Full text
PDF












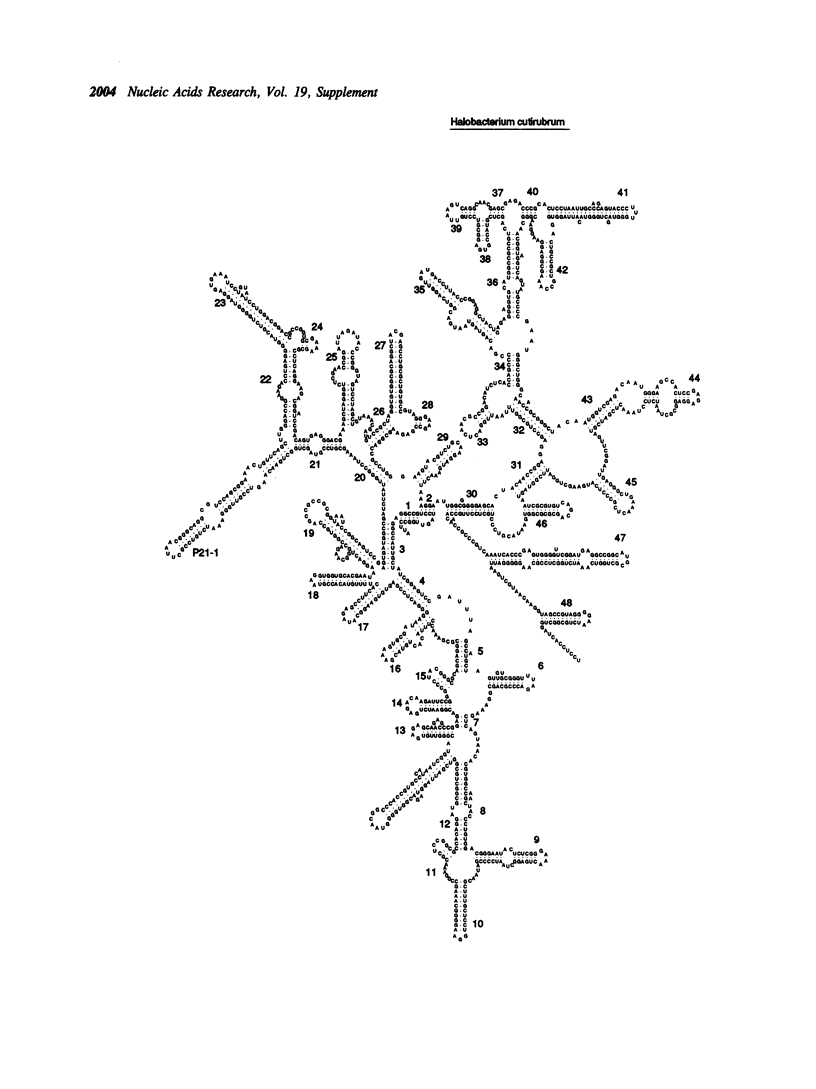
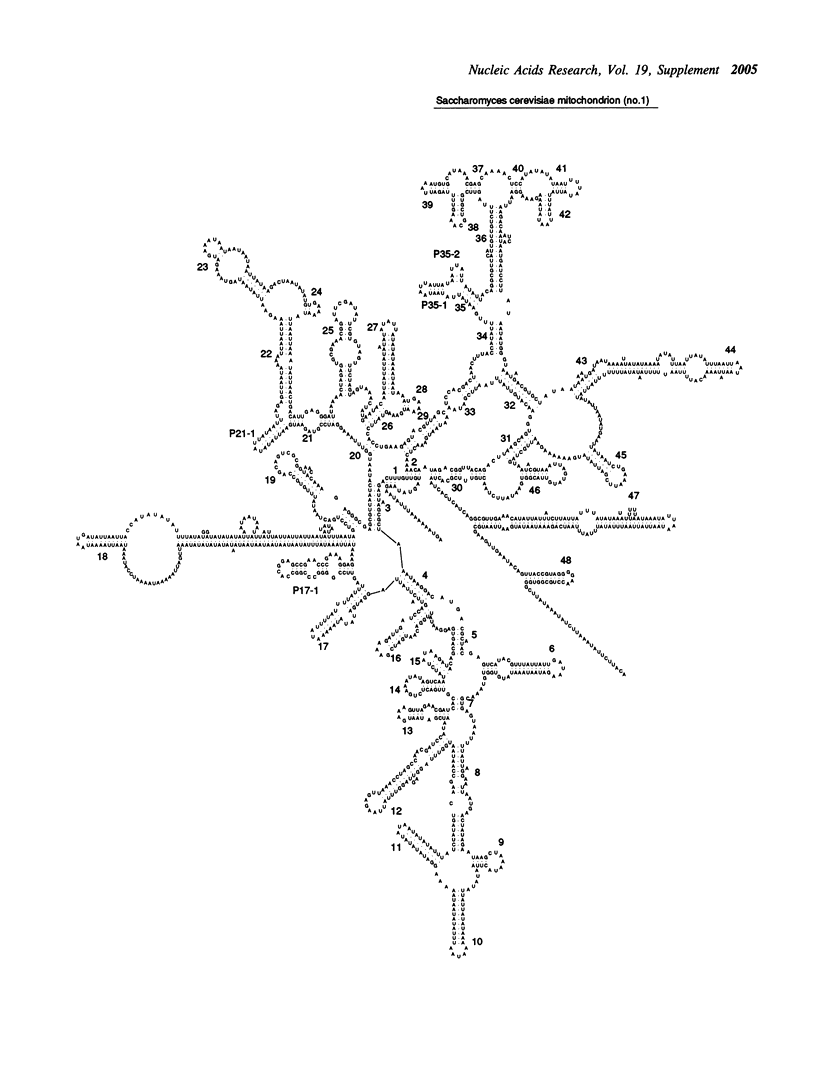

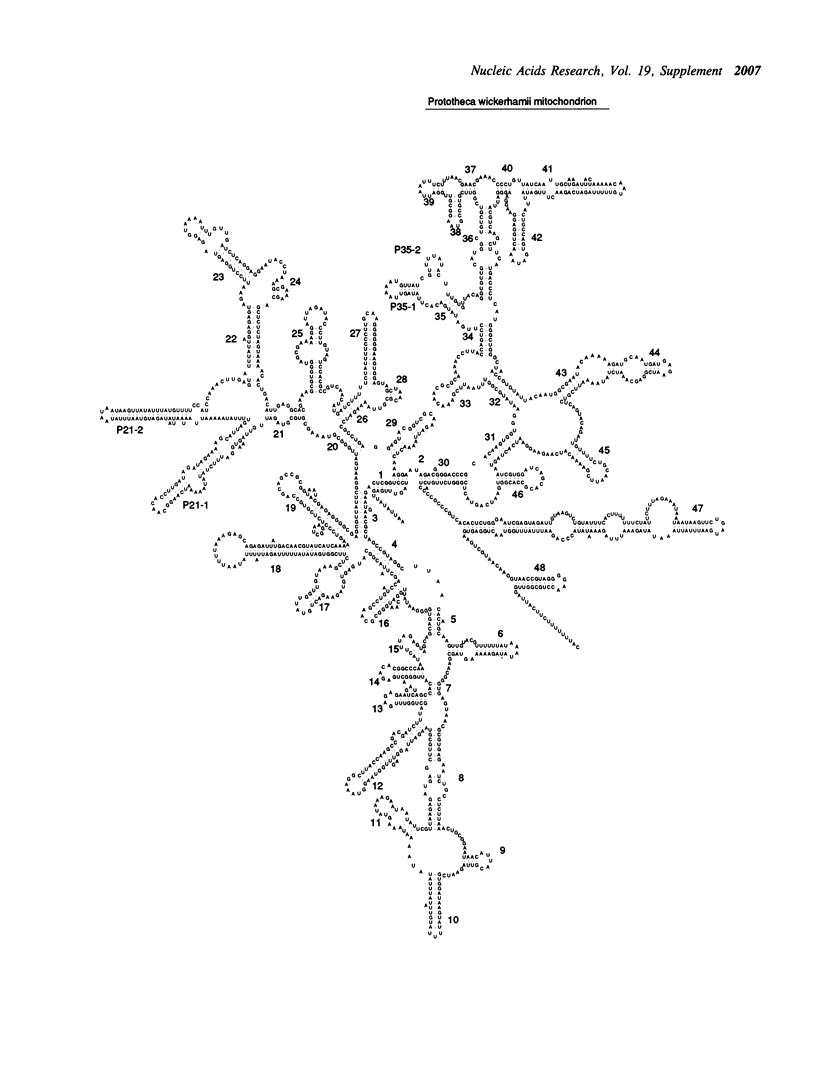


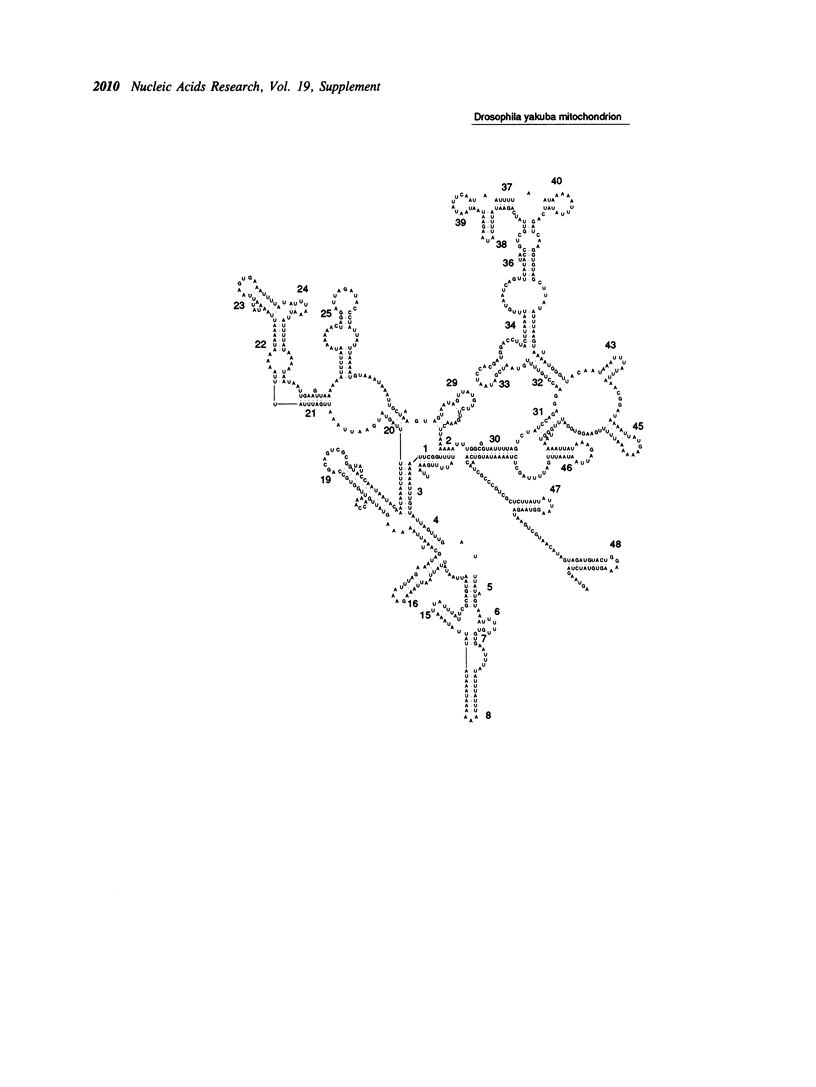
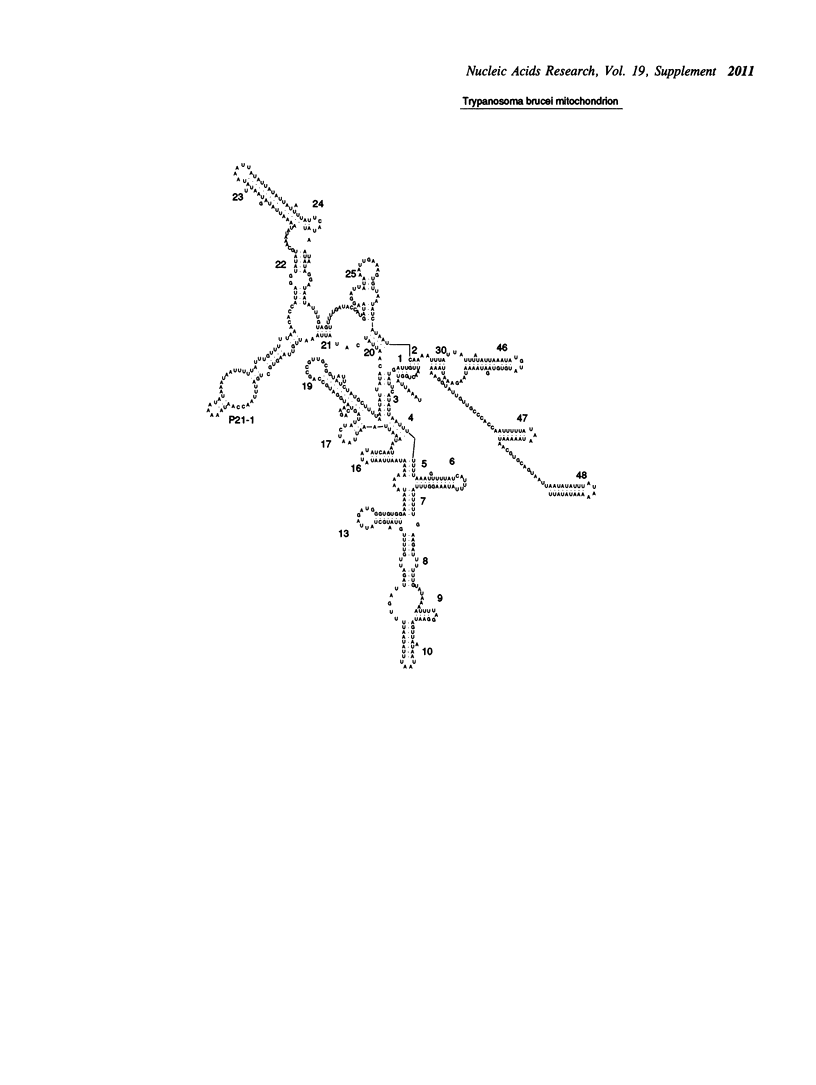
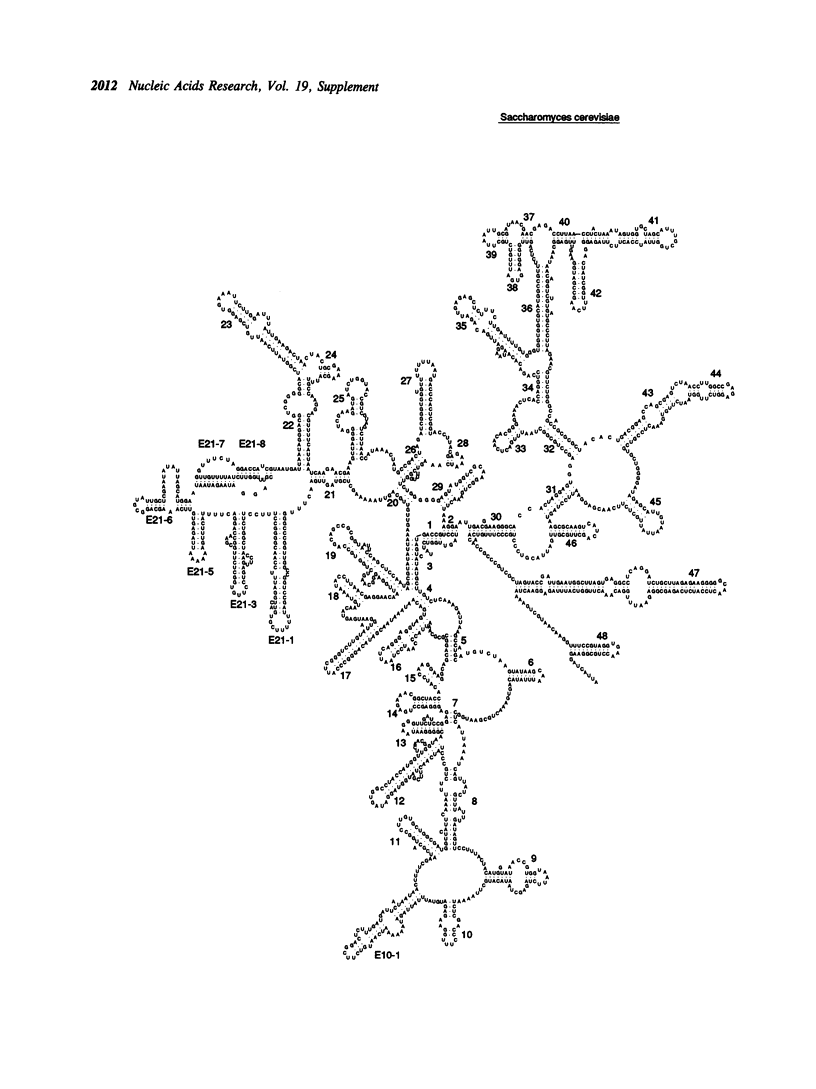
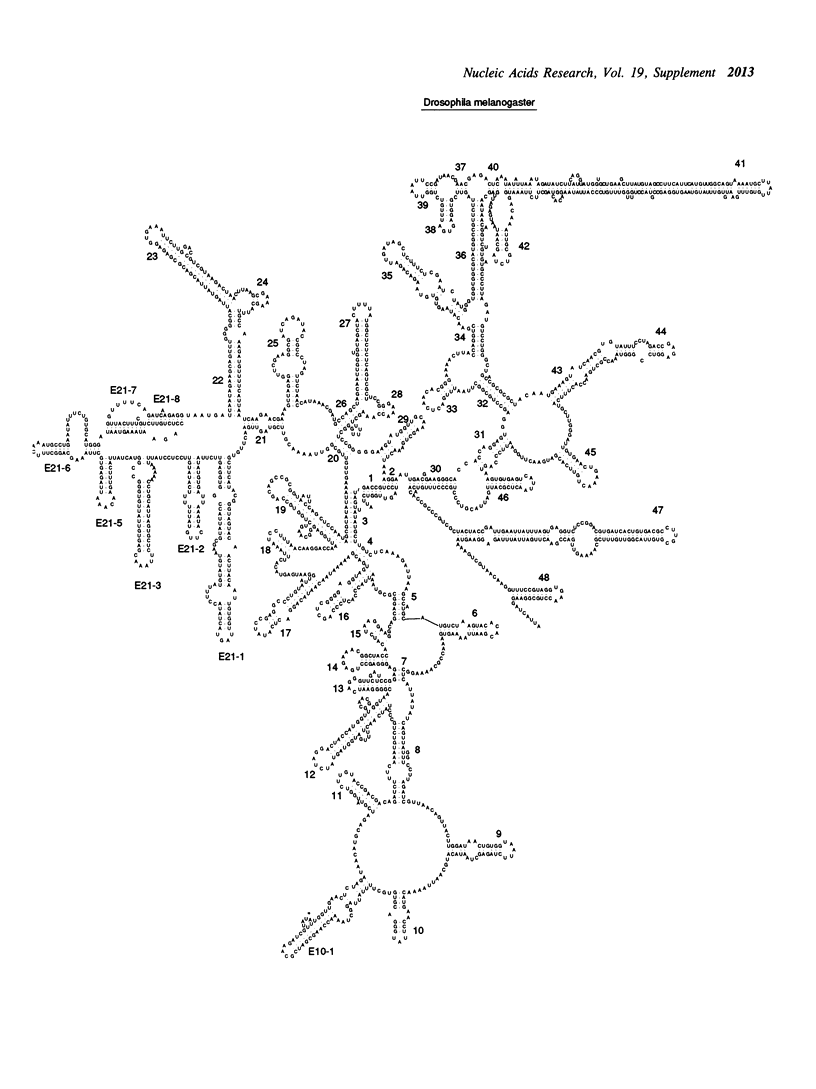
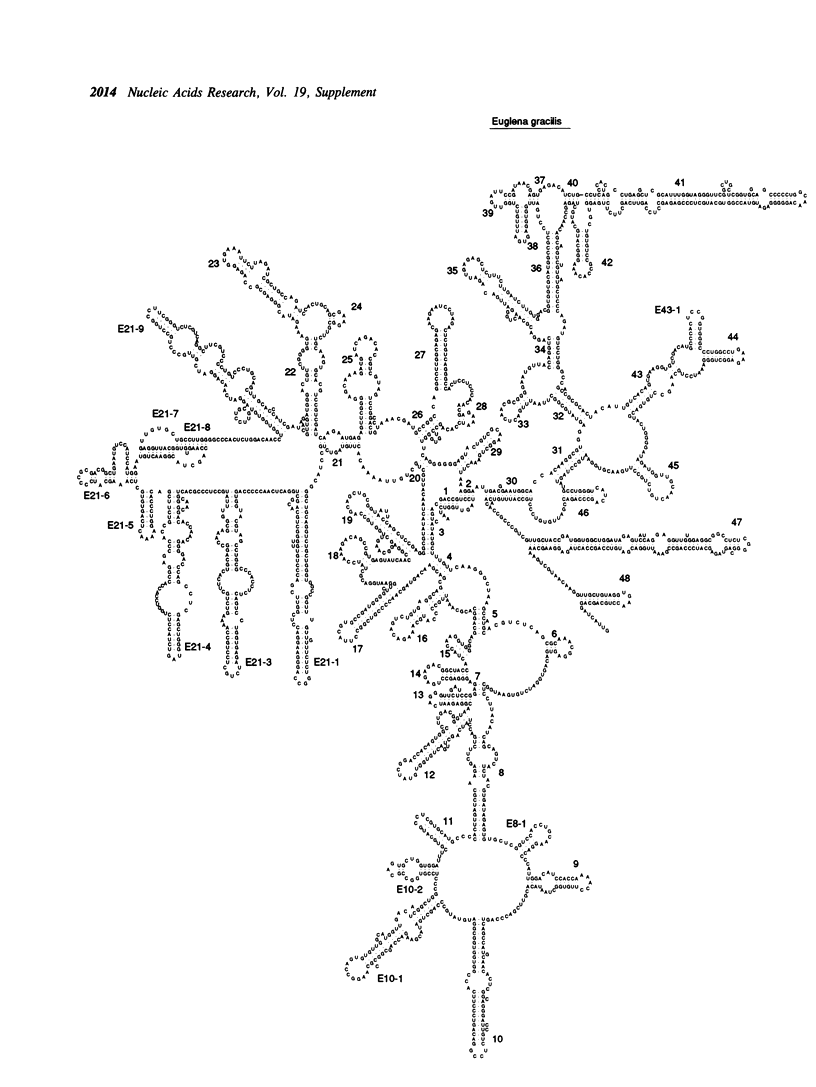
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Achenbach-Richter L., Gupta R., Zillig W., Woese C. R. Rooting the archaebacterial tree: the pivotal role of Thermococcus celer in archaebacterial evolution. Syst Appl Microbiol. 1988;10:231–240. doi: 10.1016/s0723-2020(88)80007-9. [DOI] [PubMed] [Google Scholar]
- Achenbach-Richter L., Stetter K. O., Woese C. R. A possible biochemical missing link among archaebacteria. Nature. 1987 May 28;327(6120):348–349. doi: 10.1038/327348a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Baylis H. A., Bibb M. J. The nucleotide sequence of a 16S rRNA gene from Streptomyces coelicolor A3(2) Nucleic Acids Res. 1987 Sep 11;15(17):7176–7176. doi: 10.1093/nar/15.17.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C. J., Rice E. L., Murphy C. A., Liu Q. Y., Ragan M. A. Nucleotide sequences of 18S ribosomal RNA genes from the red algae Gracilaria tikvahiae McLachlan, Gracilaria verrucosa (Hudson) Papenfuss and Gracilariopsis sp. Nucleic Acids Res. 1990 Jul 11;18(13):4023–4024. doi: 10.1093/nar/18.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Gadaleta M. N., Saccone C. The complete nucleotide sequence, gene organization, and genetic code of the mitochondrial genome of Paracentrotus lividus. J Biol Chem. 1989 Jul 5;264(19):10965–10975. [PubMed] [Google Scholar]
- Carbon P., Ebel J. P., Ehresmann C. The sequence of the ribosomal 16S RNA from Proteus vulgaris. Sequence comparison with E. coli 16S RNA and its use in secondary model building. Nucleic Acids Res. 1981 May 25;9(10):2325–2333. doi: 10.1093/nar/9.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chao S., Sederoff R., Levings C. S., 3rd Nucleotide sequence and evolution of the 18S ribosomal RNA gene in maize mitochondria. Nucleic Acids Res. 1984 Aug 24;12(16):6629–6644. doi: 10.1093/nar/12.16.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charfreitag O., Stackebrandt E. Inter- and intrageneric relationships of the genus Propionibacterium as determined by 16S rRNA sequences. J Gen Microbiol. 1989 Jul;135(7):2065–2070. doi: 10.1099/00221287-135-7-2065. [DOI] [PubMed] [Google Scholar]
- Clark C. G., Cross G. A. Small-subunit ribosomal RNA sequence from Naegleria gruberi supports the polyphyletic origin of amoebas. Mol Biol Evol. 1988 Sep;5(5):512–518. doi: 10.1093/oxfordjournals.molbev.a040516. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. Drosophila mitochondrial DNA: conserved sequences in the A + T-rich region and supporting evidence for a secondary structure model of the small ribosomal RNA. J Mol Evol. 1987;25(2):116–125. doi: 10.1007/BF02101753. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The ribosomal RNA genes of Drosophila mitochondrial DNA. Nucleic Acids Res. 1985 Jun 11;13(11):4029–4045. doi: 10.1093/nar/13.11.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Stackebrandt E. Molecular taxonomic studies on some LL-diaminopimelic acid-containing coryneforms from herbage: description of Nocardioides fastidiosa sp. nov. FEMS Microbiol Lett. 1989 Feb;57(3):289–293. doi: 10.1016/0378-1097(89)90316-9. [DOI] [PubMed] [Google Scholar]
- Corliss J. O. The kingdom Protista and its 45 phyla. Biosystems. 1984;17(2):87–126. doi: 10.1016/0303-2647(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Domenico J. M., Nelson J., Sogin M. L. DNA sequence, structure, and phylogenetic relationship of the small subunit rRNA coding region of mitochondrial DNA from Podospora anserina. J Mol Evol. 1989 Mar;28(3):232–241. doi: 10.1007/BF02102481. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Devereux R., Delaney M., Widdel F., Stahl D. A. Natural relationships among sulfate-reducing eubacteria. J Bacteriol. 1989 Dec;171(12):6689–6695. doi: 10.1128/jb.171.12.6689-6695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux R., He S. H., Doyle C. L., Orkland S., Stahl D. A., LeGall J., Whitman W. B. Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J Bacteriol. 1990 Jul;172(7):3609–3619. doi: 10.1128/jb.172.7.3609-3619.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch M., Moreno E., Stackebrandt E. Nucleotide sequence of the 16S rRNA from Brucella abortus. Nucleic Acids Res. 1989 Feb 25;17(4):1765–1765. doi: 10.1093/nar/17.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast 16S rRNA gene and its surrounding regions of Chlamydomonas reinhardii. Nucleic Acids Res. 1982 Dec 11;10(23):7609–7620. doi: 10.1093/nar/10.23.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon-Bluteau D., Brun G. The secondary structures of the Xenopus laevis and human mitochondrial small ribosomal subunit RNA are similar. FEBS Lett. 1986 Mar 31;198(2):333–338. doi: 10.1016/0014-5793(86)80431-8. [DOI] [PubMed] [Google Scholar]
- Durocher V., Gauthier A., Bellemare G., Lemieux C. An optional group I intron between the chloroplast small subunit rRNA genes of Chlamydomonas moewusii and C. eugametos. Curr Genet. 1989 Apr;15(4):277–282. doi: 10.1007/BF00447043. [DOI] [PubMed] [Google Scholar]
- Dyson N. J., Brown T. A., Waring R. B., Davies R. W. The mitochondrial ribosomal RNA molecules of Aspergillus nidulans. Gene. 1989 Jan 30;75(1):109–118. doi: 10.1016/0378-1119(89)90387-9. [DOI] [PubMed] [Google Scholar]
- Eckenrode V. K., Arnold J., Meagher R. B. Comparison of the nucleotide sequence of soybean 18S rRNA with the sequences of other small-subunit rRNAs. J Mol Evol. 1984;21(3):259–269. doi: 10.1007/BF02102358. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Edwards U., Rogall T., Blöcker H., Emde M., Böttger E. C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989 Oct 11;17(19):7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen R., Harmsen H., Geerling A., de Vos W. M. Nucleotide sequence of a 16S rRNA encoding gene from the archaebacterium Methanothrix soehngenii. Nucleic Acids Res. 1989 Nov 25;17(22):9469–9469. doi: 10.1093/nar/17.22.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. E., Sulston J. E., Coulson A. R. The rDNA of C. elegans: sequence and structure. Nucleic Acids Res. 1986 Mar 11;14(5):2345–2364. doi: 10.1093/nar/14.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood H. J., Olsen G. J., Sogin M. L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol. 1985 Sep;2(5):399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Embley T. M., Smida J., Stackebrandt E. Reverse transcriptase sequencing of 16S ribosomal RNA from Faenia rectivirgula, Pseudonocardia thermophila and Saccharopolyspora hirsuta, three wall type IV actinomycetes which lack mycolic acids. J Gen Microbiol. 1988 Apr;134(4):961–966. doi: 10.1099/00221287-134-4-961. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Janssen J. W., Hoeijmakers J. H., Borst P. The major transcripts of the kinetoplast DNA of Trypanosoma brucei are very small ribosomal RNAs. Nucleic Acids Res. 1983 Jan 11;11(1):105–125. doi: 10.1093/nar/11.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Frydenberg J., Christiansen C. The sequence of 16S rRNA from Mycoplasma strain PG50. DNA. 1985 Apr;4(2):127–137. doi: 10.1089/dna.1985.4.127. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Horie I., Okuzako N., Mifuchi I. Nucleotide sequence of a 16S rRNA gene for Leptospira interrogans serovar canicola strain Moulton. Nucleic Acids Res. 1990 Jan 25;18(2):366–366. doi: 10.1093/nar/18.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., Britschgi T. B., Moyer C. L., Field K. G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990 May 3;345(6270):60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., Britschgi T. B., Moyer C. L., Field K. G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990 May 3;345(6270):60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez I. L., Schmickel R. D. The human 18S ribosomal RNA gene: evolution and stability. Am J Hum Genet. 1986 Apr;38(4):419–427. [PMC free article] [PubMed] [Google Scholar]
- Graf L., Roux E., Stutz E., Kössel H. Nucleotide sequence of a Euglena gracilis chloroplast gene coding for the 16S rRNA: homologies to E. coli and Zea mays chloroplast 16S rRNA. Nucleic Acids Res. 1982 Oct 25;10(20):6369–6381. doi: 10.1093/nar/10.20.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Elwood H., Ingold A., Kindle K., Sogin M. L. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5823–5827. doi: 10.1073/pnas.84.16.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., Elwood H., Ingold A., Kindle K., Sogin M. L. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5823–5827. doi: 10.1073/pnas.84.16.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., McCutchan T. F., Sogin M. L. Sequence of the small subunit ribosomal RNA gene expressed in the bloodstream stages of Plasmodium berghei: evolutionary implications. J Protozool. 1986 Nov;33(4):525–529. doi: 10.1111/j.1550-7408.1986.tb05656.x. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L. Length variation in eukaryotic rRNAs: small subunit rRNAs from the protists Acanthamoeba castellanii and Euglena gracilis. Gene. 1986;44(1):63–70. doi: 10.1016/0378-1119(86)90043-0. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L., Wollett G., Hollingdale M., de la Cruz V. F., Waters A. P., McCutchan T. F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- Gupta R., Lanter J. M., Woese C. R. Sequence of the 16S Ribosomal RNA from Halobacterium volcanii, an Archaebacterium. Science. 1983 Aug 12;221(4611):656–659. doi: 10.1126/science.221.4611.656. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Haas E. S., Brown J. W., Daniels C. J., Reeve J. N. Genes encoding the 7S RNA and tRNA(Ser) are linked to one of the two rRNA operons in the genome of the extremely thermophilic archaebacterium Methanothermus fervidus. Gene. 1990 May 31;90(1):51–59. doi: 10.1016/0378-1119(90)90438-w. [DOI] [PubMed] [Google Scholar]
- Healey A., Mitchell R., Upcroft J. A., Boreham P. F., Upcroft P. Complete nucleotide sequence of the ribosomal RNA tandem repeat unit from Giardia intestinalis. Nucleic Acids Res. 1990 Jul 11;18(13):4006–4006. doi: 10.1093/nar/18.13.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks L., De Baere R., Van Broeckhoven C., De Wachter R. Primary and secondary structure of the 18 S ribosomal RNA of the insect species Tenebrio molitor. FEBS Lett. 1988 May 9;232(1):115–120. doi: 10.1016/0014-5793(88)80398-3. [DOI] [PubMed] [Google Scholar]
- Hendriks L., Goris A., De Bruyn K., De Wachter R. The nucleotide sequence of the small ribosomal subunit RNA of the yeast Torulaspora delbrueckii. Nucleic Acids Res. 1990 Aug 11;18(15):4611–4611. doi: 10.1093/nar/18.15.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks L., Van Broeckhoven C., Vandenberghe A., Van de Peer Y., De Wachter R. Primary and secondary structure of the 18S ribosomal RNA of the bird spider Eurypelma californica and evolutionary relationships among eukaryotic phyla. Eur J Biochem. 1988 Oct 15;177(1):15–20. doi: 10.1111/j.1432-1033.1988.tb14339.x. [DOI] [PubMed] [Google Scholar]
- Hendriks L., Van de Peer Y., Van Herck M., Neefs J. M., De Wachter R. The 18S ribosomal RNA sequence of the sea anemone Anemonia sulcata and its evolutionary position among other eukaryotes. FEBS Lett. 1990 Sep 3;269(2):445–449. doi: 10.1016/0014-5793(90)81212-7. [DOI] [PubMed] [Google Scholar]
- Hernández R., Rios P., Valdés A. M., Piñero D. Primary structure of Trypanosoma cruzi small-subunit ribosomal RNA coding region: comparison with other trypanosomatids. Mol Biochem Parasitol. 1990 Jun;41(2):207–212. doi: 10.1016/0166-6851(90)90183-m. [DOI] [PubMed] [Google Scholar]
- Herzog M., Maroteaux L. Dinoflagellate 17S rRNA sequence inferred from the gene sequence: Evolutionary implications. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8644–8648. doi: 10.1073/pnas.83.22.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hixson J. E., Brown W. M. A comparison of the small ribosomal RNA genes from the mitochondrial DNA of the great apes and humans: sequence, structure, evolution, and phylogenetic implications. Mol Biol Evol. 1986 Jan;3(1):1–18. doi: 10.1093/oxfordjournals.molbev.a040379. [DOI] [PubMed] [Google Scholar]
- Hui I., Dennis P. P. Characterization of the ribosomal RNA gene clusters in Halobacterium cutirubrum. J Biol Chem. 1985 Jan 25;260(2):899–906. [PubMed] [Google Scholar]
- Huss V. A., Giovannoni S. J. Primary structure of the chloroplast small subunit ribosomal RNA gene from Chlorella vulgaris. Nucleic Acids Res. 1989 Nov 25;17(22):9487–9487. doi: 10.1093/nar/17.22.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss V. A., Sogin M. L. Phylogenetic position of some Chlorella species within the chlorococcales based upon complete small-subunit ribosomal RNA sequences. J Mol Evol. 1990 Nov;31(5):432–442. doi: 10.1007/BF02106057. [DOI] [PubMed] [Google Scholar]
- Huss V. A., Sogin M. L. Primary structure of the Chlorella vulgaris small subunit ribosomal RNA coding region. Nucleic Acids Res. 1989 Feb 11;17(3):1255–1255. doi: 10.1093/nar/17.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttenhofer A., Sakai H., Weiss-Brummer B. Site-specific AT-cluster insertions in the mitochondrial 15S rRNA gene of the yeast S. cerevisiae. Nucleic Acids Res. 1988 Sep 12;16(17):8665–8674. doi: 10.1093/nar/16.17.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami M., Muto A., Yamao F., Osawa S. Nucleotide sequence of the rrnB 16S ribosomal RNA gene from Mycoplasma capricolum. Mol Gen Genet. 1984;196(2):317–322. doi: 10.1007/BF00328065. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Johansen T., Johansen S., Haugli F. B. Nucleotide sequence of the Physarum polycephalum small subunit ribosomal RNA as inferred from the gene sequence: secondary structure and evolutionary implications. Curr Genet. 1988 Sep;14(3):265–273. doi: 10.1007/BF00376747. [DOI] [PubMed] [Google Scholar]
