Abstract
Recent results have indicated that factor Xa (FXa) cleaves protease-activated receptor 2 (PAR-2) to elicit protective intracellular signaling responses in endothelial cells. In this study, we investigated the molecular determinants of the specificity of FXa interaction with PAR-2 by monitoring the cleavage of PAR-2 by FXa in endothelial cells transiently transfected with a PAR-2 cleavage reporter construct in which the extracellular domain of the receptor was fused to cDNA encoding for alkaline phosphatase. Comparison of the cleavage efficiency of PAR-2 by a series of FXa mutants containing mutations in different surface loops indicated that the acidic residues of 39-loop (Glu-36, Glu-37 and Glu-39) and the basic residues of 60-loop (Lys-62 and Arg-63), 148-loop (Arg-143, Arg-150 and Arg-154) and 162-helix (Arg-165 and Lys-169) contribute to the specificity of receptor recognition by FXa on endothelial cells. This was evidenced by significantly reduced activity of mutants toward PAR-2 expressed on transfected cells. The extent of loss in the PAR-2 cleavage activity of FXa mutants correlated with the extent of loss in their PAR-2-dependent intracellular signaling activity. Further characterization of FXa mutants indicated that, with the exception of basic residues of 162-helix, which play a role in the recognition specificity of the prothrombinase complex, none of the surface loop residues under study makes a significant contribution to the activity of FXa in the prothrombinase complex. These results provide new insight into mechanisms through which FXa specifically interacts with its macromolecular substrates in the clotting and signaling pathways.
Factor Xa (FXa)1 is a vitamin K-dependent trypsin-like serine protease in plasma that upon interaction with factor Va (FVa) on negatively charged membrane surfaces in the presence of calcium (prothrombinase) activates prothrombin to thrombin during the blood coagulation process (1–3). Thrombin cleaves fibrinogen to fibrin to form a blood clot at the site of vascular injury, thereby preventing blood loss from the injured vessel (1–3). In addition to this essential role in the clotting cascade, FXa is also known to elicit intracellular signaling responses through the activation of protease-activated receptor 2 (PAR-2) expressed on endothelial cells (4–6). PAR-2 belongs to a subfamily of G-protein coupled receptors with four members to date having been identified and characterized (PAR-1, PAR-2, PAR-3, and PAR-4) (7). Thrombin can activate PAR-1, PAR-3 and PAR-4, but not PAR-2 (7). It appears that PAR-2 is specifically cleaved by FXa and factor VIIa-tissue factor complex (5,8,9), but not by thrombin or other coagulation proteases. The mechanism by which coagulation proteases recognize their plasma substrates with a high degree of specificity has been extensively studied, however, there is far less data available on the determinants of the specificity of these proteases in interaction with cell surface receptors. Recent results have indicated that distinct variant residues in the extended binding pocket of coagulation proteases play critical roles in determining the substrate and inhibitor specificity of these proteases (10–12). Among the residues located in the active-site pocket, Asp-189 (chymotrypsin numbering (13)), which is conserved in all trypsin-like serine proteases, determines the P1-Arg binding specificity through a salt-bridge interaction with the side-chain guanidine group of this residue on the activation peptide of substrates (10–13). Since the P1 residue on the extracellular domain of all four PARs is an Arg, the primary specificity of the receptor recognition by coagulation proteases thus must also be determined through a similar salt-bridge interaction between P1-Arg of the receptor and Asp-189 of the protease (12,13). However, in addition to P1-Arg, coagulation proteases also require specific interactions with other residues surrounding the scissile bonds, in particular with those at the P3-P3’ sites (nomenclature of Schechter and Berger, (14)), in order to engage their substrates in catalytic reactions, a feature that is not shared by trypsin (10–13). For instance, while FXa prefers a Gly at P2 sites (15), thrombin exhibits a strong preference for a Pro at this position of the substrates (13). Similarly, while FXa can accommodate both basic and acidic residues at the P3 site, the occurrence of an acidic residue at this position is inhibitory for thrombin (10,16). In agreement with these observations, we recently demonstrated that exchanging the P2 and/or P3 residues between PAR-1 and PAR-2 switches the receptor specificity of coagulation proteases (17). Thus, changing the P2-Pro of PAR-1 with the P2-Gly of PAR-2, and vice versa, switched the target protease specificity of the mutant receptors so that thrombin effectively cleaved the PAR-2 but not the PAR-1 mutant, and FXa efficiently cleaved the PAR-1 but not the PAR-2 mutant (17).The molecular basis for the preference of FXa and thrombin for different P2 residues appears to be due to the presence of non-conserved variant residues at the extended P2-binding pockets (S2-subsite) of these proteases (13,15).
In addition to the variant residues within or near the active-site grooves, coagulation proteases also have surface loops removed from the catalytic pocket (exosites) which play essential roles in conferring narrower substrate specificity for these proteases (11,12,18). In the case of PAR-1 recognition by thrombin, it is known that the interaction of the basic exosite-I of thrombin with the acidic hirudin-like region of PAR-1, reminiscent of the exosite-I interaction with thrombomodulin, is required for the protease recognition of the receptor (19,20). Such an exosite-binding region has not been identified in PAR-2, and it is not known whether, similar to thrombin, FXa utilizes specific exosites to interact with the receptor. To analyze the determinant of the PAR-2 recognition specificity of FXa, we used a PAR-2-cleavage reporter construct and monitored the PAR-2 cleavage specificity of a series of FXa mutants which contain substitutions in various surface loops. The results demonstrate that acidic residues of the 39-loop and basic residues of both 60- and 148-loops contribute to the FXa recognition of PAR-2 on endothelial cells. Further biochemical analysis of the activities of the FXa mutants in a prothrombinase assay revealed that none of these surface loop residues play a role in the interaction of the protease with FVa and/or prothrombin in the activation complex.
