Summary
Background
Evidence from Europe, Asia, and North America suggests that standard three-drug regimens of a proton pump inhibitor plus amoxicillin and clarithromycin are significantly less effective for eradicating Helicobacter pylori (H. pylori) infection than five-day concomitant and ten-day sequential four-drug regimens that include a nitroimidazole. These four-drug regimens also entail fewer antibiotic doses and thus may be suitable for eradication programs in low-resource settings. Studies are limited from Latin America, however, where the burden of H. pylori-associated diseases is high.
Methods
We randomised 1463 men and women ages 21–65 selected from general populations in Chile, Colombia, Costa Rica, Honduras, Nicaragua, and Mexico (two sites) who tested positive for H. pylori by a urea breath test (UBT) to: 14 days of lansoprazole, amoxicillin, and clarithromycin (standard therapy); five days of lansoprazole, amoxicillin, clarithromycin, and metronidazole (concomitant therapy); or five days of lansoprazole and amoxicillin followed by five of lansoprazole, clarithromycin, and metronidazole (sequential therapy). Eradication was assessed by UBT six–eight weeks after randomisation.
Findings
In intention-to-treat analyses, the probability of eradication with standard therapy was 82·2%, which was 8·6% higher (95% adjusted CI: 2·6%, 14·5%) than with concomitant therapy (73·6%) and 5·6% higher (95% adjusted CI: −0·04%, 11·6%) than with sequential therapy (76·5%). In analyses limited to the 1314 participants who adhered to their assigned therapy, the probabilities of eradication were 87·1%, 78·7%, and 81·1% with standard, concomitant, and sequential therapies, respectively. Neither four-drug regimen was significantly better than standard triple therapy in any of the seven sites.
Interpretation
Standard 14-day triple-drug therapy is preferable to five-day concomitant or ten-day sequential four-drug regimens as empiric therapy for H. pylori among diverse Latin American populations.
Funding
Bill & Melinda Gates Foundation and US National Institutes of Health.
Introduction
Helicobacter pylori (H. pylori) infects most of the world’s adult population and is the principal cause of gastric cancer, accounting for an estimated 60 per cent of all cases.1–3 Gastric cancer is second only to lung cancer as a cause of cancer death worldwide, and the vast majority of the nearly one million cases and three-quarters of a million deaths each year occur in East Asia and Latin America.4 Although gastric cancer death rates have fallen in recent decades, the number of deaths has actually increased as a consequence of aging populations, and gastric cancer is projected to rank among the ten leading global causes of death by the year 2030.5–6 H. pylori is also the major cause of peptic ulcer disease, a condition responsible for an estimated 4·6 million disability adjusted life years lost annually worldwide, with most of the burden borne by populations in low- and middle-income countries.7 Population-wide eradication programs appear to offer the most direct approach to reducing the enormous human and economic consequences of H. pylori infection; however, none has been implemented to date.8
Large programs of H. pylori eradication require a practical and inexpensive antibiotic regimen that is effective in the specific locale where it will be used. Standard antibiotic regimens for H. pylori usually entail a proton pump inhibitor, amoxicillin, and clarithromycin, taken together over 7–14 days.9–11 However, the effectiveness of these triple-therapy regimens appears to have diminished over time, largely as a result of emerging resistance of the organism to clarithromycin.12–13 Recent meta-analyses have shown that regimens that add a nitroimidazole (metronidazole or tinidazole) to triple therapy and are given either sequentially for ten days or concomitantly for five days are significantly more successful at eradicating H. pylori than are triple-therapy regimens.14–16 They also require fewer doses of antibiotics and thus may be more affordable in low-resource settings. Almost all of the evidence supporting these four-drug regimens comes from Europe and Asia; there are scant data from Latin America, a region with some of the world’s highest gastric cancer mortality rates.17 We therefore undertook a randomised trial in Latin America comparing the effectiveness of four-drug regimens given concomitantly or sequentially to that of a standard 14-day regimen of triple therapy. The trial also provided insights into the feasibility of community-based programs of H. pylori eradication.
Methods
Study population, recruitment, and eligibility
The trial (SWOG S0701) was conducted at seven centres in: Chile (Santiago), Colombia (Túquerres), Costa Rica (Guanacaste), Honduras (Santa Rosa de Copán), Mexico (Ciudad Obregón; and Tapachula), and Nicaragua (León). The institutional review boards for each clinical centre and for the SWOG Statistical Center in Seattle approved the study protocol. The trial was registered with clinicaltrials.gov; registration number NCT01061437.
Between September 2009 and June 2010, study research staff recruited potential participants from the general population of adult men and women aged 21–65 years and explained the purpose and eligibility requirements of the study to them. The sites in Colombia, Costa Rica, and Nicaragua selected individuals from a census of households. The Chilean site used a list of individuals served by a large public primary care clinic. Staff in Honduras and the two sites in Mexico recruited participants by walking house-to-house within the local community or through announcements at primary care clinics. Study participants in Tapachula (Mexico), Nicaragua, and Chile were predominantly urban, and those in the other sites were from small, rural communities. Potential participants were deemed ineligible if they had been treated in the past for H. pylori, had serious illnesses that might end their lives before completing the study, or had other conditions that required or precluded therapy with antibiotics or proton pump inhibitors. They also had to agree to abstain from alcohol use for at least two weeks. Those who expressed an interest in participating and signed their informed consent then completed an interview regarding socio-economic characteristics and health history and a detailed gastrointestinal symptom history assessment using the validated Spanish language version of the Rome III Diagnostic Questionnaire for the Adult Functional Gastrointestinal Disorders (www.theromefoundation.org/).18,19
Urea breath test procedures
Participants provided a urea breath test (UBT) for H. pylori infection by exhaling into foil balloons before, and 30 minutes after, consuming a 75 mg dose of 13C-labelled urea with water. Staff at each centre analysed the breath samples using an infrared mass spectrometry device (IRIS, Wagner Analysen Technik GmbH, Bremen, Germany) that produced a computer-generated result of positive (delta over baseline ≥4·0%) or negative (delta over baseline <2·5%); intermediate values were classified as inconclusive. If a participant reported using an antibiotic or proton pump inhibitor within the past 15 days, or if the UBT result was inconclusive, the test was rescheduled for a later date.
Randomisation, interventions and blinding
Participants who had a positive UBT and met all other eligibility criteria were assigned at random, in equal proportions, to one of three treatment groups: a) standard triple therapy of lansoprazole 30 mg, amoxicillin 1,000 mg, and clarithromycin 500 mg taken twice a day for 14 days; b) concomitant therapy of lansoprazole 30 mg, amoxicillin 1,000 mg, clarithromycin 500 mg, and metronidazole 500 mg taken twice a day for five days; and c) sequential therapy of lansoprazole 30 mg and amoxicillin 1,000 mg taken twice a day for five days followed by lansoprazole 30 mg, clarithromycin 500 mg, and metronidazole 500 mg taken twice a day for five days. Each clinical centre purchased its own supply of the drugs, as generic (off-patent) preparations, from suppliers in country, except that the centres in Honduras and Nicaragua both used the same supplier in Honduras. The drug suppliers provided quality-control data regarding the content and dissolution of each medication. Randomisation was implemented through a web-based dynamic balancing procedure at the SWOG Statistical Center to assure balance of sex and age (21–40 versus 41–65) within centre across the three regimens. All participants were randomised within two weeks following their positive UBT result. The trial was not blinded.
Follow-up
Study staff contacted participants at least once during treatment to encourage adherence and to remind them to return any unused doses at their follow-up visit, which was scheduled to occur six-to-eight weeks after randomisation. During follow-up visits, participants completed another interview concerning adherence to therapy, their reasons for missing any doses of the regimens, and the occurrence of any new or worsened medical conditions that led them to seek medical attention. Study staff counted the number of medication doses returned and administered the follow-up UBT.
Statistical considerations
We designed the study to address two primary hypotheses. The first was that five-day concomitant therapy is not inferior to 14-day standard therapy, where inferior is defined as a difference in eradication probability of 5% or greater in favour of standard therapy. We reasoned that the shorter duration and lower cost associated with concomitant therapy would make it preferable to standard therapy if it were not appreciably less effective in eradicating H. pylori. Our second hypothesis was that ten-day sequential therapy would be more effective than 14-day standard therapy, because a four-drug sequential regimen would be preferred over standard three-drug therapy only if it were clearly superior in eradicating H. pylori. Assuming an eradication rate of 80% for standard therapy,10% missing follow-up UBT results, and 210 randomised participants per centre (1470 total), each treatment arm contrast would have 82% power to detect a difference of 8% or greater, based on a two-sided 0·025-level test. Although the meta-analysis indicated that concomitant therapy was about 10% better than sequential therapy,16 if the therapies were actually equivalent the trial would have 46% power to reject the inferiority of concomitant therapy, based on a one-sided, 0·025 level test.
Our primary statistical analyses adhered to the intention-to-treat principal and included all randomised eligible participants, with those lacking a definitive follow-up UBT considered to be treatment failures (UBT positive). Comparison of concomitant to standard therapy employed a two-sample z-test of the null hypothesis that the difference in the estimated probabilities of eradication was 5% or greater in favour of the standard regimen, based on a one-sided test of non-inferiority. Comparison of sequential to standard therapies was based on a two-sample z-test for no difference between eradication probabilities. Sensitivity to missing data assumptions was examined by excluding data from the participants without a conclusive follow-up UBT. To explore how lack of adherence may have affected our conclusions, we further restricted the analyses to participants with a definitive UBT who had taken at least 80% of their assigned study medications.
Secondary analyses explored variability in treatment outcomes by sex, age, presence of chronic dyspeptic symptoms, and clinical centre. Tests of interaction were calculated as deviance tests comparing logistic regression models with the treatment arm indicators, the designated covariate, and their interaction terms, to those without the interaction terms.
