Abstract
BACKGROUND AND OBJECTIVES:
Pediatric sudden cardiac death (SCD) occurs in an estimated 0.8 to 6.2 per 100 000 children annually. Screening for cardiac disorders causing SCD in asymptomatic children has public appeal because of its apparent potential to avert tragedy; however, performance of the electrocardiogram (ECG) as a screening tool is unknown. We estimated (1) phenotypic (ECG- or echocardiogram [ECHO]-based) prevalence of selected pediatric disorders associated with SCD, and (2) sensitivity, specificity, and predictive value of ECG, alone or with ECHO.
METHODS:
We systematically reviewed literature on hypertrophic cardiomyopathy (HCM), long QT syndrome (LQTS), and Wolff-Parkinson-White syndrome, the 3 most common disorders associated with SCD and detectable by ECG.
RESULTS:
We identified and screened 6954 abstracts, yielding 396 articles, and extracted data from 30. Summary phenotypic prevalences per 100 000 asymptomatic children were 45 (95% confidence interval [CI]: 10–79) for HCM, 7 (95% CI: 0–14) for LQTS, and 136 (95% CI: 55–218) for Wolff-Parkinson-White. The areas under the receiver operating characteristic curves for ECG were 0.91 for detecting HCM and 0.92 for LQTS. The negative predictive value of detecting either HCM or LQTS by using ECG was high; however, the positive predictive value varied by different sensitivity and specificity cut-points and the true prevalence of the conditions.
CONCLUSIONS:
Results provide an evidence base for evaluating pediatric screening for these disorders. ECG, alone or with ECHO, was a sensitive test for mass screening and negative predictive value was high, but positive predictive value and false-positive rates varied.
KEY WORDS: sudden cardiac death, ECG screening, hypertrophic cardiomyopathy, long QT syndrome, Wolff-Parkinson-White syndrome
Although sudden cardiac death (SCD) in children and adolescents (hereafter “children”) is rare (0.8–6.2 per 100 000 annual incidence1,2), the sudden death of a child is tragic and has widespread repercussions. Concern about SCD has raised calls for screening in primary care or school-based settings for all children; others have recommended screening for subgroups of children starting stimulants or participating in competitive athletics, both of which increase heart rate and may theoretically precipitate SCD.1,3,4
Population-based screening programs that identify children at risk for SCD have broad public appeal, as common sense suggests that presymptomatic diagnosis saves lives, and the societal cost is presumed to be the cost of the screening test itself. Japan is the sole country with published data on mass screening of school-aged children, including a targeted cardiac history and physical and electrocardiogram (ECG).5 No data regarding mass pediatric screening and associated costs are available in the United States.6
In 2008, the American Heart Association released a statement7 broadly interpreted as recommending an ECG before initiating stimulants for children with attention-deficit/hyperactivity disorder, estimated at 4% to 12% of children.8 The American Academy of Pediatrics later released a statement, in collaboration with the American Heart Association, recommending that children with attention-deficit/hyperactivity disorder be assessed with a targeted history and cardiac examination but that further evaluation, including an ECG, be obtained only if indicated.9 A recent decision analysis recommended that children participating in competitive sports undergo mass screening.3 Several studies have described screening programs for athletes. Italy uses pre-participation screening, including ECGs, for athletes aged 12 to 351 and some American universities use pre-participation screening and ECGs for college athletes. Because 10 million people in the United States may be classified as “young competitive athletes,”10 calls for screening have far-reaching implications.
Screening programs are most effective if (1) preclinical prevalence is sufficiently high in the screened population, (2) a highly discriminatory screening test is available, (3) the disease or disorder is serious, and (4) treatment while asymptomatic decreases morbidity and mortality more than treatment after symptoms develop.11 These criteria enable evaluation of the efficiency of ECG to detect the disorders that may cause SCD in asymptomatic children.
Several rare disorders cause pediatric SCD, but not all have ECG findings.12 The most common disorders detectable by ECG are hypertrophic cardiomyopathy (HCM), long QT syndrome (LQTS), and Wolff-Parkinson-White syndrome (WPW). Their estimated prevalence rates are low in otherwise healthy, asymptomatic children; moreover, the value of the ECG as a “highly discriminatory” test is not well established. The ECG should selectively identify disorders responsible for SCD in all affected patients (ie, sensitivity) and rule out these disorders in healthy children (ie, specificity). Together, low prevalence and imperfect sensitivity and specificity estimates could result in inefficient screening strategies with unanticipated societal and economic costs.
We undertook a systematic review and meta-analysis of the literature of these 3 disorders that cause SCD. Our first aim was to summarize how often ECG- or echocardiogram (ECHO)-based testing (phenotypic prevalence) suggests HCM, LQTS, or WPW among asymptomatic and undiagnosed children who could be identified by mass screening. We focused on phenotypic prevalence, rather than genetic prevalence, because genetic testing is currently impractical in mass screening programs and is limited to diagnosis or risk stratification. Our second aim was to examine the reported sensitivity and specificity of the ECG, alone or with ECHO, to detect these disorders and calculate predictive values. Together, this information on phenotypic (ECG- or ECHO-based) prevalence, sensitivity, specificity, and predictive value form an evidence base that will facilitate further evaluation of the efficiency and downstream implications of ECG screening programs for SCD.
Methods
We focused on HCM, LQTS, and WPW because they are the most common disorders potentially detectable by ECG among children. Because ECG findings in HCM are age sensitive (ie, may not be detected until late adolescence or early adulthood) and can be nonspecific, thus requiring an ECHO for diagnostic guidance, we chose to include articles that examined test characteristics of ECG alone, ECHO alone, or ECG combined with ECHO (ECG/ECHO).
Literature Searches
We performed a systematic review and searched the Medline database (1950 to December 2010) for studies reporting on HCM, LQTS, WPW, and SCD or on ECG and/or ECHO detection of these disorders. We combined keywords and Medical Subject Heading terms for hypertrophic cardiomyopathy, long QT syndrome, Wolff-Parkinson-White syndrome, sudden cardiac death, electrocardiography, echocardiography, sensitivity, and specificity. The search was limited to English-language publications of primary studies in humans with no geographic restrictions. Six reviewers screened titles and abstracts to identify relevant studies and then examined full-text articles for eligibility.
Eligibility Criteria
To summarize how often ECG- or ECHO-based testing (phenotypic prevalence) suggests HCM, LQTS, or WPW in asymptomatic children or young adults (3–25 years old), we included cross-sectional or cohort studies from the general population that used ECG or ECHO diagnostic criteria for each disorder consistent with clinical standards. Studies in which the mean age was >2 SDs from 25 years were excluded, including a recent study focused on neonates.13 We also excluded studies of highly selected subgroups that were not representative of the general population. For example, “elite” athletes who competed in competitive regional, national, or international events were excluded, but studies of normally active high school athletes were included. Studies that used sampling techniques that might result in a nonrepresentative sample (eg, convenience sampling, studies requiring informed consent from participants) were excluded. Studies assessing the frequency of genetic variations related to HCM, LQTS, or WPW were excluded, given our focus on ECG screening in asymptomatic and previously undiagnosed children.
