Abstract
Much recent work in systems neuroscience has focused on how dynamic interactions between different cortical regions underlie complex brain functions such as motor coordination, language, and emotional regulation. Various studies using neuroimaging and neurophysiologic techniques have suggested that in many neuropsychiatric disorders, these dynamic brain networks are dysregulated. Here we review the utility of combined noninvasive brain stimulation and neuroimaging approaches towards greater understanding of dynamic brain networks in health and disease. Brain stimulation techniques, such as transcranial magnetic stimulation and transcranial direct current stimulation, use electromagnetic principles to noninvasively alter brain activity, and induce focal but also network effects beyond the stimulation site. When combined with brain imaging techniques such as functional MRI, PET and EEG, these brain stimulation techniques enable a causal assessment of the interaction between different network components, and their respective functional roles. The same techniques can also be applied to explore hypotheses regarding the changes in functional connectivity that occur during task performance and in various disease states such as stroke, depression and schizophrenia. Finally, in diseases characterized by pathologic alterations in either the excitability within a single region or in the activity of distributed networks, such techniques provide a potential mechanism to alter cortical network function and architectures in a beneficial manner.
Keywords: Humans, transcranial magnetic stimulation, transcranial direct current stimulation, EEG, fMRI, functional connectivity
INTRODUCTION
Traditionally, insights into brain function have been largely derived from studying the deficits caused by specific brain lesions. The view emerging from this approach posits a simplified structure-function relationship, in which anatomically distinct brain regions perform specialized, relatively independent computations (e.g. visual cortex is responsible for early visual processing). More recently, this approach has been extended by studies using brain imaging modalities such as electroencephalography (EEG), positron emission tomography (PET), and functional MRI (fMRI) to study brain function both in the resting state (Fox & Raichle, 2007) and during performance of various behavioral tasks. It has become increasingly apparent that complex brain functions, such as coordinated movement, memory and language, depend critically on interactions between brain areas, leading to the concept of functional connectivity networks— distributed brain regions interacting (often transiently) to perform a particular neural function. Studies have suggested that abnormalities in the interactions of network components play a critical role in common neuropsychiatric disorders ranging from depression to epilepsy (Mayberg et al., 2005; Lytton, 2008), and damage to specific functional connectivity networks can lead to distinct neurological syndromes (Seeley et al., 2009). Furthermore, the deficits and functional recovery after damage from strokes or traumatic brain injury may depend on the architecture and adaptability of these networks (He et al., 2007b; Ween, 2008; Kumar et al., 2009). Consequently, there is active research exploring functional connectivity in normal subjects and in patients suffering from various neuropsychiatric disorders, with the hope that it may lead to valuable biomarkers of disease and new therapeutic approaches.
Most neuroscience techniques utilized in humans either passively measure brain activity in different ways, or require invasive procedures. However, a number of noninvasive techniques for manipulating brain activity have been developed, permitting targeted interventions on human brain function and behavior. The two most common noninvasive brain stimulation techniques, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), both rely on electromagnetic principles to influence brain activity. Combination of these brain stimulation techniques with traditional neuroimaging methods enables more sophisticated studies of the mechanisms and dynamics of brain activity, and their relationship with specific cognitive processes. Thus it becomes possible to test hypotheses regarding causal interactions between different brain regions in health and disease. Furthermore, by producing potentially long-lasting changes in cortical function, brain stimulation techniques provide a new therapeutic modality whose utility is being explored in a variety of diseases.
In this review, begin with a brief review of functional connectivity and network theory. We then explore how neuroimaging and neurophysiology are being used to study functional connectivity networks, and provide insight into the distributed nature of common brain diseases. Next, we review basic principles of noninvasive brain stimulation techniques and the evidence that these techniques have network effects beyond the stimulation site. Finally, we provide examples of how these tools can be combined to understand, and selectively manipulate functional connectivity networks. We focus on three clinical conditions (stroke, depression, and schizophrenia) to illustrate how abnormal network dynamics may underlie common brain diseases, and how manipulation of these networks through noninvasive brain stimulation represents a promising therapeutic intervention.
FUNCTIONAL CONNECTIVITY AND NETWORK THEORY
Most early studies using either neuroimaging or electrophysiology were concerned with identifying individual brain regions or cells that were modulated by a particular stimulus or task. From the electrophysiology work of Hubel and Wiesel (1962) to cognitive activation paradigms in human neuroimaging (Posner & Raichle, 1994) this approach has been very successful. However, no brain region operates in isolation. Instead, brain regions are integrated in complex, distributed neural networks, and studying the interactions between regions is proving to be just as important as understanding the response properties of individual regions. The interaction between brain regions has been termed “functional connectivity” and can refer to any examination of inter-regional correlations in neuronal variability (Friston et al., 1993; Horwitz, 2003).
Mathematically, networks can be represented as graphs, i.e. a group of interacting entities (nodes), connected by lines (edges), indicating which pairs of nodes directly interact. For our purposes these nodes can represent neurons, populations of neurons within specific anatomical brain regions, or the locations of sensors which measure neural activity (as in EEG). Certain important generic network properties turn out to depend solely on topological properties, independent of the details of individual network function. We illustrate this idea by discussing two simple intuitive properties, global and local efficiency of information transfer. For more complete discussions of network structure-function dependencies the reader is referred to several excellent recent reviews (Albert & Barabasi, 2000; Strogatz, 2001; Bassett & Bullmore, 2006; Stam & Reijneveld, 2007; Reijneveld et al., 2007; Sporns, 2010).
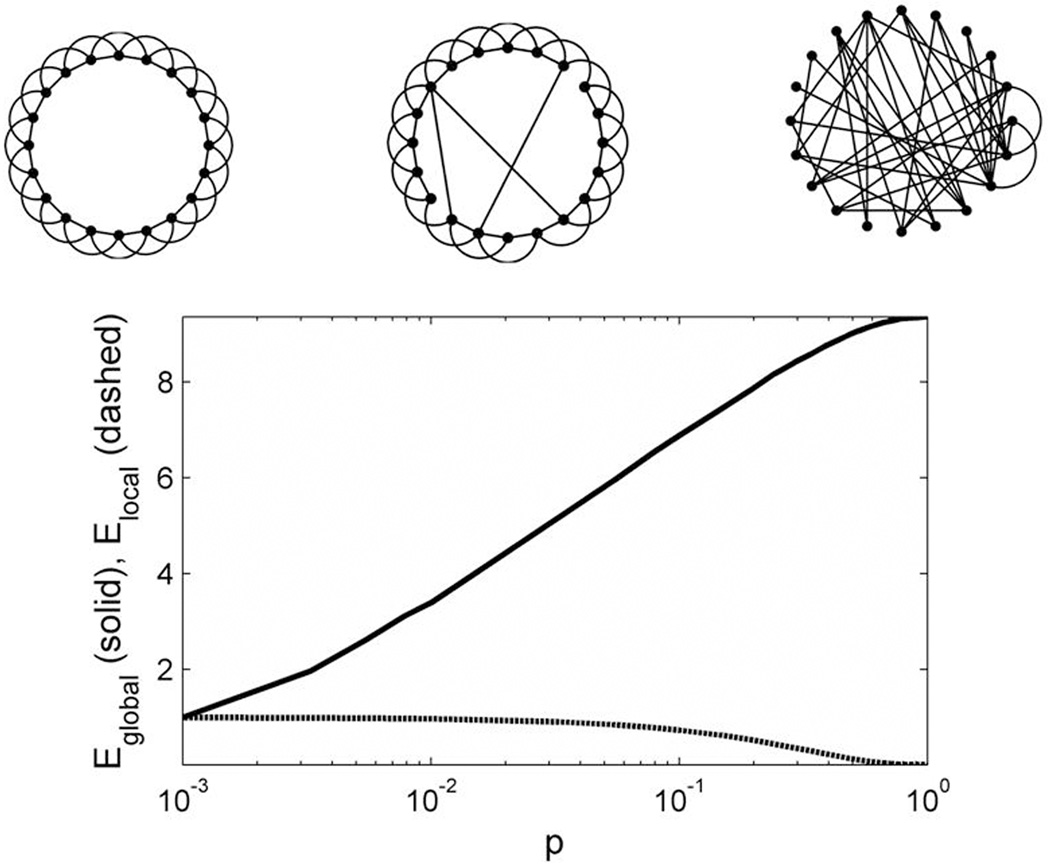
The dependency of network function on topology is most easily appreciated by considering a now-classic series of simple abstract models introduced by Watts and Strogatz (1998). Let us imagine that each node is continually exchanging information with the nodes with which it is connected (i.e. its neighbors), and that this exchange takes place at a constant rate. Consider first a regular ring network, a circular arrangement of nodes in which each node is connected by a line or edge to each of its four nearest neighbors (Figure 1A - left). This network is highly clustered, or cliquish, in that for any given node, any pair of its neighbors is likely to be connected to one another. This notion can be quantified by the clustering coefficient of a node, which ranges from 0 (none of the neighbors are connected) to 1 (all neighbors are connected). In functional terms, graphs with larger clustering coefficients support rapid local sharing of information (between neighboring nodes). Therefore, we define the local efficiency of a network as the average value of the clustering coefficients for each individual node (Latora & Marchiori, 2003; Achard & Bullmore, 2007). While such regular, highly clustered networks have high local efficiency, information must pass through a large number of short-range connections to reach nodes on the opposite side of the network, so that the average minimum path length between any two nodes will be large, and thus the global efficiency of information transfer (the average rate at which messages travel between any two randomly selected nodes) will be low. Now consider the other extreme, in which all connections are random (Figure 1A – right). In such random networks, the distance between any two nodes is likely to be small, resulting in a low minimum path length and high efficiency of global information transfer. However, local clustering (and thereby local efficiency) is also low, with the result that the potential for modular information processing is limited. In between these extremes are networks with predominantly locally structured connections, but also with a few random long-range connections (Figure 1A – center). In such graphs, known as small-world networks, the theoretical advantages of high clustering (local efficiency) that characterize regular networks are combined with the short average path-lengths (global efficiency) characteristic of random networks. Such small-world networks have high complexity, in that they are simultaneously functionally segregated (small subsets of the system can behave independently) and also functionally integrated (large subsets tend to behave coherently). (Sporns et al., 2000).
Figure 1. Network architectures and efficiency statistics.
(A) Different types of networks. Regular network in which nodes are connected only to their two nearest neighbors on either side (left). Small world network, in which a small number of local connections are replaced by long-distance connections at random locations (center). Random network, in which nodes are connected at random, with a resulting loss of local connectivity (right). (B) Global efficiency (Eglobal, solid line) and local efficiency (Elocal, dashed line) as a function of the probability of random connections.
Over the past decade, converging theoretical and experimental results have indicated that brain functional networks typically have small-world topology, with short average path length (high global efficiency) and high clustering (high local efficiency). Brain functional networks tend to be robust to random lesions, but highly vulnerable to targeted lesions, due to the existence of hubs, i.e. highly connected nodes which account for a large fraction of the graph’s overall connectivity (Achard et al., 2006; He et al., 2007c, 2007a; Xia et al., 2010). Brain functional networks are sparse, that is, only a relatively small fraction of the total number of pairs is directly connected. Finally, brain functional networks often operate in a critical dynamical state, supporting rapid reconfiguration of graph topology, a feature thought to be related to the need to rapidly switch cognitive states.
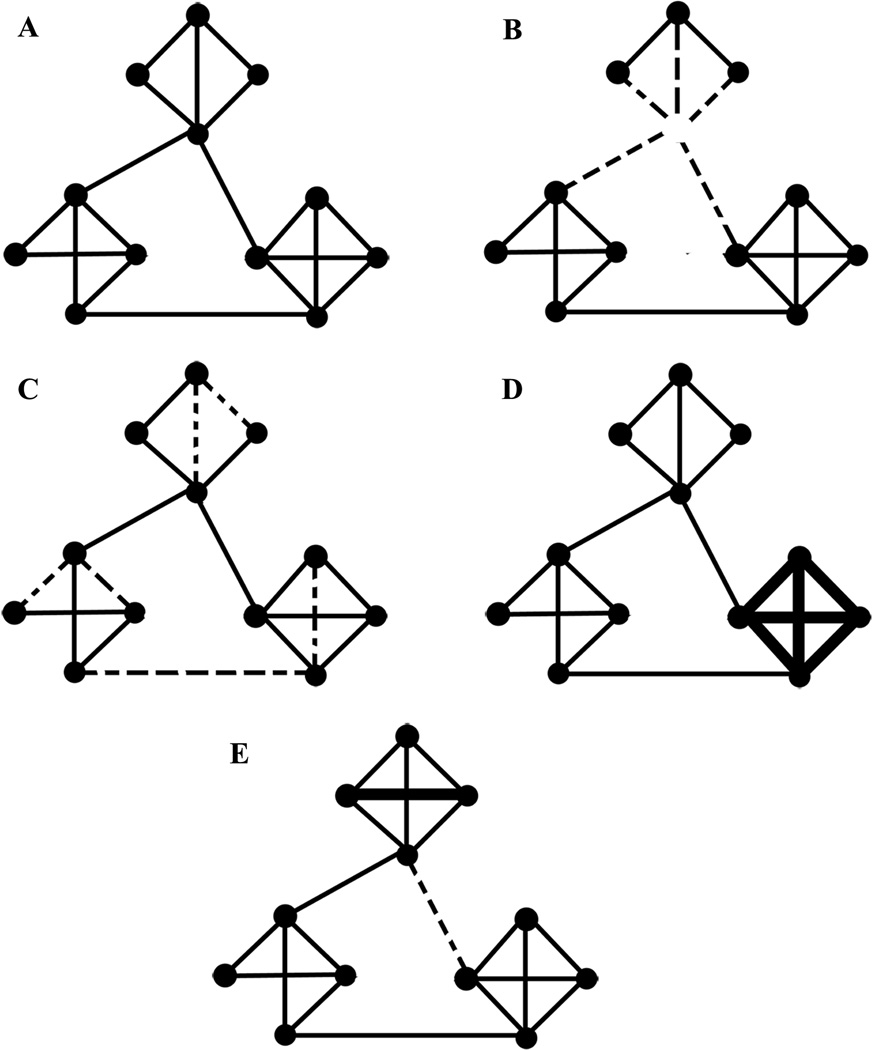
Theoretically, different neuropsychiatric disorders could result from a variety of different network pathologies. Consider a simplified network comprised of clusters of nodes with dense local connectivity and a few long-range connections (Figure 2A), consistent with the small-world topographies identified in human brains. One set of brain pathologies could result from direct elimination of node(s), with resulting network dysfunction (Figure 2B). Ischemic stroke represents a classical example of a neuropsychiatric disease with such a mechanism. Alternatively, the functional network could be disrupted by elimination of connections between different nodes (Figure 2C), as may occur in diseases in which the primary pathology is in the white matter connections between brain regions, such as multiple sclerosis. A third possibility is that the strength of the connections between nodes is altered in a manner that results in relative hypo- or hyperactivity within a specific subnetwork (Figure 2D). Epilepsy may be a paradigmatic example of a disease resulting from such a process (Bettus et al., 2008), while recent work suggests that such alterations in the strength of connectivity between different brain regions are also critical in depression and schizophrenia. A shift in the topology of network connectivity (for example, a decrease in long-distance connections with increases in local connectivity; Figure 2E) could affect the efficiency of information processing in the brain. Studies have suggested that such network topology changes might be occurring in autism (Barttfeld et al., 2011). Finally, another possibility is that network connectivity is unchanged, but the operations carried out by different subnetworks are somehow altered. It is worth emphasizing that studies focused only on anatomic pathologies (ie. structural MRI) may not detect any abnormalities in diseases with preserved structural connectivity but altered functional connectivity (such as in figure 2D), emphasizing the critical need for further studies investigating brain connectivity networks.
