Abstract
In mammalian rods and cones, light activation of the visual pigments leads to release of the chromophore, which is then recycled through a multistep enzymatic pathway, referred to as the visual or retinoid cycle. In invertebrates such as Drosophila, a visual cycle was thought not to exist since the rhodopsins are bistable photopigments, which consist of a chromophore that normally stays bound to the opsin following light activation. Nevertheless, we recently described a visual cycle in Drosophila that serves to recycle the free chromophore that is released following light-induced internalization of rhodopsin, and a retinol dehydrogenase (RDH) that catalyzes the first step of the pathway. Here, we describe the identification of a putative RDH, referred to as RDHB (retinol dehydrogenase B), which functions in the visual cycle and in de novo synthesis of the chromophore. RDHB was expressed in the retinal pigment cells (RPCs), where it promoted the final enzymatic reaction necessary for the production of the chromophore. Mutation of rdhB caused moderate light-dependent degeneration of the phototransducing compartment of the photoreceptor cells—the rhabdomeres, reminiscent of the effects of mutations in some human RDH genes. Since the first and last steps in the visual cycle take place in the RPCs, it appears that these cells are the sites of action for this entire enzymatic pathway in Drosophila.
Introduction
Rhodopsin is comprised of a protein subunit, the opsin, and a light-sensitive retinylidene chromophore (Wald, 1968; Palczewski, 2006). Light activates the visual pigments by inducing a cis- to trans-isomerization of the protein-bound chromophore, whereupon in vertebrate rod and cone photoreceptor cells, all-trans retinal is released from the protein subunit, the opsin. The 11-cis-retinal is then regenerated through an enzymatic pathway known as the visual or retinoid cycle. This regeneration pathway, as well as the de novo synthesis of the chromophore, depends on multiple retinol dehydrogenases (RDHs), which catalyze the interconversion between retinol and retinal (Lidén et al., 2003; Travis et al., 2007).
In Drosophila photoreceptor cells, the chromophore does not appear to release from the opsin following photoisomerization (Hamdorf, 1979). Rather, a second photon of light promotes the regeneration of the inactive, cis-form, which is 3-hydroxy-11-cis-retinal (3-OH-11-cis-retinal). A similar stable interaction is thought to occur between the visual pigment, melanopsin, and the chromophore in mammalian intrinsically photosensitive retinal ganglion cells (Berson, 2007; Hankins et al., 2008).
Despite the stability of the chromophore/opsin interaction in fly photoreceptor cells, an enzymatic visual cycle is used in Drosophila, and functions to recycle the chromophore that is liberated following endocytosis of rhodopsin, and degradation of the opsin (Wang et al., 2010). This enzymatic pathway allows the flies to maintain chromophore levels under conditions of nutrient deprivation when dietary carotenoids are unavailable. The first step in the Drosophila visual cycle pathway depends on a retinol dehydrogenase, PDH (pigment cell dehydrogenase), which is expressed in the retinal pigment cells (RPCs) (Wang et al., 2010). The function and juxtaposition of RPCs to photoreceptor cells is reminiscent of the cells in the mammalian retinal pigment epithelium (RPE) (Travis et al., 2007). Other than PDH, the RDHs responsible either for the visual cycle or de novo synthesis of the chromophore are unknown.
Here we report the identification of a gene encoding a protein homologous to known retinol dehydrogenases, and which is highly expressed in RPCs (RDHB, retinol dehydrogenase B). We created rdhB-null mutant flies by homologous recombination, and found that RDHB promoted the visual cycle and synthesis of 3-OH-11-cis-retinal from dietary sources. The rdhB1 flies displayed visual impairment and progressive retinal degeneration. This work allows for a refinement of the enzymes and cellular requirement for the Drosophila visual cycle (Wang et al., 2010).
Materials and Methods
Fly stocks and media for feeding.
We used flies of either sex for the feeding experiments. The following stocks were obtained from the Bloomington Stock Center: bw1;st1, ninaEI17 and the y,w;P[70FLP]11 P[70I-SceI]2B nocSco/CyO flies. We raised flies at 25°C on standard cornmeal-yeast medium under a 12 h light/dark cycle unless indicated otherwise. For retinoid feeding during the larval stages, we placed embryos for 4 d on retinoid-deficient medium (Wang et al., 2010) and transferred second instar larvae to the retinoid-deficient medium containing one type of retinoid: 5 mm β-carotene (Sigma), 500 μm all-trans-retinal (Sigma) or all-trans-retinol (Sigma). For retinoid feeding using adult flies, we reared the larvae on retinoid-free food, and then transferred newly eclosed flies to retinoid-deficient medium containing one of the following retinoids for 48–72 h before performing Western blots: 5 mm β-carotene, 500 μm all-trans-retinal or all-trans-retinol.