For our second aim we included studies with data on the sensitivity or specificity of ECG (with or without ECHO) to identify children who would have a diagnosis of HCM, LQTS, or WPW according to clinical criteria. Specifically, we deemed that an adequate reference (“gold”) standard for HCM is ECHO, genotyping, or a well-documented HCM diagnosis. For LQTS, we accepted as reference standard testing for pathogenic variations (eg, the KCNQ1, KCNH2, and SCN5A genes) or a combination of personal and family history, clinical follow-up, and ECG. For WPW, ECG is the reference standard, so sensitivity and specificity information were not collected. Based on these criteria, studies with incorporation bias (where the index test comprises part of the reference standard against which it is measured) were eligible. We included studies in which only those with positive ECG and/or ECHO were verified with the reference standard (verification bias, which may overestimate sensitivity and underestimate the specificity of the index test). For those studies that had multiple alternative sets of ECG and/or ECHO criteria, we selected the most widely used or most sensitive criteria to avoid duplication of information.
Data Extraction
Four reviewers extracted data with at least 2 independently extracting or reviewing each article. All 4 reviewers discussed and resolved any discrepancies by consensus. From studies informing on phenotypic (ECG- or ECHO-based) prevalence, we extracted information on study population (description, country), study design (prospective, retrospective), sampling technique (representative or not), age of the study sample, sample size, diagnostic criteria, and number of participants with each disorder.
From studies on the sensitivity and specificity of ECG and/or ECHO to identify HCM or LQTS, we extracted information on study population (description, country), age of the study sample, type of test (ECG and/or ECHO), diagnostic criterion and thresholds for the test, reference standard definition, true-positive, false-negative, false-positive, true-negative, and presence of verification bias. If the study provided only sensitivity and specificity, we used this information to calculate the true-positive, false-negative, false-positive, and true-negative values.
Analysis
Because of the complexities of our methods, we briefly discuss analyses in the following paragraphs and provide detailed Supplemental Information that discusses characteristics of screening tests in general (eg, sensitivity, specificity, predictive value) and analytic methods used (eg, creation of hierarchical summary receiver operating characteristic [HSROC] curve).
Analysis of Phenotypic Prevalence
Phenotypic (ECG- or ECHO-based) prevalence (per 100 000) estimates and 95% confidence intervals (CIs) for HCM, LQTS, and WPW were calculated by using the exact binomial distribution. We obtained summary estimates of phenotypic prevalence by using random effects meta-analysis of logit-transformed phenotypic prevalence.14 To assess the extent to which variation in the reported outcomes may be a result of chance alone, we used Cochran Q to test for heterogeneity (significant when P < .10) and quantified its magnitude in terms of I2,15 which ranges between 0% and 100% and expresses the proportion of between-study variability attributable to heterogeneity rather than chance. We considered I2 values exceeding 75% suggestive of substantial heterogeneity. These calculations were performed by using Stata, version 11 (StataCorp LP, College Station, TX).
Analysis of Sensitivity and Specificity
For each disorder, we summarized the relationship between sensitivity (ie, the probability of having a positive test among those with the disorder) and specificity (ie, the probability of having a negative test among those without the disorder) of ECG and/or ECHO with an extension of the HSROC model.16–18 For each disorder and screening tool combination, we plotted the HSROC curve for individual studies and the HSROC curve for the summary of the reviewed studies. These curves allow visual comparison between individual studies and the summary curve. Points along the summary curves incorporate different diagnostic criteria and do not correspond directly to specific observed study cut points for ECG and/or ECHO. Restricting the range to that observed in the data, the area under the posterior estimate of the HSROC curve (AUC) calculated by numeric integration indicates test performance. An AUC of 1.0 represents a perfect test, whereas an AUC of 0.5 represents a test that performs no better than chance.
For each summary curve, we identified 2 illustrative examples to demonstrate how changes in sensitivity and specificity resulted in different predictive values, number needed to screen, false-positives, and false-negatives. We selected 2 points on the HSROC curve: (1) the point with “maximal accuracy” (ie, maximizing the sum of the sensitivity and specificity), thereby giving equal weight to ruling in people with disease (sensitivity) and ruling out those without disease (specificity); and (2) the point with “maximal specificity” where specificity was near 1 and the corresponding sensitivity, thereby giving more weight to ruling out those without the disease (specificity). We did not select a point on the curve where sensitivity was maximized because the corresponding specificity was low (0.001). By using the 2 illustrative points, we calculated 5 parameters: (1) positive predictive value (PPV, ie, the probability of having the disorder given a positive test), (2) negative predictive value (NPV, ie, the probability of not having the disorder given a negative test), (3) number needed to screen to detect 1 case, (4) number of false-positives when detecting 1 case, and (5) number of false-negatives per 100 000 children screened. To explore the effect of alternative prevalence rates, we repeated these calculations by using oft-cited prevalences of 200 per 100 000 for HCM,19 50 per 100 000 for LQTS,13 and 200 per 100 000 for WPW (See Supplemental Information, Supplemental Figures 4-8, and Supplemental Tables 1-3 for more information on screening trade-offs in general.).20
Sensitivity Analysis
To determine whether alternative assumptions substantially affected the meta-analysis results, we performed extensive sensitivity analyses.21 For key questions related to phenotypic (ECG- or ECHO-based) prevalence, we repeated the analyses excluding studies where (1) diagnostic criteria were not specified, (2) reported phenotypic prevalence exceeded the range of often-cited prevalence rates, or (3) phenotypic prevalence was based on previously diagnosed cases and not asymptomatic cases. For key questions addressing the ability of ECG- or ECHO-based testing to diagnose people with the conditions of interest, we repeated the analyses by excluding studies that did not apply the reference standard to participants with a nonsuggestive ECG and/or ECHO (ie, verification bias).