Figure 2. Theoretical mechanisms of network pathology.
(A) The normal network, comprised of three densely connected local clusters, with a few long-range connections between clusters. (B) Loss of a node (and thus associated connections, dashed lines) in the top cluster. (C) A loss of connections (dashed lines) without a change in the nodes. (D) Increased connectivity (thick lines) within a local cluster (bottom right). (E) Increased local connectivity (thick line, top cluster) along with loss of a long-distance connection between clusters (dashed line). These changes would result in a substantial change in network information processing metrics (increased clustering coefficient and local efficiency, but also increased path length and decreased global efficiency).
STUDYING BRAIN NETWORKS IN HUMANS
A key technical question in studying brain networks is the way in which connectivity is defined and measured. Structural connectivity, the stable direct physical pathways linking spatially distinct brain regions, is distinguished from the dynamical or state-dependent functional connectivity and effective connectivity. Effective connectivity describes the directional flow of information, or more generally, the causal relationships between nodes in a graph, e.g. relationships such as “changes in the activity of A lead to changes in the activity of B”. However, the techniques for determining effective connectivity are complex, and the tools available to analyze the resulting networks are limited. It is often significantly more straightforward, and much more common, to simply compute measures of statistical dependence (correlation) between nodes, which is dubbed functional connectivity.
In humans, functional connectivity has been studied across a broad range of spatial and temporal scales. Using neuroimaging, functional connectivity has been studied using PET, near-infrared spectroscopy, and fMRI. With these methods, variability has been correlated across subjects, runs, blocks, trials, or individual blood-oxygen-level dependence (BOLD) time points and has been studied both during resting and task conditions, an ambiguity which can become confusing (Horwitz, 2003; Rogers et al., 2007). It is yet unclear if functional connectivity assessed in these various ways reflects similar phenomena (Fox & Raichle, 2007), but it is clear that these inter-regional interactions play a critical role in behavior and disease.
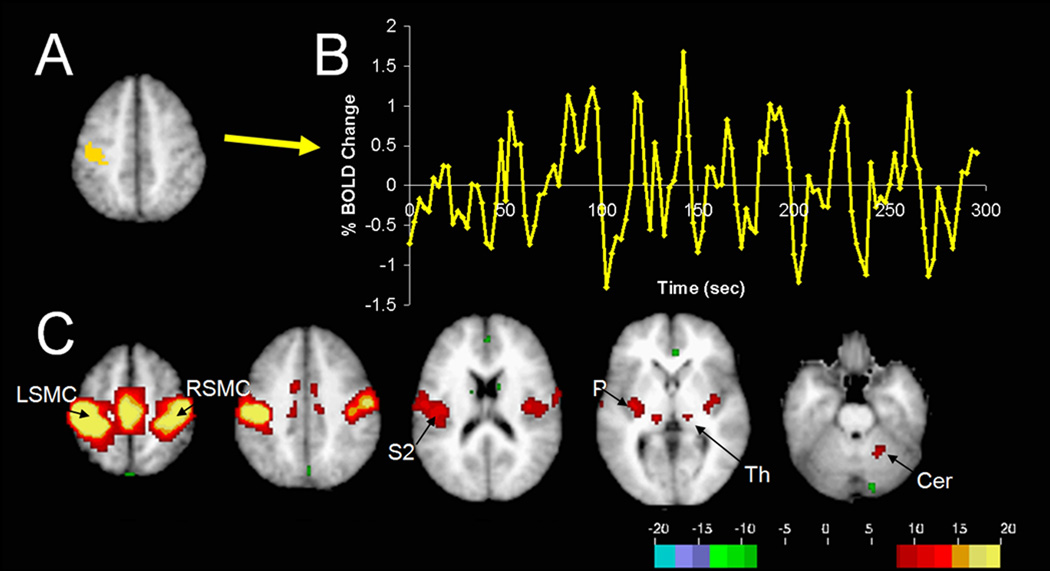
Currently, the most popular neuroimaging approach for studying functional connectivity is using fMRI to examine inter-regional correlations across individual BOLD time-points (functional connectivity MRI, or fcMRI). Often, these correlations are examined during specific tasks and have been related to individual subject’s task performance (Ranganath et al., 2005; Hampson et al., 2006b), genetics (Pezawas et al., 2005), and even personality (Pezawas et al., 2005). However, a recent advance with important clinical applications has been the discovery of robust inter-regional correlations in spontaneous BOLD fluctuations present even in the absence of an assigned task, referred to as resting state functional connectivity (for review see (Fox & Raichle, 2007)). These spontaneous fluctuations are consistently correlated between regions with similar functional properties and known anatomical connections including somatomotor, visual, auditory, language, default mode, and corticothalamic networks (Fox & Raichle, 2007). For example, one can extract the spontaneous BOLD modulations from a region such as the left somatomotor cortex and compute the correlation between this extracted signal and all other brain regions to obtain a map of the human somatomotor system (Biswal et al., 1995) (Figure 3). Anticorrelations between regions with apparent opposing functional properties have also been observed (Fox et al., 2005; Fransson, 2005). These spontaneous fluctuations predict the task-response properties of brain regions (De Luca et al., 2005; Vincent et al., 2006), identify subjects’ aptitude for different cognitive tasks (Hampson et al., 2006a; Seeley et al., 2007), facilitate refinement of neuro-anatomical models (Fox et al., 2006; Dosenbach et al., 2007), and account for trial-to-trial variability in behavior (Fox et al., 2007). Significant resting state fcMRI abnormalities have been identified across almost every major neurological and psychiatric disease (for reviews see (Greicius, 2008; Fox & Greicius, 2010; Zhang & Raichle, 2010)). As these resting state fcMRI abnormalities continue to be replicated, refined, and clarified, the next step will be translating this information into practical clinical interventions.
Figure 3. Generation of resting-state correlation maps.
(A) Seed region in the left somatomotor cortex (LSMC) is shown in yellow. (B) Time course of spontaneous BOLD activity recorded during resting fixation and extracted from the seed region. (C) Voxels significantly correlated with the extracted time course assessed using a random effects analysis across a population of ten subjects (Z score values). In addition to correlations with the right somatomotor cortex (RSMC) and medial motor areas, correlations are observed with secondary somatosensory association cortex (S2), posterior nuclei of the thalamus (Th), putamen (P), and cerebellum (Cer). Reproduced with permission from (Fox and Raichle 2007).
Neurophysiologic techniques have also been used to probe functional connectivity in the human brain. Compared with fMRI, EEG and magnetoencephalography (MEG) have poorer spatial resolution (millimeters for fMRI vs centimeters for EEG/MEG), but superior temporal resolution (milliseconds for EEG/MEG vs seconds for fMRI). Consequently, EEG and MEG permit study of temporal dynamics across a much broader bandwidth (on the order of order of 1–100Hz for EEG vs 0.001–0.5Hz for fMRI). Functional networks derived from fMRI data may thus in principle be more easily and directly related to precise anatomical structures, while EEG / MEG signals more directly reflect neuronal activity.
Intriguingly, recent studies have shown that EEG/MEG network topologies change over the course of a lifetime (Micheloyannis et al., 2009), and that individual differences in graph theoretic network properties may be related to intelligence (IQ) and cognitive performance (Micheloyannis et al., 2006b; Bassett et al., 2009). A number of recent papers have suggested that alterations in EEG network properties may be seen in various neuropsychiatric diseases. In Alzheimer’s disease, EEG functional connectivity (fcEEG) analysis has shown promise as a diagnostic aid in early stages of the disease (Dauwels et al., 2010). In another fcEEG study, the severity of cognitive dysfunction in Alzheimer’s disease was found to be a monotonically decreasing function of path length, while the average clustering coefficients were similar to control subjects, suggesting that Alzheimer’s dementia may be related to loss of “small-worldliness” (Stam et al., 2007). To a lesser degree, loss of small-worldliness and lower levels of synchronization within high frequency EEG rhythms (beta and gamma) has also been reported in normal aging (Micheloyannis et al., 2009). As another example, in patients presenting after a first seizure, mean functional connectivity within the theta band has been reported to be a predictor of subsequent epilepsy, and thus may prove useful in identifying patients at risk for epilepsy who lack other markers such as epileptic spikes (Douw et al., 2010). Enhanced fcEEG across a broad range of frequencies has also been suggested as a characteristic feature within the seizure onset zone in patients with mesial temporal lobe epilepsy (Bettus et al., 2008).
Thus, both neurophysiological techniques, such as EEG, and neuroimaging techniques, such as fMRI, have been used to assess the functional connectivity of the human brain during both the resting state and during task activity, and to explore the structure of brain activity. Furthermore, alterations in functional connectivity have been associated with several neuropsychiatric diseases. Consequently, there is a pressing need for tools that enable more precise study and manipulation of human cortical networks in vivo. Noninvasive brain stimulation techniques hold significant promise in this regard. Manipulation of diffuse neurotransmitter systems through pharmacological therapy may prove useful in normalizing altered network dynamics (Anand et al., 2005a). However, brain network dynamics in health and disease may be more directly addressed through spatially and temporally more specific and more precisely quantifiable interventions such as TMS or tDCS.
BRAIN STIMULATION TECHNIQUES
Transcranial Magnetic Stimulation (TMS)
TMS is based on the principle of electromagnetic induction; briefly, a changing electric current in the stimulation coil produces a magnetic flux, which in turn induces electric currents in brain tissue. The basic TMS stimulator design involves a capacitive high-voltage, high-current charge-discharge system connected via a switch (usually a thyristor or a silicon-controlled rectifier to prevent ringing in the circuit) to the inductor of the stimulation coil (see Wagner et al, (2007) for further review). The effect of a TMS pulse on cortical activity is dependent on a number of different factors, including the strength of the magnetic flux, the shape of the stimulation coil, the shape and duration of the pulse, the distance and angle between the coil and the cortical surface, the direction of the induced electrical currents, the precise stimulation sequence, and the underlying cortical architecture and activity. One commonly used coil design is the “figure-8” or “butterfly”, in which two round coils are placed side by side such that the currents flow in the same direction at the junction point. As a result, the induced electric fields add up to a maximum in the region below the junction of the two coils, thereby limiting the area in which the induced currents are sufficient to significantly alter neuronal activity. The precise extent of the cortical surface that is intensely stimulated has been debated, but models and some experimental data on evoked responses suggest that it is on the order of approximately 1 cm2 (Cowey & Walsh, 2000; Wagner et al., 2004).
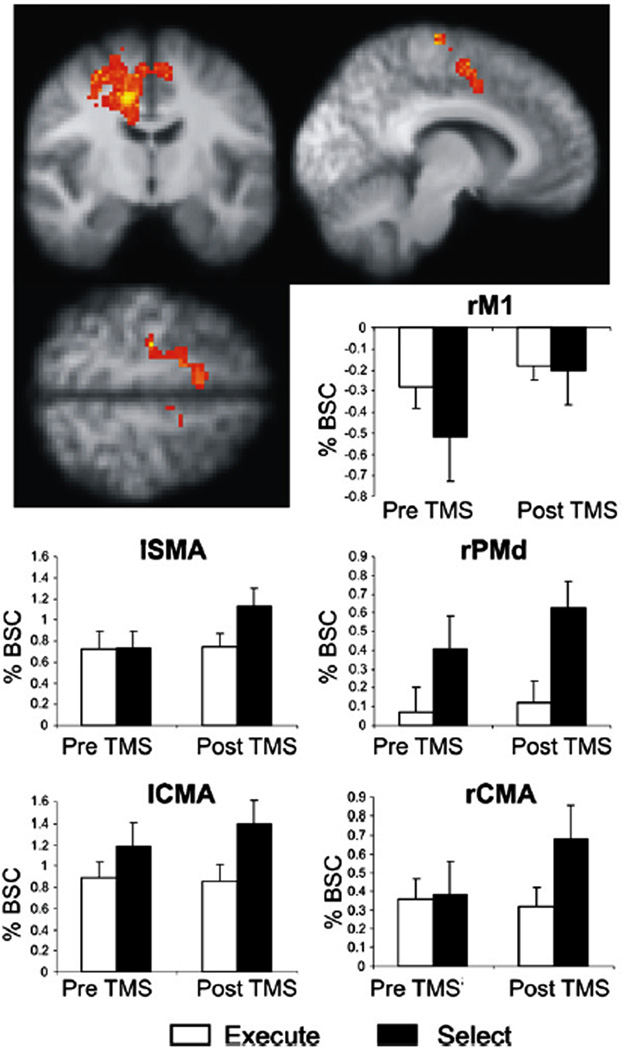
Figure 8. Compensatory activation increases in the action selection network after left dorsal premotor cortex rTMS.