All analyses were done using SAS and R statistical software. To account for the two primary comparisons, Bonferroni-adjusted 95% confidence intervals are presented and p-values less than 0·025 are considered statistically significant. No corrections for multiplicity were applied to secondary analyses, as they are considered exploratory. All p-values are two-sided except the test of non-inferiority.
Role of the funding sources
The Bill & Melinda Gates Foundation provided financial support for the trial, and the National Institutes of Health (Grant # CA037429) supported SWOG administrative and statistical infrastructure. The funding entities were not involved in the design, conduct of the trial, the analysis and interpretation of study data, or the preparation and submission of the manuscript. All authors participated in the decision to submit the manuscript and had complete access to the data.
Results
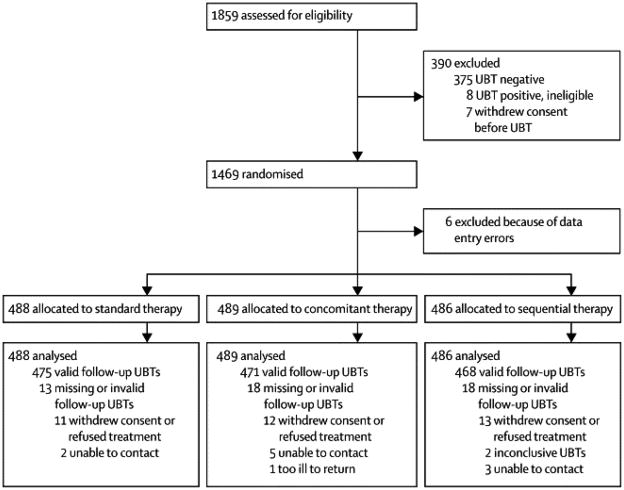
A total of 1859 potentially-eligible adults agreed to participate and completed the screening interview and intake questionnaire, but seven withdrew before the UBT. The UBT was positive for 79% (1471 of 1852) of the tested participants. Eight patients with a positive UBT were not randomised because they opted not to be treated, could not be randomised within two weeks of their UBT, or had a disqualifying condition (e.g., pregnancy). Six participants with a negative UBT who were randomised incorrectly because of data entry errors were withdrawn from the study before receiving treatment, and their data are not included in any of the following analyses. Of the eligible participants who were randomised, 59% (861 of 1463) were women, 58% (847 of 1463) were older than 40 years, and 26% (373 of 1462) had chronic dyspeptic symptoms as classified by the Rome III criteria. Participant characteristics did not differ substantially among the three treatment arms (Table 1). Forty-seven participants did not return for their follow-up UBT, and two participants had follow-up UBTs that were inconclusive after two repeat tests. Thus, we obtained a definitive follow-up UBT result for 97% (1414 of 1463) of the randomised eligible participants (Figure 1).
Table 1.
Participant Characteristics by Treatment Arm
| 14-day Standard | 5-day Concomitant | 10-day Sequential | Total | |
|---|---|---|---|---|
| N=488 | N=489 | N=486 | N=1463 | |
| Center (N, %) | ||||
| Santiago, Chile | 69 (14·1) | 70 (14·3) | 70 (14·4) | 209 (14·3) |
| Túquerres, Colombia | 71 (14·5) | 72 (14·7) | 69 (14·2) | 212 (14·5) |
| Guanacaste, Costa Rica | 70 (14·3) | 70 (14·3) | 70 (14·4) | 210 (14·4) |
| Copán, Honduras | 70 (14·3) | 72 (14·7) | 71 (14·6) | 213 (14·6) |
| Tapachula, México | 71 (14·5) | 69 (14·1) | 70 (14·4) | 210 (14·4) |
| Obregón, México | 70 (14·3) | 69 (14·1) | 71 (14·6) | 210 (14·4) |
| León, Nicaragua | 67 (13·7) | 67 (13·7) | 65 (13·4) | 199 (13·6) |
| Sex (N, %) | ||||
| Female | 287 (58·8) | 288 (58·9) | 286 (58·8) | 861 (58·9) |
| Male | 201 (41·2) | 201 (41·1) | 200 (41·2) | 602 (41·1) |
| Age (N, %) | ||||
| 20–29 | 66 (13·5) | 66 (13·5) | 91 (18·7) | 223 (15·2) |
| 30–39 | 137 (28·1) | 139 (28·4) | 117 (24·1) | 393 (26·9) |
| 40–49 | 142 (29·1) | 117 (23·9) | 127 (26·1) | 386 (26·4) |
| 50+ | 143 (29·3) | 167 (34·2) | 151 (31·1) | 461 (31·5) |
| Education (N, %) | ||||
| 4 or fewer years | 88 (18·0) | 77 (15·7) | 80 (16·5) | 245 (16·7) |
| 5–8 years | 135 (27·7) | 166 (33·9) | 143 (29·4) | 444 (30·3) |
| 9–12 years | 146 (29·9) | 135 (27·6) | 136 (28·0) | 417 (28·5) |
| 13 or more years | 71 (14·5) | 71 (14·5) | 70 (14·4) | 212 (14·5) |
| Not Reported | 48 (9·8) | 40 (8·2) | 57 (11·7) | 145 (9·9) |
| Chronic Dyspeptic Symptoms (N, %) | ||||
| Present | 125 (25·6) | 121 (24·7) | 127 (26·1) | 373 (25·5) |
| Absent | 363 (74·4) | 368 (75·3) | 359 (73·9) | 1090 (74·5) |
Figure 1.