Generation of rdhB1 knock-out flies.
We generated the rdhB knock-out flies (rdhB1) by ends-out homologous recombination (Gong and Golic, 2003). The targeting construct deleted a ∼500-bp region encompassing the second to last exon and the translation termination site. Two ∼3 kb genomic fragments were inserted into the NotI site and the BamHI site of pw35 (Gong and Golic, 2003), respectively. Transgenic flies carrying the targeting construct on the second chromosome were crossed to y,w;P[70FLP]11 P[70I-SceI]2B nocSco/CyO flies, and the progeny were screened for gene targeting by PCR.
Generation of transgenic flies.
To generate the UAS-rdhB::myc flies, we subcloned the rdhB cDNA (GH05294, BDGP DGC clone), between the NotI and XbaI sites of pUAST (Brand and Perrimon, 1993), and added the sequence encoding a 3× Myc tag to the 3′ end of the cDNA. The construct was introduced into w1118 flies by germline transformation.
Generation of recombination proteins and RDHB antibodies.
To generate RDHB antibodies, an rdhB cDNA fragment encoding the N-terminal 120 residues was subcloned into the pGEX5X-1 vector (GE Healthcare). We expressed the GST-fusion protein in Escherichia coli BL21 codon-plus (Stratagene) and purified it using glutathione agarose beads (GE Healthcare). The protein was introduced into rabbits (Covance) to create the RDHB antibodies.
Western blots.
We prepared head extracts from flies of either sex by homogenizing the heads directly in 1× SDS sample buffer with a pellet pestle (Kimble-Kontes). The extracts were fractionated by 4–10% SDS-PAGE (Bio-Rad) and transferred to Immobilon-P transfer membranes (Millipore) in Tris-glycine buffer. The primary antibodies used for probing were mouse anti-tubulin and mouse anti-Rh1(rhodopsin) from the Developmental Studies Hybridoma Bank, or rabbit anti-RDHB. The secondary antibodies used were peroxidase-conjugated anti-mouse or rabbit IgG secondary antibody (Sigma). The signals were detected using ECL reagents (GE Healthcare). For quantification of the Western blots we used LI-COR Odyssey secondary antibodies (anti-mouse IRDye 680, or anti-rabbit IRDye 800) and detected the signals using the LI-COR Odyssey Imaging System.
Immunolocalization.
Immunofluorescent stainings of dissected fly pupae retinas were performed as described previously (Walther and Pichaud, 2006). The primary antibodies were rabbit anti-RDHB. The secondary antibodies were anti-rabbit IgG conjugated with Alexa Fluor 568 (Invitrogen). The primary antibodies used for staining adult eye sections were anti-Myc and anti-Rh1. The samples were examined using a laser-scanning microscope (Zeiss LSM510-Zeta) with Plan Apochromat 20× objectives. The images were acquired using a Carl Zeiss LSM imaging system and transferred into Adobe Photoshop 7.0 for analysis.
ERG recordings.
ERG recordings were performed as described previously using flies of either sex (Wes et al., 1999). Briefly, two glass microelectrodes filled with Ringer's solution were inserted into small drops of electrode cream placed on the surfaces of the compound eye and the thorax, respectively. A Newport light projector (model 765) was used for stimulation. The ERG signals were amplified with a Warner electrometer IE-210 and recorded with a MacLab/4s A/D converter and the Chart v3.4/s program (ADInstruments). We used 5 s pulses of orange (580) or blue (480) light with 7 s intervals in the following order: orange, blue, blue, orange, orange.
HPLC.
Dissected fly heads were homogenized in a glass homogenizer in 200 μl of 2 m NH2OH, pH 6.8, and 600 μl of methanol. After 10 min, we added 600 μl of acetone, 250 μl of diethyl ether, and 250 μl of petroleum benzene. To extract retinoids, the samples were vortexed three times for 10 s, centrifuged (2000 × g, 25°C, 15 s), and the organic phases were collected. The extraction was repeated two times with 400 μl of petroleum benzene and the collected organic phases were dried under a nitrogen stream. Lipophilic compounds were dissolved in 100 μl of HPLC-solvent and subjected to quantitative HPLC analysis as described previously (Oberhauser et al., 2008). All steps were performed under a dim red safety light.
Characterization of retinal degeneration by transmission electron microscopy.
Dissected fly heads were fixed in glutaraldehyde and embedded in LR White resin as described previously (Porter et al., 1992). Thin sections (50 nm) prepared at a depth of 30 μm were examined using a Zeiss FEI Tecnai 12 transmission electron microscope. The images were acquired using a Gatan camera (model 794) and Gatan Digital Micrograph software and converted into tiff files.
Statistics.
Unpaired Student's t tests were used for quantification of Western blot data (n ≥ 3). ANOVA was used for determining significant differences in rhabdomere numbers. *p < 0.05.
Results
Expression of rdhB in retinal pigment cells
RDHs belong to the short-chain dehydrogenase/reductase (SDR) family, which also includes alcohol dehydrogenases and other proteins that act in cell types that are not associated with the visual system (Lidén et al., 2003; Travis et al., 2007). Therefore, to identify RDHs that were required for recycling or for de novo synthesis of the chromophore, we focused on SDR proteins that were expressed primarily in the eye. We took advantage of a previous genome-wide gene expression analysis that compared the relative levels of mRNA expression in heads isolated from wild-type and eyeless flies (Xu et al., 2004). The most eye-enriched gene is pdh (>220-fold) (Xu et al., 2004), which is required for recycling of the chromophore (Wang et al., 2010). The second most eye-enriched SDR family member (CG7077; 43-fold enrichment) has not been characterized functionally.
Since an RDH could function in the eye either in the photoreceptor cells or in RPCs, we examined the cellular distribution of the CG7077 protein. The fly compound eye is comprised of ∼800 ommatidia, which includes seven of the eight photoreceptor cells in coronal sections (Fig. 1A). Each photoreceptor cell contains a microvillar portion referred to as the rhabdomere, which is the functional equivalent of the outer segments of vertebrate rods and cones. The photoreceptor cells are surrounded by six secondary RPCs. The vertices of the hexagonally shaped ommatidia consist of either tertiary RPCs or mechanosensory bristle cells arranged in an alternating pattern.
Figure 1.
Spatial and temporal expression of RDHB. A, Schematic diagram of a cross-sectional view of a single ommatidium from a Drosophila compound eye. B–D, Immunostaining of a 0.5 μm head section from an rdhB-GAL4;rdhB1, UAS-rdhB::myc fly. B, Myc antibodies (red). C, Rhodopsin (Rh1) antibodies (green). D, Merged image. E, Developmental Western blots probed with antibodies to RDHB, Rh1, and tubulin (Tub). Samples were prepared from flies at the indicated pupal times and from adult flies of the indicated ages. F, G, Immunostaining of dissected pupae eyes (∼45 h after puparium formation) with rabbit anti-RDHB. F, wild-type. The arrows indicate labeling of tertiary pigment cells. G, rdhB1.
To examine the spatial distribution of CG7077, we expressed a CG7077::Myc fusion protein under control of the CG7077 promoter using the GAL4/UAS system (Brand and Perrimon, 1993) and stained coronal eye sections with anti-Myc. As a marker for the rhabdomeres of the R1–6 photoreceptor cells, we costained the sections with antibodies specific for the major rhodopsin, Rh1. The anti-Myc signal was restricted to cells situated between ommatidia, and which encircle the R1–6 rhabdomeres labeled by anti-Rh1 (Fig. 1B–D). Since this expression pattern was consistent with secondary RPCs, and CG7077 was required for chromophore production (see below), we referred to CG7077 as RDHB.
In flies, maturation of rhodopsin is dependent on binding to the chromophore, 3-OH-11-cis-retinal. Without the chromophore, rhodopsin is trapped in the endoplasmic reticulum and eventually gets degraded (Harris et al., 1977; Ozaki et al., 1993). Thus, we would expect that an RDH that is required for chromophore synthesis would be expressed at a time earlier than the initial appearance of rhodopsin, which takes place ∼80–90 h after puparium formation (APF; Fig. 1E). To characterize the temporal expression pattern of RDHB, we raised RDHB antibodies and probed a Western blot containing extracts prepared at various times during pupal development. RDHB expression initiated during early pupal development, ∼40 h APF, and continued to be expressed throughout the rest of pupal development, and in the adult (Fig. 1E). We performed immunostaining on pupal eyes, and detected the anti-RDHB signal exclusively in secondary and tertiary RPCs (Fig. 1F,G).