For additional sensitivity analyses, we back-calculated disease prevalence when applying a non-ECG reference standard to define disease. A positive screening ECG (eg, a result suggesting LQTS) can be either a true-positive (the person has LQTS) or a false-positive (the person does not and will not have LQTS). Thus, the frequency of “suggestive ECGs” is not the same as the prevalence of the disease. One can back-calculate the prevalence of LQTS from an acceptable alternative non-ECG reference standard to diagnose LQTS (eg, genetic testing for deleterious mutations in LQTS genes13), and from the proportion of positive ECG tests in a population. We performed such analyses only for LQTS, as an example to contextualize our discussion comments. As described in the Supplemental Information, we extended the Bayesian method of Joseph and colleagues.22
Results
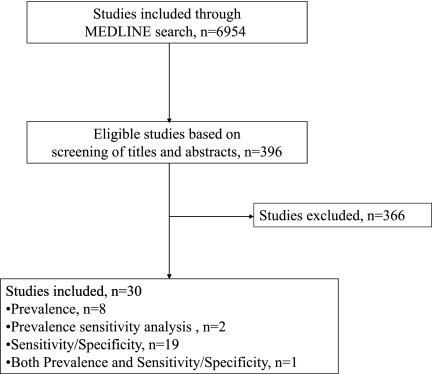
From 6954 titles and abstracts screened for eligibility, we retrieved and evaluated the full text in 396 articles, with 30 meeting eligibility criteria (Fig 1).1,19,23–50
FIGURE 1.
Literature search strategy.
Characteristics of Reviewed Studies
In the 11 primary studies1,19,23–31 that reported phenotypic (ECG- or ECHO-based) prevalence findings, study populations ranged from general to subgroups of high school athletes and military conscripts. Studies were conducted in North America, Europe, and Asia and had sample sizes ranging from 1369 to 1 336 377 (Table 1).
TABLE 1.
Articles Reporting Phenotypic (ECG- or ECHO-based) Prevalence of HCM, LQTS, and WPW
| Author | Population and Source | Location | Study Design | Sampling Technique | Age, y | Sample Size | Diagnostic Criteria |
|---|---|---|---|---|---|---|---|
| HCM, n = 9 | |||||||
| Arola (1997)23a | Medical chart review within hospitals | Finland | RC | Representative | Range: 0–20 | 1 336 377 | Interventricular septum or LV wall thickness ≥2 SD of normal |
| Colivicchi (2004)24 | Pre-participation athletic screening | Italy | PC | Representative | Mean=16.2 SD=2.4 | 7568 | LV wall thickness ≥13 mm |
| Corrado (1998)25 | Pre-participation athletic screening | Italy | PC | Representative | Mean=19 SD=5 | 33 735 | LV wall thickness ≥13 mm |
| Corrado (2006)1 | Pre-participation athletic screening | Italy | PC | Representative | Range: 12–35 | 42 386 | LV wall thickness ≥13 mm |
| Maron (1995)19 | Epidemiology study with subjects selected from general population | USA | PC | Representative | Range: 23–35 | 4111 | LV wall thickness ≥15 mm |
| Maron (1999)26a | Diagnostic testing requested by primary physician in rural community | USA | PC | Representative | Range: 16–87 | 15 137 | LV wall thickness >13 mm |
| Niimura (1989)27 | Screening of “presumably healthy” nursery school and junior high school children | Japan | PC | Representative | Ranges: 3–5, 12–14 | 930 939 | Not specified |
| Nistri (2003)28 | Screening of military recruits (males only) before mandatory military service | Italy | RC | Representative | Mean=19 SD=2 | 34 910 | LV wall thickness ≥15 mm |
| Zou (2004)29 | Epidemiology study in random sample of general population | China | PC | Representative | Range: 18–29 | 1369 | LV wall thickness ≥13 mm |
| LQTS, n = 4 | |||||||
| Chiu (2008)30 | Citywide survey of general population | Taiwan | PC | Representative | Range: 6–20 | 430 391 | QTc >450 ms |
| Corrado (2006)1 | Pre-participation athletic screening | Italy | PC | Representative | Range: 12–35 | 42 386 | Male: QTc >440 ms, Female: QTc >460 ms |
| Kobza (2009)31a | Screening of military recruits (mostly male) before mandatory military service | Switzerland | PC | Representative | Mean=19.2 SD=1.4 | 40 917 | Male: QTc >450 ms, Female: QTc >460 ms |
| Niimura (1989)27 | Screening of “presumably healthy” nursery school and junior high school children | Japan | PC | Representative | Ranges: 3–5, 12–14 | 930 939 | Not specified |
| WPW, n = 3 | |||||||
| Chiu (2008)30 | Citywide survey of general population | Taiwan | PC | Representative | Range: 6–20 | 430 391 | PR interval <120 ms; slurred upstroke of the QRS complex; QRS >120 ms |
| Corrado (1998)25 | Pre-participation athletic screening | Italy | PC | Representative | Mean=19 SD=5 | 33 735 | PR interval <0.12 s; QRS ≥0.12 s |
| Corrado (2006)1 | Pre-participation athletic screening | Italy | PC | Representative | Range: 12–35 | 42 386 | PR interval <0.12 s; QRS ≥0.12 s |
LV, left ventricular; PC, prospective cohort; QTc, corrected QT interval; RC, retrospective cohort.
These studies were included in sensitivity analysis only.
Twenty primary studies28,32–50 reported sensitivity and specificity estimates by using ECG to detect LQTS and/or HCM, ECHO to detect HCM, or ECG/ECHO to detect HCM. Populations in these studies were mainly disease probands and their relatives. The studies were conducted in North America, Europe, and Asia and had sample sizes ranging from 23 to 2770 (Table 2).
TABLE 2.