Over 92% (1315 of 1416) of participants who returned for their follow-up visit had taken at least 80% of their assigned medications, as assessed by pill count and self-report (Table 2). In all, 102 participants reported that they missed taking at least some of their medicines because of concerns about side effects; 41, 28, and 33 patients in the standard, concomitant, and sequential arms, respectively. Five participants experienced new, therapy-related symptoms (stomach discomfort, nausea/vomiting, or fatigue/weakness) that led them to seek medical attention, one in the standard treatment arm and two each in the concomitant and sequential treatment arms.
Table 2.
Adherence by treatment arm among patients that returned for 6-week follow-up
| Returned for 6-week follow-up | Standard | Concomitant | Sequential | Total |
|---|---|---|---|---|
| N=475 | N=471 | N=470 | N=1416 | |
| Amount of Medication Taken (N, %)* | ||||
| All (100%) | 427 (89·9) | 442 (93·8) | 437 (93) | 1306 (92·2) |
| Nearly all (>80%) | 7 (1·5) | 0 (0) | 2 (0·4) | 9 (0·6) |
| Most (50%–80%) | 24 (5·1) | 14 (3) | 21 (4·5) | 59 (4·2) |
| Less than half (<50%) | 10 (2·1) | 8 (1·7) | 5 (1·1) | 23 (1·6) |
| Undetermined (but not all) | 7 (1·5) | 5 (1·1) | 5 (1·1) | 17 (1·2) |
| None | 0 (0) | 2 (0·4) | 0 (0) | 2 (0·1) |
| Reasons for not taking all meds (N, %)** | ||||
| Concern about having or developing side effects | 41 (8·6) | 28 (5·9) | 33 (7·0) | 102 (7·2) |
| Unrelated illness/injury | 3 (0·6) | 1 (0·2) | 5 (1·1) | 9 (0·6) |
| Forgot or inconvenient | 36 (7·6) | 25 (5·3) | 37 (7·9) | 98 (6·9) |
| Reason not given | 0 (0) | 3 (0·6) | 2 (0·4) | 5 (0·4) |
Based on count of returned medication and self-report among participants who returned for a follow-up UBT.
Multiple responses allowed.
In intention-to-treat analyses, the estimated 82·2% (401 of 408) probability of eradicating H. pylori with 14-day standard triple therapy was 8·6% higher (95% adjusted CI: 2·6%, 14·5%) than with five-day concomitant therapy and 5·6% (95% adjusted CI: −0·04%, 11·6%) higher than with ten-day sequential therapy (Table 3). The null hypothesis of inferiority of concomitant therapy to standard therapy could not be rejected (one-sided p=0·91); thus, the data are consistent with five-day concomitant therapy being inferior to 14-day standard therapy. The difference between ten-day sequential therapy and standard therapy was not statistically significant (p=0·037) at the p=0·025 level, but the near statistical significance of this two-sided test suggests that sequential therapy was not as effective as standard therapy. Sensitivity analyses that excluded participants without a definitive follow-up UBT and those confined to participants who had adhered to the prescribed regimens showed similar patterns of treatment contrasts as the intention-to-treat analysis, but with somewhat higher estimated eradication probabilities (Table 3). Secondary analyses examining potential interactions revealed no statistically significant interactions between treatment and sex (p=0·91), age (p=0·21), presence of chronic dyspeptic symptoms at baseline (p = 0·38), or study centre (p=0·28). There were differences among centres in the overall probability of eradication in both the intention-to-treat population (Table 4) and the adherent-to-therapy population (Table 5, supplementary material), but nowhere was either four-drug regimen clearly superior to standard triple therapy.
Table 3.
H. pylori eradication by treatment arm for three definitions of analysis population
|
|
||||||
|---|---|---|---|---|---|---|
| Analysis Population | N | Eradicated
|
95% CI for Percent Eradicated | Difference from Standard Arm | Adjusted 95% CI for Difference | |
| N | Percent | |||||
| Intention to Treat (ITT) | 1463 | |||||
|
| ||||||
| 14-day Standard | 488 | 401 | 82.2% | 78.5%, 85.5% | (referent) | |
| 5-day Concomitant | 489 | 360 | 73.6% | 69.5%, 77.5% | 8.6% | 2.6%, 14.5% |
| 10-day Sequential | 486 | 372 | 76.5% | 72.5%, 80.2% | 5.6% | −0.4%, 11.6% |
|
| ||||||
| Definitive 6-week UBT | 1414 | |||||
|
| ||||||
| 14-day Standard | 475 | 401 | 84.4% | 80.8%, 87.6% | (referent) | |
| 5-day Concomitant | 471 | 360 | 76.4% | 72.3%, 80.2% | 8.0% | 2.2%, 13.7% |
| 10-day Sequential | 468 | 372 | 79.4% | 75.5%, 83.1% | 4.9% | −0.9%, 10.8% |
|
| ||||||
| Adherent to Therapy | 1314 | |||||
|
| ||||||
| 14-day Standard | 434 | 378 | 87.1% | 83.6%, 90.1% | (referent) | |
| 5-day Concomitant | 442 | 348 | 78.7% | 74.6%, 82.5% | 8.4% | 2.7%, 14.0% |
| 10-day Sequential | 438 | 355 | 81.1% | 77.1%, 84.6% | 6.0% | 0.3%, 11.8% |
Table 4.
