Abstract
Impaired revascularization of transplanted islets is a critical problem that leads to progressive islet loss. Since endothelial progenitor cells (EPCs) are known to aid neovascularization, we aimed to enhance islet engraftment by cotransplanting EPCs with islets. Porcine islets, with (islet-EPC group) or without (islet-only group) human cord blood–derived EPCs, were transplanted into diabetic nude mice. The islet-EPC group reached euglycemia by ∼11 days posttransplantation, whereas the islet-only group did not. Also, the islet-EPC group had a higher serum porcine insulin level than the islet-only group. Islets from the islet-EPC group were more rapidly revascularized at the early period of transplantation without increment of final capillary density at the fully revascularized graft. Enhanced revascularization rate in the islet-EPC group was mainly attributed to stimulating vascular endothelial growth factor-A production from the graft. The rapid revascularization by EPC cotransplantation led to better graft perfusion and recovery from hypoxia. EPC cotransplantation was also associated with greater β-cell proliferation, probably by more basement membrane production and hepatocyte growth factor secretion. In conclusion, cotransplantation of EPCs and islets induces better islet engraftment by enhancing the rate of graft revascularization. These findings might provide a directly applicable tool to enhance the efficacy of islet transplantation in clinical practice.
Type 1 diabetes mellitus (T1DM) accounts for 5–10% of diabetes and results from the destruction of the β-cells of the pancreas. Patients with T1DM become dependent on insulin for survival, and therefore strategies for replacing β-cells such as pancreas transplantation or islet transplantation have long been considered to have promise for T1DM (1). Islet transplantation is less invasive than pancreas transplantation, more physiologic than insulin injection, and may be beneficial for patients with brittle diabetes via prevention of severe hypoglycemic events (2). However, there are several hurdles with islet transplantation such as limited availability of donor islets, the immediate destruction of ∼50% of transplanted islets, and impaired revascularization of grafts (2).
Compared with the acinar cells of pancreas, islets are highly vascularized tissue, and the pivotal role of this vasculature has been well demonstrated (3–5). However, isolated islets are supplied with oxygen and nutrients solely by diffusion immediately after transplantation and are not fully revascularized until 1 month posttransplantation (6). Even after 1 month, islets suffer from chronic hypoxia and ischemia (7). Therefore, a new strategy for facilitating islet revascularization may be effective for successful islet transplantation.
Endothelial progenitor cells (EPCs) are circulating progenitor cells that enhance neovascularization in various pathophysiologic conditions (8,9). EPCs can be obtained from the patient’s own peripheral blood or from cord blood, making EPC-based strategies more accessible and safer than those using other types of stem/progenitor cells (10,11). Although there are previous reports demonstrating that bone marrow–derived endothelial cells are recruited to the pancreas (12,13) or transplanted islets (14) and that these cells contribute to angiogenesis, the decreased number and impaired function of EPCs in diabetic patients suggest that the recipient-derived EPCs may be poor adjunct cells for improvement of graft neovascularization (15,16). In this study, we attempted to demonstrate the therapeutic efficacy of ex vivo–expanded, cord blood–derived EPC cotransplantation in enhancing islet engraftment and to explain the underlying mechanism of this strategy.
RESEARCH DESIGN AND METHODS
Islet isolation and culture.
Porcine islets were isolated from modern farm pigs according to previously established protocols (17). Mouse islets were isolated according to previous reported protocols (18). In brief, pancreata were harvested, distended with University of Wisconsin solution, and digested with Liberase DL (Roche Biochemicals, Basel, Switzerland) in a modified Ricordi chamber. Islets were separated from nonislet tissue of the pancreas using University of Wisconsin/OptiPrep density gradient solution in a COBE 2991 cell processor (Gambro BCT, Lakewood, CO). Isolated porcine islets were cultured in M199 medium (Gibco-BRL, Grand Island, NY), and mouse islets were culture in RPMI-1640 medium (Gibco-BRL).
EPC isolation and culture.
Human EPCs were isolated from umbilical cord blood obtained during normal birth by the preestablished protocol (19). The institutional review board of Seoul National University Hospital approved this study protocol, and all subjects provided informed consent for EPC isolation from their cord blood. Briefly, mononuclear cord blood cells were harvested via a density gradient using Histopaque (Sigma, St. Louis, MO) and plated in cell culture dish with endothelial basal medium-2 (Lonza, Walkersville, MD). The medium was changed daily for 2–3 weeks after seeding. An EPC colony with a typical cobblestone appearance developed after 2 weeks of seeding. The colony was removed by trypsinization and reseeded in gelatin-coated plates. The EPCs from the third passage were tested for expression of previously reported EPC surface markers (i.e., positive for platelet endothelial cell adhesion molecule (PECAM)-1, vascular endothelial growth factor (VEGR) receptor (VEGFR)2, tyrosine kinase with immunoglobulin-like and endothelial growth factor-like domains [Tie]-2, vascular endothelial (VE)-cadherin, ulex europaeus agglutinin (UEA)1, CD34, and von-Willebrand factor [vWF] and negative for CD45, CD3, and CD14) by either fluorescence-activated cell sorting (BD FACSCalibur; BD-Pharmingen) or immunostaining. All of the flow cytometry antibodies were from BD Biosciences.
Islet and EPC coculture.
For in vitro experiments, 500 islets from the BALB/c mice were carefully mixed with 5 × 105 of human cord blood–derived EPCs in 3 mL medium consisting of equal volumes of RPMI-1640 and endothelial basal medium-2. The cell mixture was maintained for 2 h in a 5% CO2 cell culture incubator with careful pipetting every 15 min and was then plated on a noncoated, stirred cell culture dish to inhibit attachment of the cells to the plate. As a result, some of the EPCs became attached to the surface of the islets. The cocultured islet-EPC complexes were maintained for 48 h to allow enough time for the EPCs to cover most of the surface of the islets. As a control, 500 islets were cultivated alone under the same conditions. For the porcine islet and human EPC coculture experiments, 1.5 × 105 human EPCs were seeded on the bottom of the cell culture plate. After these EPCs had attached to the plate, the hanging-well inserts, where 2,000 islet equivalents (IEQs) of porcine islets had previously been cultivated, were inserted into the plates to permit the coculture of the porcine islets and EPCs via transwell membranes. EPCs and porcine islets were cultivated alone as controls. After 4 days of coculture, the EPCs and porcine islets from each group were harvested and analyzed for human hepatocyte growth factor (HGF) or porcine VEGF-A by quantitative RT-PCR (q-PCR).
Animal experiments.
BALB/c nude mice were purchased from Japan SLC (Shizuoka, Japan). The animal care and experimental procedures were performed with the approval of the animal care committee of the Seoul National University Hospital. All mice were maintained in a specific pathogen-free facility and fed ad libitum with a standard normal diet (PMI LabDiet; Purina Mills) and free access to water. Streptozotocin (180 mg/kg; Sigma) was injected via the peritoneum to induce diabetes in the 10-week-old mice (20). Only the mice displaying stable hyperglycemia (>500 mg/dL) for 2 weeks were selected as the transplantation recipients. Porcine islets (7,000 IEQ, with or without 5 × 105 EPCs) were transplanted by careful injection of the cells into the renal capsules of these mice (21). Random blood glucose levels were measured from the tail vein with a glucose meter (One Touch Ultra Sensor, Lifescan, Milpitas, CA), and body weight was recorded for 35 days after transplantation. Whole blood was collected from the retro-orbital sinus using a capillary tube, and serum was separated. For functional vessel evaluation, 200 µg tetramethyl rhodamine isothiocyanate (TRITC)-bandeiraea simplicifolia (BS)1-lectin (Sigma) was injected via the tail vein 1 h before harvesting the tissue. To assess the degree of tissue hypoxia, 60 mg/kg hypoxia probe (Hypoxyprobe, Burlington, MA) was injected into the tail vein 30 min before tissue harvesting. Porcine insulin was detected using an immunoradiometric assay (DIASourceImmunoAssays, Nivelles, Belgium). For the quantitative analysis of VEGF-A, insulin, and HGF in vivo, the graft site was harvested and evaluated using q-PCR with porcine-specific primers for VEGF-A and insulin, and with primers that detected human, porcine, and mouse HGF.
Immunostaining and morphometric analysis.
The mice were anesthetized, and the graft-bearing kidney was harvested after perfusion with 1% paraformaldehyde. After overnight fixation in 4% paraformaldehyde, the graft was processed for paraffin blocks. Each section was processed for hematoxylin-eosin or immunofluorescence, and digital images were obtained using either a Zeiss inverted microscope or a Zeiss LSM 710 confocal microscope (Carl Zeiss, Thornwood, NY). The entire grafts from each animal were serially cut, and 40 sections per graft were analyzed for β-cell mass and vasculature. For other immunostaining experiments, 10 sections per graft were analyzed. All antibodies used for immunohistochemical staining were tested with secondary antibody only as negative control to confirm their specificity at each experiment, and some antibodies to detect human sample specifically were also evaluated on the cross- reactivity between human, porcine, and mouse tissues (Supplementary Fig. 1). Morphometric measurements and analysis were performed using ImageJ software (http://rsb.info.nih.gov/ij). The result of the morphometric analysis was expressed as the mean signal density or integrated signal density (the summation of the mean signal density for each pixel of the intended area).
Antibodies and primer sequences.
The following antibodies were used for the experiments. Goat anti–VE-cadherin, goat anti-CD34, goat anti–PECAM-1, rabbit anti–PECAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-insulin, biotin-conjugated BS1-lectin, biotin-conjugated UEA1-lectin, TRITC-BS1-lectin, fluorescein isothiocyanate (FITC)-UEA1-lectin, mouse anti-glucagon, rabbit anti-laminin (all from Sigma), mouse anti–PECAM-1, mouse anti-vWF, guinea pig anti-insulin (Dako, Carpinteria, CA), goat anti–VEGF-A, goat anti-angiopoietin 1 (Ang1), goat anti-VEGFR2 (R&D Systems, Minneapolis, MN), rabbit anti–hypoxia probe (Hypoxyprobe), rabbit anti-Ki67 (Abcam, Cambridge, MA), and goat anticollagen IV (Col IV) (Millipore, Billerica, MA) antibodies were used as primary antibodies. All secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Hoechst and Sytox blue (Molecular probe, Eugene, OR) were used for nuclear staining. For the primer sequences, see Supplementary Table 1. For primer sequences, see Supplementary Table 1.
Statistical analysis.
Continuous and categorical variables are presented as means ± SEs. Comparisons between two groups were performed with unpaired t tests. All statistical analyses were performed with SPSS 17.0 (SPSS, Chicago, IL), and P < 0.05 was considered statistically significant.
RESULTS
Better glycemic control and islet engraftment with EPC cotransplantation.
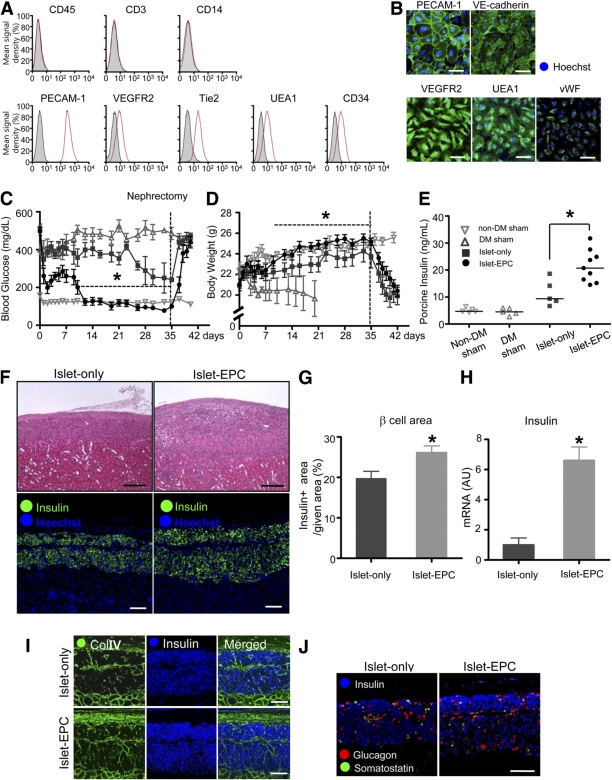
We isolated EPCs from human cord blood and confirmed them with EPC-specific surface markers (Fig. 1A–B). To evaluate whether EPC cotransplantation could enhance engraftment in islet transplantation, we transplanted 7,000 IEQ porcine islets, either with (the islet-EPC group) or without (the islet-only group) 5 × 105 human cord blood–derived EPCs, into the renal capsules of diabetic nude mice. Nondiabetic mice with sham transplantation (the non-DM sham group) and diabetic mice with sham transplantation (the DM-sham group) were used as controls. In contrast to the persistent hyperglycemia in the DM-sham group, the islet-EPC group showed a gradual decrease in blood glucose, finally reaching normoglyclemia (<200 mg/dL) at ∼11 days. Although blood glucose decreased slightly in the islet-only group, it failed to reach normoglycemia over a 5-week period. At 5 weeks after transplantation, the graft-bearing kidney was removed. The blood glucose of both the islet-only and islet-EPC groups increased to 550 mg/dL, indicating that the improved glycemic control in the islet-EPC group was derived from the transplanted islets and not from the regeneration of remnant pancreatic islets of the host (Fig. 1C). The DM-sham group gradually lost weight, eventually resulting in death. However, the incremental weight gain in the islet-EPC group was similar to that of the non-DM sham group. The islet-only group also gained weight, but there was a distinct lag behind the islet-EPC group (Fig. 1D). We investigated whether the improved glycemic control in the islet-EPC group was associated with insulin production from the transplanted islets. The islet-EPC group showed a higher serum porcine insulin level than the islet-only group under fasting conditions (islet-EPC vs. islet-only 21.5 ± 2.0 vs. 12.2 ± 2.6 ng/mL, respectively; P < 0.05) (Fig. 1E). Histological examination of the graft-bearing kidneys demonstrated larger islet mass, as assessed by the insulin-positive area per given field in the islet-EPC group compared with that of the islet-only group (islet-EPC vs. islet-only 26.1 ± 1.6 vs. 19.8 ± 1.8%; P < 0.05) (Fig. 1F and G) at day 35. Additionally, the porcine insulin mRNA level of the graft site was also found to be higher in the islet-EPC group than in the islet-only group by q-PCR (islet-EPC vs. islet-only 6.6 ± 0.9 vs. 1.0 ± 0.5-fold; P < 0.05) (Fig. 1H). This difference was not the result of the decrease in connective tissue area or other endocrine cell fractions because there were no difference in the Col IV–positive connective tissue or the glucagon/somatostatin-positive endocrine cell areas in either group (Fig. 1I and J). These findings indicate that EPC cotransplantation may be helpful for encouraging successful islet engraftment, ultimately leading to better glycemic control.
FIG. 1.
Better glycemic control and islet engraftment with EPC cotransplantation. A and B: Cord blood–derived EPCs of passage 3 were harvested and analyzed for expression of EPC markers by fluorescence-activated cell sorting (A) and immunocytostaining (B). EPCs were negative for CD45, CD3, and CD14 expression and positive for PECAM-1, VEGFR2, Tie2, UEA1, CD34, VE-cadherin, and vWF expression. C–E: Islets were transplanted into the kidney capsules of the recipient mice with or without EPCs (n = 6–9 per group). The blood glucose levels (C) and body weights (D) of each group were measured for 42 days after transplantation. E: The fasting serum porcine insulin level was measured with an immunoradiometric assay at day 35 (n = 5–8 per group). F–J: The graft-bearing kidney was harvested at day 35. F: The upper panel shows hematoxylin-eosin staining of the grafts in the islet-only and islet-EPC groups. The lower panel shows the insulin-positive area of each group, as determined by the immunostaining. G: The insulin-positive area was measured and presented as the percentage of a given area (0.72 mm2) (n = 4). H: The graft site of each mouse was analyzed for insulin by q-PCR with porcine-specific primers. The data are presented in arbitrary units (AU) after normalization to glyceraldehyde-3-phosphate dehydrogenase, with the islet-only group set to 1 (n = 4). I: Connective tissue area of the graft was visualized by Col IV together with insulin for β-cells by immunostaining. J: Endocrine cells of the graft were visualized for glucagon (α-cell), somatostatin (δ-cell), and insulin (β-cell) by immunostaining. Scale bars, 100 μm. All data are presented as means ± SE. *P < 0.05 vs. the islet-only group. (A high-quality color representation of this figure is available in the online issue.)
Rapid revascularization of the transplanted islets by EPC cotransplantation.
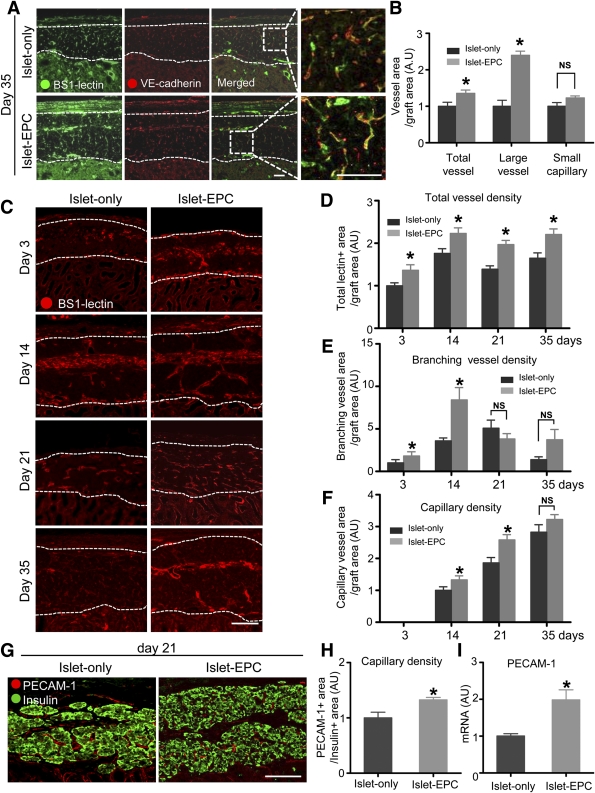
To clarify how the EPC cotransplantation enhanced the islet engraftment, we evaluated the process of neovascularization in the transplanted islets using BS1-lectin immunostaining. The BS1-lectin immunostaining was first confirmed to reach the smallest capillaries inside of the islets in porcine pancreas and islet graft (Supplementary Fig. 2) (22). The islet-EPC group exhibited a significantly greater total blood vessel density and more large vessels (≥5 µm in diameter) at 5 weeks after transplantation. VE-cadherin, another vascular marker, immunostaining of the graft site was consistent with the BS1-lectin–staining results. However, the capillary density in the graft was not different between the islet-EPC group and the islet-only group at day 35 (Fig. 2A and B). Therefore, we evaluated the rate of revascularization between the two groups from an earlier period of transplantation. Although the difference of capillary density between the two groups became insignificant by day 35, the islet-EPC group showed higher branching-vessel density at days 3 and 14 and higher capillary density at days 14 and 21 than the islet-only group (Fig. 2C–F). Quantification of the total lectin-positive area, branching-vessels area, and the capillary area in the graft site at each time point corroborated the histological analysis, suggesting that the EPC cotransplantation does not increase capillary density within the graft at full revascularization but may enhance early revascularization rate (Fig. 2D–F). For a more accurate quantification of the neovascularization, we also evaluated PECAM-1–positive capillary density per insulin-positive area at day 21 in both groups. The PECAM-1–positive intraislet capillary density was higher in the islet-EPC group than in the islet-only group, indicating that intraislet capillary formation was also hastened in the islet-EPC group (Fig. 2G and H). In addition, the level of PECAM-1 mRNA in the graft site was also higher in the islet-EPC group than in the islet-only group, indicating that the rapid revascularization found in the morphological analysis matched the gene expression characteristics (Fig. 2I).
