxide dismutase (SOD), which prevents the formation of damaging agents such as hydrogen peroxide, which in turn limits the activities of pro-apoptotic pathways such as c-Jun N-terminal kinase (JNK). NF-κB also regulates the expression of pro-survival genes such as inhibitors of apoptosis (IAPs) and B-cell lymphoma-extra large (Bcl-xL) (Pahl, 1999). Indeed, in numerous studies, expression of dominant-negative IKKβ or treatment with IKKβ inhibitors promotes death per se, enhances cell death in response to agents such as TNF-α or increases the sensitivity of cancer cells to apoptosis inducing cancer drugs (Romieu-Mourez et al., 2001). This model of NF-κB-mediated cellular survival underpins the immediate potential for therapeutic targeting of the IKKβ/NF-κB pathway in this disease (see succeeding discussion) (Escarcega et al., 2007; Lee and Hung, 2008).
More recently, it has emerged that independent of NF-κB, the IKKs may regulate cell cycle progression directly. IKKα has been shown to phosphorylate the mitotic kinase Aurora A on threonine 288 which is a key site for kinase activity (Prajapati et al., 2006). Knockdown of IKKα by siRNA impairs cell cycle progression in HeLa cells, and IKKα has been shown to regulate the M-phase of the cell cycle (Prajapati et al., 2006). Chk1 has been shown to associate with and phosphorylate IKKα during S-phase in human osteosarcoma U2OS cells, inhibiting the ability of IKKα to phosphorylate p100 (Barre and Perkins, 2007). In MCF7 cells, IKKα has been shown to regulate S-phase following oestrogen stimulation (Tu et al., 2006), further outlining the role of IKKα in the checkpoint control machinery of cell cycle progression. Recent studies have indicated that IKKα is a part of the TGFβ-Smad2/3 signalling pathway exerting control over the cell cycle in keratinocyte differentiation (Descargues et al., 2008). Since it is well recognized that Smad2/3-mediated cell cycle control is integrated with p53 function, it would not be surprising to suggest that p53 phosphorylation be mediated by IKKα. Experimental evidence however suggests that it is in fact IKKβ that phosphorylates p53 at serines 362 and 366 which leads to p53 ubiquitination and degradation by β-transducin repeat-containing protein (βTrCP) in a murine double minute 2 (Mdm2)-independent manner. This suggests that inhibition/blocking of IKKβ and/or βTrCP could result in the stabilization of p53 and enable it to retain its tumour suppressor function (Xia et al., 2009). The involvement of the individual IKKs in the cell cycle suggests that selective inhibition of these kinases may be effective in halting cell division, and therefore, in part, tumour progression.
Despite having a role in cellular survival, more recent evidence implicates the IKKs in the chronic inflammation associated with cancer development. In a transgenic adenocarcinoma of mouse prostate (TRAMP) model of prostate cancer (Gingrich et al., 1996), mice expressing an inactive mutant form of IKKα (IKKαAA/AA/TRAMP) developed fewer distant-site metastases in areas such as the liver, lung, pelvic or renal lymph nodes (Luo et al., 2007) and also displayed a delayed onset of cancer of the prostate in comparison to the WT/TRAMP mice, and a corresponding increase in survival. In this instance, tumour progression was associated with an IKKα-mediated inflammatory response involving infiltration of lymphocytes which promoted metastasis and secondary tumours (Luo et al., 2007). This pro-inflammatory input for IKKα in cancer has been further supported by experiments which demonstrated that androgen ablation caused infiltration of regressing androgen-dependent tumours in which IKK was driving the production of inflammatory cytokines to enhance hormone-free tumour survival (Ammirante et al., 2010). Independent studies observing over-expression of p65, as a function of IKKβ, suggest that this isoform plays a role in the early stages of prostate cancer, an effect likely to be linked to cell survival (Sweeney et al., 2004). Therefore, IKKα inhibition may represent a strategy to combat late-stage prostate cancer at least in part by regulating the inflammatory environment, while targeting IKKβ may be more relevant to early stages of the disease. Other studies are required to confirm if similar complimentary roles apply to different types of cancer. For example, while IKKβ is thought to play a role in melanoma, IKKα is proposed to protect cells against the development of UV-B-induced skin cancer (Luo et al., 2007; Descargues et al., 2008; Bettermann et al., 2010).
To date, IKK inhibitors have not been used therapeutically in the clinic. Nevertheless, the functional roles of IKKs in cell survival and cell cycle progression make drugs of this type promising anticancer therapies, particularly for use in combination with chemo- and radiotherapeutics. It is well documented that UV and ionizing radiation, used commonly in cancer therapy, activate the NF-κB pathway (Li and Karin, 1998) and, as for a number of genotoxic stresses, is induced via an IKKβ-dependent mechanism (Janssens and Tschopp, 2006). This relies on the nuclear translocation of NEMO scaffolding protein, its sumoylation and subsequent phosphorylation by the checkpoint kinase ataxia telangiectasia mutated (ATM) (Wu et al., 2006). With de-sumoylation and ubiquitylation of NEMO, nuclear export of a NEMO-ATM complex results in activation of the IKK complex, principally IKKβ (Wu et al., 2006). Whether this mechanism of regulation is activated in response to all forms of radiation in all cell types remains unclear. Furthermore, whether NF-κB activation in response to UV and ionizing radiation is predominantly IKKβ dependent, to the exclusion of IKKα-mediated regulation, is as yet unexplored, although the non-canonical pathway has been implicated in mediating survival of endometrial carcinoma cells post-treatment with ionizing radiation (Wu et al., 2011).
Therefore, to gain further benefit from these approaches, compromised IKK/NF-κB signalling may be required to offset stress-induced survival mechanisms involving NF-κB. This approach could also be extended to chemotherapeutics, where NF-κB has been associated with chemoresistance. Many drug-resistant cancer cell lines have been shown to have high levels of NF-κB-DNA binding activity, and inhibition of NF-κB has been shown to improve the efficacy of some current chemotherapeutics. For example, the targeted inhibition of NF-κB in combination with the chemotherapy drug doxorubicin was shown to enhance the level of apoptosis (Bednarski et al., 2009; Guo et al., 2010). Over-expression of p50 and p65 has been shown in MCF7 breast cancer cells, which correlated directly with resistance to 2',2'-difluorodeoxycytidine/gemcitabine, a pyrimidine analogue also known as gemcitabine (Guo et al., 2010). Targeted inhibition of the IKKs, as regulators of p65, may therefore offer a therapeutic advantage when used in combination with gemcitabine in this setting. In salivary gland cancer cells, 5-fluorouracil (5-FU) (also a pyrimidine analogue) induces apoptosis through suppression of NF-κB and inhibition of IKK (Azuma et al., 2001). However, in colorectal cancer (CRC) cells, NF-κB activity is reported to increase following 5-Fu treatment. The authors went further to demonstrate that this was mediated through activation of IKK in RKO cells and specifically required IKKβ (Fukuyama et al., 2007). In turn, disulfiram (DS)-mediated inhibition of NF-κB in the CRC setting enhanced the cytotoxic effects of 5-Fu. This evidence may lend weight to the notion of IKK/NF-κB inhibition as a means to decrease the expression of pro-survival genes which may help to combat chemoresistance. Therefore, collectively, the clinical treatments for cancer described above and their modes of action in cellular settings have further illustrated the potential of NF-κB to be one of a number of cellular targets for anti-cancer therapies.