- Kazda J., Stackebrandt E., Smida J., Minnikin D. E., Daffe M., Parlett J. H., Pitulle C. Mycobacterium cookii sp. nov. Int J Syst Bacteriol. 1990 Jul;40(3):217–223. doi: 10.1099/00207713-40-3-217. [DOI] [PubMed] [Google Scholar]
- Kiss T., Szkukálek A., Solymosy F. Nucleotide sequence of a 17S (18S) rRNA gene from tomato. Nucleic Acids Res. 1989 Mar 11;17(5):2127–2127. doi: 10.1093/nar/17.5.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Seki T., Yaginuma K., Koike K. Nucleotide sequences of small ribosomal RNA and adjacent transfer RNA genes in rat mitochondrial DNA. Gene. 1981 Dec;16(1-3):297–307. doi: 10.1016/0378-1119(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Labriola J., Weiss I., Zapatero J., Suyama Y. Unexpectedly long 14S ribosomal RNA gene in Tetrahymena mitochondria. Curr Genet. 1987;11(6-7):529–536. doi: 10.1007/BF00384616. [DOI] [PubMed] [Google Scholar]
- Lake J. A., de la Cruz V. F., Ferreira P. C., Morel C., Simpson L. Evolution of parasitism: kinetoplastid protozoan history reconstructed from mitochondrial rRNA gene sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4779–4783. doi: 10.1073/pnas.85.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassnig C., Dorsch M., Wolters J., Schaber E., Stöffler G., Stackebrandt E. Phylogenetic evidence for the relationship between the genera Mobiluncus and Actinomyces. FEMS Microbiol Lett. 1989 Nov;53(1-2):17–21. doi: 10.1016/0378-1097(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Leffers H., Garrett R. A. The nucleotide sequence of the 16S ribosomal RNA gene of the archaebacterium Halococcus morrhua. EMBO J. 1984 Jul;3(7):1613–1619. doi: 10.1002/j.1460-2075.1984.tb02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tzagoloff A., Underbrink-Lyon K., Martin N. C. Identification of the paromomycin-resistance mutation in the 15 S rRNA gene of yeast mitochondria. J Biol Chem. 1982 May 25;257(10):5921–5928. [PubMed] [Google Scholar]
- Liesack W., Pitulle C., Sela S., Stackebrandt E. Nucleotide sequence of the 16S rRNA from Mycobacterium leprae. Nucleic Acids Res. 1990 Sep 25;18(18):5558–5558. doi: 10.1093/nar/18.18.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. O., Sears B. B. 16S rRNA sequence indicates that plant-pathogenic mycoplasmalike organisms are evolutionarily distinct from animal mycoplasmas. J Bacteriol. 1989 Nov;171(11):5901–5906. doi: 10.1128/jb.171.11.5901-5906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker D., Miller L. A., Elwood H. J., Stickel S., Sogin M. L. Primary structure of the Leishmania donovani small subunit ribosomal RNA coding region. Nucleic Acids Res. 1988 Jul 25;16(14B):7198–7198. doi: 10.1093/nar/16.14.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W., Weizenegger M., Dorn S., Andreesen J., Schleifer K. H. The phylogenetic position of Peptococcus niger based on 16S rRNA sequence studies. FEMS Microbiol Lett. 1990 Sep 1;59(1-2):139–143. doi: 10.1111/j.1574-6968.1990.tb03812.x. [DOI] [PubMed] [Google Scholar]
- Maid U., Zetsche K. Nucleotide sequence of the plastid 16S rRNA gene of the red alga Cyanidium caldarium. Nucleic Acids Res. 1990 Jul 11;18(13):3996–3996. doi: 10.1093/nar/18.13.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleszka R., Clark-Walker G. D. Sequence of the gene for the cytoplasmic ribosomal RNA small subunit from Kluyveromyces lactis. Nucleic Acids Res. 1990 Apr 11;18(7):1889–1889. doi: 10.1093/nar/18.7.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Kagramanova V. K., Teterina N. L., Rubtsov P. M., Belova E. N., Kopylov A. M., Baratova L. A., Bogdanov A. A. The nucleotide sequence of the gene coding for the 16S rRNA from the archaebacterium Halobacterium halobium. Gene. 1985;37(1-3):181–189. doi: 10.1016/0378-1119(85)90271-9. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Skryabin K. G., Rubtsov P. M. Identification of ten additional nucleotides in the primary structure of yeast 18S rRNA. Gene. 1986;44(1):143–145. doi: 10.1016/0378-1119(86)90054-5. [DOI] [PubMed] [Google Scholar]
- Markowicz Y., Loiseaux-de Goër S., Mache R. Presence of a 16S rRNA pseudogene in the bi-molecular plastid genome of the primitive brown alga Pylaiella littoralis. Evolutionary implications. Curr Genet. 1988 Dec;14(6):599–608. doi: 10.1007/BF00434086. [DOI] [PubMed] [Google Scholar]