FIG. 2.
Rapid revascularization of the graft by EPC cotransplantation. A and B: The blood vessels of the graft site were visualized at day 35 using BS1-lectin and VE-cadherin immunostaining (n = 4). A: Representative images of the immunostaining. The areas in the white dotted rectangle are shown as magnification view in the right panel. B: The total area of all vessels, large-vessel (diameter ≥5 μm) and small-capillary (diameter <5 μm), in the graft were quantified. C–F: The BS1-lectin–positive blood vessels of the graft were shown from early to late time point (days 3, 14, 21, and 35). C: Representative images of the immunostaining. D–F: The area of total BS1-lectin–positive vessels (D), branching vessels longer than 30 μm (E), and capillary density (F) were measured in the graft site (n = 4). All quantification data of vascular density in B, D, E, and F were calculated as percent area of the total graft area and then presented as arbitrary units (AU), with the value of the islet-only group set to 1 in B, with the value of the islet-only group at day 3 set to 1 in D and E, and with the value of the islet-only group at day 14 set to 1 in F. G and H: The capillary density of the intraislet area at day 21 was visualized with anti-human and anti-pig/mouse PECAM-1 antibodies (red) together with insulin antibody (green). G: Representative images of the PECAM-1 immunostaining. H: The capillary density positive for PECAM-1 within the insulin-positive area was measured, calculated as percent area of the insulin-positive area, and then presented as arbitrary units with the value of the islet-only group set to 1 (n = 4). I: The graft sites of each mouse at day 10 were dissected, harvested, and analyzed for PECAM-1 with primers all for human, porcine, and mouse by q-PCR (n = 4). All of the q-PCR data are presented in arbitrary units after normalization to glyceraldehyde-3-phosphate dehydrogenase, with the islet-only group set to 1. The data are presented as means ± SE. *P < 0.05 vs. the islet-only group. White dotted lines show the territory of the graft site. Scale bars, 50 μm in A and 100 μm in C and G. (A high-quality color representation of this figure is available in the online issue.)
Mechanism underlying the EPC-induced neovascularization after islet transplantation.
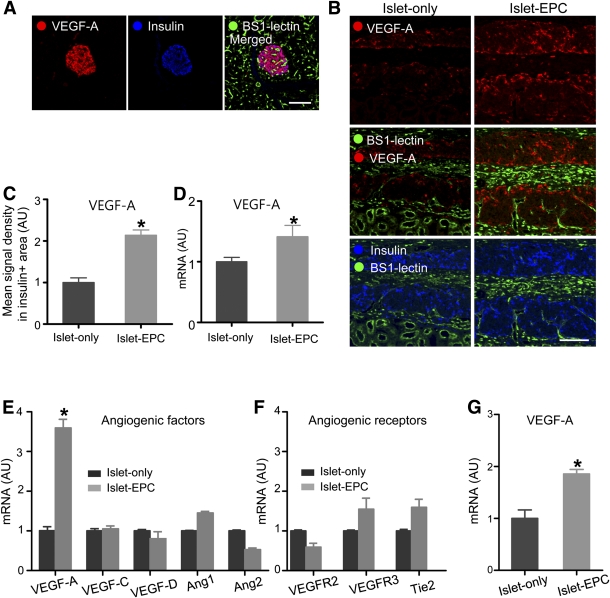
Next, we examined the mechanism of the EPC contribution to rapid islet neovascularization. We investigated whether the EPCs might induce the production of angiogenic factors, such as VEGF-A and Ang1. In the porcine pancreas, VEGF-A is expressed predominantly in islet β-cells (Fig. 3A). Even though the increase in Ang1 expression was not statistically significant (Supplementary Fig. 3), VEGF-A expression was significantly higher in the islet-EPC group than in the islet-only group at day 14 (Fig. 3B). This finding was supported by the higher mean signal density of VEGF-A in the insulin-positive area in the islet-EPC group than in the islet-only group (islet-EPC vs. islet-only 2.1 ± 0.1 vs. 1.0 ± 0.1-fold, respectively; P < 0.05) (Fig. 3C). In addition, the porcine VEGF-A mRNA expression in the graft site was elevated in the islet-EPC group compared with the islet-only group, consistent with the histological analysis (islet-EPC vs. islet-only 1.4 ± 0.2 vs. 1.0 ± 0.1-fold; P < 0.05) (Fig. 3D). To evaluate whether the EPCs directly stimulated the production of angiogenic factors from the islets, human-origin EPCs were cocultured with mouse islets, and the mRNA levels of several angiogenic factors were evaluated with mouse-specific primers. Importantly, the islets cocultured with EPCs produced significantly more VEGF-A mRNA than did the control islets (islet-EPC vs. islet-only 3.6 ± 0.2 vs. 1.0 ± 0.1-fold; P < 0.01). Other angiogenic factors, such as VEGF-C, VEGF-D, Ang1, and Ang2, showed only small changes. The receptors for angiogenic proteins, such as the VEGFR2, VEGFR3, and Tie2 receptor, showed only slight changes (Fig. 3E and F). There was also more VEGF-A production from the EPC-cocultured islets when porcine islets were used instead of mouse islets (islet-EPC vs. islet-only 1.9 ± 0.1 vs. 1.0 ± 0.2-fold; P < 0.05) (Fig. 3G). In addition, we evaluated whether EPCs could contribute to the enhanced revascularization rate by direct incorporation into the vasculature. To discriminate between the blood vessels originating from the cotransplanted EPCs and those from the islets and the recipient, we immunostained the specimens with human-specific PECAM-1 antibody for EPCs and with BS1-lectin for islets or recipient-origin endothelial cells (23,24). Among BS1-lectin–positive vessels, only a small number was positive for human-specific PECAM-1 at day 10. The proportion of these cells decreased further at day 35 (Supplementary Fig. 4). These findings suggest that direct incorporation of EPCs into the host vasculature may not play a major role in the graft revascularization. Rather, EPC cotransplantation may induce rapid revascularization at an early period after transplantation mainly by stimulation of angiogenic cytokine secretion from the graft.