A number of synthetic compounds and natural products that target NF-κB, distinct from the IKKs, have been assessed for their anti-cancer potential at the cellular level and are presently in clinical trials (Sethi et al., 2009). Some have progressed to the clinic; one of these, the proteosomal inhibitor Bortezomib (Velcade), has been found to be effective in patients presenting with head and neck squamous cell carcinoma (HNSCC) (Chen et al., 2008), multiple myeloma (Field-Smith et al., 2006) and mantle cell lymphoma (Alinari et al., 2009), acting at least in part by inhibiting NF-κB translocation/activation (Chiao et al., 2002; Singh et al., 2007). Indeed, the promising data emerging on the use of NF-κB inhibitors to combat chemoresistance (Syrovets et al., 2005; Tapia et al., 2007; Schôn et al., 2008; Gao et al., 2010) point to a therapeutic direction in which the addition of IKK inhibition may improve current treatment strategies. Similar approaches could be applied to the use of radiopharmaceuticals and existing radiotherapeutics. This awaits experimental outcomes from current ongoing studies.
Alternative strategies to target the IKK complex: protein–protein interactions and their disruption
The apparent problems associated with directly inhibiting IKK activity, in particular IKKβ, suggest that targeting protein–protein interactions may be an alternative strategy for pharmacological intervention. This strategy relies upon identifying defined areas, regions or domains of proteins that are critical for the regulation and/or (de)activation of proposed target proteins. The site of interaction between IKKα/β and NEMO, which exists as a hydrophobic pocket, contains a central conserved hexapeptide sequence, LDWSWL, that is termed the NEMO-binding domain (NBD). This domain was initially predicted by means of hydropathy plots (May et al., 2000) but was more recently detailed in an IKKα/β peptide-truncated NEMO co-crystal structure (Rushe et al., 2008). Experiments have been conducted with peptides derived from the surrounding primary amino acid sequence (11- or 12-mers), fused to membrane transduction sequences. These peptides were observed to inhibit the NF-κB pathway across a wide range of systems (Table 2), presumably by disruption of the IKKα/β-NEMO interaction.
Studies in vitro using cell-permeable peptides have also shown effective inhibition of NF-κB signalling. For example, treating human monocyte-derived dendritic cells with the NBD peptide arrested the cells in an immature state despite stimulation with LPS (Tas et al., 2005). Similarly, use of the NBD peptide in human melanoma cultures inhibited NF-κB-DNA binding and also induced apoptosis via the activation of caspase 3 (Ianaro et al., 2009). Most recently, two separate studies have shown that inhibition of the IKK complex by the NBD peptides in vivo (see Table 2) is an effective approach to the treatment of inflammatory diseases in which bone resorption plays a substantial pathological role (Dai et al., 2004; Jimi et al., 2004). The NBD peptide only blocks the induction of NF-κB activity in response to pro-inflammatory stimuli and does not inhibit basal activity. This helps limit possible side effects, for example, undesired apoptosis (Kucharczak et al., 2003). The use of peptide-based disruptors of protein–protein interactions is not confined to IKKα/β-NEMO interactions. Other examples of the use of peptides for the inhibition of the NF-κB pathway include a cell-permeable peptide targeted to the NLS of p50, which was found to inhibit LPS-stimulated nuclear translocation of NF-κB complexes (Lin et al., 1995). There was some evidence to suggest that this peptide also inhibited the nuclear translocation of other transcription factors, so a similar peptide targeting the RelB:p52 dimer (which did not have similar off-target effects) may be a better alternative (Torgerson et al., 1998; Xu et al., 2008). Interestingly, peptides targeting the IKK-binding domain of NEMO have thus far been proven to have little effect on NF-κB activity (Marienfeld et al., 2006). However, peptides designed to block NEMO oligomerization have been shown to inhibit NF-κB-dependent gene expression and increase apoptosis in a number of studies (Agou et al., 2004; Carvalho et al., 2007; Wyler et al., 2007).
As shown above, cell-permeable peptides targeting the IKK complex can efficiently inhibit NF-κB signalling, although there are several challenges. Other chemical entities that inhibit the IKKα/β-NBD-NEMO interactions in vitro have also recently been identified (Gotoh et al., 2010). The chemical structure of these ‘disruptors’ remains undisclosed, and as such, it is unknown whether these ‘compounds’ represent low-molecular-weight entities or are peptide-based. The progression of any peptide-based disruptors of protein–protein interactions into drug-like molecules brings the significant challenges of ‘in-building’ the appropriate pharmacology and desired drug-like characteristics. This will likely require the development of peptidomimetics into small drug-like molecules that work as efficiently, if not better, than the original peptide. One technique that is proving to be a popular tool in the development of this approach is virtual screening of proteins. For example, the structural determination of human Mdm2 bound to a 15 residue peptide of p53 has led to the development of a number of small non-peptidic inhibitors (Shangary and Wang, 2009). Structure-based drug design has also been used to improve the druggability of a small molecule directed at the interaction between B-cell leukaemia 2 (Bcl2)/Bcl-xl (van Montfort and Workman, 2009). A similar strategy could therefore be applied to the interactions between the IKKs within the IKKs complex, relevant to both the canonical and non-canonical axes.
Summary and future perspectives
Within the NF-κB field and in the study of the IKKs, there remain the key challenges of understanding fully the functional roles of the individual kinase isoforms. This has in part driven the quest for IKK-selective inhibitors and has been based primarily on the development of ATP-competitive agents that are more easily identified in HTS. Unfortunately, ATP mimetics have a number of limitations (Garber, 2006). Despite being of low molecular weight, being orally bioavailable and able to inhibit target proteins, they can still ‘hit’ other kinases to generate ‘off-target’/side effects (Garber, 2006). In the cancer setting, for example, it has also been observed that the strategy of using ATP-competitive inhibitors may be flawed as tumours, and kinases within them, develop mutations in the ATP-binding pocket that interferes with drug binding, negates its effects and leads to resistance; whether this is relevant to the IKKs across a number of pathophysiological settings remains to be determined. To avoid these issues, alternative strategies to targeting aberrant kinase activity are now emerging as the kinase field embarks on identifying agents that bind to, and inhibit, kinases in novel ways. Substrate-competitive inhibitors particularly are now being developed to this end (e.g. Bogoyevitch and Arthur, 2008; Licht-Murava et al., 2011) and are providing promising leads. In time, this approach will likely be applied to the IKKs.