Materials and Methods
Construction, expression and purification of recombinant proteins
Construction, expression and purification of factor X (FX) both in full-length and Gla-domainless (des-Gla-fX) forms in HEK-293 cells have been described (21,22). Expression and purification of the Glu to Gln-substitution mutants of the 39-loop (E36Q, E37Q and E39Q), Arg to Ala-substitution mutants of the 148-loop (R143A, R150A and R154A) and the Ala substitution mutants of the 162-helix (R165A and K169A) (all in the chymotrypsin numbering system (13)) has been described (21,22). Expression and purification of Lys-62 to Glu (62E), Arg-63 to Glu (63E), Glu-86 to Ala (86A), and Lys-90 to Ala (90A) has been described (22,23). The Glu-124 to Ala (124A) and Glu-129 to Ala (129A) substitution mutants of FX were constructed using the same vector system and expressed in HEK-293 cells as described (21,22). For constructing the PAR-2 cleavage reporter plasmid, the cDNA encoding for secreted human tissue non-specific alkaline phosphatase (24) lacking the last 19 COOH-terminal residues, was fused to the N-terminus of the PAR-2 cDNA (PAR-2-ALP) in the mammalian expression vector pRc/RSV (Invitrogen, San Diego, CA) as described (25).
Human plasma proteins including FXa, FVa, prothrombin, thrombin and antithrombin and the factor X-activating enzyme from Russell’s viper venom (RVV-X) were purchased from Haematologic Technologies, Inc. (Essex Junction, VT). The chromogenic substrates, Spectrozyme FXa (SpFXa) was purchased from American Diagnostica (Greenwich, CT) and S2238 was purchased from Kabi Pharmacia/Chromogenix (Franklin, OH). Phospholipid vesicles containing 80% phosphatidylcholine and 20% phosphatidylserine (PC/PS) were prepared as described (21).
Activation of factor X derivatives by RVV-X
All recombinant FX derivatives were converted to active forms by RVV-X as described (21,23). Briefly, each FX derivative (~0.5-1 mg) was incubated with RVV-X (10 nM) at 37 °C for 15–30 min in 0.1 M NaCl, 0.02 M Tris-HCl, pH 7.5 containing 5 mM Ca2+ (TBS/Ca2+). Time course analysis of the activation reactions indicated that FX zymogens have been converted to their active forms under these experimental conditions. All FXa derivatives were purified on a Mono Q ion exchange column as described (21,23). The fully γ-carboxylated proteins were eluted from the ion exchange column at ~0.40–0.45 M NaCl as described (21,26). Active-site concentrations were determined by an amidolytic activity assay using SpFXa and titrations with human antithrombin assuming a 1:1 stoichiometry as described (21,23,26). These concentrations were within 80–100% of those expected based on zymogen concentrations as determined from the absorbance at 280 nm using a published absorption coefficient (21,23,26).
Prothrombin activation
The apparent affinity of FXa derivatives for FVa and their catalytic activity toward the substrate prothrombin were evaluated on PC/PS vesicles as described (21,23). Briefly, FXa (50–100 pM) was mixed with varying concentrations of human FVa (0–10 nM) on PC/PS vesicles (25 µM) in TBS/Ca2+ containing 0.1 mg/mL BSA and 0.1% PEG 8000 at room temperature. The activation reactions were initiated in 96-well assay plates with the addition of 0.5 µM human prothrombin (final concentration) for 1 min, following which they were terminated by addition of EDTA to a final concentration of 20 mM. The rate of thrombin generation was determined by an amidolytic activity assay using S2238 (100 µM) at 405 nm by a Vmax Kinetic Microplate Reader (Molecular Devices, Menlo Park, CA) as described (21,23). The concentration of thrombin generated in each activation reaction was determined from a standard curve prepared from the cleavage rate of S2238 by known concentrations of thrombin under exactly the same conditions. The (Kd(app)) value for interaction with FVa was calculated from the hyperbolic dependence of activation rates on the concentrations of the cofactor as described (21,23). In all reactions, it was ensured that less than 10% prothrombin was activated at all concentrations of the substrate. Next, the concentration dependence of prothrombin activation in the presence of FVa on PC/PS vesicles was studied by a similar prothrombinase assay. In this case, each FXa derivative (50–100 pM) in complex with a saturating concentration of FVa (10 nM in all reactions) on PC/PS vesicles (25 µM) was incubated with varying concentrations of prothrombin (7.8–1000 nM) in TBS/Ca2+ containing 0.1 mg/mL BSA and 0.1% PEG 8000. Following 0.5–1 min incubation at room temperature, EDTA was added to a final concentration of 20 mM and the concentration of thrombin generated was determined by an amidolytic activity assay as described above.
PAR-2 Cleavage Assay
Transformed human umbilical vein endothelial (EA.hy926) cells (kindly provided by Dr. C. Edgell from University of North Carolina at Chapel Hill, NC) at 90% confluence in 24-well plates were transiently transfected with pRc/RSV containing PAR-2-ALP cDNA in antibiotic free Opti-MEM medium using Lipofectamin (Invitrogen, Carlsbad, CA). On the following day, cells were washed and incubated in serum free medium for 5 h. Cells were then incubated for an additional 1 h with varying concentrations of FXa derivatives (0–100 nM). Conditioned medium was collected and centrifuged to remove cell debris. Supernatant was collected and alkaline phosphatase activity was measured using the SensoLyte luminescent secreted alkaline phosphatase reporter gene assay kit (AnaSpec, San Jose, CA) according to the manufacture’s instruction and as described (17). Results are expressed as mean ± S.E. and all experiments were repeated three times.
Permeability assays
The barrier permeability of EA.hy926 in response to thrombin (5 nM for 15 min), following treatment with FXa (1–100 nM for 3h), was quantitated by spectrophotometric measurement of the flux of Evan’s blue-bound albumin across the cell monolayer using a modified 2-compartment chamber model as described (27). Results are expressed as mean ±S.E. and all experiments were repeated three times.