FIG. 3.
The mechanisms underlying the EPC-induced neovascularization in islet transplantation. A: The porcine pancreas was visualized with VEGF-A, insulin, and BS1-lectin immunostaining. B and C: Graft-bearing kidneys were removed at day 14, and the VEGF-A expression was visualized together with the BS1-lectin and insulin immunostaining. B: Representative images of the graft. C: The mean VEGF-A signal density from the insulin-positive area was measured and presented in arbitrary units (AU), with the value of the islet-only group set to 1 (n = 4). D: At day 10, the graft site of each mouse was dissected, harvested, and analyzed for VEGF-A by q-PCR with porcine-specific primers. The data are presented in arbitrary units after normalization to glyceraldehyde-3-phosphate dehydrogenase, with the value for the islet-only group set to 1 (n = 4). E–G: Mouse islets (E and F) or porcine islets (G) were cultivated with human EPCs for 48 h or 4 days, respectively, and harvested for RNA extraction. Mouse and porcine islets were cultivated without EPCs under the same conditions to serve as controls. The angiogenic growth factors and their receptors were evaluated by q-PCR with mouse-specific (E and F) and porcine-specific (G) primers. The data are presented in arbitrary units after normalization to mouse-specific β-actin for the mouse islets or to porcine-specific glyceraldehyde-3-phosphate dehydrogenase for the porcine islets, with the values for the islet-only group set to 1 (n = 3). The data are presented as means ± SE. *P < 0.05 vs. the islet-only group. Scale bars, 100 μm. (A high-quality color representation of this figure is available in the online issue.)
Improvement of graft perfusion and recovery of ischemia by EPC cotransplantation.
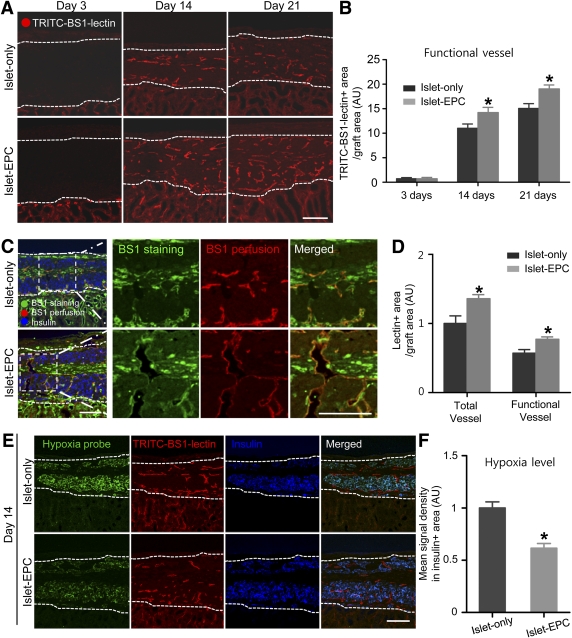
Next, we evaluated whether the rapid and effective revascularization in the islet-EPC group would lead to an actual increase in the perfusion of the transplanted islets. The mice were injected with TRITC-conjugated BS1-lectin before harvesting the kidney. At day 3, there were few TRITC-positive vessels in the grafts of either group. However, at days 14 and 21, we found more TRITC-positive functional vessels in the islet-EPC group than in the islet-only group (Fig. 4A and B). To clarify the process of functional vessel formation, we stained the TRITC-BS1-lectin–perfused graft with FITC-BS1-lectin. Similar to the data shown in Fig. 2C, the islet-EPC group showed more FITC-lectin–positive endothelial cells and branching vessels at day 14. Some of these vessels were also TRITC-lectin positive, indicating perfused vessel, as shown by the yellow color in the merged image, and also suggesting that individual endothelial cells infiltrated the graft initially and then began to form functional perfusing blood vessels (Fig. 4C and D). To determine whether the increase in functional blood vessels could lead to improvement in graft ischemia, tissue hypoxia was assessed using the Hypoxyprobe kit. At day 14, the grafts in the islet-EPC group began to recover from hypoxia, as shown by the weak-positive signal of the Hypoxyprobe, which coincided with better graft perfusion compared with the islet-only group (Fig. 4E and F). Collectively, these data demonstrate better vessel perfusion and effective relief of graft ischemia in the islet-EPC group compared with the islet-only group.
FIG. 4.
Improvement of graft perfusion and recovery from ischemia by EPC cotransplantation. A and B: The functional blood vessels in the graft were analyzed with TRITC-BS1-lectin perfusion at days 3, 14, and 21 posttransplantation. A: Representative images of the perfused vessels in the graft site. B: The TRITC-positive vessel area in A was calculated as the percentage of the total graft area, and then the values are presented in arbitrary units (AU) with the value of the islet-only group set at day 3 to 1 (n = 4). C and D: The total blood vessels were identified with FITC-BS1-lectin staining together with functional vessels identified with TRITC-BS1-lectin perfusion at day 14. C: Representative images of functional vessels among the entire vessels are presented in the left panel. The right panels show the magnification view of the insert in the white dotted rectangle. D: TRITC-positive functional vessel area and FITC-positive total vessel area were calculated as the percentage of the total graft area, and then the values were presented in arbitrary units with the value of total vessel for the islet-only group set to 1 (n = 4). E and F: The graft ischemia was evaluated by the Hypoxyprobe-positive signal at day 14 in both groups. E: Representative images of the Hypoxyprobe-positive area in the graft are shown, together with the TRITC-BS1-lectin and insulin immunostaining. F: The mean signal density of the Hypoxyprobe from the insulin-positive area was measured and presented in arbitrary units, with the value of the islet-only group set to 1 (n = 4). The data are presented as means ± SE. *P < 0.05 vs. the islet-only group. White dotted lines show the territory of the graft site. Scale bars, 100 μm. (A high-quality color representation of this figure is available in the online issue.)