1Hz (inhibitory) rTMS of left dorsal premotor cortex results in increased activation (BOLD signal) most prominently in right dorsal premotor cortex (rPMd) and right cingulate motor area (rCMA). Changes were also seen in the left supplementary motor area (lSMA), the left cingulate motor area (lCMA), and right primary motor cortex (rM1). The figures show the mean percent BOLD signal change (% BSC) when subjects performed the action selection (black bars) or the control action execution (white bars) tasks. Note that the TMS-induced activation increases occur only with action selection. (Modified with permission from O’Shea et al, 2007).
Unfortunately, relatively little is known about the precise mechanisms of TMS activation of neural tissue in vivo. One study utilizing extracellular recordings in the visual cortex of anesthetized cats assessed the effects of single-pulse TMS on neuronal activity (Moliadze et al., 2003) and demonstrated that a single TMS pulse was associated with a strong facilitation of spontaneous and visual-evoked spiking activity during the first 500ms after the TMS pulse. This was followed by a subsequent long-lasting (several second) suppression of activity, the duration of which increased with increasing stimulus strength. In another study utilizing different TMS pulse trains (1 to 4 seconds, 1 to 8 Hz), TMS increased the spontaneous activity for up to sixty seconds; in contrast, visual evoked responses were significantly decreased for approximately five minutes (Allen et al., 2007). A number of recent studies have evaluated the effect of TMS on motor cortex during epidural recordings from human patients with electrodes implanted in the spinal cord for treatment of chronic pain (see Di Lazzaro (2008) for review). These studies have demonstrated that the various TMS protocols all produce effects that are believed to be mediated primarily via trans-synaptic intracortical pathways, rather than by direct axonal activation. However, there continues to be significant uncertainty regarding the precise cellular mechanisms by which TMS exerts its effects. Furthermore, several studies have suggested that the effects of single pulses of TMS are significantly affected by the underlying pre-existing cortical state (Silvanto et al., 2008; Romei et al., 2008; Silvanto & Pascual-Leone, 2008; Sauseng et al., 2009; Thut et al., 2011). Consequently, the relationship between the local effects of TMS and the network changes that result remain almost entirely unknown. Despite this uncertainty, TMS continues to be used to probe and to alter cortical excitability in a variety of different experimental paradigms.
TMS of motor cortex produces muscle responses, termed motor-evoked potentials (MEPs), which provide a particularly useful metric for measuring cortical responses to TMS. The MEP size varies with the intensity of stimulus, with stronger TMS stimuli producing larger MEPs (van der Kamp et al., 1996). TMS-evoked MEPs are also facilitated if the subject voluntarily contracts the target muscle slightly (Hess et al., 1986, 1987; Andersen et al., 1999). Another stimulation paradigm, paired-pulse TMS, involves the application of a conditioning stimulus pulse prior to the test stimulus delivered, for example, over motor cortex. If the conditioning stimulus alters the MEP, then a functional interaction between the target of the conditioning stimulus and the location of the test stimulus is inferred.
Another important stimulation method is repetitive TMS (rTMS), which involves the delivery of trains of TMS pulses, often at high frequencies, to produce changes in cortical excitability that persist beyond the duration of the stimulus. The mechanisms through which these protocols alter excitability are unknown, but are believed to involve processes similar to synaptic long-term potentiation and long-term depression (Fitzgerald et al., 2003). In one of the earliest studies of the effects of rTMS, Pascual-Leone et al demonstrated that high-frequency (>5 Hz) rTMS trains generally increased cortical excitability, as measured via MEP size (Pascual-Leone et al., 1994). Significantly, these effects persisted for 3–4 minutes after the end of stimulation. In contrast, rTMS at frequencies of 1 Hz or below generally decreases cortical excitability (Chen et al., 1997). A recent review of studies of the effects of rTMS on cortical excitability (as measured with simultaneous EEG) notes that both low-frequency and high-frequency rTMS produce an approximately 30% change in TMS-evoked response (depression with low-frequency rTMS, and facilitation with high-frequency rTMS), with the excitability changes persisting for a mean of about 30 minutes (Thut & Pascual-Leone, 2010). Significantly however, one study demonstrated that if an identical rTMS protocol was repeated on consecutive days, the evoked change in cortical excitability was larger on day 2, implying a carryover effect (Maeda et al., 2000). More recently, Huang et al. developed a patterned repetitive stimulation protocol to rapidly induce changes in cortical plasticity (Huang et al., 2005). The “theta-burst” rTMS stimulation paradigm consists of 3 pulses at 50 Hz and intensity of 80% active motor threshold, repeated every 200 ms (ie. at 5 Hz). In the continuous protocol, a 40-second train of uninterrupted theta-burst stimulation was applied for a total of 600 pulses, resulting in a decrease in MEP amplitude of over 40%, with suppression persisting for as long as 60 minutes. In the intermittent theta burst protocol, a two-second train of theta burst stimulation was repeated every 10 seconds, also for a total of 600 pulses; the MEP amplitude was increased by up to 75%, with the facilitation lasting for about 15 to 20 minutes. In the studies of rTMS with EEG, theta-burst effects on evoked responses persisted for up to 90 minutes, longer than for conventional (fixed-rate) rTMS protocols (Thut & Pascual-Leone, 2010).
Transcranial Direct Current Stimulation (tDCS)
In tDCS, static weak polarizing electrical currents applied to the scalp penetrate cortical regions of the brain. These currents are believed to preferentially modulate the activity of neurons with axons that are oriented longitudinally in the plane of the applied electric field, producing changes in the activity of individual cortical neurons (Creutzfeldt et al., 1962; Bindman et al., 1962; Purpura & McMurtry, 1965). The induced changes in excitability occur primarily via modulation of voltage-sensitive cation channels (Lopez et al., 1991). Unlike TMS, tDCS does not directly induce cell firing, but rather modulates neuronal activity. Anodal stimulation of the cortex generally increases the excitability of underlying neurons by depolarizing cell membranes, while cathodal stimulation decreases cortical excitability via hyperpolarization (although this is not always the case (Creutzfeldt et al., 1962)). More recent studies have combined tDCS with single-pulse TMS to assess the excitability changes produced by tDCS (Nitsche & Paulus, 2000, 2001; Nitsche et al., 2003, 2005). These studies demonstrated that anodal tDCS significantly increases the size of the TMS evoked MEP, while cathodal tDCS decreases MEP size. Furthermore, these excitability changes persisted after the end of the tDCS stimulation, with the duration and magnitude of the effects varying as a function of the current intensity and duration of tDCS (Nitsche & Paulus, 2000). A subsequent study demonstrated that if tDCS is applied at 1 milli-ampere for at least 9 minutes, the induced excitability changes after cessation of stimulation were long-lasting (90 minutes when anodal tDCS was applied for 13 minutes) (Nitsche & Paulus, 2001). These long-lasting changes are believed to occur at an intracortical level, perhaps mediated through NMDA receptor activity (Liebetanz et al., 2002; Nitsche et al., 2003, 2004b, 2004a, 2005).
TRANSCRANIAL BRAIN STIMULATION AND NETWORK ANALYSIS
As summarized above (see section, “Studying Brain Networks in Humans”), much recent work has suggested that cognitive functions are carried out by a dynamic network of interacting brain regions. The integration of brain stimulation techniques and neuroimaging enables further identification and evaluation of these dynamic network interactions. TMS changes neural activity directly in a spatially and temporally focused manner. By studying how the changes induced by TMS are then propagated throughout the rest of the brain, the connectivity of the stimulated brain region can be causally assessed, and the results compared with the findings of traditional functional connectivity analysis (Pascual-Leone et al., 2000; Paus, 2005; Lee et al., 2006; O’Shea et al., 2008; Bestmann et al., 2008; Miniussi & Thut, 2010). Furthermore, since different rTMS and tDCS protocols produce somewhat long-lasting changes in neural activity in a relatively predictable manner, noninvasive brain stimulation techniques permit the directed manipulation of neural activity. The potential implications for our understanding and treatment of network dysfunction in neuropsychiatric diseases are significant.
TMS enables the assessment of dynamical changes in the interactions between cortical regions. One of the earliest uses of TMS involved producing a “virtual lesion” to assess the temporal relationship of involvement of different cortical regions in specific cognitive functions (Walsh & Pascual-Leone, 2005). For example, Amassian et al demonstrated that TMS to the occipital pole was effective in abolishing visual perception of a letter if the pulse was administered between 80 and 100ms after stimulus onset; pulses administered significantly before or after this interval had no such effect (Amassian et al., 1989). Such studies can reveal surprising results. For example, Chambers et al (2004) demonstrated that the right angular gyrus is involved in the reorienting of spatial attention at two distinctly different time points (between 90 and 120 ms after stimulus onset, and again between 210 and 240 ms after stimulus onset), suggesting that the same cortical region can be involved at different time points during a single task (Chambers et al., 2004). Furthermore, experiments with TMS can delineate the time-course of interactions between different cortical regions. As an example, Silvanto et al (2006) studied the effects of single-pulse stimulation to the frontal eye fields (FEF) on the excitability of area V5/MT (as measured by phosphene threshold, the minimum TMS intensity required to produce a phosphene). They demonstrated that FEF stimulation 20 to 40 milliseconds before stimulation of area V5/MT lowered the phosphene threshold significantly. Stimulation of the FEF at other time points had no such effects.
The paired-pulse technique has also been used to explore network connectivity and interregional interactions, particularly in the motor system (see Rothwell, 2010 for a recent review). For example, studies have shown that a conditioning stimulus applied to one motor cortex inhibits the response to a subsequent test stimulus delivered to the contralateral motor cortex (Ferbert et al., 1992; Chen et al., 2003). Similarly, TMS of motor cortex suppresses voluntary contraction of the ipsilateral hand for a short period of time (Ferbert et al., 1992; Meyer et al., 1995; Chen et al., 2003). In patients with agenesis of the corpus callosum, no such inhibition was seen (Meyer et al., 1995). Similarly, other studies have demonstrated that a conditioning TMS pulse applied to the right dorsal premotor cortex affected the MEP produced by stimulation of the contralateral primary motor cortex (Mochizuki et al., 2004), helping to confirm that the premotor cortex and motor cortex are functionally connected. In another paired-pulse study exploring cortical processing, Pascual-Leone and Walsh (2001) utilized paired-pulse protocols to demonstrate that backprojections from area V5 to V1 are important in the perception and awareness of visual motion.
Paired-pulse protocols can also be used to assess dynamic changes in functional connectivity between brain regions. For example, in an elegant experiment Davere et al (2008) showed that a conditioning stimulus applied to the ventral premotor cortex in the resting state inhibited the subsequent MEP produced by a test stimulus to the primary motor cortex. In contrast, if the conditioning stimulus was applied during a precision grasping task, the subsequent TMS-evoked MEP was facilitated, suggesting that the influence of ventral premotor cortex on motor cortex varied as a function of the task state. Thus, paired-pulse TMS can be used to not only elucidate the task-related dynamics of interhemispheric functional connectivity, but also to explore how that connectivity is altered by disease.
The combination of TMS with other neuroimaging technologies such as PET, EEG and fMRI is particularly promising for our understanding of brain network interactions. Specifically, these imaging techniques provide a richer and more sensitive toolbox for assessing the results of brain stimulation, particularly in non-eloquent areas. Furthermore, because neuroimaging data is amenable to functional connectivity and network analysis techniques, the combination of brain stimulation and neuroimaging permits the study of the effects of brain stimulation techniques on widespread networks composed of a number of different cortical regions. In addition, the time course of activity changes in these different regions can be used to assess the causal relationship between them.
One seminal early study performed PET scanning while rTMS trains of varying lengths were applied to the frontal eye fields (Paus et al., 1997) to demonstrate a significant positive relationship between blood flow and TMS in the region being stimulated (the left frontal FEF), as well as in a number of distant cortical regions, including the left medial parieto-occipital cortex, the bilateral superior parietal cortex, and the right supplementary eye field (Figure 4). Thus, TMS produced changes in cerebral blood flow not only at the site of stimulation, but in a distributed network of functionally connected regions. A subsequent study showed that the pattern of blood flow changes varies as a function of the stimulated region (Chouinard et al., 2003): rTMS to premotor cortex modulated a widespread network, including several regions in the prefrontal and parietal cortices; in contrast, rTMS to motor cortex modulated activity in a smaller number of brain regions, primarily confined to the cortical and subcortical motor systems. More recent studies combining TMS with fMRI have confirmed and extended the above findings, demonstrating that even subthreshold TMS can activate a widespread cortical and subcortical network (Bestmann et al., 2003, 2004, 2005); (Figure 5a).