- Martinez-Murcia A. J., Collins M. D. Nucleotide sequence of 16S ribosomal RNA from Lactobacillus kandleri and Lactobacillus minor. Nucleic Acids Res. 1990 Jun 11;18(11):3401–3401. doi: 10.1093/nar/18.11.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum F. S., Maden B. E. Human 18 S ribosomal RNA sequence inferred from DNA sequence. Variations in 18 S sequences and secondary modification patterns between vertebrates. Biochem J. 1985 Dec 15;232(3):725–733. doi: 10.1042/bj2320725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., de la Cruz V. F., Lal A. A., Gunderson J. H., Elwood H. J., Sogin M. L. Primary sequences of two small subunit ribosomal RNA genes from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Feb;28(1):63–68. doi: 10.1016/0166-6851(88)90181-8. [DOI] [PubMed] [Google Scholar]
- Medlin L., Elwood H. J., Stickel S., Sogin M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Carlson J., Hagen G., Rubenstein I., Oleson A. Cloning and sequencing of the ribosomal RNA genes in maize: the 17S region. DNA. 1984;3(1):31–40. doi: 10.1089/dna.1.1984.3.31. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. M., Kraus F., Ryder O. A. Phylogeny and evolution of antlered deer determined from mitochondrial DNA sequences. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6127–6131. doi: 10.1073/pnas.87.16.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzina N. V., Vorozheykina D. P., Matvienko N. I. Nucleotide sequence of Thermus thermophilus HB8 gene coding 16S rRNA. Nucleic Acids Res. 1988 Aug 25;16(16):8172–8172. doi: 10.1093/nar/16.16.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae Y., Fujii H., Yoneyama Y., Goto Y., Okazaki T. Nucleotide sequences of the Rana catesbeiana mitochondrial small (12S) and large (16S) ribosomal RNA genes. Nucleic Acids Res. 1988 Nov 11;16(21):10363–10363. doi: 10.1093/nar/16.21.10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn C. J., Ferl R. J. The complete nucleotide sequence of the small-subunit ribosomal RNA coding region for the cycad Zamia pumila: phylogenetic implications. J Mol Evol. 1988;27(2):133–141. doi: 10.1007/BF02138373. [DOI] [PubMed] [Google Scholar]
- Neefs J. M., De Wachter R. A proposal for the secondary structure of a variable area of eukaryotic small ribosomal subunit RNA involving the existence of a pseudoknot. Nucleic Acids Res. 1990 Oct 11;18(19):5695–5704. doi: 10.1093/nar/18.19.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles L., Fang B. L., Volckaert G., Vandenberghe A., De Wachter R. Nucleotide sequence of a crustacean 18S ribosomal RNA gene and secondary structure of eukaryotic small subunit ribosomal RNAs. Nucleic Acids Res. 1984 Dec 11;12(23):8749–8768. doi: 10.1093/nar/12.23.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. J., Pace N. R., Nuell M., Kaine B. P., Gupta R., Woese C. R. Sequence of the 16S rRNA gene from the thermoacidophilic archaebacterium Sulfolobus solfataricus and its evolutionary implications. J Mol Evol. 1985;22(4):301–307. doi: 10.1007/BF02115685. [DOI] [PubMed] [Google Scholar]
- Oyaizu H., Debrunner-Vossbrinck B., Mandelco L., Studier J. A., Woese C. R. The green non-sulfur bacteria: a deep branching in the eubacterial line of descent. Syst Appl Microbiol. 1987;9:47–53. doi: 10.1016/s0723-2020(87)80055-3. [DOI] [PubMed] [Google Scholar]
- Pernodet J. L., Boccard F., Alegre M. T., Gagnat J., Guérineau M. Organization and nucleotide sequence analysis of a ribosomal RNA gene cluster from Streptomyces ambofaciens. Gene. 1989 Jun 30;79(1):33–46. doi: 10.1016/0378-1119(89)90090-5. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Mahalingam R., Sable C. L., Venuti S. E., Cummings D. J. Nucleotide sequence of the mitochondrial genome of Paramecium. Nucleic Acids Res. 1990 Jan 11;18(1):173–180. doi: 10.1093/nar/18.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pütz J., Meinert F., Wyss U., Ehlers R. U., Stackebrandt E. Development and application of oligonucleotide probes for molecular identification of Xenorhabdus species. Appl Environ Microbiol. 1990 Jan;56(1):181–186. doi: 10.1128/aem.56.1.181-186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rairkar A., Rubino H. M., Lockard R. E. Revised primary structure of rabbit 18S ribosomal RNA. Nucleic Acids Res. 1988 Apr 11;16(7):3113–3113. doi: 10.1093/nar/16.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathgeber J., Capesius I. Nucleotide sequence of the intergenic spacer and the 18S ribosomal RNA gene from mustard (Sinapis alba). Nucleic Acids Res. 1990 Mar 11;18(5):1288–1288. doi: 10.1093/nar/18.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch H., Larsen N., Schmitt R. Phylogenetic relationships of the green alga Volvox carteri deduced from small-subunit ribosomal RNA comparisons. J Mol Evol. 1989 Sep;29(3):255–265. doi: 10.1007/BF02100209. [DOI] [PubMed] [Google Scholar]