Results
Expression, purification and characterization of FXa derivatives
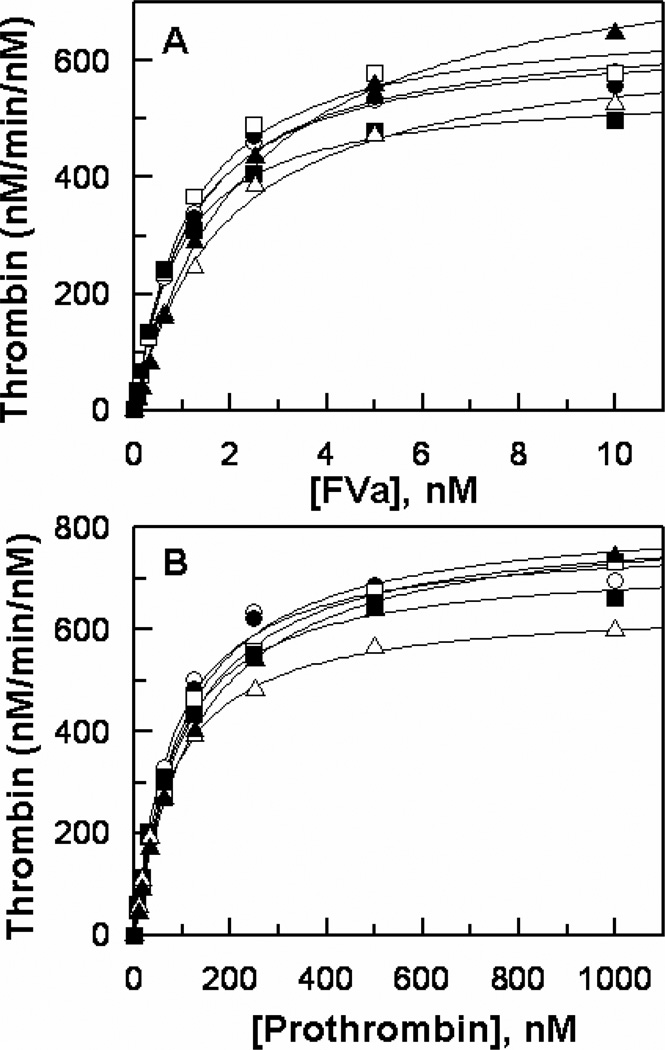
All FX derivatives were expressed in HEK-293 cells, purified to homogeneity and converted to active forms by RVV-X as described (21,23,26). The amidolytic activities of FXa derivatives, have been previously characterized and normal activities for all of them have been observed (21–23,28), suggesting that the mutagenesis of residues under study did not adversely affect the folding or the reactivity of the catalytic triad of mutant proteases. We and others have also investigated the contribution of basic residues of both the 148-loop (21) and the 162-helix (28–30) to the catalytic activity of FXa in the prothrombinase complex. The results indicated that, similar to wild-type FXa, all three mutants of the 148-loop (R143A, R150A and R154A) interact with FVa with a similar Kd(app) of 1–2 nM and exhibit a similar catalytic specificity (kcat/Km) toward prothrombin in the prothrombinase complex (21,23). On the other hand, the basic residues of the 162-helix (in particular Arg-165) appear to be critical for the activity of FXa and have been shown to provide recognition sites for FVa in the prothrombinase complex (28–30). Thus the mutagenesis of Arg-165 and Lys-169 significantly impaired the affinity of the FXa mutants for interaction with FVa as well as their catalytic activity toward prothrombin in the prothrombinase complex (28–30). However, the contribution of the acidic residues of the 39-loop and the basic residues of the 60-loop to the catalytic function of FXa in the prothrombinase complex has not been investigated. The results presented in Fig. 1A demonstrate that the mutants of both surface loops bind to FVa with near normal apparent affinities (Kd(app) = 1-2 nM) and activate prothrombin with normal catalytic efficiencies (kcat/Km = 1.3–1.5×108 M−1 s−1 for all FXa derivatives) (Fig. 1B). These results suggest that neither one of the acidic residues of the 39-loop nor the basic residues of the 60-loop make a significant contribution to the specificity of FXa interaction with either the cofactor or the substrate of the prothrombinase complex. It should be noted that all of these FXa derivatives also exhibited normal activity toward prothrombin in the absence of FVa.
Figure 1.
FVa-dependence of prothrombin activation by FXa derivatives in the prothrombinase complex. (A) The activation of prothrombin (0.5 µM) by FXa derivatives (50–100 pM each) was monitored in the presence of different concentrations of FVa on PC/PS vesicles (25 µM) in TBS/Ca2+ containing 0.1 mg/mL BSA and 0.1% PEG 8000. Following 0.5–1 min activation at room temperature, EDTA was added to a final concentration of 20 mM and the rate of thrombin generation was measured from the cleavage rate of S2238 as described under “Materials and Methods”. (B) The same as (A) except that the concentration dependence of prothrombin activation by the same FXa derivatives was monitored in the presence of a saturating concentration of FVa (10 nM). The symbols in both panels are: wild-type FXa (○), FXa-E36Q (●), FXa-E37Q (□), FXa-E39Q (■), FXa-K62E (△), and FXa-R63E (▲). Solid lines in both panels are nonlinear regression fits of kinetic data to the Michaelis-Menten equation, yielding similar Kd(app) values of 1–2 nM for FVa (panel B) and similar kcat/Km values of 1.3–1.5 × 108 M−1 s−1 (panel B) for all FXa mutants. Data are derived from at least three independent measurements ±S.D.