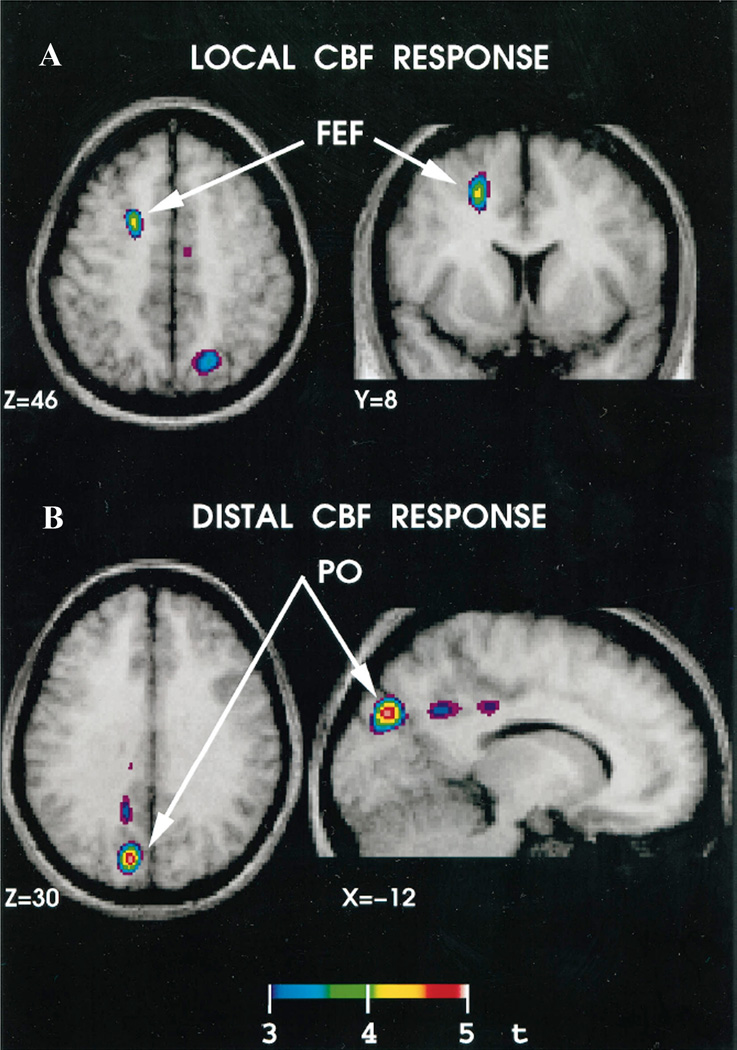
Figure 4. Brain regions with significant correlations between cerebral blood flow (CBF) and the number of TMS pulse trains in a rTMS-PET study.
(A) Significant correlation in the stimulated area, the left frontal eye field (FEF). (B) Significant correlation in a distant area, the ipsilateral parieto-occipital (PO) region. (Modified with permission from Paus et al, 1997).
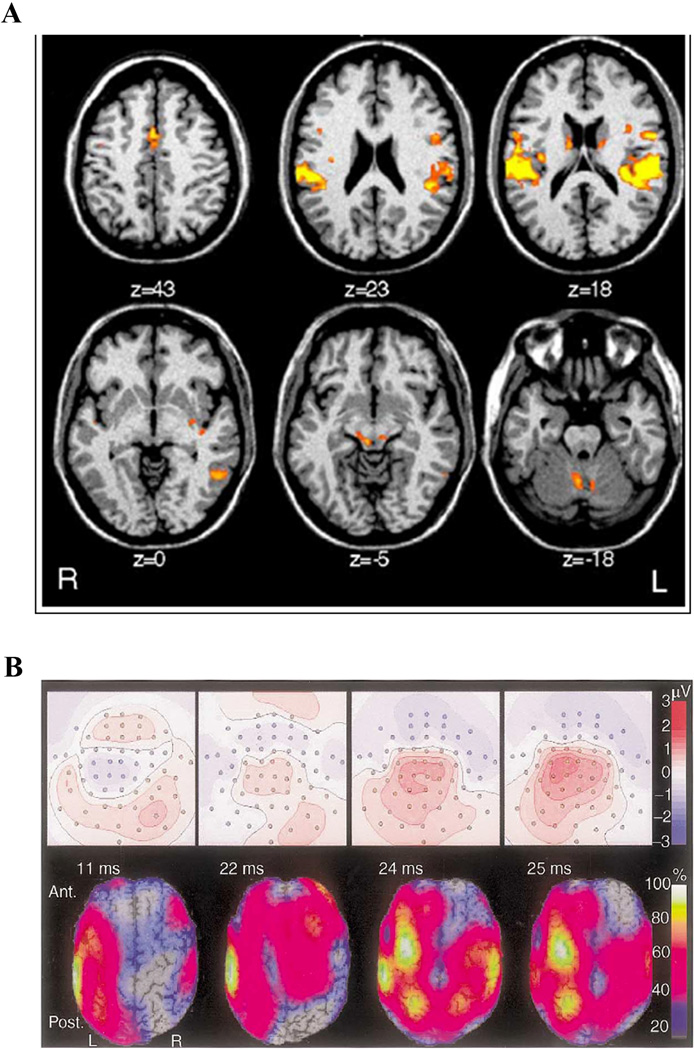
Figure 5. BOLD fMRI and EEG responses to TMS.
(A) Bold fMRI response to rTMS of left dorsal premotor cortex. Six transverse sections showing activity changes in the cingulate gyrus, ventral premotor cortex, auditory cortex, caudate nucleus, left posterior temporal lobe, medial geniculate and cerebellum. (Modified with permission from Bestmann et al, 2005). (B) EEG response to single-pulse stimulation of left sensorimotor cortex. Top panels: Scalp potential with head shown as a two dimensional projection. The contour lines depict constant potentials; positive potentials are red, negative potentials are blue. Bottom panels: Current-density distributions: the calculated current-density at each time point is depicted as a percentage of the maximum current-density at that time point. For this subject, at 11 ms, the activation had spread from below the coil center to involve the surrounding frontal and parietal cortices. Contralateral activation emerged at 22 ms, and peaked at 24 ms. (Modified with permission from Komssi et al, 2002.)
Similarly, early studies combining TMS with EEG demonstrated that single-pulse TMS to the motor cortex produced a complex sequence of successive activations, with EEG activity changes under the TMS coil occurring immediately, then spreading over a few milliseconds to ipsilateral motor, premotor and parietal regions, and then spreading several milliseconds later to the contralateral motor cortex (Ilmoniemi et al., 1997; Komssi et al., 2002), (Figure 5b). Subsequent studies utilizing fcEEG measures, such as coherence, have provided quantitative evidence that rTMS can alter the strength of the connection between different cortical regions (Jing & Takigawa, 2000; Plewnia et al., 2008); the behavioral significance of these changes is as yet unknown.
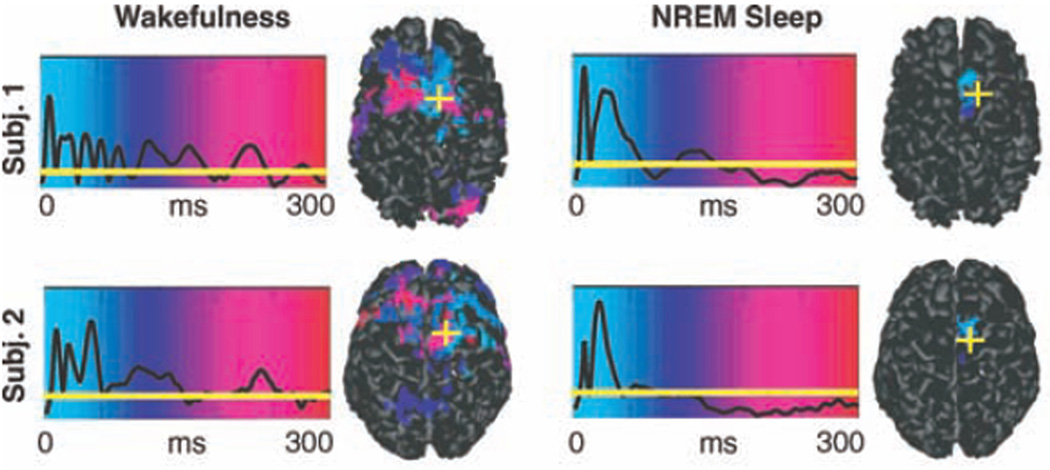
The combination of TMS with other technologies also permits more sophisticated analysis of the dynamics of interactions between different cortical regions. For example, in one novel study, the TMS-evoked response was studied using functional connectivity analysis of EEG data in the awake and sleeping state (Massimini et al., 2005). The authors hypothesized that consciousness is based on the brain’s ability to integrate information from disparate sources, which in turn is contingent on effective connectivity between different specialized regions of the thalamocortical system. As a consequence of this hypothesis, the authors predicted that effective connectivity decreases during sleep. To test this hypothesis, they applied single-pulse TMS to the frontal cortex of subjects in either wakefulness or different sleep stages, and studied the resulting TMS-evoked potential using EEG. The authors found that during wakefulness TMS induced a sustained response of recurrent waves of activity, with the underlying cortical currents shifting over time to different regions across the cortex. In contrast, during non-REM sleep, TMS induced a much larger immediate local response that then terminated rapidly. Furthermore, the TMS-evoked potential was confined to the region of stimulation, and did not propagate to any other cortical region (Figure 6). These results thus supported the hypothesis that the loss of consciousness during sleep is associated with a breakdown in effective connectivity between different cortical regions. A recent follow-up study utilizing TMS demonstrated a similar breakdown in effective connectivity during the loss of consciousness induced by midazolam anesthesia (Ferrarelli et al., 2010).
Figure 6. Spatiotemporal TMS-evoked current maps during wakefulness and NREM sleep in two subjects.
The black traces represent the global mean field power at each time point; when the black line is above the horizontal yellow line, the global power of the evoked field was significantly higher (>6 SD) than the mean prestimulus level. For each significant time sample, maximum current sources were plotted on the cortical surface and color-coded according to their latency of activation (light blue, 0 ms; red, 300 ms). The yellow cross indicates the location of the TMS target on the cortical surface. (Modified with permission from Massimini et al, 2005.)
Such combined-modality studies permit analysis of precisely how different regions interact. Because the TMS pulse produces a change in brain activity at a particular place and time, various techniques that assess how that change is propagated through the brain can be used to assess metrics of effective connectivity. For example, in a recent study, TMS was applied to the left motor hand region while brain activity was imaged with PET (Laird et al., 2008). Structural equation modeling was then applied to the PET data to evaluate the connectivity, focusing on regions known to be activated during TMS to motor cortex. Since TMS was being applied to a single (known) location at a specific time point, the sequence and direction of interactions with other cortical regions could be precisely delineated, permitting the construction of a detailed activity-path model. Following TMS of left motor cortex, activity initially propagated to five regions: the supplementary motor area, the cingulate gyrus, the left ventral nucleus of the thalamus, the right secondary somatosensory cortex, and the right cerebellum. From these initial points, activity then propagated through a number of additional regions (Figure 7).
Figure 7. Connectivity of left M1 hand region, based on structural equation modeling of PET data after TMS.
TMS is applied to the left primary motor cortex, and blood flow changes examined with PET. The connectivity is determined using structural equation modeling in regions of interest based on the timing of activity changes in these different regions. The pink connections are the first order paths, where the TMS “signal” propagates immediately after motor cortex stimulation. The second-order paths, where the activity changes propagate from the first-order regions, are illustrated in green. The third order paths are shown in blue. Regions are as follows: LMI - Left primary sensorimotor cortex; LTHvpl - Left ventral posterolateral nucleus of the thalamus; LTHvl - Left ventral lateral nucleus of the thalamus; LPPC = Left posterior parietal cortex; LPMv - Left ventral premotor area; Cing - Cingulate gyrus; SMA - Supplementary motor area; RSII - Right secondary somatosensory Cortex; LSII - Left secondary somatosensory cortex; RTHvl - Right ventrolateral thalamus; Rcer - Right cerebellum. (Modified with permission from Laird et al, 2008)
Combined-modality studies involving TMS can also be used to assess how neural functional connectivity changes during different cognitive tasks and after various interventions. In one recent study combining TMS and EEG, single-pulse TMS was applied to the human FEF while subjects performed either a face discrimination or motion discrimination task (Morishima et al., 2009). Notably, there was a significant difference between the two tasks in the TMS event-related potentials in the right parieto-occipital region. Furthermore, the TMS pulse during the motion task preferentially activated a current source in the region corresponding to area MT (known from fMRI studies to be involved in motion perception), while the fusiform face area was the preferential source of the currents evoked by the TMS pulse during the face task. Taken together, these results suggest that the activity provoked by FEF TMS propagated along different pathways depending on which visual task was being performed, and that the functional connectivity of the FEF varied dynamically as a function of the task parameters.
BRAIN STIMULATION TECHNIQUES AND NETWORK ANALYSIS IN NEUROPSYCHIATRIC DISEASE
There has been an explosion of recent research suggesting that the pathophysiology underlying a variety of different neuropsychiatric disease states is a network phenomenon. Despite this, the findings of studies of traditional EEG, fMRI and PET functional connectivity networks have had limited application in clinical neuropsychiatry, for reasons that could be substantially addressed by combining them with brain stimulation techniques. The reasons why traditional neuroimaging network techniques have not been clinically useful to date include: (1) The specific alterations in network connectivity that have been identified in different disease states tend to vary considerably across studies and depending on the precise analysis technique utilized, and therefore reliable & consistent EEG/fMRI network biomarkers of disease and recovery are not currently available. (2) The techniques currently used in EEG, fMRI and PET functional connectivity studies are essentially correlational, and the interactions they identify have not been validated in experiments that directly manipulate neural activity. Consequently, while various techniques may identify correlated activity between two different cortical regions, a direct interaction between the two can only be confirmed by direct and focal stimulation that changes the activity of one of the regions. (3) Therapeutic interventions that modulate neural networks in a specific and targeted fashion have not been developed, as most traditional pharmaceutical measures modulate the activity of entire networks rather than by targeting specific dysfunctional nodes or connections.