So, to date, despite the limitations of ATP-competitive molecules, significant advances have been made in developing inhibitors of the IKKs, particularly those that target and inhibit the intrinsic catalytic activity of IKKβ (see Section The development of novel small molecule inhibitors of the IKKs). This has been achieved again through the pursuit of HTS; however, these strategies have not delivered parallel inhibitors of IKKα. The synthesis and optimization of highly selective inhibitors of IKKα remains one of the key challenges in developing a fuller understanding of the functional role(s) of IKKα in both physiological and pathophysiological settings.
The further development of IKKα selective inhibitors (ATP competitive or otherwise), NBD-based novel IKK complex ‘disruptors’ and the refinement and reappraisal of existing IKKβ inhibitors will undoubtedly be supported by the availability of solved crystal structures for the IKK catalytic subunits. Significantly, Xu et al. (2011) have very recently reported the solved crystal structure for Xenopus-IKKβ in complex with an inhibitor, at a resolution of 3.6 Å, to identify a trimodular architecture. It may be that further successful crystallization of human IKKα and/or IKKβ requires association with similar inhibitor molecules, with IKKγ/NEMO or other interacting proteins, for example, heat-shock proteins (Hsps) (Chen et al., 2002; Broemer et al., 2004; Mohan et al., 2009), connection to IKK and SAPK (CIKS) (Mauro et al., 2003), IKK interacting protein (IKIP) (Hofer-Warbinek et al., 2004), A20 (Zhang et al., 2000; Zetoune et al., 2001) and A20-binding protein (ABIN) (Mauro et al., 2006). Further IKK-focussed proteomic experiments will likely inform on the status of differing IKK complexes in cells. The complexity of these interactions may initially limit the progress of drug design, but in the long run could give rise to the opportunity for selectivity in the disruption of distinct interactions within the different protein complexes, for example, IKKα/Aurora A or IKKβ/ABIN, which lead to different functional outcomes.
Since their discovery in the late 1990s, the IKKs remain a potentially useful therapeutic strategy for the treatment of numerous conditions. Not only in RA, IBD and cancer but also in chronic obstructive pulmonary disease (COPD) (Shuto et al., 2001), asthma (Yang et al., 1998), ischaemia reperfusion (Herrmann, 2005), atherosclerosis (Saxena et al., 1994; Reddy et al., 2002) diabetes (Arkan et al., 2005) and transplant rejection (Townsend et al., 2004). Further development of IKK inhibitors or disruptors of protein–protein interactions could make an important contribution to the future alleviation of such diseases and conditions.
Acknowledgments
The authors would like to thank Dr Oliver Sutcliffe for assistance in preparation of diagrams depicting chemical structures. Work in R. P.'s laboratory is supported by the Biotechnology and Biological Sciences Research Council (BBSRC), British Heart Foundation (BHF), Cancer Research UK (CRUK), Scottish Universities Life Science Alliance (SULSA), the Wellcome Trust and the University of Strathclyde. Work in A. P.'s laboratory is supported by the BHF, BBSRC, CRUK, SULSA and the University of Strathclyde.
Glossary
- ABIN
A20-binding protein
- ADP
adenosine diphosphate
- ATM
ataxia telangiectasia mutated
- ATP
adenosine triphosphate
- βTrCP
β-transducin repeat-containing protein
- Bcl2
B-cell leukaemia 2
- Bcl-xL
B-cell lymphoma-extra large
- CIA
collagen-induced arthritis
- CIKS
connection to IKK and SAPK
- COPD
chronic obstructive pulmonary disease
- COX
cyclo-oxygenase
- DMARD
disease-modifying anti-rheumatic drugs
- DS
disulfiram
- DSS
dextran sulphate sodium
- HNSCC
head and neck squamous cell carcinoma
- Hsps
heat-shock proteins
- HTS
high-throughput screening
- IAP
inhibitor of apoptosis
- IBD
inflammatory bowel disease
- IgG
immunoglobulin G
- IKIP
IKK interacting protein
- IKK
inhibitory kappa B kinase
- IκB
inhibitory of kappa B
- IL-1
interleukin-1
- IRFs
interferon regulatory factors
- JNK
c-Jun N-terminal kinase
- KO
knockout
- LPS
lipopolysaccharide
- MCP
monocyte chemoattractant protein
- Mdm2
murine double minute 2
- MEFs
murine embryonic fibroblasts
- MEKK
MAP or ERK kinase kinase
- MMP
matrix metalloproteinases
- NAK
NF-κB-activating kinase
- NBD
NEMO-binding domain
- NEMO
NF-κB essential modulator
- NIK
NF-κB-inducing kinase
- NF-κB
nuclear factor kappa B
- NLS
nuclear localization sequence
- NSAIDs
non-steroidal anti-inflammatory drugs
- PGE
prostaglandin E
- PMA
phorbol myristoyl acetate
- RA
rheumatoid arthritis
- RANTES
regulated upon activation, normal T cell expressed and secreted
- SAR
structure–activity relationship
- SMRT
silencing mediator for retinoic acid and thyroid hormone receptor
- SOD
superoxide dismutase
- TNBS
trinitrobenzene sulphonic acid
- TGF-β
transforming growth factor-β
- TRAFs
TNF receptor-associated factors
- TBK1
TANK-binding kinase 1
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor-α
- TRAMP
transgenic adenocarcinoma of mouse prostate
- UV
ultraviolet
Conflict of interest
The authors have no conflicts of interest with respect to the manuscript and its potential publication.
