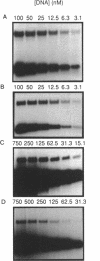
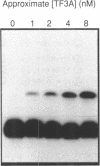
Abstract
Zinc fingers of the TFIIIA type are connected by short linker sequences between the structural units. Structural investigations by 2D NMR in solution and by X-ray crystallographic analyses of complexes with DNA point to a passive role for the linkers. We have therefore investigated the influence of the linker sequence on DNA binding using as a model the first three fingers of the protein TFIIIA. Insertion of certain heterologous linkers abolishes binding, and replacement of individual amino acids can reduce binding by factors of up to twenty-four.
Full text
PDF

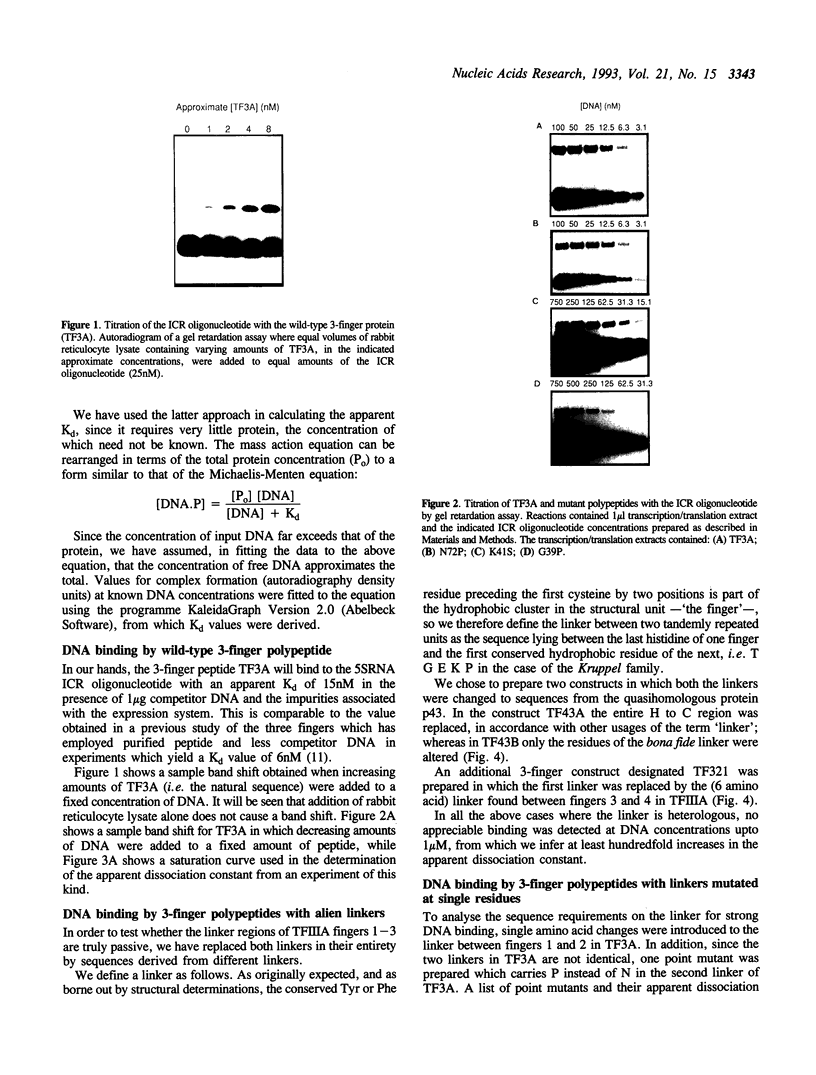


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci U S A. 1988 Jan;85(1):99–102. doi: 10.1073/pnas.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun B. R., Bartholomew B., Kassavetis G. A., Geiduschek E. P. Topography of transcription factor complexes on the Saccharomyces cerevisiae 5 S RNA gene. J Mol Biol. 1992 Dec 20;228(4):1063–1077. doi: 10.1016/0022-2836(92)90315-b. [DOI] [PubMed] [Google Scholar]
- Christensen J. H., Hansen P. K., Lillelund O., Thøgersen H. C. Sequence-specific binding of the N-terminal three-finger fragment of Xenopus transcription factor IIIA to the internal control region of a 5S RNA gene. FEBS Lett. 1991 Apr 9;281(1-2):181–184. doi: 10.1016/0014-5793(91)80388-j. [DOI] [PubMed] [Google Scholar]
- Clemens K. R., Wolf V., McBryant S. J., Zhang P., Liao X., Wright P. E., Gottesfeld J. M. Molecular basis for specific recognition of both RNA and DNA by a zinc finger protein. Science. 1993 Apr 23;260(5107):530–533. doi: 10.1126/science.8475383. [DOI] [PubMed] [Google Scholar]
- Fairall L., Harrison S. D., Travers A. A., Rhodes D. Sequence-specific DNA binding by a two zinc-finger peptide from the Drosophila melanogaster Tramtrack protein. J Mol Biol. 1992 Jul 20;226(2):349–366. doi: 10.1016/0022-2836(92)90952-g. [DOI] [PubMed] [Google Scholar]
- Fairall L., Rhodes D., Klug A. Mapping of the sites of protection on a 5 S RNA gene by the Xenopus transcription factor IIIA. A model for the interaction. J Mol Biol. 1986 Dec 5;192(3):577–591. doi: 10.1016/0022-2836(86)90278-0. [DOI] [PubMed] [Google Scholar]
- Hoovers J. M., Mannens M., John R., Bliek J., van Heyningen V., Porteous D. J., Leschot N. J., Westerveld A., Little P. F. High-resolution localization of 69 potential human zinc finger protein genes: a number are clustered. Genomics. 1992 Feb;12(2):254–263. doi: 10.1016/0888-7543(92)90372-y. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H. Determination of the base recognition positions of zinc fingers from sequence analysis. EMBO J. 1992 Dec;11(12):4507–4517. doi: 10.1002/j.1460-2075.1992.tb05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho K. E., Darby M. K., Crawford E. T., Brown D. D. A finger protein structurally similar to TFIIIA that binds exclusively to 5S RNA in Xenopus. Cell. 1990 Apr 20;61(2):293–300. doi: 10.1016/0092-8674(90)90809-s. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Gippert G. P., Soman K. V., Case D. A., Wright P. E. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989 Aug 11;245(4918):635–637. doi: 10.1126/science.2503871. [DOI] [PubMed] [Google Scholar]
- Liao X. B., Clemens K. R., Tennant L., Wright P. E., Gottesfeld J. M. Specific interaction of the first three zinc fingers of TFIIIA with the internal control region of the Xenopus 5 S RNA gene. J Mol Biol. 1992 Feb 20;223(4):857–871. doi: 10.1016/0022-2836(92)90248-i. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Bullard S., Lundquist S., Kim S., Tarkowski T. A meiotic gene conversion gradient opposite to the direction of transcription. Nature. 1992 Sep 10;359(6391):154–155. doi: 10.1038/359154a0. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaseko Y., Neuhaus D., Klug A., Rhodes D. Adjacent zinc-finger motifs in multiple zinc-finger peptides from SWI5 form structurally independent, flexibly linked domains. J Mol Biol. 1992 Nov 20;228(2):619–636. doi: 10.1016/0022-2836(92)90845-b. [DOI] [PubMed] [Google Scholar]
- Nardelli J., Gibson T. J., Vesque C., Charnay P. Base sequence discrimination by zinc-finger DNA-binding domains. Nature. 1991 Jan 10;349(6305):175–178. doi: 10.1038/349175a0. [DOI] [PubMed] [Google Scholar]
- Omichinski J. G., Clore G. M., Robien M., Sakaguchi K., Appella E., Gronenborn A. M. High-resolution solution structure of the double Cys2His2 zinc finger from the human enhancer binding protein MBP-1. Biochemistry. 1992 Apr 28;31(16):3907–3917. doi: 10.1021/bi00131a004. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Brown D. D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4170–4174. doi: 10.1073/pnas.77.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Jackson I. J., Brown D. D. Domains of the positive transcription factor specific for the Xenopus 5S RNA gene. Cell. 1984 Jun;37(2):645–652. doi: 10.1016/0092-8674(84)90396-9. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thukral S. K., Morrison M. L., Young E. T. Alanine scanning site-directed mutagenesis of the zinc fingers of transcription factor ADR1: residues that contact DNA and that transactivate. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9188–9192. doi: 10.1073/pnas.88.20.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Day M. L., Pexton T., Padgett K. A., Johnston M., Milbrandt J. In vivo mutational analysis of the NGFI-A zinc fingers. J Biol Chem. 1992 Feb 25;267(6):3718–3724. [PubMed] [Google Scholar]