β-Cell proliferation in EPC cotransplanted group associated with more basement membrane protein and HGF production.
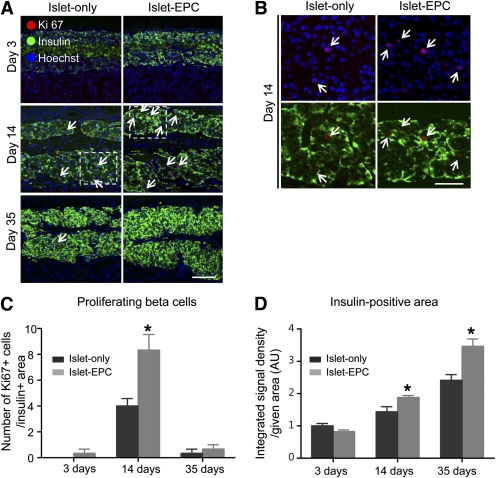
Finally, we evaluated whether the improvement in islet engraftment might have resulted from a higher proliferating capacity of the β cells caused by the EPC cotransplantation. The number of Ki67/insulin–double-positive proliferating β-cells began to increase at day 3 in the islet-EPC group, peaked at day 14, and returned to baseline at day 35. By contrast, the islet-only group showed fewer proliferating β cells from day 3 to day 35, suggesting that EPC cotransplantation enhanced β-cell proliferation (Fig. 5A–C). In addition, there were fewer transferase-mediated dUTP nick-end labeling/insulin–double-positive apoptotic cells in the islet-EPC group than in the islet-only group (Supplementary Fig. 5). The higher proliferation rate of the β cells was associated with a higher engrafted β-cell mass, as shown by the insulin-positive area at day 35 (Fig. 5D).
FIG. 5.
EPC cotransplantation is associated with β-cell proliferation. A–C: The transplanted islets were evaluated for proliferation by Ki67/insulin double staining at days 3, 14, and 35. A: The Ki67/insulin–double-positive proliferating β cells were visualized at each time point (arrow). Hoechst stain was used to distinguish the nucleus. B: Magnification views of the inserts within the white dotted line at day 14. C: The number of Ki67-positive cells among the insulin-positive cells in the total given area was counted (0.18 mm2) (n = 4). D: The integrated density of the insulin-positive area of the total given area (0.18 mm2) was measured and presented in arbitrary units (AU), with the value of day 3 for the islet-only group set to 1 (n = 4). The data are presented as means ± SE. *P < 0.05 vs. the islet-only group. Scale bars, 100 μm in A and 50 μm in B. (A high-quality color representation of this figure is available in the online issue.)
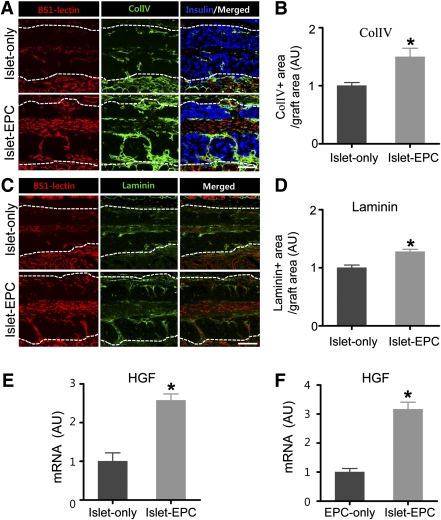
Because matrix proteins forming basement membranes are a possible factor for β-cell proliferation and survival (5,25–28), we assessed whether the EPC cotransplantation would lead to more extensive basement membrane production at day 14, when blood vessel grew more rapidly in the islet-EPC group. First, we evaluated the expression pattern of Col IV, the major basement membrane protein for porcine islets. Col IV was mainly expressed in the vicinity of the BS1-lectin–positive endothelial cells (Supplementary Fig. 6). To evaluate the formation of the basement membrane after transplantation, we visualized Col IV and laminin (another important basement membrane protein) in the graft site at day 14. Consistent with the rapid neovascularization, the Col IV or laminin deposition was more extensive in the islet-EPC group, suggesting that the EPC cotransplantation may have extended the contact area between the β cells and the basement membrane, resulting in increased β-cell proliferation (Fig. 6A–D). Because HGF is another factor that can induce β-cell proliferation (29–33) and HGF might perform such a role by communication with islet endothelial cells (32), HGF expression from EPCs was evaluated in vitro and in vivo. The HGF mRNA level was higher in the graft site of the islet-EPC group than in the islet-only group (islet-EPC vs. islet-only 2.6 ± 0.2 vs. 1.0 ± 0.2-fold, respectively; P ≤ 0.01) (Fig. 6E). To identify the source of HGF, we performed porcine islet/human EPC coculture experiments in vitro. The EPCs cocultured with islets produced more HGF than the EPCs cultured alone, suggesting that the cross-talk between islet and EPC may induce the production of key factors for β-cell proliferation, such as HGF from EPCs (islet-EPC vs. islet-only 3.2 ± 0.2 vs. 1.0 ± 0.1-fold; P ≤ 0.01) (Fig. 6F). Therefore, EPC cotransplantation may also enhance islet engraftment by providing the basement membrane and/or production of HGF that is required for β-cell proliferation.
FIG. 6.
β-Cell proliferation in the EPC-cotransplanted group is associated with more basement protein and HGF production. A–D: At day 14, the BS1-lectin–positive blood vessels and Col IV– or laminin-positive basement membrane were visualized. The insulin was immunostained to distinguish the graft site. The representative images for Col IV (A) and laminin (C) are shown. White dotted lines show the territory of the graft site. Scale bars, 100 μm. The Col IV–positive (B) and the laminin-positive (D) areas from total graft area were calculated as a percentage of the total graft area and then presented as arbitrary units (AU), with the value of the islet-only group set to 1 (n = 4). E and F: The graft sites of day 10 from each mouse in vivo (E) and human EPCs cocultured in vitro with or without porcine islets (F) were harvested. The specimens were analyzed for HGF by q-PCR using primers that functioned for all three species (human, porcine, and mouse) in E and for human exclusively in F. The data are presented in arbitrary units after normalization to glyceraldehyde-3-phosphate dehydrogenase, with the value for the islet-only group set to 1 in E and with the value for the EPC-only group set to 1 in F (n = 4). The data are presented as means ± SE. *P < 0.05 vs. the islet-only group in B, D, and E and vs. the EPC-only group in F. (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
The most important finding of this study is that EPC cotransplantation can be a novel and clinically applicable strategy for enhancing islet engraftment in islet transplantation. Cotransplanted EPCs hastened the rate of revascularization of the graft, which improved the functional status of vasculature (i.e., better perfusion and recovery from ischemia) and finally resulted in a greater engrafted β-cell mass. The transplanted EPCs actively participated in the neovascularization process mainly by stimulating VEGF-A production from the graft. Furthermore, cotransplanted EPCs induced β-cell proliferation, possibly by inducing production of matrix proteins and HGF.
Impaired and delayed revascularization of the graft is one of the main causes of graft failure after islet transplantation (2). Several attempts to overcome this problem have shown promising results but are also associated with problems that hinder their direct clinical application. The direct application of angiogenic factors, such as VEGF-A and Ang1 (34,35), and cotransplantation of mesenchymal stem cells (36) have shown improved angiogenesis, resulting in enhanced islet survival. Nevertheless, replacing angiogenic factors (either by gene transfer or direct application) is not free of safety concerns and/or is limited by the relatively short half-life of the factors (37,38). Mesenchymal stem cells may stimulate angiogenesis, but the risk of unexpected tumor development may be a concern (39,40). On the contrary, EPC cotransplantation may be a more effective, more physiological, and safer strategy for inducing neovascularization in islet transplantation.
In this study, cotransplanted EPCs hastened the rate of revascularization at the early stage of transplantation. However, the final capillary density per area in the graft site became similar between the islet-EPC and islet-only groups. This pattern of revascularization is similar to the recent findings by Berggren and colleagues, who evaluated the contribution of intraislet endothelial cells in islet revascularization (41). The contribution of exogenously transplanted EPCs in our series of experiments is rather analogous to the more intraislet endothelial cells in freshly transplanted islets. The significant difference of vascular density at an earlier time point (days 14–21), the blood glucose control, and the islet survival coinciding with this time line suggest that the EPCs may play a critical role in enhancing revascularization at an earlier time point after transplantation. Therefore, the role of EPCs can be to trigger or hasten the initial revascularization process.
An important finding of our study is that we have proposed the existence of cross-talk between the transplanted islets and the endothelial cells and that this communication can be successfully harnessed for superior results in islet transplantation. In terms of revascularization, we found that the transplanted islets were the major producers of VEGF-A (42), a well-known proangiogenic factor that attracts VEGFR2-expressing endothelial cells and circulating EPCs (43) (Fig. 3B–D). The enhanced initial islet survival by rapid revascularization with EPCs may augment islet survival, resulting in more VEGF-A production from the surviving islets, and finally enhanced graft angiogenesis. VEGF-A may also be effective at stimulating HGF production from the vessels around the islets (32), which can also stimulate angiogenesis (44–46) and β-cell proliferation (29–33). Put together, islets may actively enhance revascularization by secretion of VEGF-A. In turn, enhanced angiogenesis by VEGF-A may enhance islet engraftment, which results in more VEGF-A production from the remaining surviving islets.
Alternatively, in terms of islet survival, augmented revascularization by the EPC cotransplantation may provide survival/proliferation cues to the islets. Both endothelial cells and β cells produce matrix proteins (47), and the matrix proteins are known to enhance insulin production and β-cell proliferation (25–28). Consistent with these previous findings, significantly better angiogenesis around the graft and higher engrafted β-cells with EPC cotransplantation was associated with a significant increase in the basement membrane proteins Col IV and laminin. In addition, enhanced VEGF-A production from the islets can itself be a direct islet survival/growth factor by an autocrine effect (48). Furthermore, consistent with a previous report demonstrating the prosurvival effect of HGF on islet proliferation (29–33), our in vitro and in vivo findings showed that higher HGF production from endothelial cells was associated with higher β-cell proliferation in the islet-EPC group. Therefore, EPC cotransplantation may augment islet proliferation by multiple mechanisms: basement membrane production and VEGF-A/HGF secretion.
Our study has several unique points compared with previous works. First, we have suggested a positive feedback effect between revascularization and islet engraftment, which involves angiogenic cytokines and matrix proteins. This communication between vasculature and graft survival was enhanced when EPCs were transplanted together with the islets. Second, our study demonstrated that EPCs, which are a rather homogenous cell group compared with bone marrow–derived cells, can be another useful adjunct cell type to boost islet engraftment. Cotransplantation of bone marrow–derived cells with islets is being intensely explored as a strategy to establish microchimerism in graft recipients that could lead to the reduction of immunosuppression (49) as well as to the enhancement of revascularization (14). Our studies suggest that cotransplantation of EPCs with islets might also be effective in reducing instant blood-mediated inflammatory reaction (50) and in enhancing early revascularization, proliferation, and ultimately engraftment of islets. Third, from a more practical viewpoint for clinical application, EPCs can be obtained rather easily either from peripheral blood or from a bank, such as cord blood (10). This may be important because the EPCs of patients with diabetes are essentially defective in both number and revascularization function (15,16). Therefore, our study provides a potential strategy to improve the efficacy of islet transplantation by enhancing early revascularization rate that might be applied into clinical practice.
In conclusion, EPC cotransplantation is a new and promising therapeutic modality for enhancing islet engraftment by rapid revascularization. The results of this study may warrant future clinical trials.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Innovative Research Institute for Cell Therapy of the Republic of Korea (A062260) and by Grant R31-2008-000-10103-0 from the World Class University project of the Ministry of Education, Science, and Technology and the Korea Science and Engineering Foundation.
No potential conflicts of interest relevant to this article were reported.
S.K. designed and performed the experiments, analyzed the results, and wrote the manuscript. H.S.P. performed experiments and contributed to the discussion. A.J. performed the experiments. S.H.H. and Y.Y.L. contributed to the porcine islet isolation. H.N.L. assisted the preparation of the experiments. J.S.P., H.S.J., and S.S.C. contributed by critically reading the manuscript. K.S.P. designed the experiments, analyzed the results, contributed to the discussion, and wrote and drafted the final version of the manuscript. K.S.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 46th European Association for the Study of Diabetes Meeting, Stockholm, Sweden, 20–24 September 2010.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1492/-/DC1.
REFERENCES
- 1.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl 1.):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes 2010;59:1285–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567 [DOI] [PubMed] [Google Scholar]
- 4.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med 2003;9:661–668 [DOI] [PubMed] [Google Scholar]
- 5.Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 2006;10:397–405 [DOI] [PubMed] [Google Scholar]
- 6.Rooth P, Dawidson I, Lafferty K, et al. Prevention of detrimental effect of cyclosporin A on vascular ingrowth of transplanted pancreatic islets with verapamil. Diabetes 1989;38(Suppl 1.):202–205 [DOI] [PubMed] [Google Scholar]
- 7.Carlsson PO, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation 2000;69:761–766 [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 9.Naruse K, Hamada Y, Nakashima E, et al. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005;54:1823–1828 [DOI] [PubMed] [Google Scholar]
- 10.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004;104:2752–2760 [DOI] [PubMed] [Google Scholar]
- 11.Tendera M, Wojakowski W. Clinical trials using autologous bone marrow and peripheral blood-derived progenitor cells in patients with acute myocardial infarction. Folia Histochem Cytobiol 2005;43:233–235 [PubMed] [Google Scholar]
- 12.Mathews V, Hanson PT, Ford E, Fujita J, Polonsky KS, Graubert TA. Recruitment of bone marrow-derived endothelial cells to sites of pancreatic beta-cell injury. Diabetes 2004;53:91–98 [DOI] [PubMed] [Google Scholar]
- 13.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 2003;21:763–770 [DOI] [PubMed] [Google Scholar]
- 14.Miller R, Cirulli V, Diaferia GR, et al. Switching-on survival and repair response programs in islet transplants by bone marrow-derived vasculogenic cells. Diabetes 2008;57:2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 16.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–2786 [DOI] [PubMed] [Google Scholar]
- 17.Lee YY, Hong SH, Lee YJ, et al. Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem Biophys Res Commun 2010;397:735–739 [DOI] [PubMed] [Google Scholar]
- 18.Szot GL, Koudria P, Bluestone JA. Murine pancreatic islet isolation. J Vis Exp 2007;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004;24:288–293 [DOI] [PubMed] [Google Scholar]
- 20.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 1976;193:415–417 [DOI] [PubMed] [Google Scholar]
- 21.Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp 2007;9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattsson G, Carlsson PO, Olausson K, Jansson L. Histological markers for endothelial cells in endogenous and transplanted rodent pancreatic islets. Pancreatology 2002;2:155–162 [DOI] [PubMed] [Google Scholar]
- 23.Crisa L, Cirulli V, Smith KA, Ellisman MH, Torbett BE, Salomon DR. Human cord blood progenitors sustain thymic T-cell development and a novel form of angiogenesis. Blood 1999;94:3928–3940 [PubMed] [Google Scholar]
- 24.Yamahara K, Sone M, Itoh H, et al. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLoS One 2008;3:e1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes 2000;49:233–243 [DOI] [PubMed] [Google Scholar]
- 26.Ris F, Hammar E, Bosco D, et al. Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human beta-cells in vitro. Diabetologia 2002;45:841–850 [DOI] [PubMed] [Google Scholar]
- 27.Hammar E, Parnaud G, Bosco D, et al. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes 2004;53:2034–2041 [DOI] [PubMed] [Google Scholar]
- 28.Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. Blockade of beta1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes 2006;55:1413–1420 [DOI] [PubMed] [Google Scholar]
- 29.Dai C, Li Y, Yang J, Liu Y. Hepatocyte growth factor preserves beta cell mass and mitigates hyperglycemia in streptozotocin-induced diabetic mice. J Biol Chem 2003;278:27080–27087 [DOI] [PubMed] [Google Scholar]
- 30.García-Ocaña A, Vasavada RC, Cebrian A, et al. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes 2001;50:2752–2762 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 2000;275:1226–1232 [DOI] [PubMed] [Google Scholar]
- 32.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 2006;147:2315–2324 [DOI] [PubMed] [Google Scholar]
- 33.Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS. Growth factor/matrix-induced proliferation of human adult beta-cells. Diabetes 1995;44:1458–1460 [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Richter A, Suriawinata J, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes 2004;53:963–970 [DOI] [PubMed] [Google Scholar]
- 35.Su D, Zhang N, He J, et al. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes 2007;56:2274–2283 [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Itakura S, Todorov I, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 2010;89:1438–1445 [DOI] [PubMed] [Google Scholar]
- 37.Simons M, Bonow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation 2000;102:E73–E86 [DOI] [PubMed] [Google Scholar]
- 38.Fedak PW, Verma S, Weisel RD, Mickle DA, Li RK. Angiogenesis: protein, gene, or cell therapy? Heart Surg Forum 2001;4:301–304 [PubMed] [Google Scholar]
- 39.Scolding N, Marks D, Rice C. Autologous mesenchymal bone marrow stem cells: practical considerations. J Neurol Sci 2008;265:111–115 [DOI] [PubMed] [Google Scholar]
- 40.Siatskas C, Payne NL, Short MA, Bernard CC. A consensus statement addressing mesenchymal stem cell transplantation for multiple sclerosis: it’s time! Stem Cell Rev 2010;6:500–506 [DOI] [PubMed] [Google Scholar]
- 41.Nyqvist D, Speier S, Rodriguez-Diaz R, et al. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes 2011;60:2571–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor—a is essential for islet vascularization, revascularization, and function. Diabetes 2006;55:2974–2985 [DOI] [PubMed] [Google Scholar]
- 43.Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol 1995;9:1760–1770 [DOI] [PubMed] [Google Scholar]
- 44.Hayashi S, Morishita R, Nakamura S, et al. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation 1999;100(Suppl.):II301–II308 [DOI] [PubMed] [Google Scholar]
- 45.Morishita R, Nakamura S, Hayashi S, et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 1999;33:1379–1384 [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Morishita R, Higaki J, et al. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J Hypertens 1996;14:1067–1072 [DOI] [PubMed] [Google Scholar]
- 47.Virtanen I, Banerjee M, Palgi J, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 2008;51:1181–1191 [DOI] [PubMed] [Google Scholar]
- 48.Cross SE, Richards SK, Clark A, et al. Vascular endothelial growth factor as a survival factor for human islets: effect of immunosuppressive drugs. Diabetologia 2007;50:1423–1432 [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, Wang M, Racine JJ, et al. Induction of chimerism permits low-dose islet grafts in the liver or pancreas to reverse refractory autoimmune diabetes. Diabetes 2010;59:2228–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JH, Oh BJ, Lee HN, Park HS, Park SG, Park KS. Endothelial colony-forming cell coating of pig islets prevents xenogeneic instant blood-mediated inflammatory reaction. Cell Transplant. 7 March 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.