Articles Reporting Sensitivity and Specificity for Screening ECG and/or ECHO to detect HCM and LQTS
| Disorder & Screening Test | Author (year) | Sample | Location | Age, y | Screening Test Criteria | Definition of Reference (“Gold”) Standard | Verification Bias | Sample Size |
|---|---|---|---|---|---|---|---|---|
| HCM, n=11 | ||||||||
| ECG, n=10 | Autore (1988)32 | First-degree relatives of patients with HCM | Italy | Mean=36 SD=20 | LV hypertrophy; abnormal Q waves; negative T waves; atrial fibrillation; left or right bundle branch block | ECHO | No | 72 |
| Charron (1997)33 | Genotyped probands and first-degree relatives | France | Range: 18–29 | Q waves; LV hypertrophy, repolarization alterations; isolated left atrial enlargement; short PR interval; microvoltage; minor Q waves; bundle-branch block or hemiblock | Genotyping | No | 58 | |
| Charron (1998)34 | Children of HCM genotyped families | France | <18 | Q waves; voltage; repolarization alterations; abnormal PR interval, left and/or right atrial enlargement; atrial fibrillation; abnormal QRS axis; increased QRS duration; increased ventricular activation time; T waves; R/S ratio, rSr’ aspect; bundle branch block or hemiblock; microvoltage | Genotyping | No | 35 | |
| Charron (2003)35 | Genotyped probands and first-degree relatives | France | Mean=37.7 SD=17.9 | abnormal Q waves; T-wave inversion; LV hypertrophy | Genotyping | No | 109 | |
| Dipchand (1999)36 | Children with HCM and healthy controls | Canada | Median=3, Range: 0–19 | Q waves; R waves; S waves; T waves; QTc interval; voltage | ECHO, LV angiography | Yes | 73 | |
| Fragola (1993)37 | First-degree relatives of patients with HCM | Italy | Mean=34 SD=19 | LV and RV hypertrophy; atrial enlargement; rhythm disturbances; atrioventricular and intraventricular conduction; ST-T displacement; Q waves; R waves; QRS | ECHO | No | 116 | |
| Konno (2004)38 | Genotyped relatives of patients with HCM | Japan | <30 | Q wave; LV hypertrophy; ST-segment depression; T-wave inversion | Genotyping | No | 45 | |
| Nistri (2003)28 | Screening of military recruits (males only) | Italy | ≥17 | LV wall thickness; Q waves; ST-T waves | ECHO | No | 2770 | |
| Potter (2010)39 | Patients with HCM and healthy controls | UK, Sweden, US | Mean=48.7 SD=14.0 | RR, PR, P-wave, QRS and QT and JT intervals; P, QRS, and T-wave amplitudes; frontal plane QRS and T-wave axes; and ST-segment levels | ECHO | Yes | 181 | |
| Ryan (1995)40 | Probands and relatives | UK, Poland | Mean=47 SD=19 | R waves; S waves; Q waves; ST-T waves | Genotyping or clinical diagnosis | No | 506 | |
| ECHO, n=6 | Charron (1997)33 | Genotyped probands and first-degree relatives | France | Range: 18–29 | MWT | Genotyping | No | 58 |
| Charron (1998)34 | Children of HCM genotyped families | France | <18 | MWT; intraventricular septum/posterior wall; left atrium diameter; systolic anterior motion of mitral valve; mid-systolic aortic closure; gradient >30 mmHg; mitral valve regurgitation; E/A wave ratio | Genotyping | No | 35 | |
| Charron (2003)35 | Genotyped probands and first-degree relatives | France | Mean=37.7 SD=17.9 | MWT in anterior septum or posterior wall; MWT in posterior septum or free wall; systolic anterior motion of the mitral valve, redundant leaflets | Genotyping | No | 109 | |
| Fragola (1993)37 | First-degree relatives of patients with HCM | Italy | Mean=34 SD=19 | Increased interventricular septal thickness; posterior wall thickness | ECHO | No | 122 | |
| Ho (2002)41 | Genotyped relatives of patients with HCM and healthy controls | USA | Mean=35.6 SD=12.6 | LV ejection fraction; early diastolic myocardial velocities | Genotyping | No | 72 | |
| Ryan (1995)40 | Probands and relatives | UK, Poland | Mean=47 SD=19 | LV wall thickness | Genotyping or clinical diagnosis | No | 506 | |
| ECG/ECHO, n=4 | Charron (1997)33 | Genotyped probands and first-degree relatives | France | Range: 18–29 | See above Charron 1997 ECG and ECHO criteria | Genotyping | No | 58 |
| Charron (1998)34 | Children of HCM genotyped families | France | <18 | See above Charron 1998 ECG and ECHO criteria | Genotyping | No | 35 | |
| Charron (2003)35 | Genotyped probands and first-degree relatives | France | Mean=37.7 SD=17.9 | See above Charron 2003 ECG and ECHO criteria | Genotyping | No | 109 | |
| Ryan (1995)40 | Probands and relatives | UK, Poland | Mean=47 SD=19 | See above Ryan 1995 ECG and ECHO criteria | Genotyping or clinical diagnosis | No | 506 | |
| LQTS, n=9 | ||||||||
| ECG, n=9 | Benhorin (1990)42 | Participants in the International LQTS Registry and healthy controls | USA, Italy | Range: 17–60 | ST segment; repolarization area; T wave area symmetry | ECG: QTc > 440 ms | Yes | 352 |
| Kaufman (2001)43 | Genotyped relatives of patients with LQTS | USA | ≤13 | QTc | Genotyping | No | 38 | |
| Miller (2001)44 | Genotyped proband and first-degree relatives | USA | Range: 2–85 | QTc | Genotyping | No | 23 | |
| Moennig (2001)45 | Genotyped probands and relatives | Germany | Mean=38 Range: 31–50 | QTc | Genotyping | No | 116 | |
| Neyroud (1998)46 | Genotyped patients with LQTS and matched controls | France | Mean=31 SD=17 | QTc | Genotyping | Yes | 50 | |
| Swan (1998)47 | Genotyped probands and relatives | Finland | Range: 7–72 | QTc | Genotyping | No | 73 | |
| Vincent (1992)48 | Genotyped probands and relatives | Not specified | Range: 1.5–39.0 | QTc | Genotyping | No | 198 | |
| Viskin (2010)49 | Patients with LQTS and healthy controls | Not specified | Mean=32 SD=15 | QTc | LQTS registry or genotyping | Yes | 150 | |
| Wong (2010)50 | Genotyped probands and relatives | UK | Mean=26 SD=31 | QTc | Genotyping | No | 159 |
ECG/ECHO, ECG combined with ECHO; LV, left ventricular; MWT, maximal wall thickness; QTc, corrected QT interval; RV, right ventricular.
Hypertrophic Cardiomyopathy
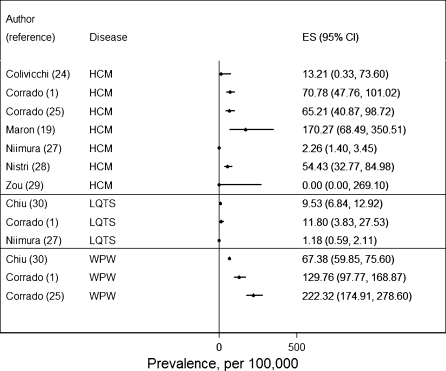
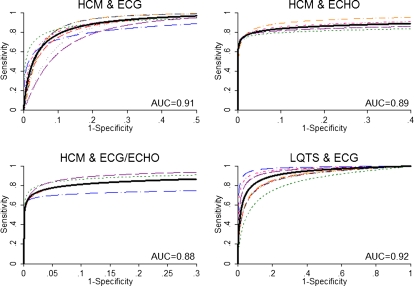
Based on 7 studies,1,19,24,25,27–29 HCM phenotypic (ECG- or ECHO-based) prevalence ranged from 0 to 170 per 100 000 (Fig 2) with a summary phenotypic prevalence rate of 45 per 100 000 (95% CI: 10–79) but with substantial variation between studies (I2 = 91%, P < .001). Inclusion of the study with a phenotypic prevalence estimate of zero29 helped inform the upper bounds of the estimate. Although 1 study27 did not specify diagnostic criteria, we included it because its exclusion had no effect on phenotypic prevalence (remained at 45 per 100 000) and this study was based on a well-established screening program in Japan. When adding 2 nonrepresentative studies23,26 for sensitivity analysis, the summary phenotypic prevalence rate decreased to 13 per 100 000 (95% CI: 7–19) with substantial heterogeneity (I2 = 92%, P < .001). Turning to screening for HCM, Fig 3 illustrates a set of HSROC curves for detection by ECG (10 studies),28,32–40 ECHO (6 studies),33–35,37,40,41 and ECG/ECHO (4 studies).33–35,40 Based on the summary HSROC curves, the AUC values were high.