Defects in chromophore synthesis during pupal stage in rdhB mutant flies
To investigate the requirements for RDHB in vivo, we generated rdhB mutant flies (rdhB1) by ends-out homologous recombination (Fig. 2A). The RDHB protein was eliminated in the rdhB1, as assessed by probing Western blots with RDHB antibodies (Fig. 2B), and by immunostaining pupal eyes (Fig. 1F,G). Since maturation of fly rhodopsin is dependent on binding to the chromophore (Ozaki et al., 1993), mutations that interfere with de novo synthesis or recycling of the chromophore cause a reduction in rhodopsin levels. To test for a requirement for RDHB for chromophore production, we raised larvae on retinoid-deficient food, with or without retinoid supplementation. We then prepared head extracts from newly eclosed flies, and probed Western blots for expression of Rh1. Wild-type or rdhB1 animals raised on retinoid-deficient food were impaired in the production of Rh1 (Fig. 2C,D). Addition of β-carotene restored production of Rh1 (Fig. 2C,D). During de novo synthesis of the chromophore carotenoids are cleaved into two copies of retinaldehyde through activity of the NINAB (Neither Inactivation Nor Afterpotential B) β,β′-carotene-15,15′-dioxygenase (von Lintig et al., 2001) (Fig. 3). Since NINAB also possesses intrinsic light-independent isomerase activity, some of the 3-OH-all-trans-retinal is directly converted into 3-OH-11-cis-retinal (Oberhauser et al., 2008; Voolstra et al., 2010), which can be used as the chromophore without a requirement for an RDH (Fig. 3). Supplementation of retinoid deficit food with either all-trans-retinal or all-trans-retinol, which are rapidly converted to their 3-OH derivatives in the eyes (Seki et al., 1998), restored the ability of wild-type animals to synthesize rhodopsin (Fig. 2C,D). However, the rdhB1 mutant was unable to produce Rh1 after feeding on food containing either all-trans-retinal or all-trans-retinol (Fig. 2C,D).
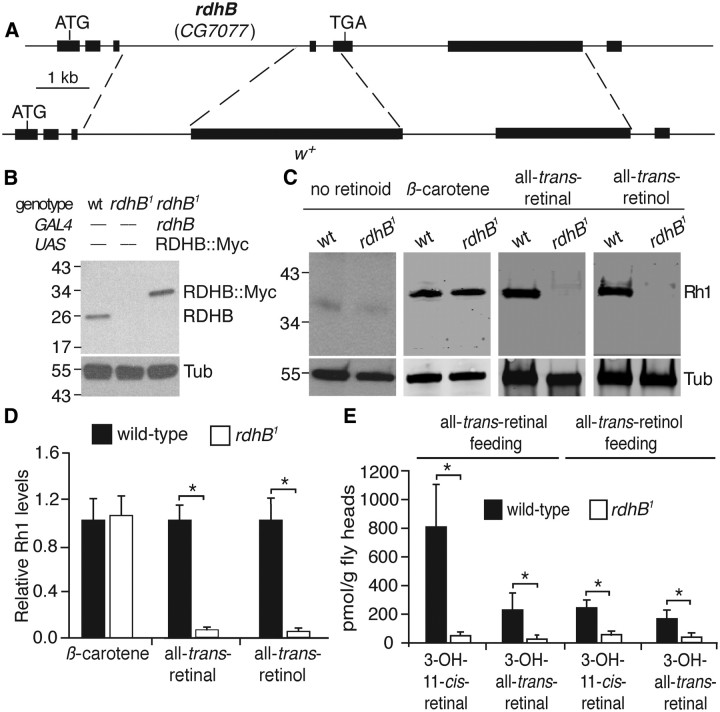
Figure 2.
Generation of rdhB1 and effects of mutation on chromophore and Rh1 production during the pupal period. A, Schematic illustration of the rdhB (CG7077) gene and generation of the rdhB knock-out (rdhB1) by ends-out homologous recombination. B, Western blot containing head extracts from the indicated flies probed with anti-RDHB and reprobed with anti-Tubulin (Tub). C, Western blot of head extracts from ≤1-d-old wild-type and rdhB1. The flies were raised from the second instar larval stage on retinoid-free medium supplemented with 5 mm β-carotene or 500 μm all-trans-retinal or all-trans-retinol. The flies were kept under a 12 h light/dark cycle. D, Quantification of Western blot results shown in C. n ≥ 3. E, Decreased chromophore levels in rdhB1 flies fed all-trans-retinal or all-trans-retinol. The flies were ≤1 d old and raised as indicated in C. n = 3. The concentration of 3-OH-11-cis-retinal and 3-OH-all-trans-retinal were measured by HPLC. Error bars represent ±SEMs. *p < 0.05. Unpaired Student's t tests.
Figure 3.

Proposed pathways for de novo synthesis of the chromophore and for the visual cycle. The proposed function for RDHB is indicated.