- Raynal F., Michot B., Bachellerie J. P. Complete nucleotide sequence of mouse 18 S rRNA gene: comparison with other available homologs. FEBS Lett. 1984 Feb 27;167(2):263–268. doi: 10.1016/0014-5793(84)80139-8. [DOI] [PubMed] [Google Scholar]
- Ree H. K., Cao K. M., Thurlow D. L., Zimmermann R. A. The structure and organization of the 16S ribosomal RNA gene from the archaebacterium Thermoplasma acidophilum. Can J Microbiol. 1989 Jan;35(1):124–133. doi: 10.1139/m89-019. [DOI] [PubMed] [Google Scholar]
- Rice E. L. Nucleotide sequence of the 18S ribosomal RNA gene from the Atlantic sea scallop Placopecten magellanicus (Gmelin, 1791). Nucleic Acids Res. 1990 Sep 25;18(18):5551–5551. doi: 10.1093/nar/18.18.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossau R., Heyndrickx L., Van Heuverswyn H. Nucleotide sequence of a 16S ribosomal RNA gene from Neisseria gonorrhoeae. Nucleic Acids Res. 1988 Jul 11;16(13):6227–6227. doi: 10.1093/nar/16.13.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salim M., Maden B. E. Nucleotide sequence of Xenopus laevis 18S ribosomal RNA inferred from gene sequence. Nature. 1981 May 21;291(5812):205–208. doi: 10.1038/291205a0. [DOI] [PubMed] [Google Scholar]
- Sargent M., Zahn R., Walters B., Gupta R., Kaine B. Nucleotide sequence of the 18S rDNA from the microalga Nanochlorum eucaryotum. Nucleic Acids Res. 1988 May 11;16(9):4156–4156. doi: 10.1093/nar/16.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Leuteritz M., Weiss N., Ludwig W., Kirchhof G., Seidel-Rüfer H. Taxonomic study of anaerobic, gram-negative, rod-shaped bacteria from breweries: emended description of Pectinatus cerevisiiphilus and description of Pectinatus frisingensis sp. nov., Selenomonas lacticifex sp. nov., Zymophilus raffinosivorans gen. nov., sp. nov., and Zymophilus paucivorans sp. nov. Int J Syst Bacteriol. 1990 Jan;40(1):19–27. doi: 10.1099/00207713-40-1-19. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Collings J. C., Gray M. W. Structure and evolution of the small subunit ribosomal RNA gene of Crithidia fasciculata. Curr Genet. 1986;10(5):405–410. doi: 10.1007/BF00418414. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Heinonen T. Y., Young P. G., Gray M. W. A discontinuous small subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1986 Apr 15;261(11):5187–5193. [PubMed] [Google Scholar]
- Seilhamer J. J., Olsen G. J., Cummings D. J. Paramecium mitochondrial genes. I. Small subunit rRNA gene sequence and microevolution. J Biol Chem. 1984 Apr 25;259(8):5167–5172. [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemeister G., Hachtel W. Organization and nucleotide sequence of ribosomal RNA genes on a circular 73 kbp DNA from the colourless flagellate Astasia longa. Curr Genet. 1990 May;17(5):433–438. doi: 10.1007/BF00334524. [DOI] [PubMed] [Google Scholar]
- Sloof P., Van den Burg J., Voogd A., Benne R., Agostinelli M., Borst P., Gutell R., Noller H. Further characterization of the extremely small mitochondrial ribosomal RNAs from trypanosomes: a detailed comparison of the 9S and 12S RNAs from Crithidia fasciculata and Trypanosoma brucei with rRNAs from other organisms. Nucleic Acids Res. 1985 Jun 11;13(11):4171–4190. doi: 10.1093/nar/13.11.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J. Primary structure of the Paramecium tetraurelia small-subunit rRNA coding region: phylogenetic relationships within the Ciliophora. J Mol Evol. 1986;23(1):53–60. doi: 10.1007/BF02100998. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Ingold A., Karlok M., Nielsen H., Engberg J. Phylogenetic evidence for the acquisition of ribosomal RNA introns subsequent to the divergence of some of the major Tetrahymena groups. EMBO J. 1986 Dec 20;5(13):3625–3630. doi: 10.1002/j.1460-2075.1986.tb04691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Miotto K., Miller L. Primary structure of the Neurospora crassa small subunit ribosomal RNA coding region. Nucleic Acids Res. 1986 Dec 9;14(23):9540–9540. doi: 10.1093/nar/14.23.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Swanton M. T., Gunderson J. H., Elwood H. J. Sequence of the small subunit ribosomal RNA gene from the hypotrichous ciliate Euplotes aediculatus. J Protozool. 1986 Feb;33(1):26–29. doi: 10.1111/j.1550-7408.1986.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Séquence nucléotidique du gène de l'ARN ribosomique 15S mitochondrial de la levure. C R Seances Acad Sci D. 1980 Dec 8;291(12):933–936. [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Spencer D. F., Schnare M. N., Gray M. W. Pronounced structural similarities between the small subunit ribosomal RNA genes of wheat mitochondria and Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):493–497. doi: 10.1073/pnas.81.