PAR-2 cleavage
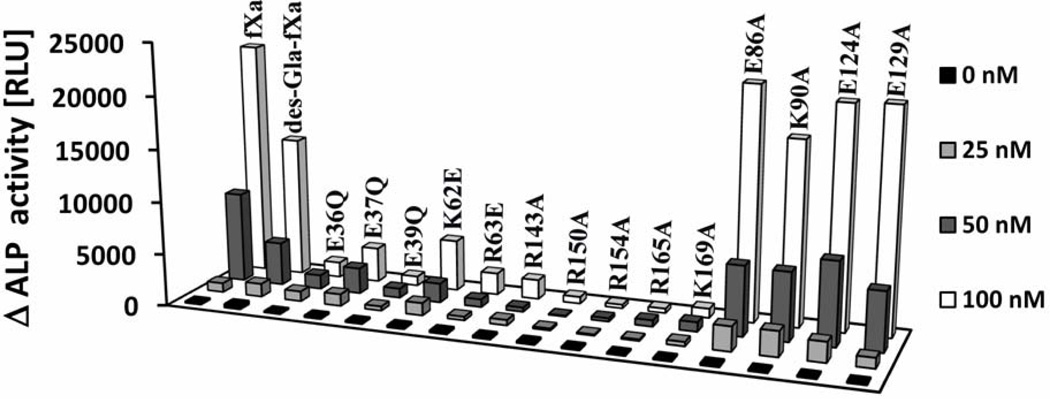
The activity profiles of FXa derivatives toward PAR-2, expressed in fusion with alkaline phosphatase (ALP) at the surface of endothelial cells, are presented in Fig. 2. In contrast to their normal amidolytic and prothrombinase activities, all mutants of the 39-, 60-, and 148-loops exhibited dramatically impaired activities in the PAR-2 cleavage assay, suggesting that these residues contribute to the specificity of PAR-2 recognition by FXa on vascular endothelial cells. Furthermore, similar to their impaired prothrombinase activities, the 162-helix mutants were also poor activators of PAR-2, suggesting that the basic residues of this loop contribute to the specificity of FXa in both the prothrombinase and PAR-2 cleavage reactions. In agreement with previous results (17), the cleavage of the cell surface PAR-2 by FXa did not require the Gla-domain of the protease since the Gla-domainless protease (des-Gla-fXa) also cleaved PAR-2 with a relatively good efficiency (Fig. 2). In light of the results that every mutant tested exhibited markedly impaired activity toward PAR-2, we decided to monitor the activity of several other mutants of FXa (E86A, K90A, E124A and E129A) to ensure that the diminished activities of FXa derivatives are not somehow non-specifically related to the mutagenesis of FXa. However, analysis of the activity of these four FXa mutants ruled out this possibility since all mutants exhibited normal activity toward PAR-2 in this assay (Fig. 2). It is worth noting that the prothrombinase activities of FXa-E86A and FXa-K90A have been previously studied and normal activities for them have been observed (23). Further studies in this manuscript suggested that both FXa-E124A and FXa-E129A also have normal affinity for FVa and wild-type-like catalytic activity in the prothrombinase complex (data not presented).
Figure 2.
PAR2-ALP cleavage profiles of FXa derivatives. EA. hy926 cells, transiently transfected with PAR2-ALP fusion construct, were incubated in the presence and absence of indicated concentration of FXa derivatives for 1 hr at 37 °C. The cleaved alkaline phosphatase (ALP) activity in the cultured medium was measured using a chemiluminescence substrate (AnaSpec). Δ ALP on y-axis represents the background-subtracted alkaline phosphatase activity in presence of proteases.
PAR-2-dependent signaling
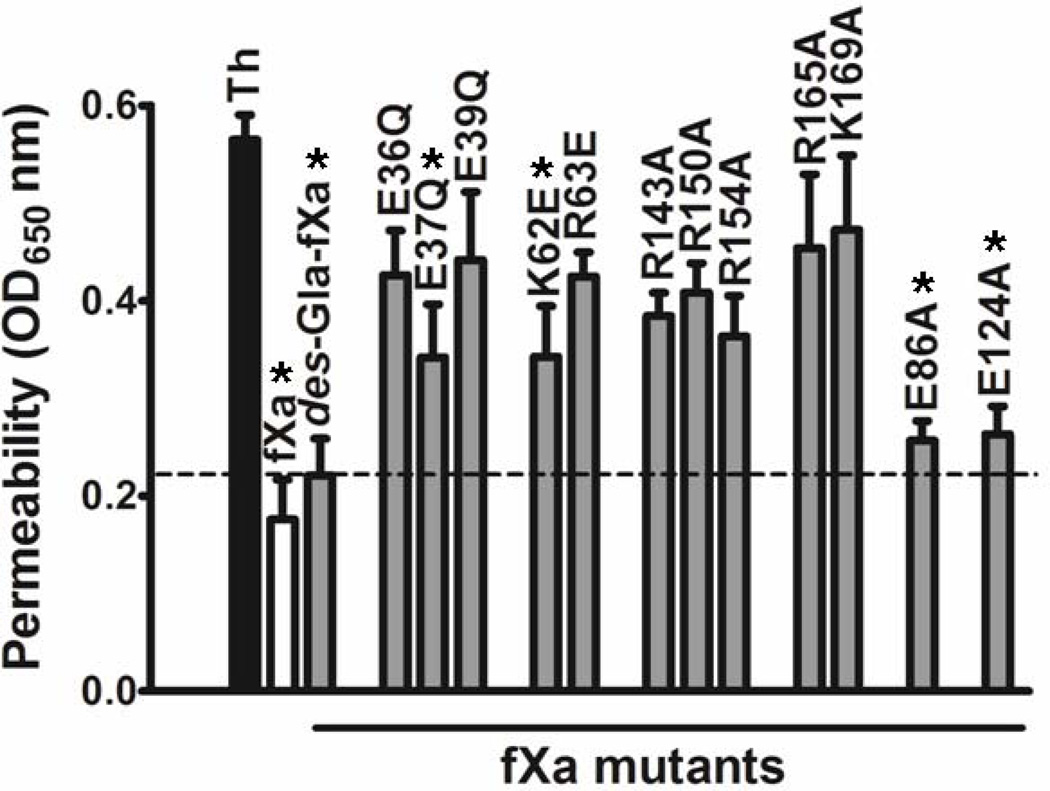
FXa is known to elicit PAR-2-dependent protective signaling responses in endothelial cells (6,17,31). To determine whether the PAR-2 cleavage profiles of mutants correlate with their ability to elicit PAR-2-dependent intracellular signaling activities, the properties of mutants were monitored in an endothelial cell permeability assay as described (17,31). Analysis of the concentration dependence of FXa activity in this assay showed that FXa elicits a barrier protective effect in thrombin-stimulated endothelial cells with an optimal concentration of ~25 nM (data not presented). The barrier protective activity profiles of FXa mutants (25 nM) in the thrombin-induced endothelial cell hyperpermeability assay are presented in Fig. 3. Consistent with their PAR-2 cleavage profiles in endothelial cells (Fig. 2), all mutants of the 39-, 60-, and 148-loops and 162-helix exhibited significantly lower protective signaling activity in endothelial cells (Fig. 3). The extent of loss of the activities of FXa mutants correlated in the two cell-based assays. Thus, among the residues of the 39-loop, the E39Q mutant had the lowest activity in the PAR-2 reporter cleavage assay (Fig. 2). The same mutant also exhibited the lowest barrier protective activity in the permeability assay (Fig. 3). The same observation was true for the 60-loop mutants when their activities were compared in the PAR-2 cleavage and permeability assays (Figs. 2 and 3). In agreement with the results presented above, the FXa mutant lacking its Gla-domain as well as the E86A and E124A mutants exhibited normal signaling activities (Fig. 3). The activities of the other two FXa mutants (K90A and E129A) were not examined in the permeability assay. The barrier protective signaling activity of FXa was dependent on the cleavage of PAR-2 since the function-blocking anti-PAR-2, but not anti-PAR-1 antibody effectively inhibited the barrier protective activity of FXa in endothelial cells (data not shown). The same anti-PAR-2 antibody also inhibited the FXa cleavage of the PAR-2-ALP fusion protein in endothelial cells transfected with this construct (data not shown).