The integration of brain stimulation techniques with traditional neuroimaging network analysis provides a unique set of tools to potentially address all of these issues. By studying the distributed changes in brain activity that can be produced by focal transcranial brain stimulation, the connectivity pathways identified by traditional network analysis techniques can be validated in both normal subjects and in different disease states. Furthermore, by directly changing the activity of a single region in a controlled manner, brain stimulation techniques enable the identification of causal interactions between different cortical areas. As such, their use has added significantly to our understanding of the pathophysiology of cortical networks in various disease states.
Because transcranial brain stimulation techniques provide a means to modulate cortical activity in a noninvasive, safe and targeted fashion, they have naturally come under investigation as potentially useful therapeutic tools. While the application of these approaches in the therapeutic realm is still in preliminary stages, early results are promising. In this section, we use the examples of motor recovery after stroke, depression and schizophrenia to illustrate how transcranial brain stimulation techniques can be used to explore and modify cortical networks in various disease states.
Motor recovery after stroke
Stroke, once the prime example of how a focal brain lesion can lead to a neurological deficit, is being increasingly recognized as a disorder of interacting brain networks (Grefkes et al., 2008; Carter et al., 2010; van Meer et al., 2010). Hemiparesis has been related to reduced interhemispheric connectivity during rest (Carter et al., 2010), as well as reduced effective connectivity between the supplementary motor area and primary motor area (M1) during hand movements, both of which are correlated with the severity of the movement deficit (Grefkes et al., 2008). Neglect has been related to decreased connectivity within the dorsal and ventral attention networks (He et al., 2007b; Carter et al., 2010). Not only does the severity of neglect correlate with these connectivity abnormalities, but recovery of neglect over time is associated with restoration of normal connectivity patterns (He et al., 2007b). Similarly, EEG studies have demonstrated changes in functional connectivity within both the ipsilesional hemisphere and the contralesional hemisphere (as well as the connections between them) after ischemic stroke (Gerloff et al., 2006; Zhu et al., 2009).
Experiments utilizing TMS have provided insights into the network mechanisms of stroke recovery, as well as factors that may inhibit this process. Intriguingly, studies using paired-pulse TMS have demonstrated that in cortical strokes, short-interval intracortical inhibition is decreased in the acute stage, whereas intracortical facilitation is unchanged, suggesting that the balance of excitability in these cortical circuits is shifted towards excitation (Cicinelli et al., 1997; Liepert et al., 2000b, 2000a; Nardone & Tezzon, 2002; Manganotti et al., 2002). However, other studies have demonstrated that the cortical silent period is initially prolonged, suggesting increased inhibition (Braune & Fritz, 1995; Traversa et al., 1997; Ahonen et al., 1998; Liepert et al., 2000a; Nardone & Tezzon, 2002); this prolongation normalizes with clinical recovery (Traversa et al., 1997; Classen et al., 1997; Cicinelli et al., 1997; Byrnes et al., 2001). Stroke patients undergoing rehabilitation also demonstrate an increase in the number of cortical sites from where an MEP of the paretic hand can be obtained (Traversa et al., 1997; Liepert et al., 1998, 2000b; Wittenberg et al., 2003). Another study demonstrated that TMS pulses to ipsilesional dorsal premotor cortex can produce much greater delays in reaction time in stroke patients with infarcts in motor cortex but preserved premotor cortices than in healthy controls (Fridman et al., 2004). Furthermore, TMS to the premotor cortex in the intact cortex produces MEPs in the ipsilateral (paretic) hand (Caramia et al., 2000), suggesting that the contralesional premotor cortex also plays a role in motor activation after stroke. The importance of the contralesional hemisphere was also demonstrated in a study by Lotze et al (2006), who evaluated the impact of inhibitory rTMS to various locations in the contralesional hemisphere in patients who had recovered fully from subcortical strokes. They found that stimulation of the contralesional M1, dorsal premotor cortex, and superior parietal lobule all produced significant decreases in performance of motor tasks by the ipsilateral hand (that was affected by the stroke). Taken together, these studies suggest that the excitability of the lesioned hemisphere is altered after a stroke, and non-primary motor cortices can be recruited to compensate for the decrease in motor cortex activity.
TMS in combination with neuroimaging techniques can be used to study the dynamic mechanisms that the brain utilizes to compensate for focal disruptions in activity. In one elegant study, O’Shea et al (2007) used repetitive TMS to induce mild, transient disruptions to a focal cortical region, and then used fMRI to study compensatory changes in the brain. They focused on the left dorsal premotor region, which shows increased activation after motor stroke and is involved in action selection. Inhibitory rTMS applied to the left dorsal premotor cortex initially resulted in a disruption in performance on an action selection task. However, within a few minutes, performance returned to baseline. fMRI demonstrated that during task performance prior to rTMS, blood flow increased to a left-hemisphere dominant premotor-parietal network. fMRI several minutes after rTMS of the left premotor cortex, after behavioral performance had recovered to baseline, demonstrated increased activation in the right premotor cortex, left supplementary motor area, and bilateral cingulate motor areas (Figure 8). Thus, recovery of task performance was associated with increased activity in multiple other cortical regions. These compensatory increases in activity were not seen when subjects performed a control motor task that did not involve the left premotor cortex, and these changes were also not seen when rTMS was applied to primary motor cortex, suggesting that the observed changes were occurring in a task- and region-specific manner. To show that this compensatory activity in right premotor cortex is behaviorally relevant, TMS was then also applied to the right premotor cortex. TMS to the right premotor cortex alone had no effect on task performance, suggesting that right premotor cortex is usually not critical for task performance. However, if right premotor cortex was stimulated after rTMS of left premotor cortex, task performance was impaired. Thus, the results suggest that the compensatory increase in right premotor activity seen after inhibitory rTMS of left premotor cortex is causally involved in behavioral recovery, a finding with significant clinical implications for motor recovery after stroke.
Similarly, another important TMS/PET study (Chouinard et al., 2006) explored the effects of physical therapy on brain connectivity, as measured via TMS-induced blood flow changes in the resting state. The authors applied rTMS trains to both ipsilesional and contralesional M1, before and after three weeks of constraint-induced movement therapy. Improvements in motor performance were negatively correlated with local cerebral blood flow changes when rTMS was delivered to both ipsilesional and contralesional M1. There were also changes in the cerebral blood flow response to rTMS in the cingulate motor area, basal ganglia and thalamus that correlated with motor performance. Thus, the authors utilized the combination of brain stimulation and PET to demonstrate that the motor performance changes produced by physical therapy are associated with changes in cortical effective connectivity.
Another clinically significant study assessed the impact of interhemispheric inhibition from the unaffected hemisphere to the affected hemisphere. In normal subjects, the amount of transcallosal inhibition from the “resting” hemisphere to the “active” hemisphere initially decreases and then becomes facilitation just before movement onset (stimulation of one hemisphere leads to a larger response in contralateral stimulation); however, in stroke patients, interhemispheric inhibition remained significant (Murase et al., 2004). Furthermore, the degree of interhemispheric inhibition to the lesioned cortex was correlated with slower performance on a finger-tapping task. Based on these results, the authors postulated that inhibition from the unaffected hemisphere might actually inhibit motor activity from the lesioned hemisphere after stroke.
These and other studies have motivated research investigating the therapeutic potential of noninvasive brain stimulation techniques in stroke recovery. A number of proof-of-principle therapeutic trials have been completed, with the results suggesting that excitatory brain stimulation to the lesioned hemisphere, or inhibitory brain stimulation to the unaffected hemisphere, may have beneficial effects in promoting recovery after stroke (Murase et al., 2004; Hummel et al., 2005; Khedr et al., 2005; Fregni et al., 2005, 2006a; Takeuchi et al., 2005; Kirton et al., 2008; Nowak et al., 2009; Emara et al., 2010).
In a particularly intriguing recent study, Grefkes et al (2010) utilized fMRI and functional connectivity analysis techniques to explore the network changes produced by rTMS of the contralesional hemisphere in stroke patients. This study was motivated by previous work that demonstrated significant disturbances in the effective connectivity between different cortical regions in stroke patients: reduced coupling between ipsilesional SMA and M1, reduced coupling between the bilateral SMAs, and increased interhemispheric inhibition from contralesional M1 to ipsilesional M1 during movements with the paretic hand (Grefkes et al., 2008). Interestingly, the weaker the coupling between ipsilesional SMA and M1, and the greater the interhemispheric inhibition from contralesional M1 to ipsilesional M1, the worse the performance was in the paretic hand. After 1Hz rTMS to the contralesional cortex, motor performance of the paretic hand improved. rTMS was also associated with an increase in the endogenous coupling of ipsilesional SMA and M1, and with a significant decrease of the pathologic inhibition from contralesional M1 to ipsilesional M1 with movement of the paretic hand. The magnitude of the reduction in this pathologic inhibition was correlated with the degree of improvement in motor performance of the paretic hand (Grefkes et al., 2010). Thus, this study demonstrated that rTMS might promote more efficient network interactions in both ipsilesional and contralesional cortex. The techniques utilized in this study hold significant potential for understanding how brain stimulation techniques affect cortical networks, and thus should enable the development of more effective therapeutic protocols.
Depression
Similar to stroke, psychiatric diseases including depression and schizophrenia are being increasingly viewed as network disorders involving abnormal interactions between multiple brain regions. However, unlike stroke, the regions and networks involved are not immediately obvious using routine clinical imaging. While this has lead to great interest in the potential of functional connectivity for revealing previously hidden pathology, there has been a large degree of heterogeneity in the networks of interest and results (Greicius, 2008; Fox & Greicius, 2010; Zhang & Raichle, 2010). Early neuroimaging studies suggested that one of the changes seen in depressed subjects is a relative hypoactivity of the left dorsal prefrontal cortex (Baxter et al., 1989; Martinot et al., 1990; Drevets, 2000), with a normalization of activity accompanying response to treatment (Bench et al., 1995; Mayberg et al., 2000). More recent studies using functional connectivity techniques have focused on the subgenual cingulate cortex (Mayberg et al., 2005), dorsolateral prefrontal cortex (for example see (Seminowicz et al., 2004)) and the default mode network (DMN). Reported functional connectivity abnormalities include decreased corticolimbic connectivity (especially with the dorsal anterior cingulate), increased connectivity within the DMN, especially in the subgenual prefrontal cortex, and decreased connectivity between DMN and caudate (Seminowicz et al., 2004; Anand et al., 2005a, 2005b, 2009; Greicius et al., 2007; James et al., 2009; Bluhm et al., 2009a). Increased subbgenual connectivity has been related to depression severity (Greicius et al., 2007), and algorithms based on functional connectivity can distinguish between depressed and control subjects (Craddock et al., 2009) and predict treatment response (Seminowicz et al., 2004). Similarly, EEG functional connectivity studies have suggested a role for a pathological global increase in functional connectivity within alpha and theta frequency bands (Fingelkurts et al., 2007), and that functional networks during sleep are topologically different in acutely depressed patients versus normal controls (Leistedt et al., 2009). Most intriguingly, a recent analysis applying graph theoretic techniques to resting-state fMRI functional connectivity data demonstrated a significant decrease in mean path length in depressed patients, primarily due to an increase in functional connectivity within a network comprised of several DMN regions (Zhang et al, 2011).
To date, the strongest support for noninvasive brain stimulation techniques in clinical neuropsychiatry (and the only FDA-approved therapeutic indication) comes from the treatment of certain forms of medication-resistant depression. The potential utility of brain stimulation techniques for treating depression was illustrated in several early studies that demonstrated that rTMS to prefrontal cortex had effects on mood (George et al., 1996; Pascual-Leone et al., 1996b). Based on these findings, one early study conducted a trial of daily high-frequency versus sham rTMS to left or right dorsolateral prefrontal cortex, with each site stimulated for five consecutive days (Pascual-Leone et al., 1996a); they showed that only high-frequency rTMS to the left dorsolateral prefrontal cortex significantly improved depression scores, with the effects lasting for approximately two weeks. A large number of subsequent trials have been carried out, with the majority finding high-frequency rTMS to the left dorsolateral prefrontal cortex to be effective in relieving symptoms of depression. Several studies have also looked at the effects of low-frequency (inhibitory) rTMS to the right prefrontal cortex, with most finding that inhibitory rTMS to the right prefrontal cortex is also efficacious in the treatment of depression (Klein et al., 1999; Januel et al., 2006; O’Reardon et al., 2007). A recent meta-analysis combined randomized trial data from 38 studies with a total of 1383 patients (Slotema et al., 2010); 28/34 studies demonstrated a benefit with rTMS, with a mean weighted effect size (mean difference / standard deviation) for all studies of 0.55 (p < 0.001). The single largest randomized placebo-controlled trial conducted to date involved the application of high-frequency (10 Hz) rTMS to the left prefrontal cortex, in daily sessions occurring five times a week for a maximum of 30 sessions over six weeks (O’Reardon et al., 2007). The authors found that active rTMS was consistently and significantly superior to sham treatment on a variety of different outcome measures.