FIGURE 2.
Forest plot of phenotypic (ECG- or ECHO-based) prevalence of HCM, LQTS, and WPW from reviewed studies. ES, effect size.
FIGURE 3.
HSROC and AUC of reviewed studies for HCM and ECG, HCM and ECHO, HCM and ECG/ECHO (ECG combined with ECHO), and LQTS and ECG. Solid black lines represent summaries of reviewed studies and other lines represent individual studies. The lines describe the relationship between (average) sensitivity and (average) specificity for varying diagnostic thresholds (eg, increasingly stringent ECG or ECHO criteria in the 2 top panels, respectively). These lines describe average test performance. It is generally not straightforward to correspond specific points on the curve to specific cut points for ECG or ECHO. The x-axis is restricted to the range of the data.
To provide clinical context for interpreting these results, we used the summary phenotypic prevalence estimate for HCM and the 2 previously described illustrative points (the maximal accuracy point and the maximal specificity point) on the HSROC curves for detection of HCM by using ECG, ECHO, and ECG/ECHO (Table 3). Regardless of whether an ECG, ECHO, or ECG/ECHO was used, both illustrative points yielded an NPV that was near 100%, but PPV, number needed to screen, false-positives, and false-negatives differed substantially. At the maximal accuracy point, the PPVs fell below 1% compared with PPVs from 2% to 21% at the maximal specificity point. The maximal accuracy point led to fewer false-negatives (16%) and a lower number needed to screen to detect 1 case of HCM (2600). In contrast, the maximal specificity point led to 40% to 96% false-negative rates and 4000 to 57 000 needed to screen to detect 1 case of HCM. Last, the maximal accuracy point led to more false-positives per true HCM case detected (400 vs 4–57) than the maximal specificity point. By using the often-cited prevalence of 200 per 100 00019 (4 times our estimate) resulted in similar NPV, a fourfold increase in PPV and false-negatives per 100 000 screened, and a decrease in the number needed to screen to detect 1 case and number of false-positives when detecting 1 case.
TABLE 3.
Implications of Screening ECG and/or ECHO on PPV, NPV, Number Needed to Screen, False-Positives, and False-Negatives for Illustrative Points on the HSROC Curve
| Prevalence per 100 000 | Sensitivity | Specificity | PPV | NPV | Number Needed to Screen to Detect 1 Case | Number of False-Positives When Detecting 1 Case | Number of False-Negatives per 100 000 Screened | |
|---|---|---|---|---|---|---|---|---|
| Illustrative point where sensitivity and specificity are equally weighted (maximal accuracy) with prevalence from meta-analysis | ||||||||
| HCM & ECG | 45 | 0.847 | 0.848 | 0.0025 (1/400) | 0.9999 | 2624 | 399 | 7 |
| HCM & ECHO | 45 | 0.851 | 0.851 | 0.0026 (1/390) | 0.9999 | 2611 | 389 | 7 |
| HCM & ECG/ECHO | 45 | 0.837 | 0.837 | 0.0023 (1/434) | 0.9999 | 2655 | 433 | 7 |
| LQTS & ECG | 7 | 0.861 | 0.860 | 0.0004 (1/2324) | 0.9999 | 16 592 | 2323 | 1 |
| WPW & ECG | 136 | 1.000 | 1.000 | 1.0000 | 1.0000 | 735 | 0 | 0 |
| Illustrative point where specificity is given more weight (maximal specificity) with prevalence from meta-analysis | ||||||||
| HCM & ECG | 45 | 0.039 | 0.999 | 0.0173 (1/58) | 0.9996 | 56 980 | 57 | 43 |
| HCM & ECHO | 45 | 0.607 | 0.999 | 0.2146 (1/5) | 0.9998 | 3661 | 4 | 18 |
| HCM & ECG/ECHO | 45 | 0.514 | 0.999 | 0.1879 (1/5) | 0.9998 | 4323 | 4 | 22 |
| LQTS & ECG | 7 | 0.106 | 0.999 | 0.0074 (1/136) | 0.9999 | 134 771 | 135 | 6 |
| WPW & ECG | 136 | 1.000 | 1.000 | 1.0000 | 1.0000 | 735 | 0 | 0 |
| Illustrative point where sensitivity and specificity are equally weighted (maximal accuracy) with oft-cited prevalence | ||||||||
| HCM & ECG | 20019 | 0.847 | 0.848 | 0.0110 (1/91) | 0.9996 | 590 | 90 | 31 |
| HCM & ECHO | 20019 | 0.851 | 0.851 | 0.0113 (1/88) | 0.9996 | 588 | 87 | 30 |
| HCM & ECG/ECHO | 20019 | 0.837 | 0.837 | 0.0102 (1/98) | 0.9996 | 597 | 97 | 33 |
| LQTS & ECG | 5013 | 0.861 | 0.860 | 0.0031 (1/326) | 0.9999 | 2323 | 325 | 7 |
| WPW & ECG | 20020 | 1.000 | 1.000 | 1.0000 | 1.0000 | 500 | 0 | 0 |
| Illustrative point where specificity is given more weight (maximal specificity) with oft-cited prevalence | ||||||||
| HCM & ECG | 20019 | 0.039 | 0.999 | 0.0725 (1/14) | 0.9981 | 12 821 | 13 | 192 |
| HCM & ECHO | 20019 | 0.607 | 0.999 | 0.5488(1/2) | 0.9992 | 824 | 1 | 79 |
| HCM & ECG/ECHO | 20019 | 0.514 | 0.999 | 0.5074 (1/2) | 0.9990 | 973 | 1 | 97 |
| LQTS & ECG | 5013 | 0.106 | 0.999 | 0.0504 (1/20) | 0.9996 | 18 868 | 19 | 45 |
| WPW & ECG | 20020 | 1.000 | 1.000 | 1.0000 | 1.0000 | 500 | 0 | 0 |
ECG/ECHO, ECG combined with ECHO.