References
- Adli M, Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell-associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281:26976–26984. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, et al. The AKT/I[kappa]B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-[kappa]B and [beta]-catenin. Oncogene. 2004;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Agou F, Courtois G, Chiaravalli J, Baleux F, Coic YM, Traincard F, et al. Inhibition of NF-kappa B activation by peptides targeting NF-kappa B essential modulator (nemo) oligomerization. J Biol Chem. 2004;279:54248–54257. doi: 10.1074/jbc.M406423200. [DOI] [PubMed] [Google Scholar]
- Alinari L, White VL, Earl CT, Ryan TP, Johnston JS, Dalton JT, et al. Combination bortezomib and rituximab treatment affects multiple survival and death pathways to promote apoptosis in mantle cell lymphoma. MAbs. 2009;1:31–40. doi: 10.4161/mabs.1.1.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreakos E, Smith C, Kiriakidis S, Monaco C, de Martin R, Brennan FM, et al. Heterogeneous requirement of IkappaB kinase 2 for inflammatory cytokine and matrix metalloproteinase production in rheumatoid arthritis: implications for therapy. Arthritis Rheum. 2003;48:1901–1912. doi: 10.1002/art.11044. [DOI] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for I[kappa]B kinase-[alpha] in NF-[kappa]B-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C. Nuclear NF-κB p65 phosphorylation at serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin Cancer Res. 2009;15:5974–5984. doi: 10.1158/1078-0432.CCR-09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamitsu K, Yamaguchi T, Nakata K, Hibi Y, Victoriano A-FB, Imai K, et al. Inhibition of human immunodeficiency virus type 1 replication by blocking IkB kinase with noraristeromycin. J Biochem. 2008;144:581–589. doi: 10.1093/jb/mvn104. [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Azuma M, Yamashita T, Aota K, Tamatani T, Sato M. 5-Fluorouracil suppression of NF-[kappa]B is mediated by the inhibition of I[kappa]B kinase activity in human salivary gland cancer cells. Biochem Biophys Res Commun. 2001;282:292–296. doi: 10.1006/bbrc.2001.4571. [DOI] [PubMed] [Google Scholar]
- Bannai M, Tokunaga K, Imanishi T, Harihara S, Fujisawa K, Juji T, et al. HLA class II alleles in Ainu living in Hidaka District, Hokkaido, northern Japan. Am J Phys Anthropol. 1996;101:1–9. doi: 10.1002/(SICI)1096-8644(199609)101:1<1::AID-AJPA1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Barre B, Perkins ND. A cell cycle regulatory network controlling NF-kappaB subunit activity and function. EMBO J. 2007;26:4841–4855. doi: 10.1038/sj.emboj.7601899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baxter A, Brough S, Cooper A, Floettmann E, Foster S, Harding C, et al. Hit-to-lead studies: the discovery of potent, orally active, thiophenecarboxamide IKK-2 inhibitors. Bioorg Med Chem Lett. 2004;14:2817–2822. doi: 10.1016/j.bmcl.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Beaulieu F, Ouellet C, Ruediger EH, Belema M, Qiu Y, Yang X, et al. Synthesis and biological evaluation of 4-amino derivatives of benzimidazoquinoxaline, benzimidazoquinoline, and benzopyrazoloquinazoline as potent IKK inhibitors. Bioorg Med Chem Lett. 2007;17:1233–1237. doi: 10.1016/j.bmcl.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Bednarski BK, Baldwin AS, Jr, Kim HJ. Addressing reported pro-apoptotic functions of NF-kB: targeted inhibition of canonical NF-kB enhances the apoptotic effects of doxorubicin. PLoS ONE. 2009;4:e6992. doi: 10.1371/journal.pone.0006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito MJ, Murphy E, Murphy EP, van den Berg WB, FitzGerald O, Bresnihan B. Increased synovial tissue NF-kappa B1 expression at sites adjacent to the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 2004;50:1781–1787. doi: 10.1002/art.20260. [DOI] [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Cervera N, Charafe-Jauffret E, Buttarelli M, Jacquemier J, et al. How different are luminal A and basal breast cancers? Int J Cancer. 2009;124:1338–1348. doi: 10.1002/ijc.24055. [DOI] [PubMed] [Google Scholar]
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, et al. TAK1 suppresses a NEMO-dependent but NF-[kappa]B-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch M, Arthur PG. Inhibitors of c-Jun N-terminal kinases – JuNK no more? Biochim Biophys Acta. 2008;1784:76–93. doi: 10.1016/j.bbapap.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafoux D, Bonar S, Christine L, Clare M, Donnelly A, Guzova J, et al. Inhibition of IKK-2 by 2-[(aminocarbonyl)amino]-5-acetylenyl-3-thiophenecarboxamides. Bioorg Med Chem Lett. 2005;15:2870–2875. doi: 10.1016/j.bmcl.2005.03.090. [DOI] [PubMed] [Google Scholar]
- Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J Pharm Pharmacol. 2002;54:453–472. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for I[kappa]B kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-[kappa]B activation. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- Buss H, Dȏrrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-kB at serine 536 is mediated by multiple protein kinases including IkB kinase (IKK)-α, IKKβ, IKKε, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- Campbell IK, Gerondakis S, O'Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000;105:1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, et al. IKK[alpha] provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- Carlsen H, Moskaug JO, Fromm SH, Blomhoff R. In vivo imaging of NF-kappa B activity. J Immunol. 2002;168:1441–1446. doi: 10.4049/jimmunol.168.3.1441. [DOI] [PubMed] [Google Scholar]
- Carvalho G, Fabre C, Braun T, Grosjean J, Ades L, Agou F, et al. Inhibition of NEMO, the regulatory subunit of the IKK complex, induces apoptosis in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007;26:2299–2307. doi: 10.1038/sj.onc.1210043. [DOI] [PubMed] [Google Scholar]
- Castro AC, Dang LC, Soucy F, Grenier L, Mazdiyasni H, Hottelet M, et al. Novel IKK inhibitors: beta-carbolines. Bioorg Med Chem Lett. 2003;13:2419–2422. doi: 10.1016/s0960-894x(03)00408-6. [DOI] [PubMed] [Google Scholar]
- Catley MC, Sukkar MB, Chung KF, Jaffee B, Liao SM, Coyle AJ, et al. Validation of the anti-inflammatory properties of small-molecule IkappaB kinase (IKK)-2 inhibitors by comparison with adenoviral-mediated delivery of dominant-negative IKK1 and IKK2 in human airways smooth muscle. Mol Pharmacol. 2006;70:697–705. doi: 10.1124/mol.106.023150. [DOI] [PubMed] [Google Scholar]