Figure 3.
Barrier protective activity of FXa derivatives in endothelial cells in response to thrombin. Confluent EA. hy926 cells in a dual chamber system were preincubated for 3h with FXa derivatives (25 nM), followed by incubation with 5 nM thrombin for 15 min to induce hyperpermeability. Cell permeability was assessed by the amount of BSA-bound Evans blue diffused into the lower chamber by measuring OD650 nm as described under “Materials and Methods”. Each point represents Mean ± SD of three independent wells. Unpaired t-test suggests statistical significance with *; P < 0.05 vs. no FXa.
Discussion
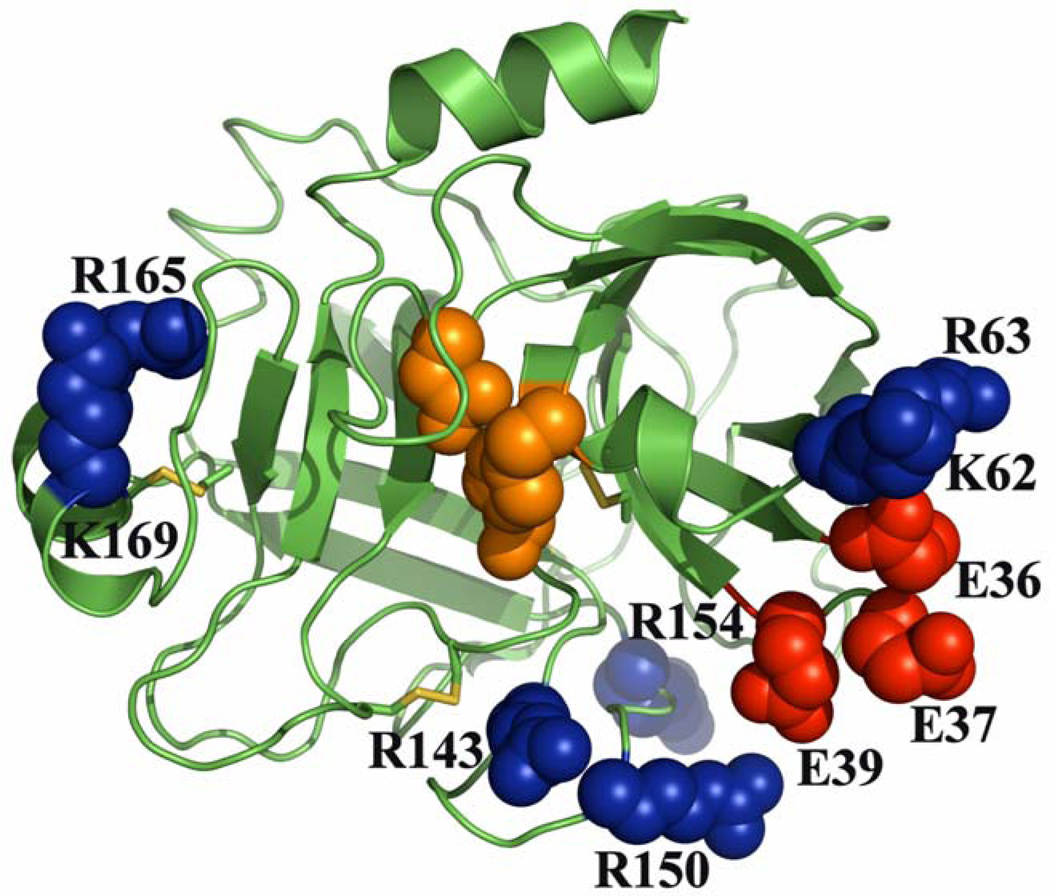
In this study, we have mapped the exosite residues of FXa that contribute to the specificity of PAR-2 recognition by the protease on endothelial cells. The relative three dimensional locations of these residues on the four surface loops of the catalytic domain of FXa are presented in Fig. 4. Among these surface loop residues, only residues of the 162-helix are essential for the catalytic function of FXa in both the prothrombinase and signaling assays. By contrast, while the charged residues of 39-, 60- and 148-loops contribute to determining the PAR-2 cleavage specificity of FXa, neither one of these residues made a significant contribution to the catalytic activity of FXa in the prothrombinase complex. This is derived from the current mutagenesis results presented above for the residues of the 39- and 60-loops as well as from the previous results we reported for the residues of the 148-loop (21). Previous studies have established that the basic residues of the 162-helix (Arg- 165 and Lys-169) make important contributions to the specificity of FXa interaction with FVa in the prothrombinase complex (28–30). In agreement with previous results, both mutants of FXa exhibited 3-4-fold weaker affinity for FVa and their catalytic activities (kcat/Km) toward prothrombin were also impaired up to 10-fold (data not presented, see also Refs. 28 and 29). In addition to their diminished activities in the prothrombinase reactions, both mutants of 162-helix also exhibited dramatically lower activity toward PAR-2 in both the cleavage and signaling assays. These results indicate that basic residues of 162-helix may contribute to the specificity of FXa in both clotting and signaling pathways. The observation that the Gla-domainless FXa (des-Gla-fXa) exhibited a normal signaling activity is consistent with our previous conclusion that the Gla-domain of FXa is not required for its barrier protective function (17), however, the Gla-domain plays an essential role in prothrombin activation by FXa in the prothrombinase complex (1–3). Taken together, these results suggest that, with the exception of the residues of 162-helix, distinct structural elements are involved in determining the specificity of FXa interaction with its substrates/receptors in the alternative clotting and signaling pathways.
Figure 4.
The x-ray crystal structure of the catalytic domain of FXa. The active site residues are highlighted in orange. Positive and negatively charged exosite residues that are critical for the interaction of FXa with PAR-2 and have been subjected to mutagenesis are respectively colored in blue and red. The coordinates obtained from Protein Data Bank accession code 1EZQ were used to prepare the figure (48).
Relative to residues of the 39, 60 and 148 surface loops, which are all located at the substrate entry site of the active-site pocket, the basic residues of 162-helix are located on the opposite side of the active-site pocket in the back of the molecule (Fig. 4). Given their three dimensional locations, it is not known whether the residues of 162-helix are directly involved in interaction with PAR-2 or if allosteric changes involving other surface loops of FXa (induced by the mutation of 162-helix) are responsible for the slower activity of the FXa mutants toward the cell surface receptor. In support of the latter possibility, it is known that the conformation of the 162-helix is allosterically linked to the sodium-binding 220-loop of FXa (32,33). Results from several laboratories have further demonstrated that an allosteric linkage between the sodium-binding loop and the S1 specificity pocket of FXa and other coagulation proteases play a decisive role in their catalytic activity (32–34). Thus, the possibility that the mutation of the 162-helix indirectly impairs the activity of FXa toward PAR-2 cannot be ruled out at this time. Another