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Charfreitag O. Partial 16S rRNA primary structure of five Actinomyces species: phylogenetic implications and development of an Actinomyces israelii-specific oligonucleotide probe. J Gen Microbiol. 1990 Jan;136(1):37–43. doi: 10.1099/00221287-136-1-37. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Fischer A., Roggentin T., Wehmeyer U., Bomar D., Smida J. A phylogenetic survey of budding, and/or prosthecate, non-phototrophic eubacteria: membership of Hyphomicrobium, Hyphomonas, Pedomicrobium, Filomicrobium, Caulobacter and "dichotomicrobium" to the alpha-subdivision of purple non-sulfur bacteria. Arch Microbiol. 1988;149(6):547–556. doi: 10.1007/BF00446759. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Urbance J. W. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990 Jan;172(1):116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nagata A., Ono Y., Yamada T. Complete nucleotide sequence of the 16S rRNA gene of Mycobacterium bovis BCG. J Bacteriol. 1988 Jun;170(6):2886–2889. doi: 10.1128/jb.170.6.2886-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Yamada T. The nucleotide sequence of 16S rRNA gene from Streptomyces lividans TK21. Nucleic Acids Res. 1988 Jan 11;16(1):370–370. doi: 10.1093/nar/16.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Oono K., Sugiura M. The complete nucleotide sequence of a rice 17S rRNA gene. Nucleic Acids Res. 1984 Jul 11;12(13):5441–5448. doi: 10.1093/nar/12.13.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschke C., Ruland K., Herrmann R. Nucleotide sequence of the 16S rRNA of Mycoplasma hyopneumoniae. Nucleic Acids Res. 1987 May 11;15(9):3918–3918. doi: 10.1093/nar/15.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Hancock J. M., Webb D. A., Tautz C., Dover G. A. Complete sequences of the rRNA genes of Drosophila melanogaster. Mol Biol Evol. 1988 Jul;5(4):366–376. doi: 10.1093/oxfordjournals.molbev.a040500. [DOI] [PubMed] [Google Scholar]
- Tohdoh N., Sugiura M. The complete nucleotide sequence of 16S ribosomal RNA gene from tobacco chloroplasts. Gene. 1982 Feb;17(2):213–218. doi: 10.1016/0378-1119(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Torczynski R. M., Fuke M., Bollon A. P. Cloning and sequencing of a human 18S ribosomal RNA gene. DNA. 1985 Aug;4(4):283–291. doi: 10.1089/dna.1985.4.283. [DOI] [PubMed] [Google Scholar]
- Torczynski R., Bollon A. P., Fuke M. The complete nucleotide sequence of the rat 18S ribosomal RNA gene and comparison with the respective yeast and frog genes. Nucleic Acids Res. 1983 Jul 25;11(14):4879–4890. doi: 10.1093/nar/11.14.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschka H. Y., Höpfl P., Ludwig W., Schleifer K. H., Ulbrich N., Erdmann V. A. Complete nucleotide sequence of a 16S ribosomal RNA gene from Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Mar 25;16(5):2348–2348. doi: 10.1093/nar/16.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkl H., Lang B. F., Wolf K. Nucleotide sequence of the gene encoding the small ribosomal RNA in the mitochondrial genome of the fission yeast Schizosaccharomyces pombe. Nucleic Acids Res. 1989 Aug 25;17(16):6730–6730. doi: 10.1093/nar/17.16.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K., Tsien H. C., Hanson R. S., DePalma S. R., Scholtz R., LaRoche S. 16S ribosomal RNA sequence analysis for determination of phylogenetic relationship among methylotrophs. J Gen Microbiol. 1990 Jan;136(1):1–10. doi: 10.1099/00221287-136-1-1. [DOI] [PubMed] [Google Scholar]
- Unfried I., Stocker U., Gruendler P. Nucleotide sequence of the 18S rRNA gene from Arabidopsis thaliana Co10. Nucleic Acids Res. 1989 Sep 25;17(18):7513–7513. doi: 10.1093/nar/17.18.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterman B. M., Baumann P., McLean D. L. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J Bacteriol. 1989 Jun;171(6):2970–2974. doi: 10.1128/jb.171.6.2970-2974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]
- Wallbanks S., Martinez-Murcia A. J., Fryer J. L., Phillips B. A., Collins M. D. 16S rRNA sequence determination for members of the genus Carnobacterium and related lactic acid bacteria and description of Vagococcus salmoninarum sp. nov. Int J Syst Bacteriol. 1990 Jul;40(3):224–230. doi: 10.1099/00207713-40-3-224. [DOI] [PubMed] [Google Scholar]