- Charalambous MP, Maihofner C, Bhambra U, Lightfoot T, Gooderham NJ. Upregulation of cyclooxygenase-2 is accompanied by increased expression of nuclear factor-kappa B and I kappa B kinase-alpha in human colorectal cancer epithelial cells. Br J Cancer. 2003;88:1598–1604. doi: 10.1038/sj.bjc.6600927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau T-L, Gioia R, Gatot J-S, Patrascu F, Carpentier I, Chapelle J-P, et al. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem Sci. 2008;33:171–180. doi: 10.1016/j.tibs.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of I[kappa]B[alpha] by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ricker JL, Malhotra PS, Nottingham L, Bagain L, Lee TL, et al. Differential bortezomib sensitivity in head and neck cancer lines corresponds to proteasome, nuclear factor-kappaB and activator protein-1 related mechanisms. Mol Cancer Ther. 2008;7:1949–1960. doi: 10.1158/1535-7163.MCT-07-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao PJ, Na R, Niu J, Sclabas GM, Dong Q, Curley SA. Role of Rel/NF-κB transcription factors in apoptosis of human hepatocellular carcinoma cells. Cancer. 2002;95:1696–1705. doi: 10.1002/cncr.10829. [DOI] [PubMed] [Google Scholar]
- Christopher JA, Avitabile BG, Bamborough P, Champigny AC, Cutler GJ, Dyos SL, et al. The discovery of 2-amino-3, 5-diarylbenzamide inhibitors of IKK-alpha and IKK-beta kinases. Bioorg Med Chem Lett. 2007;17:3972–3977. doi: 10.1016/j.bmcl.2007.04.088. [DOI] [PubMed] [Google Scholar]
- Christopher JA, Bamborough P, Alder C, Campbell A, Cutler GJ, Down K, et al. Discovery of 6-aryl-7-alkoxyisoquinoline inhibitors of IkappaB kinase-beta (IKK-beta) J Med Chem. 2009;52:3098–3102. doi: 10.1021/jm9000117. [DOI] [PubMed] [Google Scholar]
- Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-{kappa}B signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66:8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- Czabanka M, Korherr C, Brinkmann U, Vajkoczy P. Influence of TBK-1 on tumor angiogenesis and microvascular inflammation. Front Biosci. 2008;13:7243–7249. doi: 10.2741/3225. [DOI] [PubMed] [Google Scholar]
- Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- Danning CL, Illei GG, Hitchon C, Greer MR, Boumpas DT, McInnes IB. Macrophage-derived cytokine and nuclear factor kappaB p65 expression in synovial membrane and skin of patients with psoriatic arthritis. Arthritis Rheum. 2000;43:1244–1256. doi: 10.1002/1529-0131(200006)43:6<1244::AID-ANR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- Dave SH, Tilstra JS, Matsuoka K, Li F, Karrasch T, Uno JK, et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J Immunol. 2007;179:7852–7859. doi: 10.4049/jimmunol.179.11.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Schmitz ML, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, et al. The lymphotoxin-[beta] receptor induces different patterns of gene expression via two NF-[kappa]B pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Karin M. IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer. EMBO J. 2008;27:2639–2647. doi: 10.1038/emboj.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, et al. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Domenech J, Mellado B, Ferrer B, Truan D, Codony-Servat J, Sauleda S, et al. Activation of nuclear factor-[kappa]B in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer. 2005;93:1285–1294. doi: 10.1038/sj.bjc.6602851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Landesman-Bollag E, Seldin DC, et al. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65:11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- Ellis RD, Goodlad JR, Limb GA, Powell JJ, Thompson RP, Punchard NA. Activation of nuclear factor kappa B in Crohn's disease. Inflamm Res. 1998;47:440–445. doi: 10.1007/s000110050358. [DOI] [PubMed] [Google Scholar]
- Escarcega RO, Fuentes-Alexandro S, Garcia-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol) 2007;19:154–161. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497–505. doi: 10.1016/s1072-7515(02)01222-x. [DOI] [PubMed] [Google Scholar]
- Field-Smith A, Morgan GJ, Davies FE. Bortezomib (Velcade trademark) in the treatment of multiple myeloma. Ther Clin Risk Manag. 2006;2:271–279. doi: 10.2147/tcrm.2006.2.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama R, Ng KP, Cicek M, Kelleher C, Niculaita R, Casey G, et al. Role of IKK and oscillatory NFκB kinetics in MMP-9 gene expression and chemoresistance to 5-fluorouracil in RKO colorectal cancer cells. Mol Carcinog. 2007;46:402–413. doi: 10.1002/mc.20288. [DOI] [PubMed] [Google Scholar]
- Gao Z, Zhang D, Guo C. Paclitaxel efficacy is increased by parthenolide via nuclear factor-kappaB pathways in in vitro and in vivo human non-small cell lung cancer models. Curr Cancer Drug Targets. 2010;10:705–715. doi: 10.2174/156800910793605776. [DOI] [PubMed] [Google Scholar]
- Garber K. The second wave in kinase cancer drugs. Nat Biotechnol. 2006;24:127–130. doi: 10.1038/nbt0206-127. [DOI] [PubMed] [Google Scholar]
- Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, et al. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115(Pt 1):141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–6937. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- Gomez AB, MacKenzie C, Paul A, Plevin R. Selective inhibition of inhibitory kappa B kinase-beta abrogates induction of nitric oxide synthase in lipopolysaccharide-stimulated rat aortic smooth muscle cells. Br J Pharmacol. 2005;146:217–225. doi: 10.1038/sj.bjp.0706308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Nagata H, Kase H, Shimonishi M, Ido M. A homogeneous time-resolved fluorescence-based high-throughput screening system for discovery of inhibitors of IKK[beta]-NEMO interaction. Anal Biochem. 2010;405:19–27. doi: 10.1016/j.ab.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Xu B, Pandey S, Goessl E, Brown J, Armesilla AL, et al. Disulfiram/copper complex inhibiting NF[kappa]B activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer Lett. 2010;290:104–113. doi: 10.1016/j.canlet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010a;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010b;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28:197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Maeda S, Nakagawa H, Hikiba Y, Shibata W, Sakamoto K, et al. Effectiveness of IκB kinase inhibitors in murine colitis-associated tumorigenesis. J Gastroenterol. 2009;44:935–943. doi: 10.1007/s00535-009-0098-7. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, et al. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11:1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hofer-Warbinek R, Schmid JA, Mayer H, Winsauer G, Orel L, Mueller B, et al. A highly conserved proapoptotic gene, IKIP, located next to the APAF1 gene locus, is regulated by p53. Cell Death Differ. 2004;11:1317–1325. doi: 10.1038/sj.cdd.4401502. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKK[alpha] controls formation of the epidermis independently of NF-[kappa]B. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Huynh QK, Boddupalli H, Rouw SA, Koboldt CM, Hall T, Sommers C, et al. Characterization of the recombinant IKK1/IKK2 heterodimer. J Biol Chem. 2000;275:25883–25891. doi: 10.1074/jbc.M000296200. [DOI] [PubMed] [Google Scholar]