Long QT Syndrome
Phenotypic (ECG-based) prevalence rates of the 3 studies reporting on LQTS ranged from 1 to 12 per 100 000 (Fig 2),1,27,30 with a summary phenotypic prevalence rate of 7 per 100 000 (95% CI: 0–14) and substantial heterogeneity (I2 = 93%, P < .001). Although 1 study27 did not specify diagnostic criteria, we included it because its exclusion increased phenotypic prevalence to only 9 per 100 000 and this study was based on a well-established screening program in Japan. The Kobza study31 was excluded because its prevalence rate (550 per 100 000) exceeded often-cited prevalence rates of LQTS (40–50 per 100 000).13 When incorporating Kobza, the summary prevalence increased fivefold to 38 per 100 000 (95% CI: 19–58) and heterogeneity increased (I2 = 99%, P < .001).42–50 The Bayesian sensitivity analysis giving the Schwartz13 prior a low weight (1/2500) resulted in similar phenotypic prevalence of 7 per 100 000 (95% CI: 1–30), whereas giving the Schwartz prior more weight increased the phenotypic prevalence to 34 per 100 000 (95% CI: 20–54). For detecting LQTS (9 studies), Fig 3 illustrates a set of HSROC curves for ECG with a summary AUC of 0.92.
To provide clinical context, we used the summary phenotypic prevalence estimate for LQTS and 2 illustrative points (the maximal accuracy point and the maximal specificity point) on the HSROC curve for detection of LQTS by using ECG (Table 3). For both points, the NPV was near 100%. The PPV was very low (0.04% [1 in 2324]) at the maximal accuracy point, but increased slightly at the maximal specificity point (0.7% [1 in 136]). With maximal accuracy, the number needed to screen to detect 1 case of LQTS with an ECG was more than 16 000 with only 14% of those with LQTS missed (false-negatives), but more than 2000 false-positives per LQTS case detected. With maximal specificity, the number needed to screen to detect 1 case increased to 135 000 and 91% of those with LQTS would be missed, but there would be only 135 false-positives per LQTS case detected. By using the often-cited prevalence of 50 per 100 00013 (7 times our estimate) resulted in a similar NPV, a sevenfold increase in PPV and false-negatives per 100 000 screened, and a decrease in number needed to screen to detect 1 case and number of false-positives when detecting 1 case.
WPW Syndrome
Three studies1,25,30 reported phenotypic (ECG-based) prevalence rates for WPW ranging from 68 to 222 per 100 000 with a summary phenotypic prevalence rate of 136 per 100 000 (95% CI: 55–218) (Fig 2) and substantial heterogeneity (I2 = 95%, P < .001). Because ECG is considered the reference standard and no studies reported estimates of sensitivity and specificity for any other screening tests to detect WPW, we assumed that its sensitivity and specificity were one and the PPV and NPV estimates were perfect and are not discussed further. By using the often-cited prevalence estimate of 200 per 100 00020 did not substantially alter the number needed to screen.
Discussion
By using published literature, we report on phenotypic (ECG- or ECHO-based) prevalence rates of HCM, LQTS, and WPW in asymptomatic children and the test characteristics of ECG and/or ECHO in detecting these disorders. Based on our prespecified inclusion/exclusion criteria and methodology, phenotypic prevalence estimates demonstrated wide variation across studies and were lower than those in neonates (eg, Schwartz et al13) or studies examining genotypic prevalence. Consequently, we explored the effects of alternative prevalence estimates in our results. Although the AUC ranged from 0.88 to 0.92, indicating that ECG and/or ECHO are statistically acceptable screening tests for detecting the most common disorders that cause SCD, the low phenotypic prevalence substantially affected the predictive value.
Because these disorders have a very low phenotypic prevalence, choosing a point on the HSROC curve that maximizes accuracy or maximizes specificity had little impact on NPV (nearly 100% NPV for HCM and LQTS); however, the maximal specificity point resulted in improved PPV (0.74% [1 in 136] to 21%) at the cost of needing to screen more individuals to detect 1 case and missing more diseased individuals because of reduced sensitivity. With maximal accuracy, the number needed to screen to detect 1 case fell and fewer cases were missed, but at the cost of lower PPV (0.04% [1 in 2324] to 0.26% [1 in 390]) and more false-positives per case detected. These findings help define boundaries of the theoretical utility of ECG and/or ECHO as screening tests for these disorders, but are difficult to comprehend in isolation. Unlike “typical” screening programs that value ruling in those who may have the disease (ie, sensitivity), these illustrative points demonstrate that when phenotypic prevalence is low, prioritizing specificity over sensitivity can improve PPV while not affecting the NPV (similar to HIV screening51).
We performed calculations to understand how these estimates might apply to population-based ECG screening. First, assuming independence, the combined prevalence estimate of HCM, LQTS, and WPW from our meta-analysis is 188 per 100 000. When maximizing accuracy, the NPV approaches 100%, indicating a low false-reassurance rate. However, the PPV of using an ECG to screen for any of the 3 disorders is 1%, indicating a high false-alarm rate (99% of children with a positive ECG would not have any of the disorders). Conversely, when maximizing specificity, the NPV still approaches 100% (false-reassurance rate remains near 0%), but the PPV is 41% (false-alarm rate decreases to 59%). A sensitivity analysis using often-cited prevalence rates (40–50 per 100 000 for LQTS,13 200 per 100 000 for HCM,19 100–200 per 100 000 for WPW20) showed a more favorable outlook for screening (higher PPV, fewer need to screen to detect 1 case, fewer false-positives when detecting 1 case, but more false-negatives per 100 000 screened).
Although these results suggest that ECG may be considered for mass screening from a statistical perspective and from the US Preventive Services Task Force criteria,52 it does not address other components of screening programs, including changes in mortality, morbidity, cost, quality of life, and functioning that need to be weighed. Because of the very low phenotypic prevalence and inherent inaccuracy in nearly all medical tests (including pediatric cardiologists reviewing ECGs53), screening for rare disorders will lead to many false-positive tests that trigger additional diagnostic evaluations and, possibly, unnecessary therapies and physical activity restrictions. In addition, false-positives may lead to unwarranted child and parent anxiety; previous work suggests this anxiety may not dissipate immediately following a cardiac evaluation and may influence lifelong lifestyle decisions.54
Concern has been raised about increased rates of diagnosed heart disease where diagnosis may not be helpful (resulting in diagnosis of cardiac nondisease and overtreatment55). Some children may ultimately be diagnosed by other means (eg, family history, emergence of sublethal symptoms, diagnostic testing for unrelated indications) even in the absence of mass screening, and earlier diagnosis of the disorder may not provide survival benefit. Among those children detected by screening, the safety, efficacy, and acceptability of specific therapies and recommendations for prophylaxis of SCD in asymptomatic children is sometimes uncertain and often based on expert consensus as opposed to clinical evidence.6
In addition, families need to be aware that a negative ECG does not definitively rule out risk for SCD. Other rare cardiac disorders cause SCD, such as anomalous origin of the coronary artery, arrhythmogenic right ventricular cardiomyopathy, catecholaminergic ventricular tachycardia, and Brugada syndrome. With the exception of Brugada syndrome, which manifests in late adolescence, these disorders are not typically diagnosed by ECG. Also, given that clinical and ECG findings may not manifest in HCM until adolescence, programs that screen young children may miss those genetically predisposed to developing HCM, which may necessitate repeat ECGs during adolescence.