Summary of H. pylori eradication by treatment arm and selected baseline characteristics for ITT population
| Treatment Regimen | N | Eradicated
|
Difference from Standard Arm | Adjusted 95% CI | P-value for Interaction* | |
|---|---|---|---|---|---|---|
| N | Percent | |||||
| Gender | 0·91 | |||||
|
| ||||||
| Female | 861 | |||||
|
| ||||||
| 14-day Standard | 287 | 234 | 81·5% | (referent) | ||
| 5-day Concomitant | 288 | 207 | 71·9% | 9·7% | 1·8%, 17·5% | |
| 10-day Sequential | 286 | 214 | 74·8% | 6·7% | −1·4%, 14·8% | |
|
| ||||||
| Male | 602 | |||||
|
| ||||||
| 14-day Standard | 201 | 167 | 83·1% | |||
| 5-day Concomitant | 201 | 153 | 76·1% | 7·0% | −2·0%, 15·9% | |
| 10-day Sequential | 200 | 158 | 79·0% | 4·1% | −5·2%, 13·3% | |
|
| ||||||
| Age | 0·21 | |||||
|
| ||||||
| 40 years or younger | 663 | |||||
|
| ||||||
| 14-day Standard | 222 | 182 | 82·0% | |||
| 5-day Concomitant | 220 | 164 | 74·5% | 7·4% | −1·3%, 16·2% | |
| 10-day Sequential | 221 | 160 | 72·4% | 9·6% | 0·3%, 18·9% | |
|
| ||||||
| Older than 40 years | 800 | |||||
|
| ||||||
| 14-day Standard | 266 | 219 | 82·3% | |||
| 5-day Concomitant | 269 | 196 | 72·9% | 9·5% | 1·4%, 17·5% | |
| 10-day Sequential | 265 | 212 | 80·0% | 2·3% | −5·6%, 10·3% | |
|
| ||||||
| Chronic Dyspeptic Symptoms | 0·38 | |||||
|
| ||||||
| Absent | 1090 | |||||
|
| ||||||
| 14-day Standard | 363 | 297 | 81·8% | |||
| 5-day Concomitant | 368 | 277 | 75·3% | 6·5% | −0·2%, 13·3% | |
| 10-day Sequential | 359 | 281 | 78·3% | 3·5% | −3·4%, 10·5% | |
|
| ||||||
| Present | 373 | |||||
|
| ||||||
| 14-day Standard | 125 | 104 | 83·2% | |||
| 5-day Concomitant | 121 | 83 | 68·6% | 14·6% | 2·5%, 26·7% | |
| 10-day Sequential | 127 | 91 | 71·7% | 11·5% | −0·9%, 24·0% | |
|
| ||||||
| Study Site | 0·28 | |||||
|
| ||||||
| Santiago, Chile | 209 | |||||
|
| ||||||
| 14-day Standard | 69 | 59 | 85·5% | |||
| 5-day Concomitant | 70 | 46 | 65·7% | 19·8% | 3·9%, 35·7% | |
| 10-day Sequential | 70 | 59 | 84·3% | 1·2% | −13·6%, 16·1% | |
|
| ||||||
| Túquerres, Colombia | 212 | |||||
|
| ||||||
| 14-day Standard | 71 | 58 | 81·7% | |||
| 5-day Concomitant | 72 | 58 | 80·6% | 1·1% | −13·5%, 15·8% | |
| 10-day Sequential | 69 | 54 | 78·3% | 3·4% | −13·2%, 20·0% | |
|
| ||||||
| Guanacaste, Costa Rica | 210 | |||||
|
| ||||||
| 14-day Standard | 70 | 61 | 87·1% | |||
| 5-day Concomitant | 70 | 54 | 77·1% | 10·0% | −4·4%, 24·4% | |
| 10-day Sequential | 70 | 63 | 90·0% | −2·9% | −16·3%, 10·6% | |
|
| ||||||
| Copán, Honduras | 213 | |||||
|
| ||||||
| 14-day Standard | 70 | 66 | 94·3% | |||
| 5-day Concomitant | 72 | 62 | 86·1% | 8·2% | −2·9%, 19·2% | |
| 10-day Sequential | 71 | 58 | 81·7% | 12·6% | −0·8%, 26% | |
|
| ||||||
| Tapachula, México | 210 | |||||
|
| ||||||
| 14-day Standard | 71 | 55 | 77·5% | |||
| 5-day Concomitant | 69 | 50 | 72·5% | 5·0% | −11·4%, 21·4% | |
| 10-day Sequential | 70 | 47 | 67·1% | 10·4% | −7·9%, 28·5% | |
|
| ||||||
| Ciudad Obregón, México | 210 | |||||
|
| ||||||
| 14-day Standard | 70 | 54 | 77·1% | |||
| 5-day Concomitant | 69 | 47 | 68·1% | 9·0% | −7·8%, 25·9% | |
| 10-day Sequential | 71 | 47 | 66·2% | 10·9% | −7·4%, 29·2% | |
|
| ||||||
| León, Nicaragua | 199 | |||||
|
| ||||||
| 14-day Standard | 67 | 48 | 71·6% | |||
| 5-day Concomitant | 67 | 43 | 64·2% | 7·4% | −10·6%, 25·5% | |
| 10-day Sequential | 65 | 44 | 67·7% | 3·9% | −15·5%, 23·4% | |
P-value for Chi-squared drop-in-deviance test for significance of interaction with treatment regimen in a logistic regression model controlling for main effects of treatment and baseline covariate
Discussion
This multi-centre randomised trial of three regimens for eradicating H. pylori involved a large sample of adults recruited from the general populations of seven Latin American sites. The prevalence of H. pylori infection among screened participants was high, and nearly all individuals who tested UBT-positive were randomised into the trial. Participants tended to adhere closely to the protocol by returning for their follow-up UBT and taking their prescribed tablets. There was little difference among the three treatment arms in adherence and serious side effects. Our principal outcome measure, the probability of H. pylori eradication, was higher for 14-day standard triple therapy than for both four-drug regimens, and these results did not vary significantly by age, sex, study site, or history of chronic dyspeptic symptoms.