- Ianaro A, Tersigni M, Belardo G, Di Martino S, Napolitano M, Palmieri G, et al. NEMO-binding domain peptide inhibits proliferation of human melanoma cells. Cancer Lett. 2009;274:331–336. doi: 10.1016/j.canlet.2008.09.038. [DOI] [PubMed] [Google Scholar]
- Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-κB response. Cell Death Differ. 2006;13:773–784. doi: 10.1038/sj.cdd.4401843. [DOI] [PubMed] [Google Scholar]
- Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- Kamon J, Yamauchi T, Muto S, Takekawa S, Ito Y, Hada Y, et al. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun. 2004;323:242–248. doi: 10.1016/j.bbrc.2004.08.083. [DOI] [PubMed] [Google Scholar]
- Katoh M. AP1- and NF-kappaB-binding sites conserved among mammalian WNT10B orthologs elucidate the TNFalpha-WNT10B signaling loop implicated in carcinogenesis and adipogenesis. Int J Mol Med. 2007;19:699–703. [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, et al. A selective IKK-2 inhibitor blocks NF-kB-dependent gene expression in interleukin-1β-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, et al. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2003;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, et al. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Korherr C, Gille H, Schäfer R, Koenig-Hoffmann K, Dixelius J, Egland KA, et al. Identification of proangiogenic genes and pathways by high-throughput functional genomics: TBK1 and the IRF3 pathway. Proc Natl Acad Sci USA. 2006;103:4240–4245. doi: 10.1073/pnas.0511319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer-role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKK[alpha] limits macrophage NF-[kappa]B activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14:5656–5662. doi: 10.1158/1078-0432.CCR-08-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, et al. The IKKβ subunit of IkB kinase (IKK) is essential for nuclear factor kB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lu Q, Bottero V, Estepa G, Morrison L, Mercurio F, et al. Enhanced NF-kB activation and cellular function in macrophages lacking IkB kinase 1 (IKK1) Proc Natl Acad Sci USA. 2005;102:12425–12430. doi: 10.1073/pnas.0505997102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht-Murava A, Plotkin B, Eisenstein M, Eldar-Finkelman H. Elucidating substrate and inhibitor binding sites on the surface of GSK-3β and the refinement of a competitive inhibitor. J Mol Biol. 2011;408:366–378. doi: 10.1016/j.jmb.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Liddle J, Bamborough P, Barker MD, Campos S, Cousins RP, Cutler GJ, et al. 4-Phenyl-7-azaindoles as potent and selective IKK2 inhibitors. Bioorg Med Chem Lett. 2009;19:2504–2508. doi: 10.1016/j.bmcl.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Ling L, Cao Z, Goeddel DV. NF-kB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YM, Chen K, Liu XJ, Xie WR, Wang H. Cell-permeable Tat-NBD peptide attenuates rat pancreatitis and acinus cell inflammation response. World J Gastroenterol. 2009;15:561–569. doi: 10.3748/wjg.15.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J-L, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- Luqman S, Pezzuto JM. NFκB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- MacMaster JF, Dambach DM, Lee DB, Berry KK, Qiu Y, Zusi FC, et al. An inhibitor of IkappaB kinase, BMS-345541, blocks endothelial cell adhesion molecule expression and reduces the severity of dextran sulfate sodium-induced colitis in mice. Inflamm Res. 2003;52:508–511. doi: 10.1007/s00011-003-1206-4. [DOI] [PubMed] [Google Scholar]
- Malek S, Chen Y, Huxford T, Ghosh G. IkBα, but not IkBβ, functions as a classical cytoplasmic inhibitor of NF-kB dimers by masking both NF-kB nuclear localization sequences in resting cells. J Biol Chem. 2001;276:45225–45235. doi: 10.1074/jbc.M105865200. [DOI] [PubMed] [Google Scholar]
- Marienfeld RB, Palkowitsch L, Ghosh S. Dimerization of the I kappa B kinase-binding domain of NEMO is required for tumor necrosis factor alpha-induced NF-kappa B activity. Mol Cell Biol. 2006;26:9209–9219. doi: 10.1128/MCB.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa PE, Li X, Hanidu A, Siamas J, Pariali M, Pareja J, et al. Gene expression profiling in conjunction with physiological rescues of IKKα-null cells with wild type or mutant IKKα reveals distinct classes of IKKα/NF-kB-dependent genes. J Biol Chem. 2005;280:14057–14069. doi: 10.1074/jbc.M414401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A, Kaisho T, Rennert PD, Nakano H, Kurosawa K, Uchida D, et al. Essential role of nuclear factor (NF)-kB-inducing kinase and inhibitor of kb (IkB) kinase α in NF-kB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro C, Vito P, Mellone S, Pacifico F, Chariot A, Formisano S, et al. Role of the adaptor protein CIKS in the activation of the IKK complex. Biochem Biophys Res Commun. 2003;309:84–90. doi: 10.1016/s0006-291x(03)01532-8. [DOI] [PubMed] [Google Scholar]
- Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, et al. ABIN-1 binds to NEMO/IKKγ and co-operates with A20 in inhibiting NF-kB. J Biol Chem. 2006;281:18482–18488. doi: 10.1074/jbc.M601502200. [DOI] [PubMed] [Google Scholar]
- May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappa B activation by a peptide that blocks the interaction of NEMO with the Ikappa B kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- May MJ, Marienfeld RB, Ghosh S. Characterization of the IkB-kinase NEMO binding domain. J Biol Chem. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- McIntyre KW, Shuster DJ, Gillooly KM, Dambach DM, Pattoli MA, Lu P, et al. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 2003;48:2652–2659. doi: 10.1002/art.11131. [DOI] [PubMed] [Google Scholar]
- di Meglio P, Ianaro A, Ghosh S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-kappaB activation. Arthritis Rheum. 2005;52:951–958. doi: 10.1002/art.20960. [DOI] [PubMed] [Google Scholar]
- Miller BS, Zandi E. Complete reconstitution of human IkB kinase (IKK) complex in yeast. J Biol Chem. 2001;276:36320–36326. doi: 10.1074/jbc.M104051200. [DOI] [PubMed] [Google Scholar]
- Mohan S, Konopinski R, Yan B, Centonze VE, Natarajan M. High glucose-induced IKK-Hsp-90 interaction contributes to endothelial dysfunction. Am J Physiol Cell Physiol. 2009;296:C182–C192. doi: 10.1152/ajpcell.00575.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort RL, Workman P. Structure-based design of molecular cancer therapeutics. Trends Biotechnol. 2009;27:315–328. doi: 10.1016/j.tibtech.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Murata T, Shimada M, Sakakibara S, Yoshino T, Kadono H, Masuda T, et al. Discovery of novel and selective IKK-beta serine-threonine protein kinase inhibitors. Part 1. Bioorg Med Chem Lett. 2003;13:913–918. doi: 10.1016/s0960-894x(02)01046-6. [DOI] [PubMed] [Google Scholar]