This study has several limitations. First, our search was restricted to literature cataloged by Medline. Medline indexes most biomedical articles, making it unlikely that we omitted important findings. Second, our estimates may reflect publication bias, as it is conceivable that “unsuccessful” studies of diagnostic or detection interventions may not have been published. This phenomenon, if it took place, would inflate our test accuracy estimates. Third, heterogeneity existed between studies. Populations varied by age and studies varied in their screening approach and their diagnostic criteria. Fourth, our calculations omitted a targeted history and physical. Although we included medical history, physical examination, and family history in our search, we found insufficient data for further analyses. Published studies suggest that history and physical examination have low sensitivity,25 low PPV,56 and limited value from a health economics perspective.3,4 Fifth, we defined phenotypic prevalence based on results from ECG or ECHO (not genotyping) because these were the screening options considered in our analysis and because genotyping has only recently become available and is still evolving. In estimating the sensitivity and specificity of ECG and/or ECHO, however, we allowed genotypically identified cohorts because genetic testing will likely play a more prominent role in screening and diagnosing disorders that cause SCD. By using genotyping as the reference standard allows us to incorporate some variability in penetrance (leading to false-positives), which will likely be important for screening test interpretation. Finally, this study is limited by considering only 3 disorders, but these are the 3 most common disorders detectable by ECG and/or ECHO.
Despite these limitations, this study provides an important starting point for evaluating SCD screening programs. Screening programs may be gaining popularity because of availability bias in risk perception (ie, recent publicized events result in the overestimated likelihood of a similar event occurring). Given our results on the low phenotypic (ECG- or ECHO-based) prevalence and the variation in false-positive rate based on different sensitivities and specificities, further cost- or comparative-effectiveness analyses will be necessary to determine whether screening programs to detect SCD in asymptomatic children should be promoted as public health policy.
Supplementary Material
Supplemental Information
Acknowledgment
We thank Tully S. Saunders for his assistance in manuscript preparation.
Glossary
- AUC
area under the HSROC curve
- CI
confidence interval
- ECG
electrocardiogram
- ECHO
echocardiogram
- HCM
hypertrophic cardiomyopathy
- HSROC
hierarchical summary receiver operating characteristic
- LQTS
long QT syndrome
- NPV
negative predictive value
- PPV
positive predictive value
- SCD
sudden cardiac death
- WPW
Wolff-Parkinson-White syndrome
Footnotes
FINANCIAL DISCLOSURE: Dr Triedman is a consultant for Biosense Webster, Inc, and has received a speaker’s honoraria from St Jude Medical; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grant 1RC1HL100546-01 from the National Heart, Lung, and Blood Institute. Consultation from the Tufts Clinical and Translational Science Institute was supported by grant RR025752 from the National Center for Research Resources. Funded by the National Institutes of Health (NIH).
References
- 1.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296(13):1593–1601 [DOI] [PubMed] [Google Scholar]
- 2.Berger S, Utech L, Fran Hazinski M. Sudden death in children and adolescents. Pediatr Clin North Am. 2004;51(6):1653–1677, ix–x [DOI] [PubMed] [Google Scholar]
- 3.Wheeler MT, Heidenreich PA, Froelicher VF, Hlatky MA, Ashley EA. Cost-effectiveness of preparticipation screening for prevention of sudden cardiac death in young athletes. Ann Intern Med. 2010;152(5):276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denchev P, Kaltman JR, Schoenbaum M, Vitiello B. Modeled economic evaluation of alternative strategies to reduce sudden cardiac death among children treated for attention deficit/hyperactivity disorder. Circulation. 2010;121(11):1329–1337 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Yoshinaga M, Anan R, et al. Usefulness and cost effectiveness of cardiovascular screening of young adolescents. Med Sci Sports Exerc. 2006;38(1):2–6 [DOI] [PubMed] [Google Scholar]
- 6.Kaltman JR, Thompson PD, Lantos J, et al. Screening for sudden cardiac death in the young: report from a National Heart, Lung, and Blood Institute working group. Circulation. 2011;123(17):1911–1918 [DOI] [PubMed] [Google Scholar]
- 7.Vetter VL, Elia J, Erickson C, et al. American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee. American Heart Association Council on Cardiovascular Nursing . Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407–2423 [DOI] [PubMed] [Google Scholar]
- 8.Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004 -2006. National Center for Health Statistics. Vital Health Stat. 2008; 10(237) [PubMed] [Google Scholar]
- 9.Perrin JM, Friedman RA, Knilans TK, Black Box Working Group. Section on Cardiology and Cardiac Surgery . Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451–453 [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Thompson PD, Ackerman MJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism . Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115(12):1643–1655 [DOI] [PubMed] [Google Scholar]
- 11.Hennekens CH, Buring JE, Mayrent SL. Epidemiology in Medicine. 1st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1987 [Google Scholar]
- 12.Berger S, Dhala A, Friedberg DZ. Sudden cardiac death in infants, children, and adolescents. Pediatr Clin North Am. 1999;46(2):221–234 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120(18):1761–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558 [DOI] [PubMed] [Google Scholar]
- 16.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–2884 [DOI] [PubMed] [Google Scholar]
- 17.Gatsonis C, Paliwal P. Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer. AJR Am J Roentgenol. 2006;187(2):271–281 [DOI] [PubMed] [Google Scholar]
- 18.Dukic V, Gatsonis C. Meta-analysis of diagnostic test accuracy assessment studies with varying number of thresholds. Biometrics. 2003;59(4):936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–789 [DOI] [PubMed] [Google Scholar]
- 20.Triedman JK. Management of asymptomatic Wolff-Parkinson-White syndrome. Heart. 2009;95(19):1628–1634 [DOI] [PubMed] [Google Scholar]