The neural mechanisms by which rTMS modulates depression are unknown, but two (non-exclusive) hypotheses are that 1) rTMS directly modulates activity (e.g. via synaptic plasticity mechanisms) in the frontocingulate network that is associated with depression, or 2) rTMS may facilitate monoaminergic transmission, with a likely diverse impact on the neurochemical milieu (Paus & Barrett, 2004). Indeed, several studies have suggested that prefrontal rTMS affects serotonin synthesis and dopamine release in a number of other cortical regions (Pogarell et al., 2006; Sibon et al., 2007; Cho & Strafella, 2009). To explore how rTMS of frontal cortex affects cortical activity in other regions, Paus et al. (2001) conducted a study combining rTMS of dorsolateral prefrontal cortex with PET. Intriguingly, the authors demonstrated that an initial test stimulus (double-pulse TMS) caused decreased blood flow in both the area being stimulated and in a number of other regions (including the anterior cingulate, implicated in the functional connectivity studies above). After excitatory rTMS, the same double-pulse TMS now caused an increase in blood flow in the same regions, thereby demonstrating that rTMS modulates activity in a widespread cortical network. Another study evaluated changes in regional blood flow in depressed patients after 10 daily treatments of either 20-Hz or 1-Hz rTMS to the left dorsolateral prefrontal cortex (Speer et al., 2000). As predicted, 20-Hz rTMS increased blood flow in a widespread network including the L>R prefrontal cortex, the L>R cingulate gyrus, limbic cortex, thalamus and cerebellum (Figure 9). In contrast, low-frequency rTMS caused significant decreases in blood flow in right prefrontal cortex, left mesial temporal lobe, left basal ganglia and left amygdala. Importantly, patients whose mood improved after 20-Hz rTMS had worsening of their mood after 1-Hz rTMS – and for uncertain reasons, the reverse pattern was also observed in some patients. In a follow-up study (Speer et al., 2009), it was demonstrated that depressed patients with global baseline hypoperfusion had improvement after 20-Hz rTMS and worsening after 1-Hz rTMS; conversely, patients with hyperperfusion in specific cortical regions showed improvement after 1-Hz rTMS (no relationship was found for 20-Hz rTMS in this subpopulation). Another study looking at blood flow changes after rTMS also demonstrated relatively increased blood flow in prefrontal cortex after high-frequency stimulation, and relatively decreased blood flow after low-frequency stimulation (Loo et al., 2003). However, the pattern of changes in other cortical regions after high or low frequency rTMS was complex, with increases in some regions and decreases in others. Fregni et al (2006b) used SPECT to study the effects of rTMS of left prefrontal cortex versus an SSRI (fluoxetine) in patients with Parkinson’s Disease and comorbid depression. rTMS produced blood flow changes in a widespread cortical network involving the prefrontal and temporal cortices, as well as the posterior cingulate. Importantly, the clinical improvement in depression was significantly correlated with the rTMS-induced blood flow changes. Thus, these studies all demonstrated that prefrontal rTMS modulates the activity of a widespread network involving regions known from prior functional connectivity studies to be involved in depression. These studies also suggest that rTMS0 may exert its effects via a normalization of abnormal network activity. Approaches combining noninvasive brain stimulation with neurophysiologic and neuroimaging functional network analysis promise to enable more individually-tailored stimulation protocols that may enhance the efficacy of rTMS.
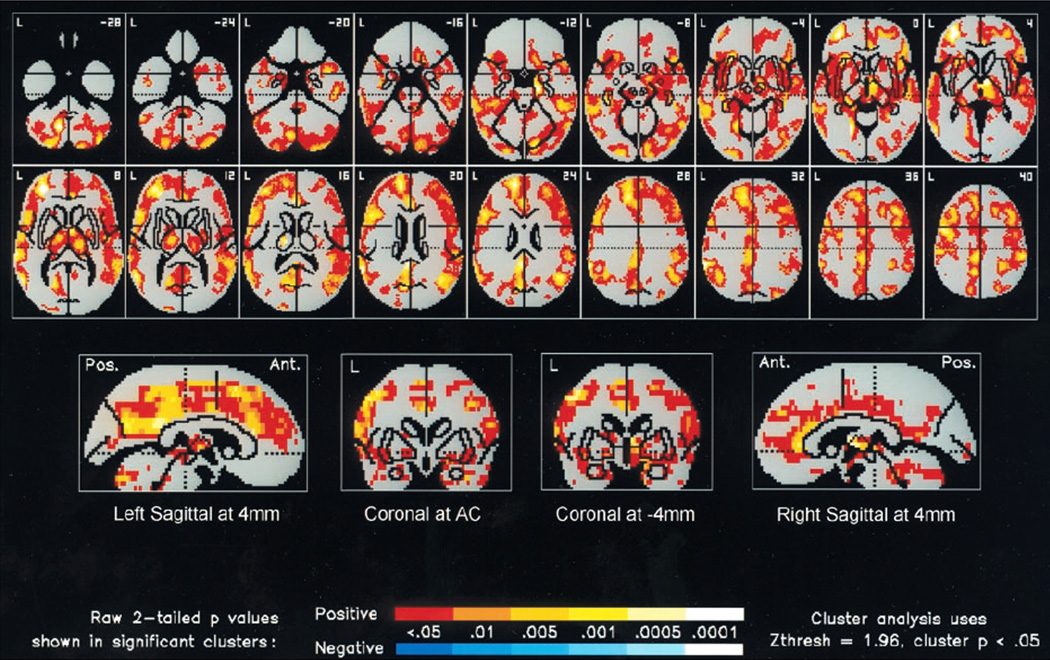
Figure 9. Changes in cerebral blood flow after rTMS for treatment of depression.
The figure shows the significant increases in absolute regional cerebral blood flow (rCBF), relative to the pretreatment baseline, 72 hours after 2 weeks of 20-Hz rTMS at 100% of motor threshold over the left prefrontal cortex in a group of 10 depressed patients. A statistical parametric map shows voxels that occur within significant clusters and is color coded according to their raw p value. Increases in rCBF are displayed with a red– orange–yellow color scale. The number in the top right corner of each horizontal section (top two rows) indicates its position in mm with respect to the anterior commissure (AC)–posterior commissure plane. Twenty-hertz rTMS resulted in widespread increases in rCBF in the following regions: prefrontal cortex (L > R), cingulate gyrus (L >> R), bilateral insula, basal ganglia, uncus, hippocampus, parahippocampus, thalamus, cerebellum, and left amygdale. (Modified with permission from Speer et al, 2000).
Schizophrenia
Functional connectivity abnormalities in schizophrenia have received even more attention than depression, but with the result of even greater heterogeneity in the reported abnormalities (Greicius, 2008; Fox & Greicius, 2010; Zhang & Raichle, 2010). Reported functional connectivity abnormalities include decreased correlations between the left temporoparietal junction and the right homotope of Broca, decreased or increased correlations within the DMN, and decreased, increased or unchanged correlations and anticorrelations between the DMN and other systems (Liu et al., 2006, 2008; Liang et al., 2006; Salvador et al., 2007; Zhou et al., 2007; Bluhm et al., 2007, 2009b; Jafri et al., 2008; Whitfield-Gabrieli et al., 2009; Vercammen et al., 2010a). Decreased correlations between activity in the posterior cingulate cortex and the rest of the DMN have been related to the severity of positive symptoms (Bluhm et al., 2007), while reduced coupling between the left temporoparietal junction and the bilateral anterior cingulate as well as the bilateral amygdala was associated with worse auditory hallucinations (Vercammen et al., 2010a). With regards to the underlying cerebral pathology, the most consistent abnormalities have been noted in the posterior superior temporal cortex of the dominant left hemisphere. However, structural/functional abnormalities have also been noted in a distributed network of brain regions, including Broca’s area and the amygdala-hippocampal network (Allen et al., 2008). fcEEG analysis has suggested that in schizophrenic patients, brain networks resemble random graphs, with relatively small ratios of clustering-coefficients (local efficiency) to path-length values, compared to the larger ratios characteristic of the small-world networks which are seen in healthy controls (Micheloyannis et al., 2006a; Rubinov et al., 2009), suggesting a relative breakdown of local processing efficiency.
Thus, while neuroimaging techniques have indicated that there are significant alterations in functional connectivity in schizophrenic patients, the precise abnormalities and their relationship to disease expression are uncertain. For this reason, over the past decade there have been a number of studies utilizing brain stimulation techniques to explore some of these altered connectivity patterns and to treat the associated symptoms. The current data suggests that low-frequency rTMS to the left temporo-parietal junction is useful in the treatment of auditory hallucinations, while high-frequency stimulation of the left dorsolateral prefrontal cortex may be beneficial for treatment of negative symptoms (Freitas et al., 2009; Dlabac-de Lange et al., 2010; Matheson et al., 2010).
One recent study (Horacek et al., 2007) combined brain imaging with PET and EEG analysis in patients receiving rTMS for auditory hallucinations. Importantly, PET and EEG were done in the resting state before and after rTMS therapy, which consisted of ten 20-minute sessions of rTMS at 0.9 Hz delivered to the left temporoparietal region. The authors found that rTMS significantly improved auditory hallucinations. The analysis of the PET data revealed that rTMS caused a pronounced decrease in metabolic activity in the left temporal cortex and cerebellum, and an increase in metabolism in the bilateral middle frontal gyrus and in the right temporo-occipital cortex, suggesting that the improvement in auditory hallucinations might be secondary to a relative increase in frontal executive control and interhemispheric inhibition from the contralateral cortex. The authors then explored how the metabolism of different brain regions covaried with metabolism in the left superior temporal gyrus. Prior to rTMS, metabolism within the left superior temporal gyrus was positively correlated with a large distributed network including the bilateral temporal cortices and anterior cingulate, and negatively correlated with a number of regions including the inferior parietal lobule, precuneus, and primary sensorimotor cortices. After rTMS, the size of both the positive and negatively correlated regions decreased, suggesting that rTMS was decreasing the functional connectivity of the stimulated region (Figure 10). The EEG analysis revealed increased delta power in the anterior cingulate bilaterally, and decreased beta power in the left temporal cortex. Intriguingly, beta activity was increased in the contralateral (right) temporal lobe and inferior parietal lobule, again raising the possibility of increased interhemispheric inhibition to the pathologically hyperactive cortex. Thus, this study also supported the notion that rTMS alters activity in a widespread cortical network, with the pattern of changes (a decrease in functional connectivity from the left temporoparietal junction and an increase in functional connectivity in the contralateral cortex and frontal areas) suggesting a mechanism for observed behavioral effects. A more recent study using resting-state fMRI (Vercammen et al., 2010b) also found that rTMS altered brain connectivity by significantly increasing the functional connectivity between the targeted left temporo-parietal junction and the right insula. However, there was no change in the strength of the specific connections that were previously shown to be correlated with symptom severity (Vercammen et al., 2010a), suggesting that further work needs to be done to determine the role of these different interactions in the pathophysiology of schizophrenia.
Figure 10. Changes in covariation between brain regions after rTMS treatment for auditory hallucinations in schizophrenic patients.
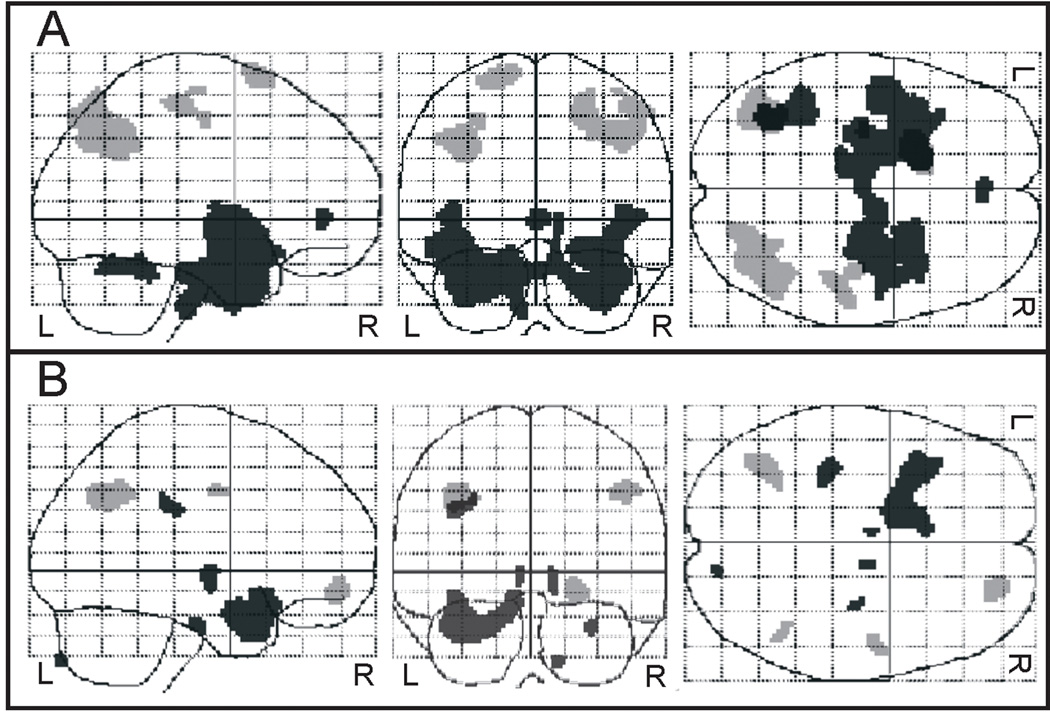
Low-frequency (0.9 Hz) rTMS was applied to the left temporoparietal region. The figure shows the positive (black) and negative (gray) covariation between mean FDG uptake in the left superior temporal cortex before (A) and after (B) rTMS treatment. Before rTMS, there was positive covariation with a large cluster consisting of the bilateral inferior, middle, and superior temporal gyri, parahippocampal gyrus, uncus, insula, anterior cingulate and left fusiform gyrus. Negative covariation was seen with the right inferior parietal lobule, precuneus, postcentral and precentral gyrus, and left precentral gyrus, superior frontal gyrus and precuneus. After rTMS, the regions of both positive and negative covariation were diminished in size. (Modified with permission from Horacek et al, 2007).