- Murata T, Shimada M, Kadono H, Sakakibara S, Yoshino T, Masuda T, et al. Synthesis and structure–activity relationships of novel IKK-beta inhibitors. Part 2: improvement of in vitro activity. Bioorg Med Chem Lett. 2004a;14:4013–4017. doi: 10.1016/j.bmcl.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Murata T, Shimada M, Sakakibara S, Yoshino T, Masuda T, Shintani T, et al. Synthesis and structure–activity relationships of novel IKK-beta inhibitors. Part 3: orally active anti-inflammatory agents. Bioorg Med Chem Lett. 2004b;14:4019–4022. doi: 10.1016/j.bmcl.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Mustapha S, Kirschner A, De Moissac D. A direct requirement of NFkappaB for suppression of apoptosis in ventricular myocytes. Am J Physiol. 2000;279:H939–H945. doi: 10.1152/ajpheart.2000.279.3.H939. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Azuma M, Muto S, Nishioka Y, Honjo A, Tezuka T, et al. IkappaB kinase beta inhibitor IMD-0354 suppresses airway remodelling in a Dermatophagoides pteronyssinus-sensitized mouse model of chronic asthma. Clin Exp Allergy. 2011;41:104–115. doi: 10.1111/j.1365-2222.2010.03564.x. [DOI] [PubMed] [Google Scholar]
- Olsen LS, Hjarnaa PJ, Latini S, Holm PK, Larsson R, Bramm E, et al. Anticancer agent CHS 828 suppresses nuclear factor-kappa B activity in cancer cells through downregulation of IKK activity. Int J Cancer. 2004;111:198–205. doi: 10.1002/ijc.20255. [DOI] [PubMed] [Google Scholar]
- O'Mahony A, Lin X, Geleziunas R, Greene WC. Activation of the heterodimeric Ikappa B kinase alpha (IKKalpha)-IKKbeta complex is directional: IKKalpha regulates IKKbeta under both basal and stimulated conditions. Mol Cell Biol. 2000;20:1170–1178. doi: 10.1128/mcb.20.4.1170-1178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai Y, Suzuki J, Kakuta T, Maejima Y, Haraguchi G, Fukasawa H, et al. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;63:51–59. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Onai Y, Suzuki J, Maejima Y, Haraguchi G, Muto S, Itai A, et al. Inhibition of NF-{kappa}B improves left ventricular remodeling and cardiac dysfunction after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H530–H538. doi: 10.1152/ajpheart.00549.2006. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell proliferation. J Pharmacol Exp Ther. 2005;312:373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Prajapati S, Tu Z, Yamamoto Y, Gaynor RB. IKKalpha regulates the mitotic phase of the cell cycle by modulating Aurora A phosphorylation. Cell Cycle. 2006;5:2371–2380. doi: 10.4161/cc.5.20.3359. [DOI] [PubMed] [Google Scholar]
- Reddy ST, Grijalva V, Ng C, Hassan K, Hama S, Mottahedeh R, et al. Identification of genes induced by oxidized phospholipids in human aortic endothelial cells. Vascul Pharmacol. 2002;38:211–218. doi: 10.1016/s1537-1891(02)00171-4. [DOI] [PubMed] [Google Scholar]
- Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Traish AM, Mercurio F, Sonenshein GE. Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-kB in breast cancer. Cancer Res. 2001;61:3810–3818. [PubMed] [Google Scholar]
- Roshak AK, Jackson JR, McGough K, Chabot-Fletcher M, Mochan E, Marshall LA. Manipulation of distinct NFkappaB proteins alters interleukin-1beta-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J Biol Chem. 1996;271:31496–31501. doi: 10.1074/jbc.271.49.31496. [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-[gamma] is an essential regulatory subunit of the I[kappa]B kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- Rushe M, Silvian L, Bixler S, Chen LL, Cheung A, Bowes S, et al. Structure of a NEMO/IKK-associating domain reveals architecture of the interaction site. Structure. 2008;16:798–808. doi: 10.1016/j.str.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkB kinases phosphorylate NF-kB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Saxena U, Goldberg IJ. Endothelial cells and atherosclerosis: lipoprotein metabolism, matrix interactions, and monocyte recruitment. Curr Opin Lipidol. 1994;5:316–322. doi: 10.1097/00041433-199410000-00002. [DOI] [PubMed] [Google Scholar]
- Schôn M, Wienrich BG, Kneitz S, Sennefelder H, Amschler K, Vôhringer V, et al. KINK-1, a novel small-molecule inhibitor of IKKβ, and the susceptibility of melanoma cells to antitumoral treatment. J Natl Cancer Inst. 2008;100:862–875. doi: 10.1093/jnci/djn174. [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peng B, Li Z, Sclabas GM, Fujioka S, Niu J, et al. Mechanisms of proinflammatory cytokine-induced biphasic NF-[kappa]B activation. Mol Cell. 2003;12:1287–1300. doi: 10.1016/s1097-2765(03)00390-3. [DOI] [PubMed] [Google Scholar]
- Schopf L, Savinainen A, Anderson K, Kujawa J, DuPont M, Silva M, et al. IKKβ inhibition protects against bone and cartilage destruction in a rat model of rheumatoid arthritis. Arthritis Rheum. 2006;54:3163–3173. doi: 10.1002/art.22081. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Sethi G, Sung B, Kunnumakkara AB, Aggarwal BB. Targeting TNF for treatment of cancer and autoimmunity. Adv Exp Med Biol. 2009;647:37–51. doi: 10.1007/978-0-387-89520-8_3. [DOI] [PubMed] [Google Scholar]
- Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein–protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, et al. Cutting edge: the IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IκB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- Shuto T, Xu H, Wang B, Han J, Kai H, Gu XX, et al. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha/beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci USA. 2001;98:8774–8779. doi: 10.1073/pnas.151236098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Singh S, Shi Q, Bailey ST, Palczewski MJ, Pardee AB, Iglehart JD, et al. Nuclear factor-kB activation: a molecular therapeutic target for estrogen receptor negative and epidermal growth factor receptor family receptor positive human breast cancer. Mol Cancer Ther. 2007;6:1973–1982. doi: 10.1158/1535-7163.MCT-07-0063. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- Sugita A, Ogawa H, Azuma M, Muto S, Honjo A, Yanagawa H, et al. Antiallergic and anti-inflammatory effects of a novel I kappaB kinase beta inhibitor, IMD-0354, in a mouse model of allergic inflammation. Int Arch Allergy Immunol. 2009;148:186–198. doi: 10.1159/000161579. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, et al. Nuclear factor-kB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Gschwend E, Jr, Büchele B, Laumonnier Y, Zugmaier W, Genze F, et al. Inhibition of IκB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J Biol Chem. 2005;280:6170–6180. doi: 10.1074/jbc.M409477200. [DOI] [PubMed] [Google Scholar]