To directly assay the effect of deleting the rdhB gene on chromophore synthesis during the pupal stage, we profiled chromophore levels in wild-type and rdhB1 flies using HPLC. Beginning during the second instar period, we raised the animals on retinoid-deficient food that was supplemented with either all-trans-retinal or all-trans-retinol, and assayed retinoid levels in 1-d-old flies. We detected only a small amount of chromophore in head extracts isolated from rdhB1 flies (Fig. 2E). These data indicate that RDHB functions subsequent to the generation of all-trans-retinol.
RDHB functioned in adult flies for rhodopsin production
To address whether RDHB contributed to chromophore biosynthesis in adult flies, we raised larvae on retinoid-free food, and then supplied newly eclosed flies with β-carotene, all-trans-retinol, or all-trans-retinal. Wild-type adult flies were able to use any of these supplements to synthesize Rh1 (Fig. 4A). The rdhB1 flies also synthesized normal levels of Rh1 after being placed on the diet supplemented with β-carotene (Fig. 4A). However, in contrast to wild-type, the levels of Rh1 were reduced significantly in rdhB1 after feeding on either all-trans-retinal or all-trans-retinol (Fig. 4A). These results indicate that RDHB also functions in adult flies for chromophore biosynthesis.
Figure 4.
RDHB was required to maintain Rh1 levels in adult flies. A, Western blot of head extracts from 2- to 3-d-old wild-type and rdhB1 flies. The flies were raised on retinoid-free medium during the larval stages. Newly eclosed flies were maintained on retinoid-free medium supplemented with 5 mm β-carotene, 500 μm all-trans-retinal or all-trans-retinol or. The flies were kept under a 12 h light/dark cycle for 48 h before performing the Western blots. B, Western blot of head extracts obtained from wild-type and rdhB1 flies of the indicated ages, and under the indicated light conditions. The flies were raised on normal retinoid-containing fly food (corn meal and molasses) and kept under a 12 h light/dark cycle. C, D, Quantification of Western blot data shown in A and B. Error bars represent ±SEMs. *p < 0.05. Unpaired Student's t test. n ≥ 3.
Adult flies rely on the visual cycle as the principle pathway for maintaining chromophore levels in light-exposed flies (Wang et al., 2010). This pathway is not necessary in the dark since it is used to recycle the chromophore that is released from light-induced internalized rhodopsin. If RDHB functions in the visual cycle, we would anticipate there would be an age-dependent reduction in rhodopsin in rdhB1 if the mutants were maintained under a light/dark cycle, but not in the dark. Therefore, we raised wild-type and rdhB1 flies on retinoid-containing corn meal food, and quantified Rh1 levels after they were exposed to a light/dark cycle or kept in the dark. After 1 d of a light/dark cycle the Rh1 levels in wild-type and rdhB1 were similar (Fig. 4B). However, there was a subsequent age- and light-dependent decline in Rh1 in rdhB1. After 7 and 25 d under a light/dark cycle the concentration of Rh1 in rdhB1 was reduced 2.3- and 11.2-fold, respectively, while the Rh1 levels were maintained if the mutant flies were dark-reared for 25 d (Fig. 4B).
Visual impairment and retinal degeneration in rdhB1 flies
A reduction in functional rhodopsin levels causes an electrophysiological phenotype that is revealed by performing ERG recordings. ERGs are extracellular recordings that measure the summed light response of the retina. After exposure to bright blue light and conversion of the inactive cis-retinal to the active all-trans form, the light-stimulated metarhodopsin remains in the active state in the dark. This causes a prolonged depolarization afterpotential (PDA) since the active all-trans-retinal does not absorb blue light, but must absorb a second photon (e.g., orange light) to restore the cis-retinal and terminate the PDA. In addition, metarhodopsin activity is quenched by binding to arrestin, which is present at ∼20% the concentration of Rh1 in wild-type flies (Dolph et al., 1993). A PDA can only be produced when functional rhodopsin is in a molar excess over arrestin (Dolph et al., 1993), as in 2-d-old wild-type and rdhB1 flies, and in 25-d-old wild-type (Fig. 5A–C). Consistent with the 90% decrease in Rh1 in 25-d-old rdhB1 (Fig. 4B,D), bright blue light did not produce a PDA in the mutant (Fig. 5D). We rescued this phenotype by expression of a wild-type rdhB cDNA under the control of the rdhB promoter (Fig. 5E).
Figure 5.