H. pylori presents a major global health challenge that probably can best be addressed through practical, inexpensive, and population-based interventions in the resource-limited countries where it is most prevalent. From this perspective our trial’s size, technical simplicity, broad geographic coverage within Latin America, community-based population, use of locally-sourced generic drugs, and statistically robust findings across important subgroups reflect the realities of a region where H. pylori-associated diseases are especially burdensome. The trial results are important in that they contradict those of meta-analyses that indicated that four-drug regimens (triple therapy plus a nitroimidazole) given concomitantly or sequentially were clearly superior to triple therapy,14–16 and they suggest that findings based primarily on data from Europe and other high-income regions may not be readily generalizable to lower-income countries.
We observed probabilities of H. pylori eradication of less than 80% with five-day concomitant and ten-day sequential regimens by the intention-to-treat analysis, whereas meta-analyses had reported probabilities greater than 90% for both.14–16 A more recent trial from Taiwan comparing-day regimens of concomitant versus sequential four-drug therapies also reported that both regimens were more than 90% successful.20 The estimated effectiveness of our three-drug regimen (82%) in the intention-to-treat analysis was only modestly greater than found in the meta-analyses (77%–79%). Thus, the divergence between our findings and those of the meta-analyses largely represents the substantially worse performance of the four-drug regimens.
Geographic variations in the pattern of H. pylori resistance to antibiotics might account for some of these discrepancies in results. In clinical series from several of the countries where our trial was conducted, clarithromycin resistance in H. pylori isolates has been reported to be less prevalent than in Europe, and metronidazole resistance substantially more prevalent (as high as 80%).21–24 Clarithromycin resistance strongly diminishes the effectiveness of triple therapy, so a better outcome with triple therapy would be expected if the prevalence of resistance in our trial population actually was low. 13,21,25 A high prevalence of resistance to metronidazole in our study population is a plausible, but less certain, explanation for the worse-than-expected success of the four-drug regimens.25 Results according to antibiotic resistance are available from only two trials of sequential therapy versus triple therapy, and the combined data showed that sequential therapy was successful in 96% (68 of 71) of patients with metronidazole-resistant organisms compared to 78% (46 of 59) for triple therapy.16,26 There are no comparable data from trials of concomitant versus triple therapy. The presence of organisms resistant to both antibiotics would likely cause treatment to fail for all three studied regimens, but there are only scant data on this topic from Latin America.
We employed a 14-day regimen of triple therapy, whereas prior trials of the four-drug regimens generally compared them to seven or ten days of triple therapy. In one meta-analysis 14-day triple therapy regimens were slightly, but statistically significantly, superior to those of seven days or ten days; thus, longer duration conceivably enhanced the performance of triple therapy in our trial.27 Success with the four-drug regimens perhaps would also improve with greater duration;13,20,28 however, this would increase their cost.
We drew our participants from the general population of adults in the community, whilst prior trials studied patients with gastrointestinal symptoms.14–16 The success of H. pylori treatment with triple therapy or sequential therapy has not been shown to be affected by a diagnosis of peptic ulcer disease or dyspepsia, and the superiority of triple therapy over the four-drug regimens in our trial did not vary significantly according to history of chronic dyspeptic symptoms.14 Although our trial was not blinded, the outcome measure (UBT) was objectively determined and seems unlikely to be biased. We used locally-available generic sources of drugs, but the differences in treatment response among centres cannot be easily attributed to variable drug quality, since some of the greatest differences were seen between Nicaragua and Honduras, centres that obtained their drugs from the same source.
For individuals with H. pylori infection in much of Latin America, 14 days of triple therapy is probably the preferred empiric treatment. Nevertheless, the 87% eradication success in participants who adhered to the regimen is suboptimal in the clinical setting, and its effectiveness may decrease over time as clarithromycin resistance increases. Better H. pylori treatment regimens for Latin American populations conceivably could be designed based on local antibiotic resistance data. However, the relatively small number of published reports on antibiotic resistance in this region generally pertain to H. pylori isolates obtained from symptomatic patients undergoing endoscopy in urban, academic centres, and thus their results might not be applicable to broader populations.21 Lacking representative data on resistance, and given the daunting technical and financial challenges of obtaining these data, treatment guidelines for Latin America and other regions with limited resources may have to rely primarily on the results of large, simple clinical trials of empiric therapies in the specific populations to which the guidelines would apply. These data could be supplemented by subsequent monitoring of effectiveness in practice over time and by the results of antibiotic resistance testing in selected patients, where feasible.
We designed our study as a preliminary step towards implementing programs of gastric cancer prevention in Latin America. H. pylori-associated gastric cancer results from a decades-long progression from normal mucosa to invasive cancer.3,29 The most promising preventive approach appears to be eradication of H. pylori before cancer develops, and a number of randomised clinical trials have evaluated this strategy. The results show that eradicating H. pylori slows or reverses progression of pre-malignant histological lesions, but no trial has been large enough to show a definitive cancer-preventive effect.30,31 Nevertheless, analyses have indicated that H. pylori eradication programs would be cost-effective over the long term if they prevented only 10% of gastric cancer deaths; over the short term they would reduce costs of care for peptic ulcers and dyspepsia symptoms.32–34 Eradication programs are potentially even more cost-effective in regions such as Latin America, where the burden of H. pylori-associated diseases is greater.
Our trial experience suggests that population-wide clinical trials or public health programs of H. pylori eradication are feasible in Latin America. UBT-positive individuals readily agreed to be randomised to antibiotic treatment, and all three regimens of generic drugs resulted in probabilities of eradication comparable to those obtained in prior prevention programs.31 The 14-day triple-drug regimen had superior results, but the lower cost of the shorter-duration regimens may make them acceptable for use in preventive programs where resources are particularly scarce. Other considerations, including risks of recrudescence and re-infection following eradication, will also be important considerations.
Panel: Research in context
Systematic review
Consensus groups that represent both global and Latin American perspectives have designated triple-drug regimens of a proton pump inhibitor plus amoxicillin and clarithromycin taken for 7–14 days as a standard approach for eradicating H. pylori.10,11 However, the effectiveness of these regimens appears to have diminished to unacceptably low levels over time, and recent meta-analyses of clinical trials from Europe and Asia indicate that four-drug regimens that add metronidazole or tinidazole to triple therapy achieve superior results.12–16 A Medline search revealed no reports of relevant trials from Latin America, an area where H. pylori-associated diseases are common and where wide-scale eradication programs may be indicated.
Interpretation
Our findings show that in the Latin American populations we studied, in contrast to many European and some Asian populations, 14-day standard triple therapy is more effective than five-day concomitant or ten-day sequential four-drug regimens that include metronidazole for eradicating H. pylori. One thus cannot safely assume that the observed effectiveness of H. pylori eradication regimens in one area will apply equally well elsewhere.
Supplementary Material
Table 5 (Supplement): Summary of H. pylori eradication by treatment arm and study centre for adherent population.
Acknowledgments
The authors thank the trial participants for making this study possible and recognise the many contributions of the investigative team members: SWOG Statistical Center: Vanessa Bolejack, Susie Carlin, Dacia Christin, Evonne Lackey, and Rachael Sexton; Division of Gastroenterology, University of North Carolina, Chapel Hill: Paris Heidt; Universidad del Valle, Cali, Colombia: Luz Stella García, Yolanda Mora; Hospital Regional de Occidente, Santa Rosa de Copán, Honduras: Dr. Jean Paul Higuero, Dr. Glenda Euceda, Lesby Castellanos; Pontificia Universidad Católica de Chile, Santiago, Chile: María Paz Cook, Drs. Paul Harris, Antonio Rollán; Fundación INCIENSA, San José, Costa Rica: Silvia Jiménez, Dr. Paula González, Dr. Ana Cecilia Rodríguez, Lidiana Morera, Dr. Blanca Cruz; Instituto Nacional de Salud Pública, Cuernavaca, Mexico: Dr. Rogelio Danis, Dr. Erika Hurtado, Pilar Hernández; Instituto Tecnológico de Sonora, Ciudad Obregón, Mexico: Myriam Bringas, Araceli Molina, Claudia Osorio, María de Jesús López Valenzuela; Centro de Investigación en Demografía y Salud, León, Nicaragua: Yesenia Zapata. We also thank Drs. Charles A. Coltman, David S. Alberts and Jesse Nodora for their help during the course of the study.
Footnotes
Contributors: All of the authors participated in the design and oversight of the clinical trial and in the interpretation and reporting of results, and all have seen and approved the final manuscript. LEB, RLD, CF, RH, MMM, RP, and ES-M directed the clinical activities at the study centres. GLA had principal responsibility for the statistical analyses of the data.
Conflict of Interest: DRM has submitted a patent application through the University of North Carolina in the area of a molecular endoscopy for cancer detection in the gastrointestinal tract, and has received funding from Axcan for his participation in a speakers’ bureau. He has also received a research grant from AstraZeneca for a proton pump inhibitor study in US Hispanic populations, and from Given Imaging for ongoing efficacy studies of colon endocapsule efficacy. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Infection with Helicobacter pylori. Schistosomes, liver flukes and Helicobacter pylori. Vol. 61. Lyon, France: International Agency for Research on Cancer; 1994. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans; pp. 177–240. [PMC free article] [PubMed] [Google Scholar]
- 2.Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull. 2008;85:87–100. doi: 10.1093/bmb/ldn007. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Marugame T, Dongmei Q. Comparison of time trends in stomach cancer incidence (1973–1997) in East Asia, Europe and USA, from Cancer Incidence in Five Continents Vol. IV–VIII. Jpn J Clin Oncol. 2007;37:242–3. doi: 10.1093/jjco/hym014. [DOI] [PubMed] [Google Scholar]
- 6.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Report. [Accessed Dec 21, 2010];Statistical Annex, Table 3: sex and mortality stratum in WHO regions, estimates. 2003 at http://www.who.int/whr/2003/en/Annex3-en.pdf.
- 8.Graham DY, Shiotani A. The time to eradicate gastric cancer is now. Gut. 2005;54:735–738. doi: 10.1136/gut.2004.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Mégraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection–The Maastricht III Consensus Report. Gut. 2007;56:772–8. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho LG, León-Barúa R, Quigley EM. Latin-American Consensus Conference on Helicobacter pylori infection. Latin-American National Gastroenterological Societies affiliated with the Inter-American Association of Gastroenterology (AIGE) Am J Gastroenterol. 2000;95:2688–91. doi: 10.1111/j.1572-0241.2000.03174.x. [DOI] [PubMed] [Google Scholar]
- 11.Chey WD, Wong BCY Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology Practice Guidelines on the Management of the Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Selgrad M. Helicobacter pylori infection and current clinical areas of contention. Curr Opin Gastroenterol. 2010;26:618–23. doi: 10.1097/MOG.0b013e32833efede. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 14.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 15.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–79. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 16.Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109–18. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–73. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 18.Morgan DR, Squella FE, Pena E, Mearin F, et al. Multinational validation of the Spanish Rome III questionnaire: Comparable sensitivity and specificity to the English instrument. Gastroenterology. 2010;138:S–386. [Google Scholar]
- 19.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 20.Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374–84. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres J, Camorlinga-Ponce M, Pérez-Pérez G, et al. Increasing multidrug resistance in Helicobacter pylori strains isolated from children and adults in Mexico. J Clin Microbiol. 2001;39:2677–80. doi: 10.1128/JCM.39.7.2677-2680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez A, Moncayo JI, Santacruz JJ, Santacoloma M, Corredor LF, Reinosa E. Antimicrobial susceptibility and mutations involved in clarithromycin resistance in Helicobacter pylori isolates from patients in the western central region of Colombia. Antimicrob Agents Chemother. 2009;53:4022–4. doi: 10.1128/AAC.00145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallejos C, Garrido L, Cáceres D, et al. Prevalencia de la resistencia a metrodinazol, claritromicina y tetraciclina en Helicobacter pylori aislado de pacientes de la Región Metropolitana. Rev Med Chil. 2007;135:287–93. doi: 10.4067/s0034-98872007000300002. [DOI] [PubMed] [Google Scholar]
- 25.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 26.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–7. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–62. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 28.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–31. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–72. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Parsonnet J, Forman D. Helicobacter pylori infection and gastric cancer--for want of more outcomes. JAMA. 2004;291:244–5. doi: 10.1001/jama.291.2.244. [DOI] [PubMed] [Google Scholar]
- 31.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–8. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 32.Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150–54. doi: 10.1016/s0140-6736(96)01501-2. [DOI] [PubMed] [Google Scholar]
- 33.Mason J, Axon AT, Forman D Leeds HELP Study Group. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther. 2002;16:559–68. doi: 10.1046/j.1365-2036.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 34.Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. A community screening program for Helicobacter pylori saves money: 10-year follow-up of a randomized controlled trial. Gastroenterology. 2005;129:1910–7. doi: 10.1053/j.gastro.2005.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 5 (Supplement): Summary of H. pylori eradication by treatment arm and study centre for adherent population.