- Tak PP, Smeets TJ, Boyle DL, Kraan MC, Shi Y, Zhuang S, et al. p53 overexpression in synovial tissue from patients with early and longstanding rheumatoid arthritis compared with patients with reactive arthritis and osteoarthritis. Arthritis Rheum. 1999;42:948–953. doi: 10.1002/1529-0131(199905)42:5<948::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, et al. Limb and skin abnormalities in mice lacking IKK. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, et al. Embryonic lethality, liver degeneration, and impaired NF-[kappa]B activation in IKK-[beta]-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, et al. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- Tapia MA, Gonzalez-Navarrete I, Dalmases A, Bosch M, Rodriguez-Fanjul V, Rolfe M, et al. Inhibition of the canonical IKK/NF kappa B pathway sensitizes human cancer cells to doxorubicin. Cell Cycle. 2007;6:2284–2292. doi: 10.4161/cc.6.18.4721. [DOI] [PubMed] [Google Scholar]
- Tas SW, de Jong EC, Hajji N, May MJ, Ghosh S, Vervoordeldonk MJ, et al. Selective inhibition of NF-kappaB in dendritic cells by the NEMO-binding domain peptide blocks maturation and prevents T cell proliferation and polarization. Eur J Immunol. 2005;35:1164–1174. doi: 10.1002/eji.200425956. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- Thiele K, Bierhaus A, Autschbach F, Hofmann M, Stremmel W, Thiele H, et al. Cell specific effects of glucocorticoid treatment on the NF-kappaBp65/IkappaBalpha system in patients with Crohn's disease. Gut. 1999;45:693–704. doi: 10.1136/gut.45.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, et al. NAK is an I[kappa]B kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Colosia AD, Donahue JP, Lin YZ, Hawiger J. Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J Immunol. 1998;161:6084–6092. [PubMed] [Google Scholar]
- Townsend RM, Postelnek J, Susulic V, McIntyre KW, Shuster DJ, Qiu Y, et al. A highly selective inhibitor of IkappaB kinase, BMS-345541, augments graft survival mediated by suboptimal immunosuppression in a murine model of cardiac graft rejection. Transplantation. 2004;77:1090–1094. doi: 10.1097/01.tp.0000118407.05205.05. [DOI] [PubMed] [Google Scholar]
- Tsao PW, Suzuki T, Totsuka R, Murata T, Takagi T, Ohmachi Y, et al. The effect of dexamethasone on the expression of activated NF-kappa B in adjuvant arthritis. Clin Immunol Immunopathol. 1997;83:173–178. doi: 10.1006/clin.1997.4333. [DOI] [PubMed] [Google Scholar]
- Tu Z, Prajapati S, Park KJ, Kelly NJ, Yamamoto Y, Gaynor RB. IKK alpha regulates estrogen-induced cell cycle progression by modulating E2F1 expression. J Biol Chem. 2006;281:6699–6706. doi: 10.1074/jbc.M512439200. [DOI] [PubMed] [Google Scholar]
- Tysnes BB. Tumor-initiating and -propagating cells: cells that we would like to identify and control. Neoplasia. 2010;12:506–515. doi: 10.1593/neo.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R, Dawson C, Hu C, Shah K, Owen T, Date K, et al. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol Cancer. 2010;9:1. doi: 10.1186/1476-4598-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Nong Y, Morgan JG, Gangurde P, Bielecki A, Dasilva J, et al. A selective small molecule IkappaB kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001. doi: 10.1124/jpet.105.097584. [DOI] [PubMed] [Google Scholar]
- Wharry CE, Haines KM, Carroll RG, May MJ. Constitutive non-canonical NFkappaB signaling in pancreatic cancer cells. Cancer Biol Ther. 2009;8:1567–1576. doi: 10.4161/cbt.8.16.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek C, Cleaver CS, Ludbrook V, Wilde J, White J, Bell DJ, et al. IkB kinase β interacts with p52 and promotes transactivation via p65. J Biol Chem. 2006;281:34973–34981. doi: 10.1074/jbc.M607018200. [DOI] [PubMed] [Google Scholar]
- Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. I{kappa}B kinase-: NF-B activation and complex formation with IB kinase- and NIK. Science. 1997;278:866–870. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-κB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- Wu L, Shao L, An N, Wang J, Pazhanisamy S, Feng W, et al. IKKβ regulates the repair of DNA double-strand breaks induced by ionizing radiation in MCF-7 breast cancer cells. PLoS ONE. 2011;6:e18447. doi: 10.1371/journal.pone.0018447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler E, Kaminska M, Coic YM, Baleux F, Veron M, Agou F. Inhibition of NF-kappaB activation with designed ankyrin-repeat proteins targeting the ubiquitin-binding/oligomerization domain of NEMO. Protein Sci. 2007;16:2013–2022. doi: 10.1110/ps.072924907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci USA. 2009;106:2629–2634. doi: 10.1073/pnas.0812256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Fang F, St Clair DK, Sompol P, Josson S, St Clair WH. SN52, a novel nuclear factor-kappaB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol Cancer Ther. 2008;7:2367–2376. doi: 10.1158/1535-7163.MCT-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Lo YC, Li Q, Napolitano G, Wu X, Jiang X, et al. Crystal structure of inhibitor of κB kinase β. Nature. 2011;472:325–330. doi: 10.1038/nature09853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak Y-T, Gaynor RB. Histone H3 phosphorylation by IKK-[alpha] is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, et al. Complementation cloning of NEMO, a component of the I[kappa]B kinase complex essential for NF-[kappa]B activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Kawakami A, Nakashima T, Nakamura H, Kamachi M, Honda S, et al. Importance of NF-kappaB in rheumatoid synovial tissues: in situ NF-kappaB expression and in vitro study using cultured synovial cells. Ann Rheum Dis. 2001;60:678–684. doi: 10.1136/ard.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, et al. The essential role of MEKK3 in TNF-induced NF-[kappa]B activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan WH, Clawson GA, Richmond A. Systemic targeting inhibitor of kappaB kinase inhibits melanoma tumor growth. Cancer Res. 2007;67:3127–3134. doi: 10.1158/0008-5472.CAN-06-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Zetoune FS, Murthy AR, Shao Z, Hlaing T, Zeidler MG, Li Y, et al. A20 inhibits NF-[kappa]B activation downstream of multiple MAP3 KINASES and interacts with the I[kappa]B signalosome. Cytokine. 2001;15:282–298. doi: 10.1006/cyto.2001.0921. [DOI] [PubMed] [Google Scholar]
- Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKK[gamma]) upon receptor stimulation. Immunity. 2000;12:301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T, et al. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol. 2005;145:178–192. doi: 10.1038/sj.bjp.0706176. [DOI] [PMC free article] [PubMed] [Google Scholar]