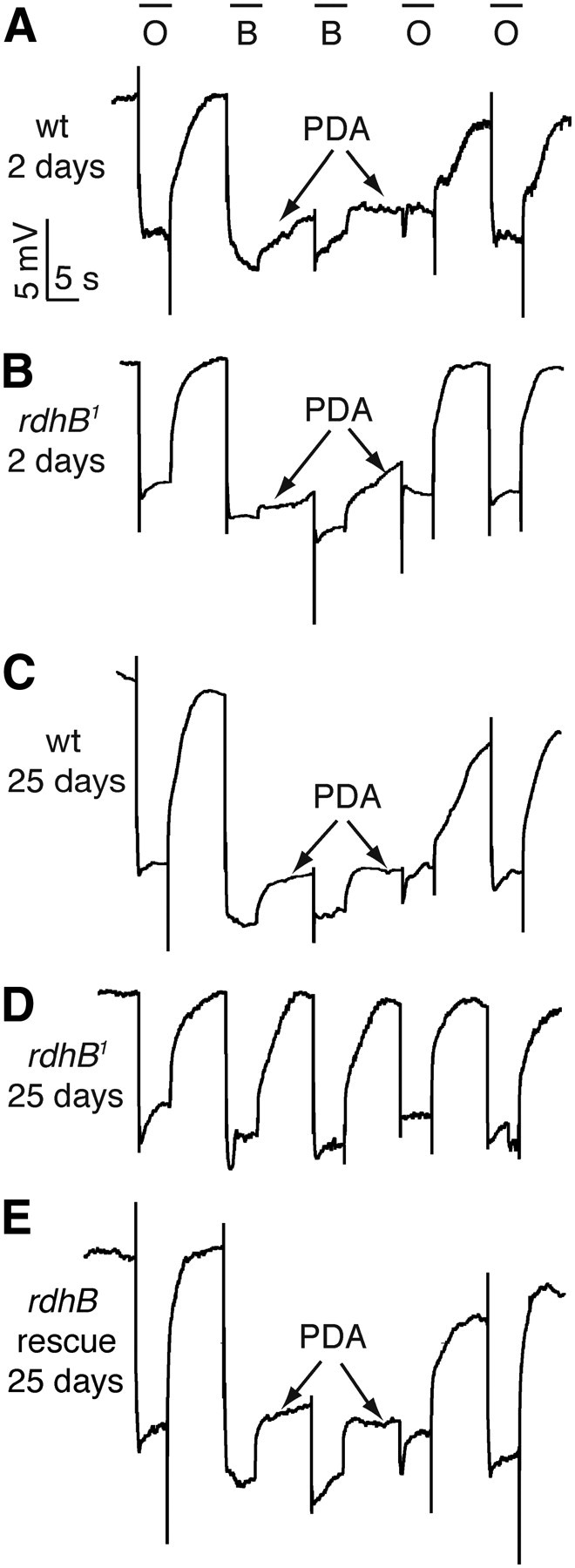
Testing for a PDA in rdhB1 flies by performing ERG recordings. Flies of the indicated genotypes and ages were dark-adapted for 1 min and subsequently exposed to 5 s pulses of orange (580 nm) light (O) or blue (480 nm) light (B) interspersed by 7 s. Arrows indicate the PDAs induced by blue light. The PDAs were terminated by orange light. A, Wild-type (wt), 2 d old. B, rdhB1, 2 d old. C, wt, 25 d old. D, rdhB1, 25 d old. E, rdhB1, 25 d old, expressing a rdhB+ transgene: rdhB-gal4;rdhB1, UAS-rdhB::myc.
To determine whether the rdhB1 mutation caused retinal degeneration, we aged flies under a light/dark cycle or in complete darkness, and examined tangential sections by transmission EM. Wild-type flies displayed the full set of seven intact rhabdomeres even after 30 d under a light/dark cycle (Fig. 6A,D). When the rdhB1 mutant was incubated for 30 d under a light/dark cycle, the rhabdomeres were either smaller than in wild-type or were missing (4.5 ± 0.4 rhabdomeres/ommatidium; Fig. 6B,D). All seven rhabdomeres were present if the mutant flies were incubated in the dark for 30 d (Fig. 6C,D). These data demonstrated that the rdhB1 caused light- and age-dependent retinal degeneration.
Figure 6.