possibility worth mentioning is that 162-helix may constitute an interactive-site for an unknown cofactor/co-receptor that facilitates the interaction of the protease with the cell surface receptor. In this context, it has been demonstrated that FXa recruits other cell surface receptors to elicit PAR-dependent intracellular signaling responses in endothelial cells (31,35,36). Thus, further studies will be required to understand the molecular basis for the diminished activity of the 162-helix mutants of FXa toward PAR-2 in endothelial cells. It is also worth noting that in a previous study we demonstrated a similar essential role for the two acidic residues of the homologous 162-helix of activated protein C in the PAR-1-dependent protective signaling activity of the protease in endothelial cells (37). There is also some evidence in support of a critical role for the residues of 162-helix in the catalytic function of other coagulation proteases. For instance, in the case of factors VIIa and IXa, residues of 162-helix are known to constitute interactive-sites for the cofactors, tissue factor and factor VIIIa, respectively, in the respective physiological activation complexes (38–40). In thrombin, basic residues of 162-helix are part of the heparin-binding exosite of the protease which is also known as exosite-II (41). This exosite is known to play a key role in mediating the specific interaction of thrombin with various physiological ligands (11–13). Thus, residues of 162-helix contribute to determining the substrate/cofactor specificity of all coagulation proteases.
Unlike residues of the 162-helix, which provide interactive-sites for the cofactors in the coagulation activation complexes, the residues of other surface loops under study (39, 60 and 148 loops) do not interact with protein or polysaccharide cofactors, but instead influence protease specificity by directly interacting with residues in the vicinity and/or surrounding the scissile bonds. Thus, the residues of 39- and 148-loops play insignificant roles in binding to FVa and/or in recognition of prothrombin in the prothrombinase complex, but they do contribute to the specificity of FXa interaction with antithrombin and tissue factor pathway inhibitor as previously demonstrated (21,22). These inhibitors regulate the proteolytic activity of FXa in the clotting cascade. A similar role for the same loops have been reported in the specific interaction of factor IXa with antithrombin (42,43). In the case of thrombin, both loops are also involved in interaction with various substrates and inhibitors (44,45). Unlike the 60-loop of FXa, which plays no apparent role in interaction with antithrombin or recognition of prothrombin, the 60-loop of thrombin, containing 8 insertion residues (13), plays a critical role in the specific interaction of thrombin with its divergent physiological substrates and inhibitors (46,47). Taken together, these results suggest that residues of the surface loops, those surrounding the substrate entry site of the catalytic pocket of FXa (Fig. 4), are all involved in determining the PAR-2 recognition specificity of the protease in the signaling pathway. Nevertheless, these residues do not appear to have a role in the substrate specificity of FXa in the prothrombinase complex. These residues are therefore critical for the interaction of FXa with its physiological substrates and inhibitors excluding prothrombin. In the case of prothrombin, it appears that complex formation with FVa in the prothrombinase complex, which enhances the catalytic efficiency of FXa toward prothrombin by more than five orders of magnitude (11), diminishes the importance of the P3-P3’ binding specificity of the protease in activation of the substrate. Consistent with this hypothesis, exchanging the P2 and P3 binding pocket residues of FXa with activated protein C switched the chromogenic substrate and inhibitor specificity of the mutant protease in free form, but not when the mutant protease was assembled into the prothrombinase complex (16). Similarly, exchanging P2 and P3 residues of PAR-1 with the corresponding residues of PAR-2 switched the receptor specificity of FXa so that the protease cleaved the PAR-1 mutant with the same efficiency as it did the wild-type PAR-2 (17), suggesting that the residues of the three surface loops surrounding the active site pocket of FXa primarily determine the specificity of P and/or P’ residues that are located in the immediate vicinity of the P1-Arg on the extracellular domain of PAR-2.