- 21.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; West Sussex, England: John Wiley & Sons Ltd; 2009 [Google Scholar]
- 22.Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol. 1995;141(3):263–272 [DOI] [PubMed] [Google Scholar]
- 23.Arola A, Jokinen E, Ruuskanen O, et al. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am J Epidemiol. 1997;146(5):385–393 [DOI] [PubMed] [Google Scholar]
- 24.Colivicchi F, Ammirati F, Santini M. Epidemiology and prognostic implications of syncope in young competing athletes. Eur Heart J. 2004;25(19):1749–1753 [DOI] [PubMed] [Google Scholar]
- 25.Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339(6):364–369 [DOI] [PubMed] [Google Scholar]
- 26.Maron BJ, Mathenge R, Casey SA, Poliac LC, Longe TF. Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J Am Coll Cardiol. 1999;33(6):1590–1595 [DOI] [PubMed] [Google Scholar]
- 27.Niimura I, Maki T. Sudden cardiac death in childhood. Jpn Circ J. 1989;53(12):1571–1580 [DOI] [PubMed] [Google Scholar]
- 28.Nistri S, Thiene G, Basso C, Corrado D, Vitolo A, Maron BJ. Screening for hypertrophic cardiomyopathy in a young male military population. Am J Cardiol. 2003;91(8):1021–1023, A8 [DOI] [PubMed] [Google Scholar]
- 29.Zou Y, Song L, Wang Z, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am J Med. 2004;116(1):14–18 [DOI] [PubMed] [Google Scholar]
- 30.Chiu SN, Wang JK, Wu MH, et al. Taipei Pediatric Cardiology Working Group . Cardiac conduction disturbance detected in a pediatric population. J Pediatr. 2008;152(1):85–89 [DOI] [PubMed] [Google Scholar]
- 31.Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm. 2009;6(5):652–657 [DOI] [PubMed] [Google Scholar]
- 32.Autore C, Fragola PV, Picelli A, et al. Equivocal and borderline myocardial hypertrophy in relatives of patients with hypertrophic cardiomyopathy: possible implications in genetics of the disease. Cardiology. 1988;75(5):348–356 [DOI] [PubMed] [Google Scholar]
- 33.Charron P, Dubourg O, Desnos M, et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation. 1997;96(1):214–219 [DOI] [PubMed] [Google Scholar]
- 34.Charron P, Dubourg O, Desnos M, et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in genotyped children. Eur Heart J. 1998;19(9):1377–1382 [DOI] [PubMed] [Google Scholar]
- 35.Charron P, Forissier JF, Amara ME, et al. Accuracy of European diagnostic criteria for familial hypertrophic cardiomyopathy in a genotyped population. Int J Cardiol. 2003;90(1):33–38, discussion 38–40 [DOI] [PubMed] [Google Scholar]
- 36.Dipchand AI, McCrindle BW, Gow RM, Freedom RM, Hamilton RM. Accuracy of surface electrocardiograms for differentiating children with hypertrophic cardiomyopathy from normal children. Am J Cardiol. 1999;83(4):628–630, A10 [DOI] [PubMed] [Google Scholar]
- 37.Fragola PV, Borzi M, Cannata D. The spectrum of echocardiographic and electrocardiographic abnormalities in nonaffected relatives of patients with hypertrophic cardiomyopathy: a transverse and longitudinal study. Cardiology. 1993;83(5-6):289–297 [DOI] [PubMed] [Google Scholar]
- 38.Konno T, Shimizu M, Ino H, et al. Diagnostic value of abnormal Q waves for identification of preclinical carriers of hypertrophic cardiomyopathy based on a molecular genetic diagnosis. Eur Heart J. 2004;25(3):246–251 [DOI] [PubMed] [Google Scholar]
- 39.Potter SL, Holmqvist F, Platonov PG, et al. Detection of hypertrophic cardiomyopathy is improved when using advanced rather than strictly conventional 12-lead electrocardiogram. J Electrocardiol. 2010;43(6):713–718 [DOI] [PubMed] [Google Scholar]
- 40.Ryan MP, Cleland JG, French JA, et al. The standard electrocardiogram as a screening test for hypertrophic cardiomyopathy. Am J Cardiol. 1995;76(10):689–694 [DOI] [PubMed] [Google Scholar]
- 41.Ho CY, Sweitzer NK, McDonough B, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105(25):2992–2997 [DOI] [PubMed] [Google Scholar]
- 42.Benhorin J, Merri M, Alberti M, et al. Long QT syndrome. New electrocardiographic characteristics. Circulation. 1990;82(2):521–527 [DOI] [PubMed] [Google Scholar]
- 43.Kaufman ES, Priori SG, Napolitano C, et al. Electrocardiographic prediction of abnormal genotype in congenital long QT syndrome: experience in 101 related family members. J Cardiovasc Electrophysiol. 2001;12(4):455–461 [DOI] [PubMed] [Google Scholar]
- 44.Miller MD, Porter CB, Ackerman MJ. Diagnostic accuracy of screening electrocardiograms in long QT syndrome I. Pediatrics. 2001;108(1):8–12 [DOI] [PubMed] [Google Scholar]
- 45.Moennig G, Schulze-Bahr E, Wedekind H, et al. Clinical value of electrocardiographic parameters in genotyped individuals with familial long QT syndrome. Pacing Clin Electrophysiol. 2001;24(4 pt 1):406–415 [DOI] [PubMed] [Google Scholar]
- 46.Neyroud N, Maison-Blanche P, Denjoy I, et al. Diagnostic performance of QT interval variables from 24-h electrocardiography in the long QT syndrome. Eur Heart J. 1998;19(1):158–165 [DOI] [PubMed] [Google Scholar]
- 47.Swan H, Saarinen K, Kontula K, Toivonen L, Viitasalo M. Evaluation of QT interval duration and dispersion and proposed clinical criteria in diagnosis of long QT syndrome in patients with a genetically uniform type of LQT1. J Am Coll Cardiol. 1998;32(2):486–491 [DOI] [PubMed] [Google Scholar]
- 48.Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med. 1992;327(12):846–852 [DOI] [PubMed] [Google Scholar]
- 49.Viskin S, Postema PG, Bhuiyan ZA, et al. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55(18):1955–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol. 2010;3(2):120–125 [DOI] [PubMed] [Google Scholar]
- 51.Meyer KB, Pauker SG. Screening for HIV: can we afford the false positive rate? N Engl J Med. 1987;317(4):238–241 [DOI] [PubMed] [Google Scholar]
- 52.Harris RP, Helfand M, Woolf SH, et al. Methods Work Group, Third US Preventive Services Task Force . Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(suppl 3):21–35 [DOI] [PubMed] [Google Scholar]
- 53.Hill AC, Miyake CY, Grady S, Dubin AM. Accuracy of interpretation of preparticipation screening electrocardiograms. J Pediatr. 2011;159(5):783–788 [DOI] [PubMed]
- 54.Young PC. The morbidity of cardiac nondisease revisited. Is there lingering concern associated with an innocent murmur? Am J Dis Child. 1993;147(9):975–977 [DOI] [PubMed] [Google Scholar]
- 55.Bergman AB, Stamm SJ. The morbidity of cardiac nondisease in schoolchildren. N Engl J Med. 1967;276(18):1008–1013 [DOI] [PubMed] [Google Scholar]
- 56.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276(3):199–204 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information