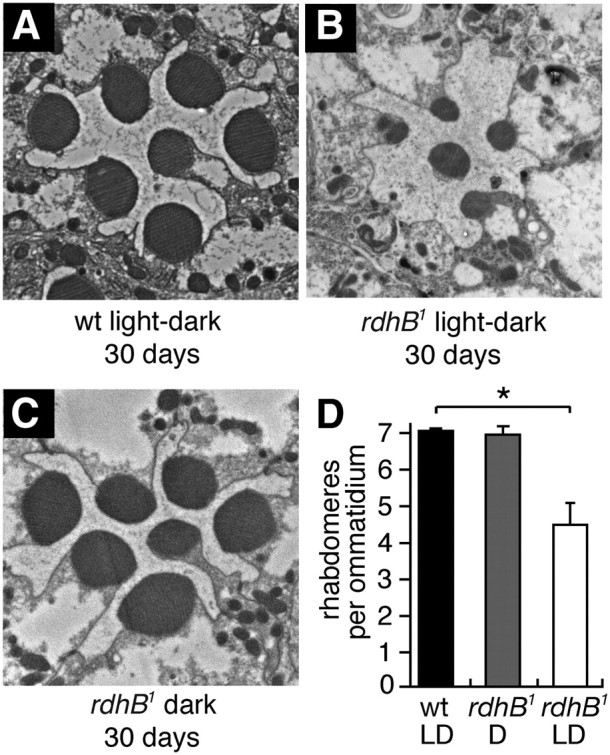
Transmission EM images of cross-sections from adult retinas. The age in days and the light conditions are indicated. The light/dark cycles were 12 h each. A, wt, 30 d under light/dark cycles. B, rdhB1, 30 d under a light/dark cycle. C, rdhB1, 30 d under constant darkness. D, Quantification of the numbers of rhabdomeres per ommatidium based on analyses of thin EM sections after 30 d under a light/dark cycle, or in the dark. Sixty ommatidia from 3 flies were counted for each condition. Error bars represent ±SEMs. *p < 0.05. ANOVA.
Discussion
Dual role for RDHB in the visual cycle and for de novo chromophore synthesis
An enzymatic visual cycle is used in flies, and is the main mode for maintaining chromophore levels in the adult (Wang et al., 2010). This pathway is required for recycling the released chromophore after rhodopsin undergoes light-induced endocytosis, and the opsin is degraded. Thus, in the dark there is no need for this enzymatic cycle. We propose that RDHB functions in the visual cycle since RDHB is expressed in adult RPCs, and elimination of RDHB causes an age-dependent decline in rhodopsin in the adult, but only if the flies are maintained under a light/dark cycle. This loss of rhodopsin occurs in the presence of carotenoid-rich food, highlighting the greater importance of the visual cycle over de novo synthesis in adult flies.
The first step in the visual cycle, the reduction of 3-OH-all-trans-retinal to all-trans-retinol, requires an RDH, referred to as PDH. As a result, pdh mutants can synthesize the chromophore from all-trans-retinol but not all-trans-retinal (Wang et al., 2010). We suggest that RDHB promotes the last step in this pathway, the oxidation of 3-OH-11-cis-retinol to 3-OH-11-cis-retinal. This step is the only RDH-dependent step that appears to be required for both de novo synthesis of the chromophore from all-trans-retinoids as well as for the regeneration of the chromophore through the visual cycle (Fig. 3). Accordingly, our analyses showed that RDHB was also required for de novo synthesis, which is the primary mechanism for generating the chromophore during the pupal stage. In further support of the proposal that RDHB functions in the final oxidation step, chromophore production in rdhB1 was severely diminished if the dietary retinoids were limited to either all-trans-retinal or all-trans-retinol. The other step that is common to the visual cycle and de novo synthesis is isomerization of 3-OH-all-trans-retinol to 3-OH-11-cis-retinol. We suggest that RDHB catalyzes the final oxidation step rather than the isomerization since the protein is homologous to known RDHs but not to retinoid isomerases.
An additional RDH may function in concert with RDHB during the visual cycle
The rdhB1 mutant undergoes light- and age-dependent retinal degeneration, consistent with a role for RDHB in the visual cycle. However the retinal degeneration in rdhB1 was mild compared with that in pdh mutant flies (Wang et al., 2010). The relatively weak retinal degeneration associated with rdhB1, combined with the finding that the concentration of Rh1 is reduced but not eliminated in 25-d-old rdhB1 mutant flies suggests that there exist one or more RDHs that are partially redundant with RDHB. A similar phenomenon of functional redundancy occurs in the mouse retina, as the severity of the retinal degeneration associated with single mutants affecting some RDH genes is less severe than the combined mutations (Maeda et al., 2007). Additional genes encoding SDRs are highly expressed in the eye relative to other tissues (e.g., CG40485 and CG40486), and therefore represent candidate RDHs. It will be of interest to generate mutations in these genes, and characterize the phenotypes individually and in combination with rdhB1.
Entire visual cycle may function in the RPCs
RDHB could function either in the RPCs or the photoreceptor cells. RDHB is most likely required in RPCs since we detected expression of the rdhB reporter (rdhB-GAL4) and the RDHB protein in secondary and tertiary pigment cells and not in photoreceptor cells. Moreover, introduction of wild-type rdhB transgene in RPCs rescues the mutant phenotype. Thus, rdhB acts cell autonomously in RPCs. These data also support the model that the oxidation of 3-OH-11-cis-retinol takes place in the RPCs.