A complementary study by Fitzgerald et al (2007) combined rTMS with fMRI to evaluate the effects of 1Hz rTMS for the treatment of auditory hallucinations on verbal task-induced brain activation. They scanned 3 patients while performing a word generation task, before and after receiving rTMS. Four control subjects were also scanned during task performance (but did not receive rTMS). The authors found that hallucination severity was substantially reduced in all three patients, with increases in task-evoked brain activity noted in various brain areas including the left temporoparietal junction, the left frontal-precentral cortex, and the left inferior frontal gyrus. There was also a significant decrease in task-evoked activity in the right middle occipital gyrus. Intriguingly, before treatment patients showed decreased task-evoked activation compared with controls in a number of cortical regions, including bilateral anterior cingulate, left fronto-temporal regions, left frontal-precentral gyrus, among others. Following rTMS, the areas of reduced activation (in comparison with controls) were significantly smaller, suggesting a normalization of pathologic distributed networks.
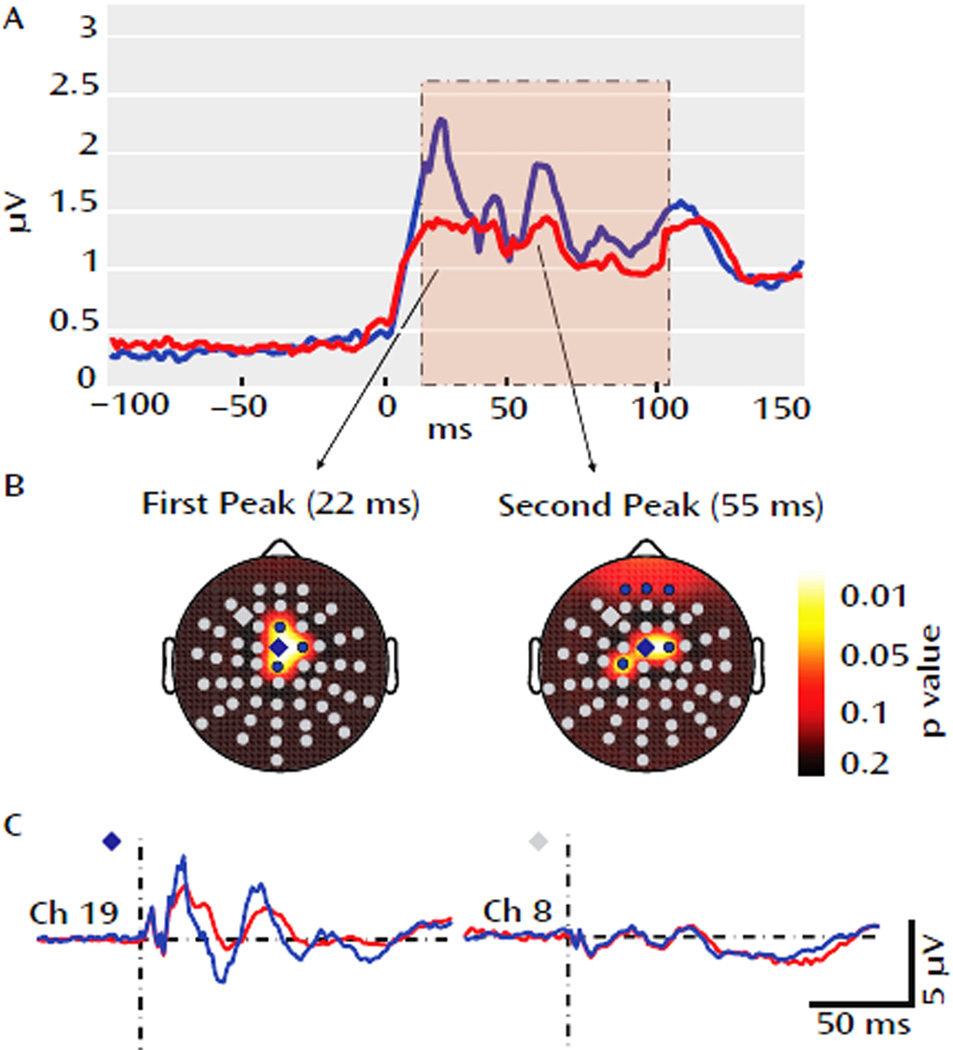
A recent study combining TMS with simultaneous EEG also showed intriguing network pathology in schizophrenic patients (Ferrarelli et al., 2008). The authors applied single TMS pulses to the right premotor cortex, and assessed differences in the resulting TMS-evoked potential between schizophrenic patients and healthy controls. They found that the total brain activation evoked by TMS, as measured via the global mean-field power, was significantly decreased for schizophrenic patients between 12 and 100ms after each stimulus pulse, with the maximum decrease occurring at the peaks of two TMS-evoked gamma oscillations, 22 and 55 ms after the TMS pulse. In schizophrenic patients, the amplitude of these peaks was significantly reduced in a subset of frontocentral electrodes (Figure 11). The authors then demonstrated that this decrease was due to both decreased amplitude and decreased phase-locking of the TMS-evoked gamma activity. Using source analysis techniques the authors demonstrated that in healthy subjects, the current maxima shifted rapidly from premotor cortex to right sensorimotor cortex and then left premotor and sensorimotor regions, whereas, in schizophrenic patients, cortical activation was more localized, shifting slowly between premotor and motor areas along the midline. Taken together, these results suggest that effective connectivity in schizophrenic patients is impaired, especially with regards to the capacity to produce and synchronize gamma activity. These results mesh well with the findings of Fitzgerald et al (2007) and Vercammen et al (2010a), which also suggested decreased functional connectivity.
Figure 11. EEG response to TMS stimulation in schizophrenic patients and healthy controls.
(A). The global mean field power derived from all 60 electrodes. Relative to controls (blue), the global mean field power was decreased in schizophrenic patients (red) between 12 and 100 ms following TMS (pink area). The decrease peaked at 22 and 55ms. (B) The electrode topography of the two peaks, demonstrating the electrodes with significantly different TMS-induced activity between healthy subjects and controls (blue electrodes). There are four centrally located electrodes with differential activity at 22ms, and 6 electrodes (3 central, 3 frontal) with differential activity at 55 ms. (C) Grand averages for a significant electrode (blue diamond) and nonsignificant electrode (gray diamond) in schizophrenic patients (red) and controls (blue). (Modified with permission from Ferrarelli et al, 2008).
BRAIN STIMULATION TECHNIQUES AND ADVANCED NETWORK ANALYSES
More recently, studies have begun to integrate brain stimulation techniques with some of the more advanced network analysis techniques, including resting-state network analysis and graph theoretical analysis. For example default-mode network activity was assessed after sham and real low-frequency rTMS to dorsolateral prefrontal cortex (van der Werf et al., 2010). The authors demonstrated that real rTMS decreased the default-mode network activity in the lateral temporal cortices and bilateral hippocampi as compared to sham, while increasing activity in the right caudate nucleus. Another recent study (Eldaief et al., 2011) demonstrated the ability of brain stimulation techniques to inform the results of these new neuroimaging network analysis techniques. rTMS at low and high frequencies was applied at low and high frequencies (on separate days) to the left inferior parietal lobule, an integral component of the default mode network, to study the effect on functional connectivity with the other elements of the default mode network. Following low-frequency (1 Hz) stimulation, functional connectivity between the targeted region and the bilateral hippocampal formations was significantly increased. However, following high-frequency stimulation, functional connectivity with the hippocampal formation was essentially unchanged, while functional connectivity with the medial prefrontal cortex, posterior cingulate cortex and contralateral inferior parietal lobule was decreased. Thus, while traditional fMRI resting state analysis suggested that the default-mode network is comprised of an integrated system encompassing all of these regions, the perturbational approach enabled by TMS demonstrated that the default mode network may actually be comprised of two distinct subsystems. Given the abnormal resting state networks identified in psychiatric diseases such as major depression (Greicius et al., 2007; Sheline et al., 2009), and the clinical utility of rTMS as a treatment for depression (see above), this study has important theoretical and practical implications.
Recent work has also begun to utilize the tools of graph theoretical analysis to characterize the effects of brain stimulation techniques on cortical network topology. Polania et al (2010) applied facilitatory anodal tDCS to the left primary motor cortex with the cathode situated over the contralateral orbit, and applied real versus sham stimulation. EEG was recorded before and after tDCS, during both resting state and performance of a simple motor task, and fcEEG between all electrode pairs was assessed using the synchronization likelihood connectivity measure (Stam & Dijk, 2002). The authors demonstrated that in the resting state networks, tDCS produced an increase in synchronization between the frontal areas within multiple frequency bands. A comparison of brain activity during performance of the motor task demonstrated increased synchronization within parieto-occipital and frontal regions of the left hemisphere in the θ and α bands, no change in the β and low-γ bands, and increased synchronization within the left premotor, motor and sensorimotor regions in the high-γ range. There was also significant interhemispheric desynchronization in the α, β and high-γ bands (Figure 12). Another study combining left motor tDCS with fMRI resting state activity analysis (Polanía et al., 2011) showed increased connectivity within the left posterior cingulate cortex and the right dorsolateral prefrontal cortex, and an increase in mean path length within the left sensorimotor cortex. Intriguingly, seeded functional connectivity analysis of the left sensorimotor cortex revealed increased functional connectivity with left motor and premotor cortex, and with part of the superior parietal cortex, and no regions of decreased functional connectivity, suggesting that the increase in path length may be due to a strengthening of the motor network at the relative (but not absolute) expense of connections with other parts of the cortex. Functional connectivity analysis of the right dorsolateral prefrontal cortex showed increased connectivity with the right anterior insula, while that of the posterior cingulate demonstrated activation within regions corresponding to the default-mode network. Thus, these studies together demonstrate that tDCS produces widespread changes in the topology of brain functional connectivity, and that these changes can be studied using the tools developed for analysis of fMRI and EEG functional connectivity networks.
Figure 12. Changes in EEG synchronization as a function of task state and tDCS in different frequency bands.
Shows EEG channels that become significantly more synchronized (red) or desynchronized (blue) in different frequency bands. Columns from left to right demonstrate the following comparisons: (1) Task before stimulation – rest before stimulation; (2) Task after stimulation – rest before stimulation; (3) Rest after stimulation – rest before stimulation; and (4) Task after stimulation – task before stimulation. (Modified with permission from Polania et al, 2010a).
The finding that brain stimulation techniques modify the activity of entire networks argues against the view that noninvasive brain stimulation techniques produce largely locally restricted modifications in cortical excitability. Rather, noninvasive brain stimulation techniques modify the activity and connectivity of distributed cortical networks, extending well beyond the region of direct stimulation. Recognition of this fact implies that the findings of previous studies utilizing brain stimulation techniques to probe the function of specific regions may need to be reinterpreted. Also, more effective utilization of noninvasive brain stimulation techniques in both research and clinical therapeutics will require exploration and evaluation of how focal brain stimulation modifies network activities as a function of baseline state, stimulation protocol, baseline functional connectivity, task, and region of stimulation.
CONCLUSIONS
In recent decades, a range of noninvasive techniques for studying and manipulating brain activity have been developed. EEG, PET and fMRI are complementary methods of assessing neural activity. A number of analytic techniques have been applied to data collected using these methods to help delineate the brain’s functional connectivity, i.e. the interactions between different brain regions both at rest and during performance of various tasks, and their use has led to the understanding that cognitive functions are carried out by widespread cortical networks. The same decades have witnessed the introduction of TMS and tDCS, two techniques which rely on electromagnetic principles to noninvasively modulate brain activity. A number of interesting studies have combined brain stimulation techniques with neuroimaging modalities to evaluate and modify brain activity. As such, they provide powerful tools for probing the connectivity of different cortical regions, and for causally investigating the role of network activity in various cognitive functions. These studies have also demonstrated that TMS and tDCS affect not only the focal region to which stimulation is being applied, but also affect widespread cortical areas that are connected to the target region. Consequently, these techniques permit the modulation of functional connectivity networks. The application of these tools has provided unique insights into the network dysfunctions underlying human neuropsychiatric disease, and there have been a number of recent studies focusing on understanding and modulating these networks for therapeutic benefit, with promising results in diseases such as depression, stroke recovery and schizophrenia. Thus, noninvasive brain stimulation in combination with neuroimaging techniques offer considerable potential to further our understanding and treatment of the network activity underlying human brain function and pathology.
Acknowledgements
Work on this study was supported by grants from the National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758), Center for Integration of Medicine and Innovative Technology (CIMIT), the Sidney R. Baer, Jr. Foundation, and Nexstim to APL. MS was supported by funds from the National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758), and the Center for Integration of Medicine and Innovative Technology (CIMIT). MBW was supported by NIH Grant R01-NS062092. MDF was supported by NIH Grant R25-NS065743.
Abbreviations
- EEG
electroencephalography
- PET
positron emission tomography
- fMRI
functional MRI
- TMS
transcranial magnetic stimulation
- tDCS
transcranial direct current stimulation
- BOLD
blood-oxygen-level dependence
- fcMRI
functional connectivity MRI
- MEG
magnetoencephalography
- fcEEG
functional connectivity EEG
- MEP
motor-evoked potentials
- rTMS
repetitive transcranial magnetic stimulation
- FEF
frontal eye fields
- M1
primary motor area
- DMN
default mode network
Footnotes
Conflict of interest
APL serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and is an inventor on patents and patent applications related to noninvasive brain stimulation and the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. MMS, MBW, and MDF declare no conflicts.