Acknowledgements
We would like to thank Dr. Soumendra Rana for technical assistance with cell-based assays and Audrey Rezaie for proofreading the manuscript.
The research discussed herein was supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL 101917 and HL 68571 to ARR).
Footnotes
Abbreviations – FXa, activated factor X; FVa, thrombin activated factor V; PAR, protease-activated receptor; FXa-E36Q, FXa, E37Q, FXa-E39Q, FXa-K62E, FXa-R63E, FXa-R143A, FXa-R150A, FXa-R154A, FX-R165A, FXa-K169A, FXa-E86A, FXa-K90A, FXa-E124A, FXa-E129A, activated factor X mutants in which residues at the identified positions in the chymotrypsin numbering system (13) have been substituted with Gln or Ala; PEG, polyethylene glycol; BSA, bovine serum albumin.
References
- 1.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Ann. Rev. Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 2.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 3.Jackson CM, Nemerson Y. Blood Coagulation. Ann. Rev. Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- 4.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J. Thromb. Haemost. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 5.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc. Natl. Acad. Sci. (USA) 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feistritzer C, Lenta R, Riewald M. Protease-activated receptor-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J. Thromb. Haemost. 2005;3:2798–2805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 8.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. (USA) 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao LV, Pendurthi UR. Tissue factor-factor VIIa signaling. Arterioscler. Thromb. Vasc. Biol. 2005;25:47–56. doi: 10.1161/01.ATV.0000151624.45775.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezaie AR, Esmon CT. Molecular basis of residue 192 participation in determination of coagulation protease specificity. Eur. J. Biochem. 1996;242:477–484. doi: 10.1111/j.1432-1033.1996.477rr.x. [DOI] [PubMed] [Google Scholar]
- 11.Krishnaswamy S. Exosite-driven substrate specificity and function in coagulation. J. Thromb. Haemost. 2005;3:54–67. doi: 10.1111/j.1538-7836.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- 12.Krem MM, Rose T, Di Cera E. Sequence determinants of function and evolution in serine proteases. Trends Cardiovasc. Med. 2000;10:171–176. doi: 10.1016/s1050-1738(00)00068-2. [DOI] [PubMed] [Google Scholar]
- 13.Bode W, Mayr I, Baumann U, Huber R, Stone SR, Hofsteenge J. The refined 1.9 Å crystal structure of human α-thrombin: interaction with D-Phe-Pro-Arg chlorometheylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 15.Padmanabhan K, Padmanabhan KP, Tulinsky A, Park CH, Bode W, Huber R, Blankenship DT, Cardin AD, Kisiel W. Structure of human des(1–45) factor Xa at 2.2 A resolution. J. Mol. Biol. 1993;232:947–966. doi: 10.1006/jmbi.1993.1441. [DOI] [PubMed] [Google Scholar]
- 16.Rezaie AR. Role of residue 99 at the S2 subsite of factor Xa and activated protein C in enzyme Specificity. J. Biol. Chem. 1996;271:23807–23814. doi: 10.1074/jbc.271.39.23807. [DOI] [PubMed] [Google Scholar]
- 17.Rana S, Yang L, Hassanian SM, Rezaie AR. Determinants of the specificity of protease activated receptors 1 and 2 signaling by factor Xa and thrombin. J. Cell. Biochem. 2012;113:977–984. doi: 10.1002/jcb.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furie B, Bing DH, Feldmann RJ, Robison DJ, Burnier JP, Furie BC. Computer-generated models of blood coagulation factor Xa, factor IXa, and thrombin based upon structural homology with other serine proteases. J. Biol. Chem. 1982;257:3875–3882. [PubMed] [Google Scholar]
- 19.Liu LW, Vu TK, Esmon CT, Coughlin SR. The region of the thrombin receptor resembling hirudin binds to thrombin and alters enzyme specificity. J. Biol. Chem. 1991;266:16977–16980. [PubMed] [Google Scholar]
- 20.Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106 doi: 10.1182/blood-2005-04-1710. 2605-2012. [DOI] [PubMed] [Google Scholar]
- 21.Manithody C, Yang L, Rezaie AR. Role of basic residues of the autolysis loop in the catalytic function of factor Xa. Biochemistry. 2002;41:6780–6788. doi: 10.1021/bi0255367. [DOI] [PubMed] [Google Scholar]
- 22.Rezaie AR, Yang L, Manithody C. Mutagenesis studies toward understanding the mechanism of differential reactivity of factor Xa with the native and heparin-activated antithrombin. Biochemistry. 2004;43:2898–2905. doi: 10.1021/bi036145a. [DOI] [PubMed] [Google Scholar]
- 23.Manithody C, Rezaie AR. Functional mapping of charged residues of the 82–116 sequence in factor Xa: Evidence that lysine 96 is a factor Va independent recognition site for prothrombin in the prothrombinase complex. Biochemistry. 2005;44:10063–10070. doi: 10.1021/bi0508791. [DOI] [PubMed] [Google Scholar]
- 24.Weiss MJ, Henthorn PS, Lafferty MA, Slaughter C, Raducha M, Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. (USA) 1986;83:7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J-S, Yang L, Rezaie AR. lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J. Thromb. Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Manithody C, Yang L, Rezaie AR. Zymogenic and enzymatic properties of the 70–80 loop mutants of factor X/Xa. Protein Sci. 2004;13:431–442. doi: 10.1110/ps.03406904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae J-S, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the PAR-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaie AR. Identification of basic residues in the heparin-binding exosite of factor Xa critical for heparin and factor Va binding. J. Biol. Chem. 2000;275:3320–3327. doi: 10.1074/jbc.275.5.3320. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph AE, Porche-Sorbet R, Miletich JP. Definition of a factor Va binding site in factor Xa. J. Biol. Chem. 2001;276:5123–5128. doi: 10.1074/jbc.M006961200. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi SH, Yang L, Manithody C, Rezaie AR. Membrane-dependent interaction of factor Xa and prothrombin with factor Va in the prothrombinase complex. Biochemistry. 2009;48:5034–5041. doi: 10.1021/bi900240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae J-S, Yang L, Rezaie AR. Factor X/Xa elicits protective signaling responses in endothelial cells directly via PAR-2 and indirectly via endothelial protein C receptor-dependent recruitment of PAR-1. J. Biol. Chem. 2010;285:34803–34812. doi: 10.1074/jbc.M110.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezaie AR, He X. Sodium binding site of factor Xa: role of sodium in the prothrombinase complex. Biochemistry. 2000;39:1817–1825. doi: 10.1021/bi992006a. [DOI] [PubMed] [Google Scholar]
- 33.Camire RM. Prothrombinase assembly and S1 site occupation restore the catalytic activity of FXa impaired by mutation at the sodium-binding site. J. Biol. Chem. 2002;277:37863–37870. doi: 10.1074/jbc.M203692200. [DOI] [PubMed] [Google Scholar]
- 34.Dang QD, Di Cera E. Residue 225 determines the Na(+)-induced allosteric regulation of catalytic activity in serine proteases. Proc. Natl. Acad. Sci. (USA) 1996;93:10653–10656. doi: 10.1073/pnas.93.20.10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharjee G, Ahamed J, Pawlinski R, Liu C, Mackman N, Ruf W, Edgington TS. Factor Xa binding to annexin 2 mediates signal transduction via protease-activated receptor 1. Circ. Res. 2008;102:457–464. doi: 10.1161/CIRCRESAHA.107.167759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, Teyton L, Petersen LC, Ruf W. The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors. J. Biol. Chem. 2011;286:5756–5767. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Bae J-S, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease- activated receptor 1. J. Biol. Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 38.Banner DW, D'Arcy A, Chène C, Winkler FK, Guha A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 39.Dickinson CD, Kelly CR, Ruf W. Identification of surface residues mediating tissue factor binding and catalytic function of the serine protease factor VIIa. Proc. Natl. Acad. Sci. (USA) 1996;93:14379–14384. doi: 10.1073/pnas.93.25.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathur A, Bajaj SP. Protease and EGF1 domains of factor IXa play distinct roles in binding to factor VIIIa. Importance of helix 330 (helix 162 in chymotrypsin) of protease domain of factor IXa in its interaction with factor VIIIa. J. Biol. Chem. 1999;274:18477–18486. doi: 10.1074/jbc.274.26.18477. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan JP, Sadler JE. Molecular mapping of the heparin-binding exosite of thrombin. Proc. Natl. Acad. Sci. (USA) 1994;91:5518–5522. doi: 10.1073/pnas.91.12.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Manithody C, Olson ST, Rezaie AR. Contribution of basic residues of the autolysis loop to the substrate and inhibitor specificity of factor IXa. J. Biol. Chem. 2003;278:25032–25038. doi: 10.1074/jbc.M302174200. [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Manithody C, Qureshi SH, Rezaie AR. Role of the residues of the 39-loop in determining the substrate and inhibitor specificity of factor IXa. J. Biol. Chem. 2010;285:28488–28495. doi: 10.1074/jbc.M110.143321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Bonniec BF, Betz A, Guinto ER, Esmon CT, Stone SR. Mapping of the thrombin des-ETW conformation by using site-directed mutants of hirudin. Evidence for the induction of nonlocal modifications by mutagenesis. Biochemistry. 1994;33:3959–3966. doi: 10.1021/bi00179a023. [DOI] [PubMed] [Google Scholar]
- 45.Le Bonniec BF, MacGillivray RT, Esmon CT. Thrombin Glu-39 restricts the P'3 specificity to nonacidic residues. J. Biol. Chem. 1991;266:13796–13803. [PubMed] [Google Scholar]
- 46.Le Bonniec BF, Guinto ER, MacGillivray RT, Stone SR, Esmon CT. The role of thrombin's Tyr-Pro-Pro-Trp motif in the interaction with fibrinogen, thrombomodulin, protein C, antithrombin III, and the Kunitz inhibitors. J. Biol. Chem. 1993;268:19055–19061. [PubMed] [Google Scholar]
- 47.Rezaie AR, Yang L. Deletion of the 60-loop provides new insights into the substrate and inhibitor specificity of thrombin. Thromb. Haemost. 2005;93:1047–1054. doi: 10.1160/TH04-11-0730. [DOI] [PubMed] [Google Scholar]
- 48.Maignan S, Guilloteau JP, Pouzieux S, Choi-Sledeski YM, Becker MR, Klein SI, Ewing WR, Pauls HW, Spada AP, Mikol V. Crystal structures of human factor Xa complexed with potent inhibitors. J. Med. Chem. 2000;43:3226–3232. doi: 10.1021/jm000940u. [DOI] [PubMed] [Google Scholar]