- Waters A. P., McCutchan T. F. Partial sequence of the asexually expressed SU rRNA gene of Plasmodium vivax. Nucleic Acids Res. 1989 Mar 11;17(5):2135–2135. doi: 10.1093/nar/17.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A. P., Unnasch T. R., Wirth D. F., McCutchan T. F. Sequence of a small ribosomal RNA gene from Plasmodium lophurae. Nucleic Acids Res. 1989 Feb 25;17(4):1763–1763. doi: 10.1093/nar/17.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Giovannoni S. J., Woese C. R. The Deinococcus-Thermus phylum and the effect of rRNA composition on phylogenetic tree construction. Syst Appl Microbiol. 1989;11:128–134. doi: 10.1016/s0723-2020(89)80051-7. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Oyaizu Y., Oyaizu H., Woese C. R. Natural relationship between bacteroides and flavobacteria. J Bacteriol. 1985 Oct;164(1):230–236. doi: 10.1128/jb.164.1.230-236.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Woese C. R., Dobson M. E., Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985 Nov 1;230(4725):556–558. doi: 10.1126/science.3931222. [DOI] [PubMed] [Google Scholar]
- Whiley R. A., Fraser H. Y., Douglas C. W., Hardie J. M., Williams A. M., Collins M. D. Streptococcus parasanguis sp. nov., an atypical viridans Streptococcus from human clinical specimens. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):115–121. doi: 10.1111/j.1574-6968.1990.tb04133.x. [DOI] [PubMed] [Google Scholar]
- Williams A. M., Collins M. D. Molecular taxonomic studies on Streptococcus uberis types I and II. Description of Streptococcus parauberis sp. nov. J Appl Bacteriol. 1990 May;68(5):485–490. doi: 10.1111/j.1365-2672.1990.tb02900.x. [DOI] [PubMed] [Google Scholar]
- Williams A. M., Fryer J. L., Collins M. D. Lactococcus piscium sp. nov. a new Lactococcus species from salmonid fish. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):109–113. doi: 10.1016/0378-1097(90)90134-c. [DOI] [PubMed] [Google Scholar]
- Witt D., Bergstein-Ben Dan T., Stackebrandt E. Nucleotide sequence of 16S rRNA and phylogenetic position of the green sulfur bacterium Clathrochloris sulfurica. Arch Microbiol. 1989;152(2):206–208. doi: 10.1007/BF00456103. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Debrunner-Vossbrinck B. A., Oyaizu H., Stackebrandt E., Ludwig W. Gram-positive bacteria: possible photosynthetic ancestry. Science. 1985;229:762–765. doi: 10.1126/science.11539659. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gutell R. R. Evidence for several higher order structural elements in ribosomal RNA. Proc Natl Acad Sci U S A. 1989 May;86(9):3119–3122. doi: 10.1073/pnas.86.9.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Mandelco L., Yang D., Gherna R., Madigan M. T. The case for relationship of the flavobacteria and their relatives to the green sulfur bacteria. Syst Appl Microbiol. 1990;13:258–262. doi: 10.1016/s0723-2020(11)80196-7. [DOI] [PubMed] [Google Scholar]
- Wolff G., Kück U. The structural analysis of the mitochondrial SSUrRNA implies a close phylogenetic relationship between mitochondria from plants and from the heterotrophic alga Prototheca wickerhamii. Curr Genet. 1990 Apr;17(4):347–351. doi: 10.1007/BF00314883. [DOI] [PubMed] [Google Scholar]
- Wong O. C., Clark-Walker G. D. Sequence of the gene for the cytoplasmic ribosomal RNA small subunit from Candida (Torulopsis) glabrata. Nucleic Acids Res. 1990 Apr 11;18(7):1888–1888. doi: 10.1093/nar/18.7.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T. Nucleotide sequence of the chloroplast 16S rRNA gene from the unicellular green alga Chlorella ellipsoidea. Nucleic Acids Res. 1988 Oct 25;16(20):9865–9865. doi: 10.1093/nar/16.20.9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. X., Yang D. C., Woese C. R., Bryant M. P. Assignment of Clostridium bryantii to Syntrophospora bryantii gen. nov., comb. nov. on the basis of a 16S rRNA sequence analysis of its crotonate-grown pure culture. Int J Syst Bacteriol. 1990 Jan;40(1):40–44. doi: 10.1099/00207713-40-1-40. [DOI] [PubMed] [Google Scholar]
- de Boer L., Dijkhuizen L., Grobben G., Goodfellow M., Stackebrandt E., Parlett J. H., Whitehead D., Witt D. Amycolatopsis methanolica sp. nov., a facultatively methylotrophic actinomycete. Int J Syst Bacteriol. 1990 Apr;40(2):194–204. doi: 10.1099/00207713-40-2-194. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lake J. A., Simpson A. M., Simpson L. A minimal ribosomal RNA: sequence and secondary structure of the 9S kinetoplast ribosomal RNA from Leishmania tarentolae. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1401–1405. doi: 10.1073/pnas.82.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen J. M., Stutz E. The soybean chloroplast genome: nucleotide sequence of a region containing tRNA-Val (GAC) and 16S rRNA gene. Nucleic Acids Res. 1988 Feb 11;16(3):1200–1200. doi: 10.1093/nar/16.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]