REFERENCES
- Achard S, Bullmore E. Efficiency and Cost of Economical Brain Functional Networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen JP, Jehkonen M, Dastidar P, Molnár G, Häkkinen V. Cortical silent period evoked by transcranial magnetic stimulation in ischemic stroke. Electroencephalogr Clin Neurophysiol. 1998;109:224–229. doi: 10.1016/s0924-980x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Albert, Barabasi Topology of evolving networks: local events and universality. Phys Rev Lett. 2000;85:5234–5237. doi: 10.1103/PhysRevLett.85.5234. [DOI] [PubMed] [Google Scholar]
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial Magnetic Stimulation Elicits Coupled Neural and Hemodynamic Consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neuroscience & Biobehavioral Reviews. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005a;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005b;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Andersen B, Rösler KM, Lauritzen M. Nonspecific facilitation of responses to transcranial magnetic stimulation. Muscle Nerve. 1999;22:857–863. doi: 10.1002/(sici)1097-4598(199907)22:7<857::aid-mus7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 2011;49:254–263. doi: 10.1016/j.neuropsychologia.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci USA. 2009;106:11747–11752. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995;25:247–261. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20:1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Blankenburg F, Weiskopf N, Driver J, Rothwell JC. Mapping causal interregional influences with concurrent TMS-fMRI. Exp Brain Res. 2008;191:383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Régis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Research. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents. Nature. 1962;196:584–585. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Théberge J, Densmore M, Bartha R, Neufeld R, Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009a;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, Théberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, Théberge J, Schaefer B, Williamson PC. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009b;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Braune HJ, Fritz C. Transcranial magnetic stimulation-evoked inhibition of voluntary muscle activity (silent period) is impaired in patients with ischemic hemispheric lesion. Stroke. 1995;26:550–553. doi: 10.1161/01.str.26.4.550. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889:278–287. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Palmieri MG, Giacomini P, Iani C, Dally L, Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol. 2000;111:1990–1996. doi: 10.1016/s1388-2457(00)00430-2. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DLW, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nat Neurosci. 2004;7:217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. Changes in effective connectivity of the primary motor cortex in stroke patients after rehabilitative therapy. Exp Neurol. 2006;201:375–387. doi: 10.1016/j.expneurol.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120(Pt 4):605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- Cowey A, Walsh V. Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport. 2000;11:3269–3273. doi: 10.1097/00001756-200009280-00044. [DOI] [PubMed] [Google Scholar]
- Craddock RC, Holtzheimer PE, Hu XP, Mayberg HS. Disease state prediction from resting state functional connectivity. Magn Reson Med. 2009;62:1619–1628. doi: 10.1002/mrm.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Musha T, Cichocki A. A comparative study of synchrony measures for the early diagnosis of Alzheimer’s disease based on EEG. NeuroImage. 2010;49:668–693. doi: 10.1016/j.neuroimage.2009.06.056. [DOI] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol (Lond) 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71:411–418. doi: 10.4088/JCP.08r04808yel. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L, de Groot M, van Dellen E, Heimans JJ, Ronner HE, Stam CJ, Reijneveld JC. “Functional Connectivity” Is a Sensitive Predictor of Epilepsy Diagnosis after the First Seizure. In: Sporns O, editor. PLoS ONE. Vol. 5. 2010. p. e10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara TH, Moustafa RR, Elnahas NM, Elganzoury AM, Abdo TA, Mohamed SA, Eletribi MA. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010 doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol (Lond) 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, Tononi G, Pearce RA. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Rytsälä H, Suominen K, Isometsä E, Kähkönen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum Brain Mapp. 2007;28:247–261. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NAU, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Sritharan A, Benitez J, Daskalakis ZJ, Jackson G, Kulkarni J, Egan GF. A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res. 2007;155:83–88. doi: 10.1016/j.pscychresns.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJL, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJL, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006a;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Fregni F, Ono CR, Santos CM, Bermpohl F, Buchpiguel C, Barbosa ER, Marcolin MA, Pascual-Leone A, Valente KD. Effects of antidepressant treatment with rTMS and fluoxetine on brain perfusion in PD. Neurology. 2006b;66:1629–1637. doi: 10.1212/01.wnl.0000218194.12054.60. [DOI] [PubMed] [Google Scholar]
- Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108:11–24. doi: 10.1016/j.schres.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Steppel J, Pascual-Leone A, Basser P, Hallett M, Post RM. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8:172–180. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Küst J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006a;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage. 2006b;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Current Opinion in Neurology. 2007a;20:655–660. doi: 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007b;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of Functional Connectivity in Frontoparietal Networks Underlies Behavioral Deficits in Spatial Neglect. Neuron. 2007c;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Responses in small hand muscles from magnetic stimulation of the human brain. The Journal of Physiology. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NMF. Magnetic stimulation of the human brain: Facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neuroscience Letters. 1986;71:235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- Horacek J, Brunovsky M, Novak T, Skrdlantova L, Klirova M, Bubenikova-Valesova V, Krajca V, Tislerova B, Kopecek M, Spaniel F, Mohr P, Höschl C. Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology. 2007;55:132–142. doi: 10.1159/000106055. [DOI] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol (Lond) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu W-H, Gerloff C, Cohen LG. Effects of noninvasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Näätänen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Kelley ME, Craddock RC, Holtzheimer PE, Dunlop BW, Nemeroff CB, Mayberg HS, Hu XP. Exploratory structural equation modeling of resting-state fMRI: applicability of group models to individual subjects. Neuroimage. 2009;45:778–787. doi: 10.1016/j.neuroimage.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januel D, Dumortier G, Verdon C-M, Stamatiadis L, Saba G, Cabaret W, Benadhira R, Rocamora J-F, Braha S, Kalalou K, Vicaut PE, Fermanian J. A double-blind sham controlled study of right prefrontal repetitive transcranial magnetic stimulation (rTMS): therapeutic and cognitive effect in medication free unipolar depression during 4 weeks. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:126–130. doi: 10.1016/j.pnpbp.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- van der Kamp W, Zwinderman AH, Ferrari MD, van Dijk JG. Cortical excitability and response variability of transcranial magnetic stimulation. J Clin Neurophysiol. 1996;13:164–171. doi: 10.1097/00004691-199603000-00007. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon A-M, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008;7:507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, Ben-Shachar D, Feinsod M. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–320. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- Komssi S, Aronen HJ, Huttunen J, Kesäniemi M, Soinne L, Nikouline VV, Ollikainen M, Roine RO, Karhu J, Savolainen S, Ilmoniemi RJ. Ipsi- and contralateral EEG reactions to transcranial magnetic stimulation. Clin Neurophysiol. 2002;113:175–184. doi: 10.1016/s1388-2457(01)00721-0. [DOI] [PubMed] [Google Scholar]
- Kumar S, Rao SL, Chandramouli BA, Pillai SV. Reduction of functional brain connectivity in mild traumatic brain injury during working memory. J Neurotrauma. 2009;26:665–675. doi: 10.1089/neu.2008.0644. [DOI] [PubMed] [Google Scholar]
- Laird AR, Robbins JM, Li K, Price LR, Cykowski MD, Narayana S, Laird RW, Franklin C, Fox PT. Modeling motor connectivity using TMS/PET and structural equation modeling. Neuroimage. 2008;41:424–436. doi: 10.1016/j.neuroimage.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Economic small-world behavior in weighted networks. The European Physical Journal B - Condensed Matter. 2003;32:249–263. [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Lee L, Siebner H, Bestmann S. Rapid modulation of distributed brain activity by Transcranial Magnetic Stimulation of human motor cortex. Behav Neurol. 2006;17:135–148. doi: 10.1155/2006/287276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistedt SJJ, Coumans N, Dumont M, Lanquart J-P, Stam CJ, Linkowski P. Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp. 2009;30:2207–2219. doi: 10.1002/hbm.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000a;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000b;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Loo CK, Sachdev PS, Haindl W, Wen W, Mitchell PB, Croker VM, Malhi GS. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychol Med. 2003;33:997–1006. doi: 10.1017/s0033291703007955. [DOI] [PubMed] [Google Scholar]
- Lopez L, Chan CY, Okada YC, Nicholson C. Multimodal characterization of population responses evoked by applied electric field in vitro: extracellular potential, magnetic evoked field, transmembrane potential, and current-source density analysis. J Neurosci. 1991;11:1998–2010. doi: 10.1523/JNEUROSCI.11-07-01998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Lytton WW. Computer modelling of epilepsy. Nat Rev Neurosci. 2008;9:626–637. doi: 10.1038/nrn2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Hardy P, Feline A, Huret JD, Mazoyer B, Attar-Levy D, Pappata S, Syrota A. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry. 1990;147:1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Matheson SL, Green MJ, Loo C, Carr VJ. A change in the conclusions of a recent systematic meta-review: repetitive transcranial magnetic stimulation is effective for the negative symptoms of schizophrenia. Schizophr Res. 2010;122:276–277. doi: 10.1016/j.schres.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- van Meer MPA, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TAP, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(Pt 2):429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006a;87:60–66. doi: 10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Vourkas M, Erimaki S, Tsirka V. Using graph theoretical analysis of multi channel EEG to evaluate the neural efficiency hypothesis. Neurosci Lett. 2006b;402:273–277. doi: 10.1016/j.neulet.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Vourkas M, Tsirka V, Karakonstantaki E, Kanatsouli K, Stam CJ. The influence of ageing on complex brain networks: a graph theoretical analysis. Hum Brain Mapp. 2009;30:200–208. doi: 10.1002/hbm.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Thut G. Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr. 2010;22:249–256. doi: 10.1007/s10548-009-0083-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang Y-Z, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol (Lond) 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol (Lond) 2003;553:665–679. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nardone R, Tezzon F. Inhibitory and excitatory circuits of cerebral cortex after ischaemic stroke: prognostic value of the transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 2002;42:131–136. [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (Lond) 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004a;14:1240–1245. doi: 10.1093/cercor/bhh085. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004b;29:1573–1578. doi: 10.1038/sj.npp.1300517. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol (Lond) 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MFS. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Taylor PCJ, Rushworth MFS. Imaging causal interactions during sensorimotor processing. Cortex. 2008;44:598–608. doi: 10.1016/j.cortex.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Catalá MD, Pascual-Leone Pascual A. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology. 1996a;46:499–502. doi: 10.1212/wnl.46.2.499. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996b;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philos Trans R Soc Lond, B, Biol Sci. 2005;360:1109–1114. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Barrett J. Transcranial magnetic stimulation (TMS) of the human frontal cortex: implications for repetitive TMS treatment of depression. J Psychiatry Neurosci. 2004;29:268–279. [PMC free article] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Rilk AJ, Soekadar SR, Arfeller C, Huber HS, Sauseng P, Hummel F, Gerloff C. Enhancement of long-range EEG coherence by synchronous bifocal transcranial magnetic stimulation. Eur J Neurosci. 2008;27:1577–1583. doi: 10.1111/j.1460-9568.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Koch W, Pöpperl G, Tatsch K, Jakob F, Zwanzger P, Mulert C, Rupprecht R, Möller H-J, Hegerl U, Padberg F. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res. 2006;40:307–314. doi: 10.1016/j.jpsychires.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21104. DOI: 10.1002/hbm.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Paulus W, Antal A, Nitsche MA. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. Neuroimage. 2011;54:2287–2296. doi: 10.1016/j.neuroimage.2010.09.085. [DOI] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. Images of Mind. Scientific American Library. 1994 [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Reijneveld JC, Ponten SC, Berendse HW, Stam CJ. The application of graph theoretical analysis to complex networks in the brain. Clinical Neurophysiology. 2007;118:2317–2331. doi: 10.1016/j.clinph.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AWF, Williams LM, Breakspear M. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30:403–416. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Martínez A, Pomarol-Clotet E, Sarró S, Suckling J, Bullmore E. Frequency based mutual information measures between clusters of brain regions in functional magnetic resonance imaging. Neuroimage. 2007;35:83–88. doi: 10.1016/j.neuroimage.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, Hummel FC. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia. 2009;47:284–288. doi: 10.1016/j.neuropsychologia.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon I, Strafella AP, Gravel P, Ko JH, Booij L, Soucy JP, Leyton M, Diksic M, Benkelfat C. Acute prefrontal cortex TMS in healthy volunteers: effects on brain 11C-alphaMtrp trapping. Neuroimage. 2007;34:1658–1664. doi: 10.1016/j.neuroimage.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J Neurophysiol. 2008;99:2725–2730. doi: 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, Sommer IEC. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? a meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010 doi: 10.4088/JCP.08m04872gre. DOI: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Speer AM, Benson BE, Kimbrell TK, Wassermann EM, Willis MW, Herscovitch P, Post RM. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. J Affect Disord. 2009;115:386–394. doi: 10.1016/j.jad.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EM, D Repella J, Willis MW, Herscovitch P, Post RM. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. 1st edn. The MIT Press; 2010. [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Theoretical neuroanatomy: relating anatomical and functional connectivity in graphs and cortical connection matrices. Cereb Cortex. 2000;10:127–141. doi: 10.1093/cercor/10.2.127. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Dijk BW van. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Phys D. 2002;163:236–251. [Google Scholar]
- Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1:3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17:92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010a;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010b;44:725–731. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wagner TA, Zahn M, Grodzinsky AJ, Pascual-Leone A. Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Trans Biomed Eng. 2004;51:1586–1598. doi: 10.1109/TBME.2004.827925. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. New edition. The MIT Press; 2005. [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Ween JE. Functional imaging of stroke recovery: an ecological review from a neural network perspective with an emphasis on motor systems. J Neuroimaging. 2008;18:227–236. doi: 10.1111/j.1552-6569.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Sanz-Arigita EJ, Menning S, van den Heuvel OA. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci. 2010;11:145. doi: 10.1186/1471-2202-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, Taub E, Gerber LH, Hallett M, Cohen LG. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- Xia Y, Fan J, Hill D. Cascading failure in Watts–Strogatz small-world networks. Physica A: Statistical Mechanics and its Applications. 2010;389:1281–1285. [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zhu C, Guo X, Wu W, Jin Z, Qiu Y, Zhu Y, Tong S. Influence of subcortical ischemic stroke on cortical neural network; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 6818–6821. [DOI] [PubMed] [Google Scholar]