Given that PDH is also expressed and required in RPCs (Wang et al., 2010), we propose that all of the enzymatic steps in the visual cycle occur in the RPCs. The mammalian RPE also functions in de novo synthesis of the chromophore, and in the visual cycle, further indicating functional similarities between the RPCs and the RPE. However, in contrast to the Drosophila pathway, the visual cycle in the mammalian rods and cones includes enzymatic reactions in both photoreceptor cells and the RPE or Müller cells, respectively (Travis et al., 2007).
The finding that flies use a visual cycle, and that the enzymatic steps appear to take place exclusively in RPCs, raises questions concerning the physiology of mammalian melanopsin. This latter visual pigment, which has non-image-forming functions in photosensitive retinal ganglion cells, bears greater sequence and biophysical similarities to the Drosophila rhodopsins than rod and cone visual pigments (Berson, 2007; Hankins et al., 2008). As is the case for the fly rhodopsins, melanopsin may be a bistable photopigment (Berson, 2007; Hankins et al., 2008). However, it remains to be determined whether melanopsin depends on a visual cycle, and if so, the cellular sites for this pathway.
Footnotes
This work was supported by a grant to C.M. from the National Eye Institute (NEI) (EY08117) and by a grant to J.v.L. from the NEI (EY020551). We thank the Bloomington Stock Center for fly stocks, FlyBase for critical gene information, and M. Delannoy for help with the electron microscopy.
The authors declare no competing financial interests.
References
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260:1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci U S A. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdorf K. The physiology of invertebrate visual pigment. In: Autrum H, editor. Handbook of sensory physiology. Vol VII/6A. Berlin: Springer; 1979. pp. 145–224. [Google Scholar]
- Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Harris WA, Ready DF, Lipson ED, Hudspeth AJ, Stark WS. Vitamin A deprivation and Drosophila photopigments. Nature. 1977;266:648–650. doi: 10.1038/266648a0. [DOI] [PubMed] [Google Scholar]
- Lidén M, Tryggvason K, Eriksson U. Structure and function of retinol dehydrogenases of the short chain dehydrogenase/reductase family. Mol Aspects Med. 2003;24:403–409. doi: 10.1016/s0098-2997(03)00036-0. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci U S A. 2008;105:19000–19005. doi: 10.1073/pnas.0807805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Nagatani H, Ozaki M, Tokunaga F. Maturation of major Drosophila rhodopsin, ninaE, requires chromophore 3-hydroxyretinal. Neuron. 1993;10:1113–1119. doi: 10.1016/0896-6273(93)90059-z. [DOI] [PubMed] [Google Scholar]
- Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Hicks JL, Williams DS, Montell C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J Cell Biol. 1992;116:683–693. doi: 10.1083/jcb.116.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Isono K, Ozaki K, Tsukahara Y, Shibata-Katsuta Y, Ito M, Irie T, Katagiri M. The metabolic pathway of visual pigment chromophore formation in Drosophila melanogaster–all-trans (3S)-3-hydroxyretinal is formed from all-trans retinal via (3R)-3-hydroxyretinal in the dark. Eur J Biochem. 1998;257:522–527. doi: 10.1046/j.1432-1327.1998.2570522.x. [DOI] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, Dreher A, Kiefer C, Wernet MF, Vogt K. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation in vivo. Proc Natl Acad Sci U S A. 2001;98:1130–1135. doi: 10.1073/pnas.031576398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra O, Oberhauser V, Sumser E, Meyer NE, Maguire ME, Huber A, von Lintig J. NinaB is essential for Drosophila vision but induces retinal degeneration in opsin-deficient photoreceptors. J Biol Chem. 2010;285:2130–2139. doi: 10.1074/jbc.M109.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Walther RF, Pichaud F. Immunofluorescent staining and imaging of the pupal and adult Drosophila visual system. Nat Protoc. 2006;1:2635–2642. doi: 10.1038/nprot.2006.379. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang T, Jiao Y, von Lintig J, Montell C. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol. 2010;20:93–102. doi: 10.1016/j.cub.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Xu XZ, Li HS, Chien F, Doberstein SK, Montell C. Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat Neurosci. 1999;2:447–453. doi: 10.1038/8116. [DOI] [PubMed] [Google Scholar]
- Xu H, Lee SJ, Suzuki E, Dugan KD, Stoddard A, Li HS, Chodosh LA, Montell C. A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. EMBO J. 2004;23:811–822. doi: 10.1038/sj.emboj.7600112. [DOI] [PMC free article] [PubMed] [Google Scholar]