It is well known that C-peptide fulfills an important function in the synthesis of insulin. After cleavage of proinsulin in the pancreatic β-cells, the 31-amino acid C-peptide is secreted into the portal circulation in equimolar concentrations with insulin. After its discovery in 1967 (1), it was believed that C-peptide might exert physiological effects similar to those of insulin. However, no influence on glucose or lipid metabolism could be demonstrated, and C-peptide was subsequently regarded as a waste product of insulin synthesis. Nevertheless, it was found to be useful as an indicator of β-cell function, and since the mid-1970s, C-peptide has been used as a surrogate marker for monitoring the course of type 1 and type 2 diabetes and determining the effects of interventions designed to preserve and improve residual β-cell function.
Several studies demonstrate that patients with type 1 diabetes who show a degree of remaining β-cell activity are considerably less prone to develop microvascular complications than those who are totally C-peptide deficient (2). The possibility that C-peptide may exert direct effects of its own was reevaluated in the early 1990s. A series of studies was undertaken involving administration of the peptide to patients with type 1 diabetes, who lack C-peptide. This approach gave positive results, and it became apparent that replacement of C-peptide in physiological concentrations resulted in significant improvements in several diabetes-induced functional abnormalities (3–7). These surprising findings prompted a renewed interest in C-peptide physiology, and during the past 15 years, a steadily increasing number of reports on new aspects of C-peptide physiology have emerged. The information available today includes studies of the peptide’s interaction with cell membranes and its intracellular signaling properties (8). In vivo studies in animal models of type 1 diabetes have defined a beneficial influence of C-peptide on diabetes-induced functional and structural abnormalities of the kidney, peripheral nerves, and central nervous system (9,10). In addition, several studies in type 1 diabetic patients describing positive effects of C-peptide replacement therapy on nerve and kidney function have appeared (9,11). The wealth of information now available supports the notion that C-peptide administration, together with regular insulin therapy, may be beneficial in the prevention and treatment of microvascular complications. The purpose of this review is to focus on C-peptide physiology and its potential role in the treatment of type 1 diabetes complications.
CELL MEMBRANE INTERACTION
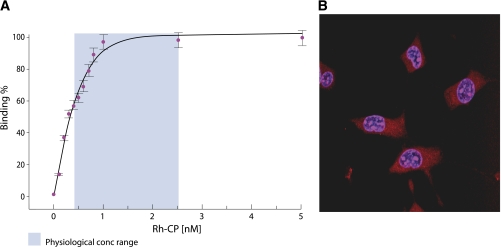
Specific binding of C-peptide to cultured rat pancreatic adenoma β-cells was described as early as 1986 (12), and further studies have demonstrated stereospecific binding to several human cell types, such as endothelial cells, skin fibroblasts, and renal tubular cells, using rhodamine-labeled C-peptide and fluorescence correlation spectroscopy (13). Bound C-peptide was displaced by excess unlabeled C-peptide and its COOH-terminal pentapeptide but not by insulin, proinsulin, IGF-I, IGF-II, or an all d-amino acid C-peptide, attesting to the specificity of the binding. Full saturation of the C-peptide binding occurred at a concentration of 0.9 nmol/L (Fig. 1A); this finding is in keeping with the notion that at physiological concentrations of C-peptide, all binding sites can be expected to be fully occupied. Consequently, in healthy subjects with normal ambient C-peptide levels, no further response is to be expected after administration of exogenous C-peptide (3). The cell membrane structure with which C-peptide interacts appears to depend on the function of a G-protein–coupled receptor in most tissues, since pretreatment with pertussis toxin modifies the membrane binding (13) and inhibits the downstream signaling effects by C-peptide (14,15). Using [35S]GTPγS binding, it has been shown that G-αi proteins are specifically activated as C-peptide interacts with renal tubular cell membranes (16). All in all, the available studies of the full-length C-peptide and its COOH-terminal pentapeptide reveal properties typical of a peptide ligand interacting with a specific G-protein–coupled receptor, but attempts at its identification using gene cloning and proteomic approaches have not yielded positive results to date. It has also been proposed that the C-peptide membrane interaction might occur via a nonspecific mechanism independent of the peptide’s direction or chirality (17), but such a mechanism has not been confirmed in further studies (18).
FIG. 1.
A: Binding of rhodamine (Rh)-labeled C-peptide (CP) to membranes of human renal tubular cells. Fractional saturation of the membrane-bound ligand is presented as a function of the C-peptide concentration in the surrounding medium. The light blue area represents the physiological concentration (conc) range. Data are from Ref. 13. B: Intracellular distribution of Rh-labeled C-peptide examined with confocal microscopy. Swiss 3T3 fibroblasts were incubated with Rh-labeled C-peptide (1 μmol/L) for 30 min at 37°C. Overlay graph of nuclear staining with Hoechst dye 33342 (1 μg/mL, blue) and Rh-labeled C-peptide staining (red). Treatment with Rh alone did not result in cytosolic or nuclear staining. Reprinted with permission from Lindahl et al. (19). (A high-quality digital representation of this figure is available in the online issue.)
Several studies have reported internalization of C-peptide after binding to the cell membrane. Uptake of rhodamine-labeled C-peptide has been demonstrated for mouse fibroblast Swiss 373 and human embryonic kidney 293 cells (19) (Fig. 1B). Internalization has also been shown in human aortic endothelial cells and umbilical artery smooth muscle cells with initial localization to early endosomes before degradation in lysosomes (20). Endosomes are important sorting stations for internalized peptides and may provide a platform for intracellular C-peptide signaling. An additional fate of intracellular C-peptide is its localization to nucleoli, where it promotes transcription of genes encoding for ribosomal RNA (21). These observations provide a mechanism by which C-peptide can exert transcriptional effects, and the findings demonstrate that the peptide may exert growth factor activity (21,22).
INTRACELLULAR MECHANISMS OF C-PEPTIDE ACTION
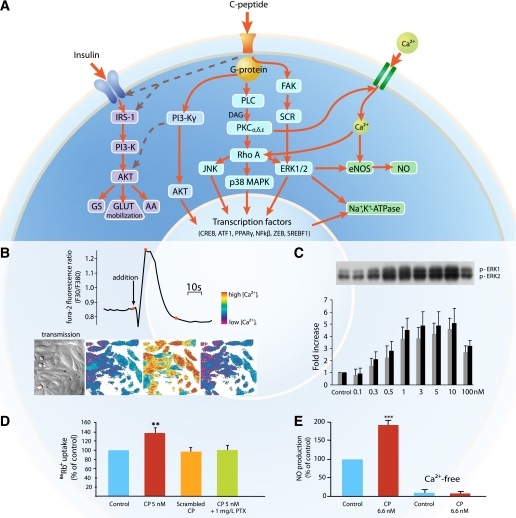
A variety of cell types respond to C-peptide exposure by activation of specific intracellular signaling pathways (see Fig. 2A for a schematic overview). Application of physiological concentrations of C-peptide to renal tubular cells and endothelial cells results in a prompt elevation of intracellular Ca2+ concentrations ([Ca2+]i) (14) (Fig. 2B). C-peptide also elicits phosphorylation of phospholipase C (PLC), several protein kinase C (PKC) isoforms, and phosphatidylinositol 3-kinase (15,23). Activation of one or several components of the mitogen-activated protein kinase (MAPK) system (15,24), frequently involving translocation of Ras homolog gene family, member A (RhoA) from the cytoplasm to the cell membrane, is consistently observed in a concentration-dependent manner for all examined cell types after exposure to C-peptide (Fig. 2C). An important cellular end effect elicited by C-peptide is its regulatory influence on Na+,K+-ATPase mediated via PLC; PKC isoforms α, δ, and ε; and RhoA-activated MAPK signaling (25,26). Thus, studies of ouabain-sensitive uptake of 86Rb+, a marker of Na+,K+-ATPase activity, in human renal tubular cells in primary culture show a stimulatory effect of C-peptide on Na+,K+-ATPase activity in the physiological concentration range (Fig. 2D). Pretreatment with pertussis toxin or incubation of the cells in a Ca2+-free medium abrogates the C-peptide mediated effect. Moreover, it has been shown that C-peptide augments the expression of Na+,K+-ATPase via activation of the transcription factor zinc-finger E-box binding protein (ZEB) (27).
FIG. 2.
A: General overview of intracellular signaling by C-peptide. C-peptide interaction with cell membranes is accompanied by activation of a pertussis toxin–sensitive G-protein. Subsequently, there is influx of Ca2+ and activation of eNOS, resulting in NO formation. PLC and specific isomers of PKC are also activated, as well as the MAPK complex. As a result, there is activation and induction of Na+,K+-ATPase, as well as DNA binding of several transcription factors, resulting in augmented eNOS mRNA formation and increased eNOS protein synthesis. Phosphoinositide 3-kinase (PI3-K)γ is also activated, giving rise to PPAR-γ–mediated transcriptional activity. In addition, there is evidence to indicate that C-peptide may interact synergistically with the insulin signaling pathway, as indicated by dashed lines. IRS-1, insulin receptor substrate 1; GS, glycogen synthesis; AA, amino acid uptake; DAG, diacylglycerol; FAK, focal adhesion kinase. B: Representation of [Ca2+]i in fura-2/AM-loaded human renal tubular cells stimulated with 5 nmol/L human C-peptide. Top panel: Tracing of the 340:380 fluorescence ratio before and during C-peptide exposure; bottom panel: cell images in transmission light (first panel) and in color code (next three panels) representing [Ca2+]i at the time points shown by the spot indicators (red) in the trace above. Reprinted with permission from Shafqat et al. (14). C: Effect of varying concentrations of C-peptide on ERK1/2 phosphorylation. Top panel: Human renal tubular cells were serum starved overnight and stimulated with human C-peptide, and cell lysates were subjected to Western blot analyses to determine ERK1/2 phosphorylation. Amount of phosphorylated (p-)ERK1/2 in the densitometric quantification is expressed as fold increase vs. control. Reprinted with permission from Zhong et al. (15). D: Effect of C-peptide and inhibitors on ouabain-sensitive 86Rb+ uptake indicating Na+,K+-ATPase activity. Primary human renal tubular cells were incubated with C-peptide (red bar), scrambled C-peptide (orange bar), or C-peptide plus pertussis toxin (PTX) (green bar) for 10 min. **P < 0.01 vs. control (blue bar). Data are from Ref. 25. E: Effect of C-peptide on the NO release from bovine aortic endothelial cells in the presence and absence of Ca2+. ***P < 0.001 vs. control. Data are from Ref. 28. (A high-quality digital representation of this figure is available in the online issue.)
A second important cellular end effect of C-peptide is its influence on endothelial nitric oxide synthase (eNOS). Reduced eNOS activity in diabetes, resulting in altered microcirculation, is considered a pathogenic factor for development of microvascular complications. C-peptide in the physiological range has been found to activate eNOS and concentration-dependently increase the synthesis of nitric oxide (NO) in endothelial cells (28). There was no C-peptide–mediated stimulation of NO production in pertussis toxin–treated cells or when a calcium-binding agent was added to the medium (Fig. 2E). C-peptide has also been shown to stimulate gene transcription of eNOS via the extracellular signal-regulated kinase (ERK)1/2, resulting in increased cellular expression of eNOS protein (29). Together, these data demonstrate that C-peptide is capable of eliciting endothelial NO release, resulting in vascular smooth muscle relaxation and increased blood flow.
In addition to its effects on the expression of Na+,K+-ATPase and eNOS, C-peptide is reported to elicit stimulation of a number of cell transcription factors (e.g., cAMP-responsive element–binding protein [CREB], activating transcription factors 1 and 2 (ATF-1 and ATF-2), Bcl-2, peroxisome proliferator–activated receptor [PPAR]-γ, ZEB, activator protein 1, sterol regulatory element–binding transcription factor [SREBF]-1, and nuclear factor [NF]-κB), all of which are of fundamental importance for cell processes such as cell growth, migration, inflammatory responses, and apoptosis (see Hills and Brunskill for a review) (8). Finally, C-peptide activates insulin receptor tyrosine kinase, insulin receptor substrate 1 tyrosine phosphorylation, and glycogen synthase kinase 3 phosphorylation (30,31), with downstream effects leading to GLUT mobilization, promotion of amino acid uptake, and glycogen synthesis (30), suggesting that C-peptide signaling may cross-talk with the insulin pathway at the level of the insulin receptor.
ANTI-INFLAMMATORY EFFECTS
Inflammation is increasingly being recognized as an important factor contributing to vascular damage in diabetes (32). There is evidence indicating that hyperglycemia may precipitate an inflammatory vascular response and play a significant role in the development of endothelial dysfunction. Patients with type 1 diabetes and microvascular complications show increased levels of several inflammatory markers as compared with patients without complications (33).
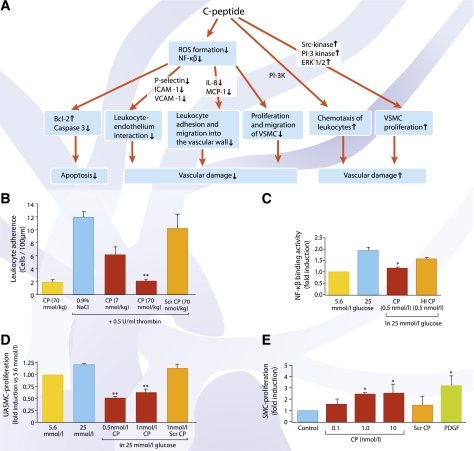
The multiple effects of C-peptide on inflammatory processes are outlined in Fig. 3A. An early event in the development of diabetes-related vascular damage is the adhesion of circulating leukocytes to endothelial cells and their migration into the vessel wall. C-peptide has been found to reduce endothelial cell surface expression of adhesion molecules, such as P-selectin, intercellular adhesion molecule 1, and vascular adhesion molecule 1 (34,35). In this way, C-peptide may attenuate leukocyte-endothelium interaction both in vitro and in vivo (34,35) (Fig. 3B). A later step in the development of vascular lesions is the secretion of chemokines that induce leukocyte adhesion and migration into the vascular wall. In this regard, C-peptide has been demonstrated to reduce glucose-induced and endotoxin-mediated secretion of the chemokines interleukin (IL)-6, IL-8, and monocyte chemoattractant protein 1 via suppression of nuclear translocation of NF-κB of both endothelial cells (35) (Fig. 3C) and monocytes (36). Yet another important step toward formation of atherosclerotic lesions is proliferation and migration of vascular smooth muscle cells. Here, several reports have demonstrated that C-peptide in the physiological concentration range exerts a protective effect against migration of vascular smooth muscle cells induced by either high glucose or elevated insulin (37–39) (Fig. 3D). More specific, it has been shown that C-peptide is capable of preventing insulin-induced neointima formation in human saphenous vein grafts via an inhibitory effect on SREBF-1 (38), indicating that C-peptide can prevent or limit the detrimental effects of insulin on vascular smooth muscle cell function. Other studies, however, report contrasting evidence to suggest that increased C-peptide levels may stimulate proliferation of aortic smooth muscle cells (40) (Fig. 3E) and induce chemotactic activity similar to the effect of known chemokines (41). Further studies will be required to reconcile these opposing findings to better understand if elevated C-peptide concentrations may contribute to the development of diabetes-induced macrovascular abnormalities.
FIG. 3.
A: Overview of C-peptide’s cytoprotective, antiapoptotic, and anti-inflammatory effects. B: Leukocyte adherence in the rat mesenteric microvasculature after superfusion of the mesentery with 0.5 units/mL thrombin. Bars show number of adhering leukocytes per 100 μm postcapillary venular endothelium after an intravenous bolus administration of C-peptide (red bars) or scrambled C-peptide (orange bar). Data are means ± SE for number of adherent cells observed at 120 min in each group (n = 6). **P < 0.01 vs. control (blue bar). Data are from Ref. 34. C: Effect of C-peptide on binding activity of NF-κB p50. Human aortic endothelial cells were cultured in low (5.6 mmol/L) or high (25 mmol/L) glucose in the presence or absence of 0.5 nmol/L C-peptide. In cells exposed to high glucose (blue bar), there was a twofold increase in NF-κB p50 nuclear translocation compared with cells in low glucose (yellow bar). A decrease in NF-κB p50 nuclear translocation was observed in the presence of C-peptide (red bar) (*P < 0.05 vs. high glucose alone), while heat-inactivated C-peptide (orange bar) had no significant effect. Data are from Ref. 35. D: C-peptide reduces high glucose–induced proliferation of umbilical artery smooth muscle cells. Cells were incubated with 5.6 or 25 mmol/L glucose (yellow and blue bars, respectively) in the presence or absence of C-peptide and assayed for cell proliferation. C-peptide (red bars) reduced high glucose–induced umbilical artery smooth muscle cell proliferation (**P < 0.01 vs. high glucose), while addition of scrambled C-peptide (orange bar) had no significant effect. Data are from Ref. 37. E: C-peptide induces vascular smooth muscle proliferation. Human aortic smooth muscle cells were stimulated with different concentrations of human C-peptide (red bars) for 24 h before cell proliferation was assessed by [3H]thymidine incorporation. Scrambled C-peptide (10 nmol/L; orange bar) was used as a negative and platelet-derived growth factor (10 ng/mL) as a positive control. Data are expressed as fold induction of unstimulated cells. *P < 0.05 vs. control. Data are from Ref. 40. ROS, reactive oxygen species; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; VSMC, vascular smooth muscle cell; CP, C-peptide; Scr, scrambled; HI, heat inactivated; UASMC, umbilical artery smooth muscle cells; VSM, vascular smooth muscle; PDGF, platelet-derived growth factor; PI-3K, phosphoinositide 3-kinase.
Apart from fueling inflammatory processes, activation of NF-κB has also been shown to accelerate apoptosis in human endothelial cells. A recent report demonstrates that C-peptide is able to suppress both glucose-induced and tumor necrosis factor (TNF)-α–mediated activation of NF-κB via reduced generation of reactive oxygen species (42). Downstream effects involve decreased levels of pro-apoptotic caspase-3, elevated levels of antiapoptotic Bcl-2, and subsequently attenuated apoptosis. In summary, C-peptide has been shown capable of antagonizing adhesion molecule expression, inflammatory cytokine secretion, and reactive oxygen species formation in endothelial cells and leukocytes exposed to a variety of inflammatory insults.
CIRCULATORY EFFECTS
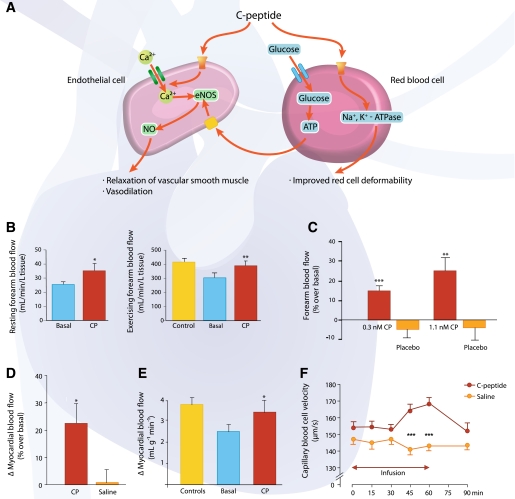
Endothelial dysfunction and compromised microvascular circulation are common denominators in the development of microvascular complications of type 1 diabetes. Impaired release of NO from the vascular endothelium and altered blood rheology secondary to diminished erythrocyte deformability are thought to be important in impeding the microcirculation in type 1 diabetes. Both of these factors can be influenced by C-peptide (Fig. 4A). Thus, physiological concentrations of the peptide have been shown to activate and induce eNOS and to elicit augmented NO release from endothelial cells (28,29), as discussed above. Moreover, C-peptide reduces or normalizes the abnormality of erythrocyte elasticity that is characteristic of type 1 diabetes. In vitro data show that erythrocyte deformability is improved by C-peptide over a range of physiological shear stress, mediated via a stimulatory influence on the Na+,K+-ATPase of the erythrocyte (43). This effect can be elicited both by the full-length native peptide and by its COOH-terminal penta- or hexapeptide segments but not by a scrambled C-peptide (44). The effect is abolished by the addition of ouabain or EDTA (43), and it is currently being discussed whether metal ions, primarily presence of Zn2+, are required (45). In addition, the interaction between C-peptide and erythrocytes extends beyond a beneficial effect on erythrocyte deformability in that the peptide has been shown to increase glucose uptake by erythrocytes and elicit release of ATP (46). Intravascular release of ATP is reported to stimulate NO synthesis in endothelial cells via purinergic receptor signaling, with subsequent dilation of resistance vessels and increase in regional blood flow (46).
FIG. 4.
A: Circulatory effects of C-peptide. C-peptide stimulates Ca2+ uptake by endothelial cells, leading to increased expression and activity of eNOS and increased production of NO. NO elicits relaxation of vascular smooth muscle cells and vasodilation. In erythrocytes, C-peptide increases uptake of glucose and subsequent release of ATP, which in turn stimulates NO production in endothelial cells. In addition, C-peptide improves the erythrocyte deformability by enhancing Na+,K+-ATPase activity. B: Resting forearm blood flow measured by a plethysmographic method in 11 patients with type 1 diabetes. Left panel: C-peptide infusion (6 pmol/kg/min; red bar) increased basal blood flow (blue bar) by 38% (*P < 0.05). Data are from Ref. 48. Right panel: Exercising forearm blood flow determined by indicator diffusion technique before and during C-peptide administration. Forearm blood flow during exercise was reduced in diabetic patients (blue bar) compared with healthy control subjects (yellow bar). When exercise was repeated during intravenous C-peptide infusion (6 pmol/kg/min), blood flow was found to rise by 28% in the diabetic patients (n = 12, **P < 0.01 vs. basal state) and become similar to that of healthy control subjects. Data are from Ref. 4. C: Forearm blood flow in 10 type 1 diabetic patients was measured using venous occlusion plethysmography during 60-min intravenous infusion of C-peptide at increasing rate (red bars) or NaCl (placebo; orange bar). In the C-peptide–treated groups, forearm blood flow increased by 15 ± 3% (0.3 nmol/L C-peptide, ***P < 0.001 vs. baseline) and 25 ± 6% (1.1 nmol/L C-peptide, **P < 0.01 vs. baseline), respectively. Data are from Ref. 49. D: Effect of C-peptide on myocardial blood flow in patients with type 1 diabetes (n = 8). The figure shows percent increase in resting myocardial blood flow, as estimated by contrast echocardiography, during infusion of C-peptide (red bar) or saline (orange bar). *P < 0.05. Data are from Ref. 50. E: Effect of C-peptide on adenosine-stimulated myocardial vasodilation in 10 type 1 diabetic and 10 healthy individuals. The graph indicates the difference between basal and adenosine-stimulated myocardial blood flow measured by positron emission tomography technique in patients before (blue bar) and during (red bar) C-peptide infusion and in healthy subjects (yellow bar). *P < 0.05. Data are from Ref. 51. F: Erythrocyte capillary velocity in skin circulation of type 1 diabetic patients (n = 19) receiving C-peptide infusion (8 pmol/kg/min) vs. saline infusion. ***P < 0.001. Reprinted with permission from Forst et al. (52). CP, C-peptide.
Administration of C-peptide in animal models and in type 1 diabetic patients results in a prompt increase in blood flow in several tissues. Direct measurements of sciatic nerve blood flow in animal models of type 1 diabetes have demonstrated augmented endoneurial blood flow in response to C-peptide administration (47). Skeletal muscle blood flow (forearm) at rest (48) and during exercise (4) increases in response to C-peptide in a concentration-dependent manner across the 0–1 nmol/L range (49) (Fig. 4B and C) as a consequence of resistance vessel relaxation and augmented capillary recruitment. Higher concentrations of C-peptide elicit no further rise in blood flow, and no effect of C-peptide is seen in healthy nondiabetic subjects (4). C-peptide infusion in replacement dose in type 1 diabetic patients gives rise to an ∼30% increase in left ventricular myocardial blood flow both under basal conditions and during pharmacological stimulation (Fig. 4D and E). The patients also show improved rates of left ventricular contraction rate and increased ejection fraction, plus a shortening of the QT interval during C-peptide administration (50,51). In addition, there is an augmented skin capillary erythrocyte velocity and a redistribution of blood flow from thermoregulatory shunts to nutritive skin capillaries when C-peptide is administered in type 1 patients (52) (Fig. 4F).
C-PEPTIDE AND RENAL FUNCTION IN DIABETES
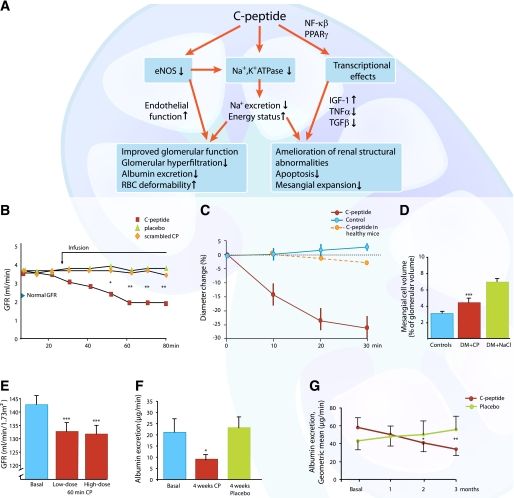
Early signs of diabetic kidney disease include glomerular hyperfiltration and glomerular enlargement, primarily as a result of mesangial matrix expansion and urinary albumin leakage. A number of studies show that C-peptide elicits beneficial effects on different aspects of diabetes-related renal dysfunction (for an overview, see Fig. 5A). C-peptide and renal function in diabetes have been studied in streptozotocin (STZ)-induced diabetic rats for periods from 2 h up to 28 days using replacement doses of the peptide. The glomerular hyperfiltration, present at onset of the studies, was promptly and almost completely prevented by C-peptide, and urinary albumin excretion was reduced by 70–100% in comparison with placebo-treated rats (53) (Fig. 5B). Regarding the mechanism(s) underlying the observed beneficial effects, direct measurements have shown that C-peptide elicits a constriction of the afferent glomerular arteriole (Fig. 5C) and possibly a relaxation of the efferent arteriole in STZ-induced diabetic mice, resulting in a reduction of intraglomerular pressure (54). These effects by C-peptide in the diabetic state are different from those observed under conditions of normoglycemia and represent an inhibitory rather than stimulatory influence by the peptide on renal arteriolar eNOS (54,55) and glomerular and tubular Na+,K+-ATPase (53--56). Further studies are required to determine the background to these surprising observations, but the findings help explain the C-peptide mediated reduction of glomerular hyperfiltration and decrease of urinary albumin excretion in the diabetic state. It is notable that renal function in healthy animals was unresponsive to C-peptide.
FIG. 5.
A: Schematic overview of C-peptide’s effect on diabetes-induced functional and structural renal abnormalities. B: Mean GFR in STZ-induced diabetic rats treated with C-peptide, placebo, or scrambled C-peptide. The arrow indicates the start of infusions of either C-peptide (0.5 nmol/min/kg) or scrambled C-peptide. *P < 0.05, **P < 0.01 vs. placebo. Data are from Ref. 53. C: The effect of C-peptide (5 nmol/L) on the diameter of afferent glomerular arterioles in healthy and hyperglycemic STZ-induced diabetic mice compared with control animals. Data are means ± SE. Data are from Ref. 54. D: Effect of C-peptide administration (50 pmol/kg/min) for 4 weeks on mesangial matrix volume in STZ-induced diabetic rats (DM). The placebo-treated group (green bar) showed a mesangial matrix fraction that was more than twice that in the control group (blue bar). C-peptide treatment significantly reduced the mesangial matrix fraction compared with placebo (red bar; ***P < 0.001). Data are from Ref. 57. E: C-peptide effects on glomerular hyperfiltration in 11 patients with early stage type 1 diabetes. C-peptide was given intravenously for 1 h (bolus of 25 pmol/kg/min for 1.5 min followed by 10 pmol/kg/min for 6.5 min and ending with 5 pmol/kg/min for 52 min; low dose) and then for an additional hour using a bolus and infusion rates six times those of the first infusion period (high dose). Mean values ± SEM for basal state (blue bar) and C-peptide infusion (red bars) after 60 min (red bars) are indicated. ***P < 0.001. Data are from Ref. 3. F: Effects of C-peptide administration for 4 weeks on urinary albumin excretion in 18 type 1 diabetic patients with microalbuminuria. The graph presents data for the basal state compared with C-peptide (red bar) and placebo (green bar) after 4 weeks. *P < 0.05 compared with basal state. Data are from Ref. 5. G: Effects of C-peptide administration for 3 months on urinary albumin excretion in 21 type 1 diabetic patients and microalbuminuria. Patients received C-peptide (600 nmol/24 h) or placebo for 3 months in a crossover design. Albumin excretion was significantly different between C-peptide- and placebo-treated patients after 2 months (*P < 0.05) and 3 months (**P < 0.01). Data are from Ref. 6. CP, C-peptide.
It has also been demonstrated that C-peptide alleviates diabetes-induced structural changes of the glomeruli. When given to STZ-induced diabetic rats in physiological concentrations, it prevents or attenuates early glomerular enlargement. Thus, for STZ-induced diabetic rats given replacement doses of C-peptide subcutaneously for 4 weeks (starting 4 weeks after onset of diabetes), 65% of the increase in fractional volume of the glomerular mesangial matrix that occurred in untreated animals was inhibited by C-peptide (57) (Fig. 5D). The mechanism involved may relate to the finding that C-peptide antagonizes the effects of the major disease mediators transforming growth factor (TGF)-β1 (58) and TNF-α (16). Thus, in vitro studies show that the peptide reduces the expression of the profibrotic cytokine TGF-β1 and the glomerular accumulation of type IV collagen (59). In addition, C-peptide has been shown to protect against TNF-α–induced apoptosis of renal cells by induction, via NF-κB, of survival genes (16,58).
Several studies evaluating the effects of C-peptide on renal function in patients with type 1 diabetes have been undertaken. In a double-blind placebo–controlled study, C-peptide was infused intravenously for 2 h, reaching plasma concentrations in the physiological range (3). All patients presented with increased glomerular filtration rate (GFR) in the basal state, and GFR decreased by 7% after C-peptide administration but was unchanged in the placebo group (Fig. 5E). In a 1-month double-blind randomized study, type 1 patients with incipient nephropathy received replacement doses of C-peptide administered by subcutaneous infusion via a pump together with the patients’ regular insulin therapy (5). All patients showed elevated GFR and mild microalbuminuria at the start of the study. In the C-peptide–treated patients, GFR fell by 6% and the urinary albumin excretion decreased by >50%, whereas no significant change was observed in the control subjects (Fig. 5F). In a further study involving type 1 diabetic patients with early stage nephropathy and microalbuminuria, C-peptide was administered in replacement dose for 3 months in a double-blind placebo–controlled cross-over design (6). Prestudy urinary albumin excretion was on average 55 ± 10 μg/min, and measurements were undertaken after 1, 2, and 3 months. The albumin excretion decreased progressively throughout the study period and had fallen by ∼40% at the end of the study in the patients receiving C-peptide. In contrast, the average albumin excretion remained unchanged or increased slightly in the placebo-treated patients (Fig. 5G). Indices of glycemic control were similar in the C-peptide group and the control group, and all patients were normotensive throughout the study, demonstrating that the diminished albumin excretion was directly related to the C-peptide administration. The in vivo animal observations and the clinical studies in type 1 diabetic patients provide support for a role for C-peptide in alleviating the diabetes-induced functional and structural abnormalities of the kidneys.
EFFECTS OF C-PEPTIDE ON DIABETIC NEUROPATHY
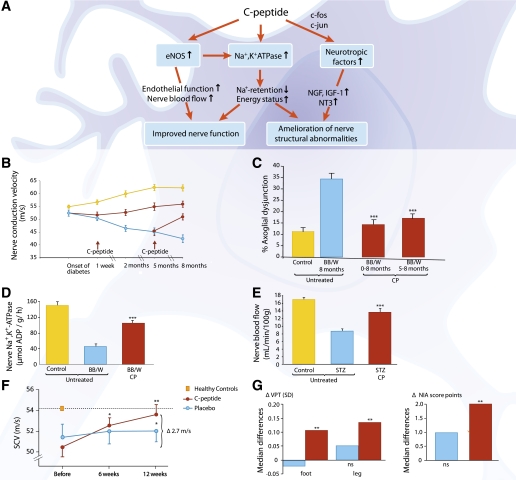
Diabetic peripheral neuropathy (DPN), the most common complication of diabetes, is manifested as sensory loss in the extremities with severe clinical implications potentially leading to foot ulceration and limb amputation. Several clinical studies, with C-peptide replacement in patients with type 1 diabetes, show beneficial effects on somatic and autonomic DPN. Figure 6A summarizes the effects of C-peptide on diabetic neuropathy. Studies in spontaneously diabetic BB/Wor rats show that C-peptide in replacement doses has the ability to improve peripheral nerve function and prevent or reverse the development of nerve structural changes. Thus, C-peptide administration for 2 months in diabetic BB/Wor rats, starting 1 week after onset of diabetes, reduced the development of the nerve conduction velocity (NCV) defect by 60% compared with control animals (60) (Fig. 6B). In rats treated for 8 months, C-peptide reduced the NCV defect by 70% and exerted a beneficial effect on the nerve structural changes that otherwise occur (60). The latter effect included prevention of diabetes-induced axonal atrophy, axoglial dysjunction, and paranodal demyelination of sural nerve fibers (Fig. 6C). C-peptide treatment has also been started after 5 months of diabetes, when the neuropathy has become established. C-peptide administration for 3 months then led to not only a significant improvement in NCV but also a reduction of diabetes-induced axonal degeneration and loss, as well as an increase in nerve fiber regeneration (60,61).
FIG. 6.
A: Schematic overview of functional and structural effects of C-peptide on diabetic neuropathy. B: C-peptide and NCV in diabetic BB/Wor rats. NCV in healthy (blue line) and diabetic BB/Wor rats given rat C-peptide in replacement dose (75 nmol C-peptide/kg/24 h) by subcutaneous pump infusion starting 1 week after onset of diabetes or after 5 months of diabetes (red lines, start of infusion indicated by arrows). The NCV declined progressively in the untreated diabetic animals (yellow line, P < 0.01 vs. controls) but increased in the C-peptide–infused animals (P < 0.05 vs. untreated BB/Wor rats). Data are from Ref. 60. C: C-peptide and the development of axoglial dysjunction in diabetic BB/Wor rats. Axoglial dysjunction was increased 3.5-fold in untreated diabetic rats (blue bar) compared with control rats (yellow bar). C-peptide (0–8 months and 5–8 months) prevented and reversed axoglial dysjunction (red bars; ***P < 0.001 vs. untreated diabetic rats). Data are from Ref. 60. D: C-peptide replacement results in a partial correction of the acute Na+,K+-ATPase defect in diabetic BB/Wor rats. Sciatic nerve Na+,K+-ATPase activity in healthy control rats (yellow bar), untreated BB/Wor diabetic rats (blue bar), and animals that received C-peptide for 2 months after diabetes onset (75 nmol/kg/24 h) shown. ***P < 0.001 vs. untreated diabetic rats. Data are from Ref. 60. E: C-peptide and nerve blood flow in diabetic rats. Endoneurial blood flow in STZ-induced diabetic rats was measured using a hydrogen elimination technique. Rats were treated for 2 weeks starting 6 weeks after onset of diabetes. Results are shown for healthy control rats (yellow bar), diabetic untreated rats (blue bar), and diabetic rats treated with C-peptide (red bar; 50 pmol/kg/min). ***P < 0.001 vs. untreated diabetic rats. Data are from Ref. 47. F: C-peptide and sensory nerve conduction velocity (SCV) in patients with type 1 diabetes and early stage neuropathy. Change in SCV after 6 (*P < 0.05) and 12 (**P < 0.001) weeks of C-peptide treatment (600 nmol/24 h, n = 26) or placebo administration (n = 20). The difference between the C-peptide and placebo groups at 12 weeks was statistically significant (2.1 m/s, *P < 0.05). Data are from Ref. 70. G: Effect of C-peptide on vibration perception (VPT) and clinical neurologic impairment (NIA score) after 6 months of C-peptide administration in type 1 diabetic neuropathy. Within the C-peptide groups (1.5 or 4.5 mg/day divided into four s.c. doses during 6 months, n = 92, red bars), both VPT and NIA score were significantly improved (**P < 0.01). The placebo group (n = 47, blue bars) showed no significant change in VPT or NIA. Data are from Ref. 71. BB/W, BB/Wor rats; CP, C-peptide; NT3, neurotrophin 3.
Effects of C-peptide on the molecular and functional abnormalities underpinning the progressive nature of DPN have been studied in animal models of type 1 diabetes. Impaired nerve Na+,K+-ATPase activity and diminished NO availability are key metabolic abnormalities responsible for the reduction in NCV. The Na+,K+-ATPase defect influences the permeation of Na+ at the node of Ranvier, resulting in decreased transmembranous potentials, intra-axonal Na+ accumulation, and decreased NCV (62,63). A further mechanism implicated in the acute nerve conduction slowing is decreased endoneurial blood flow as a result of impaired endothelial NO release (47,64). C-peptide replacement in type 1 diabetic rats restores Na+,K+-ATPase activity dose dependently (Fig. 6D) and improves NO availability with subsequent beneficial effects on endoneurial blood flow and NCV (Fig. 6E), even in the presence of highly elevated glucose levels (47,65). Further effects ascribed to C-peptide include gene-regulatory effects via the transcription factors c-jun, c-fos, NF-κB, and CREB (66), resulting in correction of several neurotrophic factors and their receptors, such as IGF-I, nerve growth factor (NGF), neurotrophin 3, and the insulin receptor (22,67). Impaired neurotrophic support affects the synthesis and posttranslational modifications of cytoskeletal proteins, such as neurofilaments and tubulins, contributing to axonal dysfunction, degeneration, and loss and impaired nerve regeneration. Corrective effects of C-peptide have also been demonstrated for several phosphorylating stress kinases, as well as on synthesis of neuroskeletal proteins (67), all of which help explain the peptide’s beneficial effects on axonal function and axonal atrophy and its stimulatory effect on nerve fiber regeneration.
Nodal and paranodal degenerative changes are characteristic of type 1 DPN in humans and animal models and have severe functional implications (68). Progressive degeneration of paranodal tight junctions, which secure the localization of α-Na+ channels to the node of Ranvier, underlies in part the NCV defect in type 1 DPN. The tight junctions are made up of adhesive proteins such as caspr, contactin, neurofascin, and RPTP-β. In type 1 diabetes, these proteins and their interactive regulations become progressively compromised with disruption of the ion-channel barrier, lateral migration of Na channels, and consequently diminished nodal Na+ permeability and decreased NCV (68,69). C-peptide replacement in diabetic BB/Wor rats has been shown to restore the expression of the adhesive proteins and their interactive regulations, thereby significantly improving the integrity of the paranodal barrier and nerve function (69).
The beneficial effects of C-peptide on impaired nerve function induced by type 1 diabetes have been confirmed in clinical studies. A double-blind placebo–controlled study in early stage neuropathy patients with C-peptide replacement during a 3-month period showed a gradual increase in sural NCV reaching 2.7 m/s that amounted to 80% correction of the initial nerve conduction deficit (Fig. 6F), as well as improvements in vibration perception (70). These results were confirmed in a larger study of patients with type 1 diabetes and manifest DPN (71). Six months of C-peptide replacement resulted in improvements in sensory NCV, vibration perception, and clinical scores of neuropathy impairment (71) (Fig. 6G), as well as erectile dysfunction (72). Levels of glycemic control were similar in C-peptide– and placebo-treated patients. Improvements in heart rate variability and other indices of cardiac autonomic innervation have also been observed in patients with mild to moderate autonomic dysfunction after both short-term (7) and 3-month (6) administration of C-peptide in replacement doses. In summary, the available clinical and experimental evidence demonstrates that C-peptide exerts a multitude of beneficial effects on the pathogenetic mechanisms underlying DPN in type 1 diabetes.
ENCEPHALOPATHY
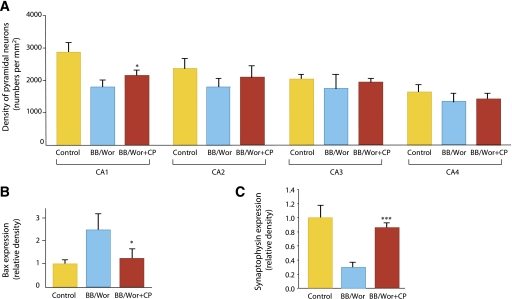
Encephalopathy is now becoming a recognized complication of type 1 diabetes (10). Neurobehavioral examinations of children with type 1 diabetes indicate the presence of deficits in attention, executive function, intelligence, and memory, which parallel lower IQs and impaired school performances (73). Contrary to earlier beliefs, cognitive deficits do not seem to correlate with previous hypoglycemic episodes. Volumetric magnetic resonance imaging and voxel-based morphometric analysis reveal significant deficits in limbic gray and white matter volumes, and neuropathology examination shows significant neuronal loss in hippocampus and frontal cortex in type 1 diabetic patients (for a review, see Sima et al. [10]). Studies in the type 1 diabetic BB/Wor rat indicate several underlying mechanisms and their sequential progression to neurobehavioral deficits and gray and white matter changes. Such changes are significantly prevented and in some instances reversed by C-peptide administration. Thus, replacement of C-peptide in the diabetic BB/Wor rat partially prevented the development of deficits in spatial learning and memory as evaluated by the radial arm maze. