SUMMARY
B cells infected by Epstein-Barr-Virus (EBV), a transforming virus endemic in humans, are rapidly cleared by the immune system, but some cells harboring the virus persist for life. Under conditions of immunosuppression EBV can spread from these cells and cause life threatening pathologies. We have generated mice expressing the transforming EBV latent membrane protein 1 (LMP1), mimicking a constitutively active CD40 coreceptor, specifically in B cells. Like human EBV infected cells, LMP1+ B cells were efficiently eliminated by T cells, and breaking immune surveillance resulted in rapid, fatal lymphoproliferation and lymphomagenesis. The lymphoma cells expressed ligands for a natural killer (NK) cell receptor, NKG2D, and could be targeted by an NKG2D-Fc fusion protein. These experiments indicate a central role for LMP1 in the surveillance and transformation of EBV infected B cells in vivo, establish a pre-clinical model for B cell lymphomagenesis in immunosuppressed patients, and validate a novel therapeutic approach.
INTRODUCTION
EBV is a γ-Herpes virus infecting and potentially transforming human B-lymphocytes and establishing a life-long latent infection in more than 90% of human beings. Because EBV-infected B cells are rapidly eliminated by the immune system, EBV infection in childhood causes no or only mild symptoms, and primary infection occurring in young adults can lead to a self-limiting lymphoproliferative disorder known as infectious mononucleosis (Kutok and Wang, 2006). T cells play a main role in this immune surveillance mechanism, but NK cells also participate (Hislop et al., 2007; Orange, 2006). In infected individuals, EBV acquires a “dormant” state in a minute fraction of B cells and persists for life. Under conditions of immunosuppression, the virus can spread from these few cells, resulting in explosive expansion of infected B cells and their malignant transformation, as seen in pathologies such as post-transplant lymphoproliferative disorder (PTLD) and AIDS-associated B cell lymphoma (Kutok and Wang, 2006). EBV is also associated with other B cell lymphomas including Hodgkin’s disease (HD) and Burkitt lymphoma (BL) (Kutok and Wang, 2006), although the strategies used by the transformed cells in these diseases to evade immune surveillance are still elusive. A likely clue to this problem lies in the various states of latency, which EBV can assume, ranging from a highly restricted pattern of viral gene expression in BL (latency I) to the expression of just a few EBV genes in HD (latency II) and that of all latent genes in PTLD and AIDS-associated B cell lymphoma (latency III) (Capello et al., 2003; Kuppers, 2003).
The EBV-encoded proteins LMP1 and LMP2A share functions with receptors on normal B cells. LMP2A mimics a constitutively active B cell receptor (BCR), which it can replace in B cell development (Caldwell et al., 1998; Casola et al., 2004). LMP1 is a functional homologue of constitutively active CD40, the major co-stimulatory receptor on B cells (Thorley-Lawson, 2001). LMP1 signals through tumor necrosis factor receptor (TNFR)-associated factors (TRAFs) or TNFR-associated death domain proteins (TRADD) to activate c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), p38, and canonical and noncanonical nuclear factor-κB (NF-κB), promoting cell growth and survival (Soni et al., 2007).
LMP1 expression is essential for the transformation of human B cells by EBV (Kaye et al., 1993) and can by itself induce oncogenic transformation of rodent fibroblasts (Wang et al., 1985). It has been reported that LMP1 positive B cell lymphomas sporadically develop in aged LMP1 transgenic mice. In these animals, LMP1 expression was barely detectable at young age, a phenomenon not yet understood (Kulwichit et al., 1998).
Here we describe a mouse model of conditional LMP1 expression, which can be used to study EBV induced immune surveillance and lymphomagenesis. This system also allowed us to validate a new therapeutic approach, potentially targeting EBV driven malignancies in immunosuppressed patients.
RESULTS
LMP1 Promotes B Cell Growth In Vitro
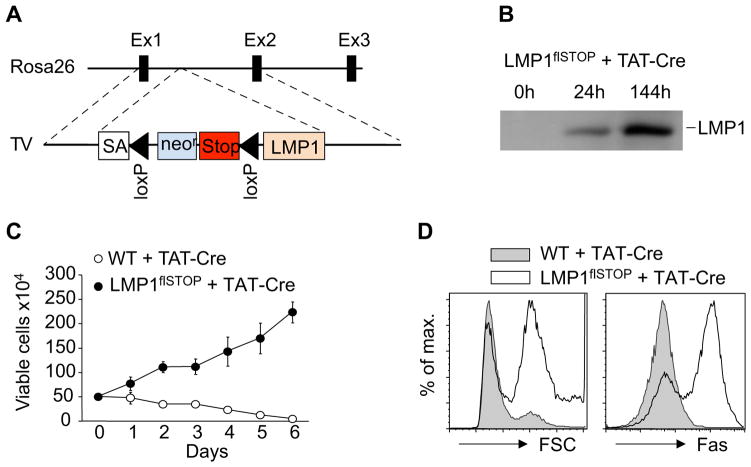
To model EBV-associated B cell transformation, we generated a Rosa26 allele allowing expression of LMP1 through excision of a transcriptional/translational STOP cassette via Cre/loxP-mediated recombination (LMP1flSTOP; Figure 1A). B cells isolated from LMP1flSTOP mice expressed LMP1 following treatment with TAT-Cre (Peitz et al., 2002) (Figure 1B) and proliferated in cell culture, whereas TAT-Cre treated wild-type (WT) B cells died over time (Figure 1C). The induction of LMP1 was accompanied by an increase in cell size and the upregulation of CD95/Fas, as expected from earlier work (Le Clorennec et al., 2006; Uchida et al., 1999) (Figure 1D). We subsequently used Fas as a reporter for LMP1 expression in B cells.
Figure 1. Expression of Transgenic LMP1 Promotes B Cell Growth In Vitro.
(A) Targeting the conditional LMP1 allele into the Rosa26 locus. TV, targeting vector; SA, splice acceptor.
(B) Immunoblotting of LMP1 in CD43-depleted splenic B cells from LMP1flSTOP mice at the indicated time points following TAT-Cre treatment.
(C) Proliferation of TAT-Cre treated splenic B cells from LMP1flSTOP and wild-type (WT) mice in culture. Data represent means of 4 parallel measurements ± s.d.
(D) FACS analysis of cell size (left panel) and Fas expression (right panel) of such cells two days after TAT-Cre treatment.
Data in (C) and (D) are representative of two independent experiments.
Elimination of LMP1+ B Cells and Activation of T Cells In Vivo
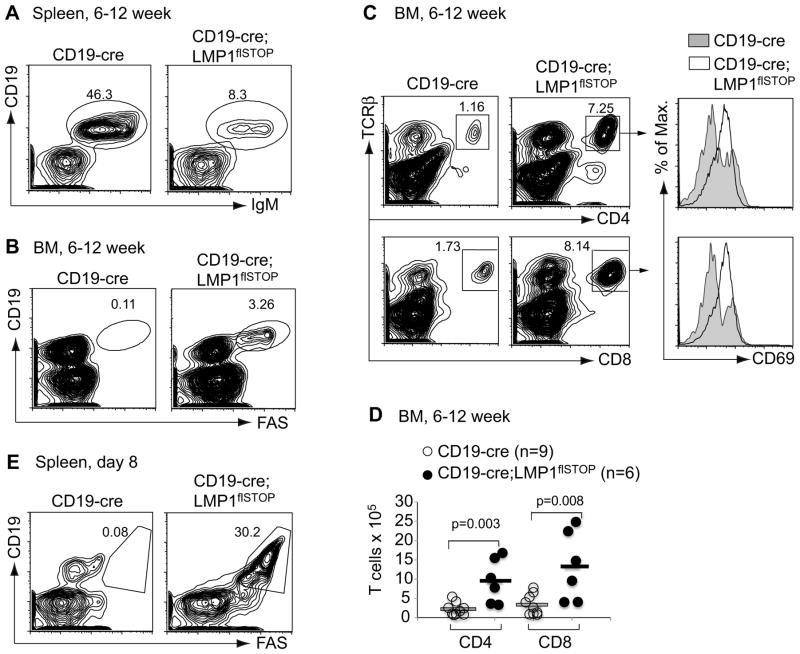
LMP1flSTOP mice were crossed to CD19-cre mice to induce LMP1 expression in B cells from the pro/pre-B cell stage (Rickert et al., 1995). Unexpectedly, the B cell compartment in the spleen of adult CD19-cre;LMP1flSTOP mice was significantly reduced compared to CD19-cre controls (Figure 2A and Figure S1A). The remaining B cells in the mutant mice had escaped deletion of the STOP cassette (Figure S1B). No Fas-expressing B cells were detected in the spleen (data not shown), although a small fraction of CD19+Fas+ B cells (LMP1+ B cells) were seen in the bone marrow (BM; Figure 2B). B cell development in the BM of the mutant mice was disrupted, with an increase of pro-B and decrease of pre-B, immature and mature B cells (Figures S1C–S1E). Since LMP1+ B cells survived and proliferated in cell culture (Figure 1C), their counterselection in vivo is unlikely a consequence of LMP1 toxicity. Considering that EBV-infected human B cells are cleared by the host immune system, we sought for a similar immune surveillance mechanism in the mutant mice. Indeed, we detected increased populations of activated CD4+ and CD8+ T cells in the BM of the mutants (Figures 2C, 2D and Figure S1F). In addition, on day 8 after birth we found a significant population of CD19+Fas+ B cells in their spleen (Figure 2E). The dynamics of CD19+Fas+ B cells and activated CD4+ and CD8+ T cells in the mutant mice between day 3 and 8 after birth suggest that a T cell immune response is induced within this time period (Figures S2A–S2C).
Figure 2. Elimination of LMP1+ B Cells and Activation of T Cells in CD19- cre;LMP1flSTOP Mice.
(A and B) Representative FACS analysis of spleen and BM cells from 6–12 week old CD19-cre and CD19-cre;LMP1flSTOP mice, respectively.
(C) Representative FACS analysis of T cells and their activation status (CD69 expression) in the BM of these mice. Boxed, percentage within lymphocyte gate.
(D) Numbers of CD4+TCRβ+ and CD8+TCRβ+ T cells in the BM of these mice. Bars show the respective mean values.
(E) Representative FACS analysis of spleen cells from CD19-cre and CD19-cre;LMP1flSTOP mice on day 8 after birth. CD19+Fas+ indicates LMP1+ B cells.
Disruption of Immune Surveillance Leads to Rapid, Fatal Expansion of LMP1+ B cell Blasts in the Mutant Mice
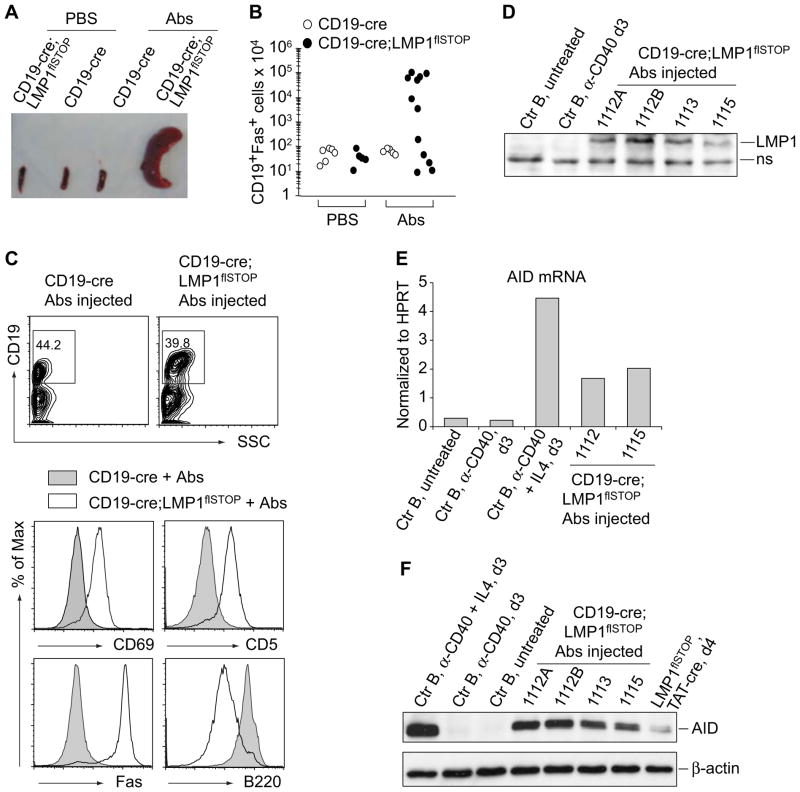
To assess whether activated T cells are responsible for the elimination of LMP1+ B cells, we injected a cocktail of depleting antibodies (Abs) containing anti-CD4, -CD8, and -Thy1 into adult CD19-cre;LMP1flSTOP and CD19-cre control animals at 3 to 4-day intervals. Two weeks after the initiation of this treatment, the majority of the mutant mice, but not the controls, became terminally ill presenting with splenomegaly, due to marked expansion of LMP1+ B cells (CD19+Fas+; Figures 3A and 3B). These cells were largely confined to peripheral lymphoid organs and the BM (Figure S3A), although infiltrations into the liver and rarely into lung and kidney were occasionally seen (data not shown). No outgrowth of LMP1+ B cells was seen in mutant mice treated with anti-CD4, -CD8, or -Thy1 alone or a combination of anti-CD4 and -CD8 (Figure S3A and data not shown). The latter depleted TCRαβ T cells as efficiently as the combination of anti-CD4, -CD8, and -Thy1 (data not shown), but the anti-Thy1 antibody might deplete TCRγδ T cells, activated NK, and natural killer T (NKT) cells in addition. Outgrowth of LMP1+ B cells was also not seen upon treatment of the animals with anti-TNF-α and/or anti-IFN-γ blocking antibodies (data not shown; see Extended Experimental Procedures for experimental details), although both of these cytokines have been implicated in anti-tumor immunity (Balkwill, 2009; Blankenstein and Qin, 2003; Ikeda et al., 2002; Koebel et al., 2007). The activated T cells in the bone marrow of CD19-cre;LMP1flSTOP mice contained normal proportions of cells expressing IFNγ, TNFα, IL4 and IL17, except for a 2 fold increase of the former in the CD8+ compartment (Figure S3B). The LMP1+ cells were also not eliminated solely because of Fas expression, as breeding CD19-cre;LMP1flSTOP mice on a Fas-deficient background (lpr/lpr) did not lead to rescue of LMP1-expressing B cells (data not shown).
Figure 3. Disruption of Immune Surveillance Leads to Rapid, Fatal Lymphoproliferation in CD19-cre;LMP1flSTOP Mice.
(A) Representative spleens of CD19-cre and CD19-cre;LMP1flSTOP mice repetitively treated with anti-CD4, -CD8 and -Thy1 antibodies (Abs) or PBS for 13–16 days.
(B) Numbers of CD19+Fas+ splenic B cells from mice as in (A). Each dot indicates one mouse.
(C) FACS analysis of splenocytes from indicated mice. In lower panels, analysis was on CD19+ cells.
(D) Immunoblotting of LMP1. Ctr B, CD43-depleted splenic B cells from CD19-cre mice; d3, day 3; the 4 lanes from the right, splenic B cells from antibody-injected CD19-cre;LMP1flSTOP mice; ns, non-specific.
(E and F) Real-time RT-PCR (E) and immunoblotting (F) of AID in splenic B cells treated as indicated or isolated from antibody-injected CD19-cre;LMP1flSTOP mice, respectively. Ctr B, CD43-depleted splenic B cells from C57BL/6 mice; d3, day 3; the most right lane in (F), splenic B cells from LMP1flSTOP mice cultured for 4 days after TAT-Cre treatment.
See also Figure S3.
The Fas+ B cells arising in antibody-treated CD19-cre;LMP1flSTOP mice resembled activated B cell blasts (CD19+, B220low, Fas+, CD69+, CD5+, surface IgMlow, intracellular IgMhi, CD138− ; Figure 3C and data not shown). They had deleted the STOP cassette and expressed LMP1 (Figure 3D and data not shown) as well as high levels of activation-induced cytidine deaminase (AID) transcripts and protein (Figures 3E and 3F), likely due to LMP1 signaling (He et al., 2003). Compared to WT B cells and consistent with the signaling properties of LMP1 (Soni et al., 2007), the LMP1+ B cells exhibited higher levels of p52 and lower levels of IκBα, indicating activation of the alternative and canonical NF-κB pathways; and they displayed higher levels of phospho-ERK and phospho-JNK (p46), but not phospho-p38 (Figure S3C). ~5% of the cells were in the S or G2 phase of the cell cycle, in contrast to < 1% of control B cells (Figure S3D).
T Cell Control of LMP1+ B Cells
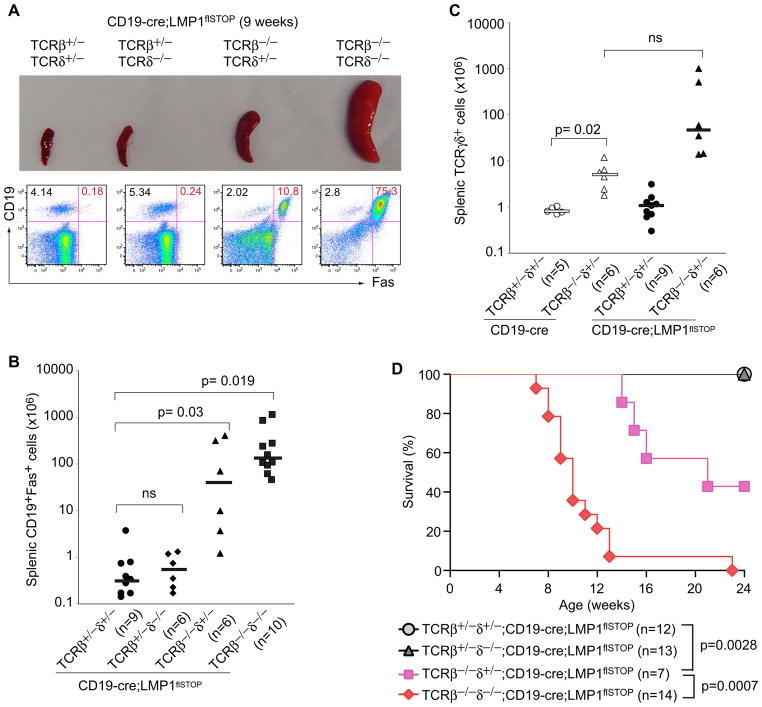
To unambiguously identify T cells as central players in the surveillance of LMP1+ B cells, we crossed CD19-cre;LMP1flSTOP to TCRβ−/−;TCRδ−/− mice. At 8–11 weeks of age, no CD19+Fas+ B cells were detectable in the spleens of CD19-cre;LMP1flSTOP mice on a TCRβ+/−;TCRδ+/− or TCRβ+/−;TCRδ−/− background, while some such cells were present on the TCRβ−/−;TCRδ+/−, and substantial numbers on the TCRβ−/−;TCRδ−/− background (Figures 4A and 4B). Thus, TCRαβ T cells are major effectors in controlling LMP1+ B cells, but TCRγδ T cells, which are indeed expanded in the absence of TCRαβ T cells (Figure 4C), also contribute. Further supporting this, essentially all of the CD19-cre;LMP1flSTOP mice on a TCRβ−/−;TCRδ−/− background died over a period of 6–12 weeks. TCRγδ T cells provided some protection, and in the presence of TCRαβ T cells the animals readily survived the 24 week period of observation (Figure 4D).
Figure 4. TCRαβ T Cells Are Major Effectors in Controlling LMP1-Expressing B Cells, and TCRγδ T Cells also Contribute.
(A) Spleens from 9 week old CD19-cre;LMP1flSTOP mice of the indicated genotypes for TCRβ and -δ alleles. The lower panel shows the FACS analysis of the corresponding splenic lymphocytes. CD19+Fas+ cells represent LMP1+ B cells.
(B) Numbers of CD19+Fas+ cells (LMP1+ B cells) in spleens of 8–11 week old CD19-cre;LMP1flSTOP mice of the indicated genotypes for TCRβ and -δ alleles.
(C) Numbers of TCRγδ+ cells in spleens of 8–11 week old CD19-cre and CD19-cre;LMP1flSTOP mice of the indicated genotypes for TCRβ and -δ alleles.
Bars in (B) and (C) show the respective median values.
(D) Survival of CD19-cre;LMP1flSTOP mice of the indicated genotypes for TCRβ and -δ alleles.
LMP1+ Tumors Arise in T-Cell Ablated Mice
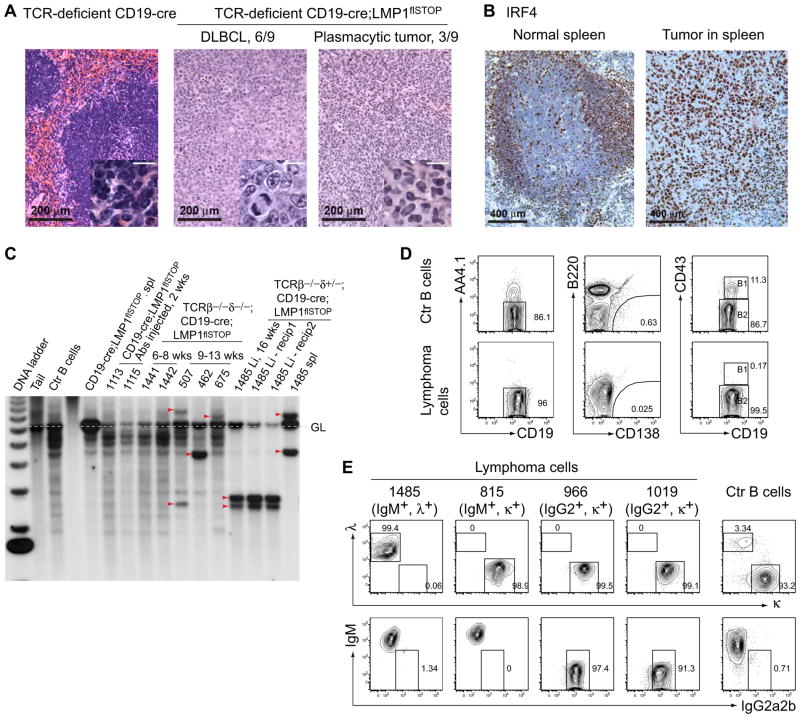
When terminally ill T cell deficient CD19-cre;LMP1flSTOP mice (Figure 4D) were examined for the cause of death, they all presented with splenomegaly and, occasionally, hepatomegaly. Most of these mice developed tumors histologically resembling Diffuse Large B Cell Lymphomas (DLBCL; 6/9 cases), while plasmacytic tumors were seen in the others (3/9 cases) (Figure 5A). Immunohistochemically, all tumors analyzed were positive for interferon regulatory factor 4 (IRF4; 5/5 cases, Figure 5B; Table S1), a target of the NF-κB pathway (Saito et al., 2007). Indeed, NF-κB activity in tumor cells was comparable to the signals detected in control B cells upon overnight CD40 stimulation, activating predominantly the alternative pathway (Figure S4A). Analysis of immunoglobulin heavy chain (IgH) gene rearrangements by Southern blotting indicated that mice dying before 8 weeks of age displayed polyclonal B cell expansion, while older animals usually developed clonal B-cell lymphomas, which could be propagated in immunodeficient (Rag2−/−γc−/−) but not WT ((C57BL/6×BALB/c) F1) animals (Figure 5C, Figure S4B, and data not shown). The tumor cells (CD19+Fas+) were AA4.1−, CD138−, CD43−, CD21+, and CD23+, and thus resembled mature follicular B cells (Figure 5D, Table S1, and data not shown). Of the 4 tumors analyzed for surface immunoglobulins (Igs), one was IgM+λ+, one IgM+κ+, and the other two were IgG2+κ+ (Figure 5E). Class switch recombination (CSR) in the latter tumors may have been due to LMP1 induced AID expression (He et al., 2003; Rastelli et al., 2008), which was indeed detectable in all LMP1+ lymphomas analyzed (Figure S4C). The rearranged V region genes of these tumors, however, were not modified by somatic hypermutation (SHM; data not shown).
Figure 5. Clonal B-Cell Lymphomas Arising in TCR-Deficient CD19-cre;LMP1flSTOP Mice.
(A) H&E staining of spleen sections from a healthy control animal (left) and two terminally ill mice (middle and right) of the indicated genotypes. 6/9 animals developed Diffuse Large B-cell Lymphoma (DLBCL) and 3/9 plasmacytic tumors, as judged by their histological appearance. Scale bars in insets, 20 μm.
(B) Immunohistochemical staining of IRF4 on spleen sections from a control animal (left) and a TCR-deficient CD19-cre;LMP1flSTOP animal carrying a tumor (right). 5/5 tumors were IRF4 positive.
(C) Southern blot analysis of rearranged Ig VDJ and DJ gene segments in B cells from the indicated mice. The 4 lanes on the right depict mouse #1485, where separate tumors developed in spleen (spl) and liver (Li). Tumors could be transplanted into immunodeficient recipients (Rag2−/−γc−/−) as exemplified for the #1485 tumor in the liver. Dashed line, germline IgH configuration; wks, weeks.
(D and E) FACS analysis of four tumor lines. Data in (D) show representative analysis of one tumor line (966); Ctr B cells from the spleen of a (C57BL/6×BALB/c) F1 mouse.
Immune Surveillance of LMP1 Driven Lymphomas
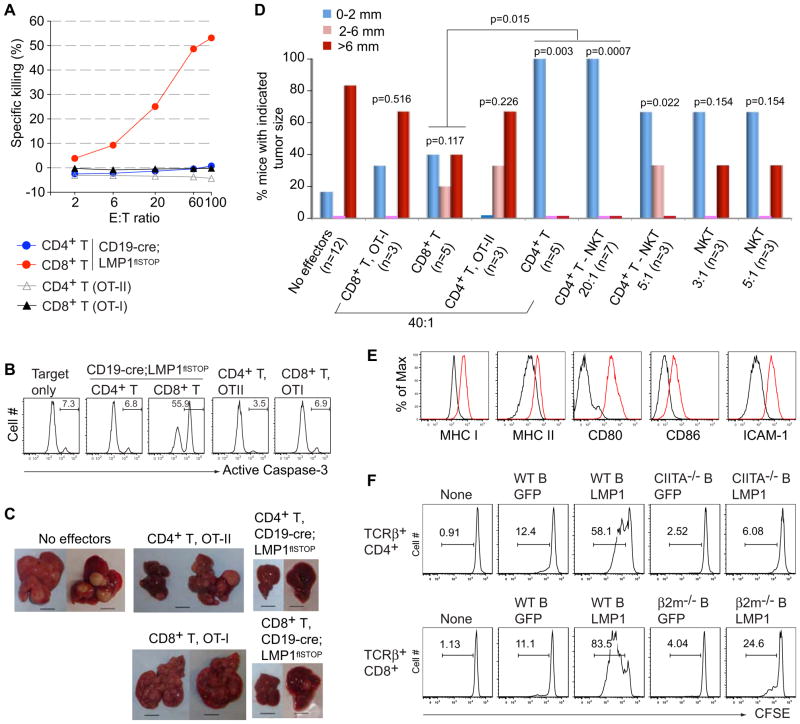
To address whether the immune surveillance of LMP1+ B cells extends to the LMP1 driven lymphomas arising in the T cell deficient animals, we established clonal cell lines from several LMP1+ tumors. In vitro killing assays with one such line revealed efficient killing of the tumor cells by CD8+ T cells from CD19-cre;LMP1flSTOP mice, whereas CD4+ T cells from the same mice as well as activated ovalbumin-specific CD4+ or CD8+ T cells from OT-II (Barnden et al., 1998) or OT-I (Hogquist et al., 1994) transgenic mice, respectively, had no effect (Figures 6A and 6B). However, when assayed for their capacity to prevent tumor outgrowth in an in vivo cell transfer system, the CD4+ T cells from CD19-cre;LMP1flSTOP mice efficiently suppressed LMP1+ tumor growth, while the anti-tumor activity of CD8+ T cells did not reach statistical significance (Figures 6C and 6D; CD4+ and CD8+ T cells from OT-II and OT-I mice showed no effect in this assay). Nevertheless, both types of cells eliminated non-transformed LMP1+ B cells upon transfer (Figure S5A).
Figure 6. CD4+ and CD8+ T Cells Control LMP1+ Tumor Cells.
(A) In vitro killing of LMP1+ tumor cells (line 966) by CD8+ T cells from CD19-cre;LMP1flSTOP mice, but not by CD4+ T cells from these mice or CD8+ and CD4+ T cells from OT-I and OT-II transgenic mice, respectively. E:T ratio, effector:target cell ratios.
(B) Representative active Caspase-3 staining on the target cells (CD19+, tumor cells) is shown for a set of killing assays at an effector:target ratio of 60. Active Caspase-3 staining indicates apoptotic cells.
(C) Representative pictures showing tumor nodules in livers of Rag2−/−γc−/− animals transplanted with 1 × 104 lymphoma cells (line 966) either alone or together with 4 × 105 of the indicated T cells. Mice were analyzed on day 30 after transplantation. Scale bars, 10 mm.
(D) Summary of tumor nodule sizes in Rag2−/−γc−/− animals transplanted with 1 × 104 lymphoma cells either alone or together with the indicated effectors at various effector:tumor cell ratios. All effector cells were derived from CD19-cre;LMP1flSTOP mice except as indicated. Numbers (n) of animals for each group are shown in the parentheses; CD4+ T-NKT, conventional CD4+ T cells. p values were calculated using Fisher’s exact test comparing the various groups to the “No effectors” control, or as indicated. Analysis of recipient mice on day 30 post-transfer revealed the absence of contaminating CD8+ cells in recipients of CD4+ cells and vice versa (Figure S5C and data not shown).
(E) FACS analysis of the indicated antigens on CD19+ cells from the spleen of C57BL/6 mice (black line) and CD19+Fas+ (LMP1+) cells from the spleen of TCRβ−/−δ−/−;CD19-cre;LMP1flSTOP mice (red line).
(F) Proliferation, monitored by CFSE dilution, of CD4+ and CD8+ T cells from CD19-cre;LMP1flSTOP mice cultured either alone or together with GFP or LMP1-expressing B cells from the indicated mice.
Data in (A) are representative of three independent experiments, (C–D) are from at least two experiments, except for the OT-II CD4+ and OT-I CD8+ T cells, (E) are representative of 2–5 mice of each genotype, and (F) of two experiments.
See also Figure S5.
The superior efficiency of CD4+ T cells in tumor elimination was unexpected. Their anti-tumor activity was not mediated by IFNγ and/or TNFα production, as it was completely preserved in the presence of antibodies blocking these cytokines (see Extended Experimental Procedures). There was also only a minor increase of T cells secreting these cytokines upon co-culture with tumor cells (Figure S5B). NKT cells, representing 10–20% of the CD4+ T cells in the bone marrow preparations, could not account for the anti-tumor activity of the CD4+ T cells, because elimination of these cells by FACS on the basis of CD1d-tetramer staining had no effect (Figure 6D). We also tested NKT cells purified from the bone marrow of CD19-cre;LMP1flSTOP mice for anti-tumor activity in the cell transfer system. Although limited by cell numbers, we were able to transfer numbers of NKT cells corresponding to those in a population of CD4+ T cells which would control tumor growth. While at least some of the cells persisted in the transplanted animals (Figure S5C), their anti-tumor activity was below the level of significance (Figure 6D, right panels).
Similar to T cell recognition of EBV infected B cells in humans (Khanna and Burrows, 2000; Rickinson and Moss, 1997), the CD4+ and CD8+ T cells from the bone marrow of CD19-cre;LMP1flSTOP mice may recognize LMP1 expressing B cells in an MHC restricted manner. As shown in Figure 6E, these cells express high levels of MHC I and II in conjunction with the co-stimulatory and adhesion molecules CD80, CD86, and CD54 (ICAM-1). To address this issue, we performed co-culture experiments with activated B cells infected with an LMP1 encoding retrovirus and lacking either MHC II or MHC I antigen expression. While both types of T cells proliferated in the presence of LMP1 expressing (but not mock-infected) activated wild-type B cells, the CD4+ T cells failed to respond to CIITA deficient B cells that lack MHC II antigen expression, and the CD8+ T cells showed severely impaired response to β2 microglobulin (β2M) and thus MHC I deficient B cells (Figure 6F). These results suggest that both types of T cells recognize the LMP1 expressing B cell blasts and, by extension, the LMP1 driven lymphoma cells by their TCR, through MHC-bound antigenic peptides. We attempted to pinpoint LMP1-derived peptides involved in this process by screening overlapping 20mers from every 10th amino acid position of LMP1 in a CD4+ T cell proliferation assay using activated B cell for presentation, but these attempts were unsuccessful (see Extended Experimental Procedures).
Exploiting NKG2D for Immunotherapy
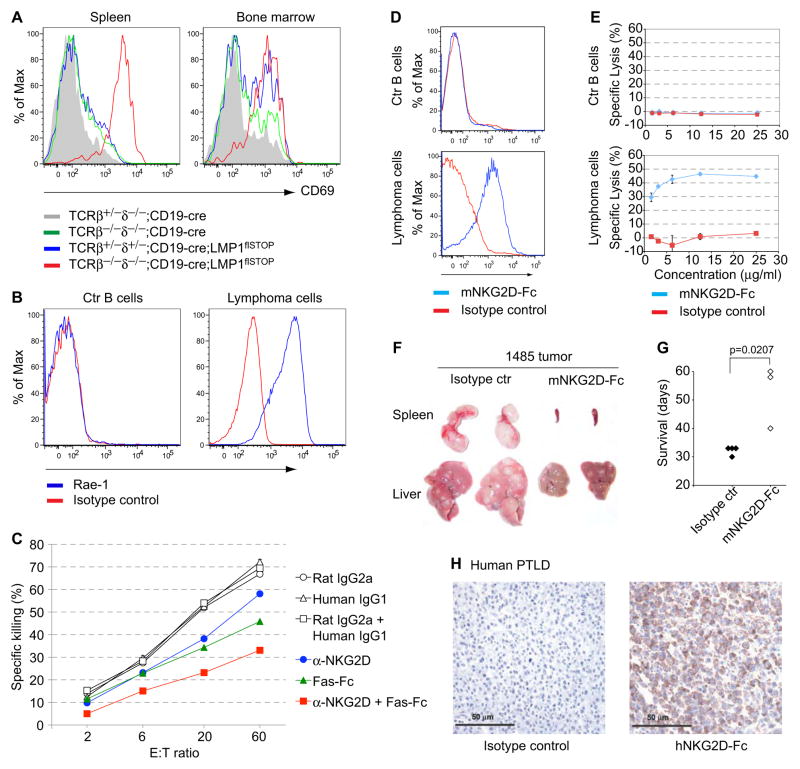
Extrapolating from the human (Hislop et al., 2007; Orange, 2006), we speculated that the tumor cells may also be recognized by NK cells (Vivier et al., 2008). Indeed, we found that in spleen and BM of CD19-cre;LMP1flSTOP mice a fraction of the NK cells were activated; and that this activation encompassed the entire NK cell population on a T cell deficient background, where LMP1+ B cells gradually expand (Figure 7A). In addition, the stress antigen Rae-1, a ligand of a major NK activating receptor termed NKG2D (Diefenbach et al., 2001), was highly expressed on the surface of the LMP1+ lymphoma cells (Figure 7B). This could be due to AID expression as in Abelson murine leukemia virus-infected B cells, allowing their recognition by NK cells (Gourzi et al., 2006). Accordingly, NK cells from CD19-cre;LMP1flSTOP mice were able to kill LMP1+ lymphoma cells in vitro, and killing could be partially inhibited by an NKG2D blocking antibody (Figure 7C). Recognition of CD95/Fas, abundantly expressed on the tumor cells, also seemed to play a role in the killing reaction, as killing was also partially inhibited by a Fas-Fc fusion protein. Addition of both antibodies had an additive effect. Although NK cells can thus recognize and attack LMP1+ B lymphoma cells, they seem to play only a minor role in the in vivo surveillance of these tumors, given that T cell deficient CD19-cre;LMP1flSTOP mice rapidly succumb to LMP1-driven lymphomas. Further supporting and extending this notion is our finding that CD19-cre;LMP1flSTOP mice on a NKG2D deficient background are perfectly protected against LMP1-driven lymphomagenesis (Figure S6A). Thus, NKG2D receptors, which are expressed not only on NK, but also several classes of activated T cells (Raulet, 2003), are not critical for tumor surveillance in this system. Accordingly, like EBV-driven PTLD in the human (see below), the LMP1+ lymphomas in the present mouse model are not selected against NKG2D ligand expression, making them ideal targets for NKG2D-based therapy. Following this reasoning, we assessed whether these tumors can be targeted by an NKG2D-Fc fusion protein, constructed by fusing the NKG2D extracellular domain to an Fc fragment of mouse IgG2a, which can activate killing by complement and cell-mediated cytotoxicity. Previous work showed that antibodies to MHC class I chain-related protein A (MICA), a human NKG2D ligand, stimulated killing of MICA expressing tumor cells through complement and cell dependent cytotoxicity (Jinushi et al., 2006; Jinushi et al., 2008). Thus, a murine NKG2D-Fc fusion protein might couple broad recognition of NKG2D ligand expressing tumor cells to Fc mediated killing mechanisms. As shown by flow cytometry, this protein readily bound to LMP1+ tumor cells (Figure 7D), and the cells were efficiently lysed upon incubation with NKG2D-Fc and complement (Figure 7E). When tumor cells were transferred into immunodeficient recipient mice, treatment of the latter with moderate doses of NKG2D-Fc significantly reduced tumor growth and prolonged the survival of the animals (Figures 7F and 7G and Figures S6B–S6E). Although the NKG2D-Fc treated recipients eventually succumbed to tumor outgrowth, the tumor cells continued to express Rae-1 at the same (high) levels as those in animals treated with control antibody (data not shown). Optimizing the dose of NKG2D-Fc and combining it with cytokines such as granulocyte macrophage colony-stimulating factor to promote antibody-dependent cell-mediated cytotoxicity (Yu et al., 2010) will therefore be important considerations in attempts to applying this therapeutic approach in the clinic. Supporting the therapeutic potential of NKG2D-Fc fusion proteins in human EBV driven pathologies, we found that 6/6 human EBV+ PTLDs examined expressed high levels of NKG2D ligands (Figure 7H).
Figure 7. Reduced Tumor Growth in Transplanted Animals Receiving an NKG2D-Fc Fusion Protein.
(A) Staining for the activation marker CD69 on NK cells (DX5+NK1.1+CD3−) from the indicated mice.
(B) Staining of normal CD19+ B cells (Ctr B cells) from a TCRβ+/−δ−/−;C D19-cre mouse and primary lymphoma cells from a T cell-deficient CD19-cre;LMP1flSTOP mouse for expression of Rae-1.
(C) Killing of LMP1+ tumor cells (line 966) by NK cells from CD19-cre;LMP1flSTOP mice in the presence of the indicated antibodies. NKG2D blocking antibody, MI6; Fas-Fc, Fas-ligand neutralizing fusion protein; rat IgG2a and human IgG1 served as controls for MI6 and Fas-Fc, respectively.
Data in (A) represent three mice of each genotype, in (B) two primary lymphomas and three established lymphoma lines, and in (C) three experiments.
(D) Staining of the Ctr B cells and lymphoma cell lines for binding of mNKG2D-Fc fusion protein.
(E) Lysis of these cells upon incubation with rabbit complement plus mNKG2D-Fc fusion protein or isotype control (mouse IgG2a) for 2 hours. Data indicate means ± s.e.m.
(F) Spleens and livers from Rag2−/−γc−/− animals transplanted with lymphoma cells (line 1485), repetitively treated with mNKG2D-Fc or isotype control, and analyzed on day 33 after tumor transplantation.
(G) Survival of mice transplanted with lymphoma cells and treated as in (F). Each dot indicates one mouse.
Data in (D–G) are representative of three tumor lines tested.
(H) A representative human EBV+ PTLD sample stained with a hNKG2D-Fc fusion protein or isotype control mouse IgG2a. 6/6 EBV+ PTLDs were stained with similar results.
See also Figure S6.
DISCUSSION
We demonstrate that expression of a single EBV protein, LMP1, in B cells is sufficient to induce immune surveillance of the LMP1+ cells by cells of the adaptive immune system, and that weakening of this surveillance mechanism results in the rapid generation of LMP1 driven B cell lymphomas. Thus, this mouse model recapitulates two salient features of EBV infection in humans and offers itself for preclinical studies relating to the pathogenesis and therapy of EBV driven malignancies under conditions of immunosuppression. In addition, the efficient immune surveillance of LMP1 expressing B cells likely explains the limited success of a previous attempt to induce B cell lymphomas through an LMP1 transgene (Kulwichit et al., 1998).
EBV driven lymphomas in immunosuppressed patients seem typically to arise from post-GC B cells expressing somatically mutated antibodies (Vakiani et al., 2008), as most of the virus in latently infected individuals seems to reside in such cells (Souza et al., 2005). In contrast, the present mouse model is based on the transformation of newly generated B cells, in a setting in which due to constitutive T cell deficiency GC B cells are not generated. Still, the tumors arising in the T cell deficient LMP1 transgenic mice are remarkably similar to EBV-driven lymphomas in immunosuppressed patients. They are derived from phenotypically mature B cells (CD19+, AA4.1−, CD43−, CD21+, CD23+, and surface Ig+), and in most cases morphologically resemble human DLBCL as is typical for monomorphic PTLD (Harris NL, 2001). As do the latter tumors (Craig et al., 2007; Vakiani et al., 2008), and consistent with active LMP1 signaling, they express the NF-κB target IRF4 (Saito et al., 2007) and AID (He et al., 2003), the enzyme controlling CSR and SHM in normal B cell development. The expression of CSR dependent antibody isotypes by some of the tumors is another feature shared by lymphomas arising in immunosuppressed patients (Vakiani et al., 2008). Thus, although PTLD and AIDS-associated B cell lymphoma express multiple EBV latency genes, our results underscore a central pathogenic role for LMP1 alone. A striking result of the present work is the efficiency and tempo of LMP1 induced B cell lymphomagenesis in the LMP1 transgenic, T cell deficient animals. Three months after birth essentially all such animals have died from aggressive, monoclonal B cell tumors. This not only highlights the potential of LMP1 to transform B cells in vivo, but also suggests that LMP1 expression induces in the cells secondary oncogenic events, resulting in the rapid outgrowth of monoclonal lymphomas. A candidate pathway in this respect is the LMP1 induced expression of AID (He et al., 2003), given the known oncogenic properties of AID in terms of mediating chromosomal translocations and introducing point mutations into non-Ig loci (Pasqualucci et al., 2001; Ramiro et al., 2004). A search for secondary oncogenic events in the LMP1 induced tumors is ongoing and should be directly relevant for the pathogenesis of EBV driven lymphomas in immunosuppressed patients.
The efficient immune surveillance of LMP1-expressing B cells and of the resulting lymphomas resembles that of EBV infected B cells in the human and points to a major role of LMP1 in the latter context. In both settings, TCRαβ cells are clearly the major players, with the present data indicating no more than an ancillary role for TCRγδ cells. However, overall our data do suggest some participation of TCRγδ cells, NKT and activated NK cells in the surveillance of the LMP1+ cells, a notion also supported by the need for anti-Thy1 in addition to anti-CD4 and -CD8 antibodies in the breaking of immune surveillance.
In vivo, activated CD4+ and CD8+ T cells appeared at the site of generation of LMP1+ B cells, namely in the BM, and at least some of these cells were able to specifically recognize the LMP1+ B cells, as demonstrated by in vitro killing and in vivo protection assays. For both classes of T cells recognition of the LMP1+ B cell blasts was MHC restricted and thus indicative of TCR-based recognition of peptide-MHC complexes. The nature of the peptides involved in this process is presently a matter of speculation. While our failure to pinpoint LMP1 derived T cell epitopes does not exclude their existence, it is in line with the absence of detectable immune surveillance of mouse B cells expressing reciprocal LMP1-CD40 fusion proteins (Homig-Holzel et al., 2008; Rastelli et al., 2008). The recognition of the LMP1+ B cells could involve a wide range of tumor specific and self-antigens that have been identified as T cell targets on tumor cells (Boon et al., 2006). In human EBV infected B cells these would include peptides from a variety of EBV proteins, prominently the EBV nuclear antigens, with LMP1 peptides playing a minor, if any role (Hislop et al., 2007). While the recognition of EBV infected and transformed B cells thus likely involves a multitude of peptide-MHC complexes, the present results indicate that LMP1 plays a crucial role in the induction of immune surveillance. We suggest that this is due to the strong and chronic activation of B cells by LMP1 through signaling pathways resembling those initiated by CD40 ligation. Like CD40-activated B cells, B cells expressing LMP1 display high levels of co-stimulatory and adhesion molecules on their surface, which have been shown to critically enhance the immunogenicity of human B cell lymphomas through effective presentation of tumor alloantigens to T cells (Schultze et al., 1995) (Schultze et al., 1997).
Surprisingly, in the in vivo tumor protection assays CD4+ cells were superior to CD8+ cytotoxic cells, despite their inability to kill the tumor cells in vitro and functional independence of IFNγ or TNFα production. CD4+ T cells have been shown in another context to exhibit anti-tumor activity in vivo, by developing into cytotoxic effectors (Quezada et al., 2010). Current immunotherapy of EBV driven diseases in transplant recipients includes infusion of EBV-specific cytotoxic T lymphocytes (CTLs) generated from autologous or donor-derived peripheral-blood mononuclear cells (Gottschalk et al., 2005; Savoldo et al., 2006). In most cases, these are predominantly CD8+ T cells, but sometimes CD4+ T cells predominate and seem to display similar therapeutic efficacy. The present results draw attention to the potential therapeutic value of EBV specific CD4+ T cells in the treatment of EBV associated malignancies in the human.
A new aspect of the surveillance of LMP1 transformed B cells emerging from the present work comes from the expression of stress antigens on these cells, likely due to LMP1 induced AID activity (Gourzi et al., 2006). These stress antigens, which we found also highly expressed on human PTLDs, are recognized by activating NK cell receptors, which are also expressed on activated CD8+ and NKT cells (Raulet, 2003). While NK cells by themselves were unable to prevent LMP1 driven lymphomagenesis, we have developed a therapeutic approach for the treatment of these tumors using the antigen binding domain of the activating NK receptor NKG2D as a soluble protein, fused to the Fc region of an antibody. We show in a proof-of-principle experiment that injection of this fusion protein into recipients of lymphoma cells strongly impedes the outgrowth of these highly aggressive tumors in our preclinical mouse model. Future studies will examine the relative contributions of complement-dependent and phagocyte-mediated killing to the anti-tumor effects. Because NKG2D ligands show limited expression in healthy tissues, the NKG2D-Fc fusion protein might achieve a favorable therapeutic index in PTLD patients. Indeed, high titers of anti-MICA antibodies are associated with clinically meaningful responses to cancer immunotherapy in the absence of toxicity (Jinushi et al., 2006). While our preclinical evaluation of the NKG2D-Fc fusion protein focused on early tumors, reflecting the rapid growth kinetics of the transplantable lines, future clinical investigations should explore the optimal timing of NKG2D-Fc administration, and a potential integration with other forms of lymphoma therapy. Finally, as NKG2D ligands are expressed by diverse hematologic and solid malignancies, the NKG2D-Fc fusion protein might also prove applicable to other clinical settings. Efforts to translate this therapeutic strategy to testing in patients are underway.
EXPERIMENTAL PROCEDURES
Mice and Depleting Antibody Treatment
To generate conditional LMP1-transgenic mice, the LMP1 coding sequence derived from the EBV B95–8 strain, preceded by a loxP-flanked Neor-STOP cassette, was cloned into the ubiquitously expressed Rosa26 locus (Sasaki et al., 2006). Transgenic mice (LMP1flSTOP) were generated from BALB/c-derived embryonic stem (ES) cells. In some experiments a similar strain recently generated from C57BL/6 ES cells was used, as indicated in the text. Other mouse strains, all on the C57BL/6 background, include CD19-cre mice; TCRβ−/−, TCRδ−/−, OT-II, OT-I, β2M−/−, and CIITA−/− mice from the Jackson Laboratory. lpr/lpr mice on the MRL/MpJ background were purchased from the Jackson Laboratory, and BALB/c mice from Taconic. Some CIITA deficient mice were kindly given to us by Dr. Arlene Sharpe, and NKG2D−/− mice by Dr. David Raulet. Homozygous CD19-cre mice were crossed with either BALB/c or LMP1flSTOP mice to generate CD19-cre or CD19-cre;LMP1flSTOP mice on a (C57BL/6×BALB/c) F1 background. For antibody-mediated cell depletion, mice were injected intraperitoneally (i.p.) two times with a 24 hour-interval, followed by injections every 3 to 4 days with 600 μg of the following depleting antibodies: anti-CD4 (YTS 191.1.2), anti-CD8 (YTS 169.4.2.1), anti-Thy-1 (YTS 154.7.7.10), either alone or in combination. All animals were maintained in specific pathogen free conditions and handled according to protocols approved by the Harvard University Institutional Animal Care and Use Committee and by the Immune Disease Institute.
Tumor and Immune Cells Transfer
In one set of experiments, 1×104 tumor cells (line 966) were transferred (i.v.) into Rag2−/−common γ chain−/− mice (Rag2−/−γc−/−, lacking B, T, and NK cells) either alone or together with 4×105 TCRβ+CD4+ or TCRβ+CD8+ cells (containing 50% activated, CD69+ cells) sorted from the BM of CD19-cre;LMP1flSTOP or the spleen of OT-II and OT-I mice (all on the (C57BL/6×BALB/c) F1 background). Recipients were analyzed on day 30 after transplantation for tumor growth. In another set of experiments, Rag2−/−γc−/− recipients were transplanted with the tumor cells either alone or together with indicated numbers of TCRβ+CD1dTetramer−CD4+ (conventional CD4+ T) or TCRβ+CD1dTetramer+CD4+/− (NKT) cells sorted from the BM of CD19-cre;LMP1flSTOP mice.
MHC Restriction
CD43− splenic B cells were purified from WT, CIITA−/−, or β2 −/− C57BL/6 mice, activated by LPS at 20 μg/ml for 24 hrs, and then infected with retroviruses, MSCV-IRES-GFP or MSCV-LMP1-IRES-GFP (kind gifts of Drs. Ellen Cahir-McFarland and Elliott Kieff). 48 hrs post-infection, 5×105 GFP+ B cells were co-cultured with 2.5×105 CFSE-labeled TCRβ+CD4+ or TCRβ+CD8+ cells sorted from the BM of CD19-cre;LMP1flSTOP mice (on a C57BL/6 background) in 96-well U-bottom plate with fresh medium added daily for 4 days, and the cells were stained with anti-TCRβ, -CD4, -CD8, and -CD19, and analyzed for CFSE levels.
NKG2D-Fc Fusion Proteins
Expression vectors were prepared with In-Fusion (Clontech) assembly, using previously reported procedures and modular cassettes (Zhu et al., 2007). Because NKG2D is a type II transmembrane protein, the DNA constructs were composed of an optimized IL-2 signal sequence (Zhang et al., 2005), the hinge-CH2-CH3 domains of mouse IgG2a beginning with EPKGP, an IEGR linker, and the extra-cellular domain of either murine (amino acids 94–232; #AF054819) or human (amino acids 78–216) NKG2D (designated mNKG2D-Fc and hNKG2D-Fc, respectively). Plasmids were electroporated into Chinese hamster ovary DG44 cells, and stable clones were generated with methotrexate selection. High producers were identified through assaying culture supernatants for mIgG2a with an ELISA. Large-scale preparation of the mNKG2D-Fc protein was performed commercially (Bio × Cell). The hNKG2D-Fc protein was purified from conditioned supernatants with recombinant protein G agarose (Invitrogen), according to the manufacturer’s protocols. For FACS analysis, cells were stained with 10 μg/ml of mNKG2D-Fc or control mIgG2a followed with a PE-conjugated goat anti-mouse IgG (Southern Biotechnology). For complement dependent lysis, tumor or control B cells were suspended in cytotoxicity medium (RPMI plus 0.3% BSA) at 4×106 cells/ml and then incubated for 2 hours at 37°C in varying concentrations of mNKG2D-Fc or control mIgG2a, and rabbit complement (Cedarlane Laboratories) diluted with 7-AAD (BD Biosciences). After washing, the cells were analyzed on a FACSCanto II. Specific lysis was calculated as: (7-AAD+% in experimental well - 7-AAD+% in complement only well)/(100 - 7-AAD+% in complement only well). For in vivo treatment of transplanted tumors, tumor cells (lines 815, 966 and 1485, all from T cell-deficient CD19-cre;LMP1flSTOP mice) were transferred (i.v.; 1×105, 1×104, and 1×106 per mouse, respectively) into Rag2−/−γc−/− mice and beginning one day later, recipients received i.v. injections of 250 μg of mNKG2D-Fc or control mIgG2a every 2 to 3 days for 7 times. The mice were monitored for tumor growth and analyzed when terminally ill.
Statistical Analysis
Data were analyzed using unpaired two-tailed Student’s t-test, except where indicated; a p value >0.05 was considered not statistically significant (ns). Survival curves were compared using the log rank test.
Supplementary Material
Acknowledgments
We thank Dr. S. Cobbold for the anti-CD4, -CD8, and -Thy1 hybridomas, Dr. L. Lanier for the CX5 hybridoma, Dr. R. Schreiber for anti-TNFα (TN3–19.12) and anti-IFNγ (H22) antibodies, Drs. E. Cahir-McFarland and E. Kieff for MSCV-IRES-GFP and MSCV-LMP1-IRES-GFP expressing vectors, Dr. D. Raulet for NKG2D−/− mice, Mr. P. Sage and Dr. A. Sharpe for CIITA−/− mice, Drs. U. Basu, F. Meng and F. Alt for the anti-AID antibody, and Dr. M. Schmidt-Supprian for the JH probe. We thank the NIH Tetramer Facility at Emory University for the PE-conjugated mCD1d-PBS57 tetramer. We are grateful to M. Bamberg, J. Xia, D. Ghitza, C. Grosse, X. Chen, A. Pellerin, J. Grundy, and J. Wang for technical assistance, M. Ottaviano for administrative assistance, S. Koralov for help with sequence analysis and intracellular cytokine staining, S. Peng for HPRT primers, K. Köchert for advice on statistics, and D. Calado, M. Janz, K. Wucherpfennig and all Rajewsky lab members for critical comments and suggestions. This work was supported by NIH grants CA098285, CA078378, AI56299, and a Leukemia and Lymphoma SCOR. B.Z. is supported by a postdoctoral fellowship of the Leukemia and Lymphoma Society. T.Y is supported by a JSPS Postdoctoral Fellowship for Research Abroad and by the Astellas Foundation for Research on Metabolic Disorders.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148–154. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Capello D, Cerri M, Muti G, Berra E, Oreste P, Deambrogi C, Rossi D, Dotti G, Conconi A, Vigano M, et al. Molecular histogenesis of posttransplantation lymphoproliferative disorders. Blood. 2003;102:3775–3785. doi: 10.1182/blood-2003-05-1683. [DOI] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Craig FE, Johnson LR, Harvey SA, Nalesnik MA, Luo JH, Bhattacharya SD, Swerdlow SH. Gene expression profiling of Epstein-Barr virus-positive and -negative monomorphic B-cell posttransplant lymphoproliferative disorders. Diagn Mol Pathol. 2007;16:158–168. doi: 10.1097/PDM.0b013e31804f54a9. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- Gourzi P, Leonova T, Papavasiliou FN. A role for activation-induced cytidine deaminase in the host response against a transforming retrovirus. Immunity. 2006;24:779–786. doi: 10.1016/j.immuni.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Harris NLSS, Frizzera G, Knowles DM. Post-transplant lymphoproliferative disorders. Lyon: IARC Press; 2001. [Google Scholar]
- He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171:5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Homig-Holzel C, Hojer C, Rastelli J, Casola S, Strobl LJ, Muller W, Quintanilla-Martinez L, Gewies A, Ruland J, Rajewsky K, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. J Exp Med. 2008;205:1317–1329. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;103:9190–9195. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A. 2008;105:1285–1290. doi: 10.1073/pnas.0711293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci U S A. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3:801–812. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404. doi: 10.1146/annurev.pathol.1.110304.100209. [DOI] [PubMed] [Google Scholar]
- Le Clorennec C, Youlyouz-Marfak I, Adriaenssens E, Coll J, Bornkamm GW, Feuillard J. EBV latency III immortalization program sensitizes B cells to induction of CD95-mediated apoptosis via LMP1: role of NF-kappaB, STAT1, and p53. Blood. 2006;107:2070–2078. doi: 10.1182/blood-2005-05-2053. [DOI] [PubMed] [Google Scholar]
- Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R, Dalla-Favera R. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc Natl Acad Sci U S A. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro AR, Jankovic M, Eisenreich T, Difilippantonio S, Chen-Kiang S, Muramatsu M, Honjo T, Nussenzweig A, Nussenzweig MC. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Rastelli J, Homig-Holzel C, Seagal J, Muller W, Hermann AC, Rajewsky K, Zimber-Strobl U. LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood. 2008;111:1448–1455. doi: 10.1182/blood-2007-10-117655. [DOI] [PubMed] [Google Scholar]
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, Pernis A, Pasqualucci L, Dalla-Favera R. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, Lin YF, Quiros-Tejeira RE, Reinke P, Schubert S, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, Gribben JG, Nadler LM. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci U S A. 1995;92:8200–8204. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze JL, Seamon MJ, Michalak S, Gribben JG, Nadler LM. Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood. 1997;89:3806–3816. [PubMed] [Google Scholar]
- Soni V, Cahir-McFarland E, Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol. 2007;597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- Souza TA, Stollar BD, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Peripheral B cells latently infected with Epstein-Barr virus display molecular hallmarks of classical antigen-selected memory B cells. Proc Natl Acad Sci U S A. 2005;102:18093–18098. doi: 10.1073/pnas.0509311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, Kikutani H. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- Vakiani E, Basso K, Klein U, Mansukhani MM, Narayan G, Smith PM, Murty VV, Dalla-Favera R, Pasqualucci L, Bhagat G. Genetic and phenotypic analysis of B-cell post-transplant lymphoproliferative disorders provides insights into disease biology. Hematol Oncol. 2008;26:199–211. doi: 10.1002/hon.859. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Leng Q, Mixson AJ. Alteration in the IL-2 signal peptide affects secretion of proteins in vitro and in vivo. J Gene Med. 2005;7:354–365. doi: 10.1002/jgm.677. [DOI] [PubMed] [Google Scholar]
- Zhu B, Cai G, Hall EO, Freeman GJ. In-fusion assembly: seamless engineering of multidomain fusion proteins, modular vectors, and mutations. Biotechniques. 2007;43:354–359. doi: 10.2144/000112536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.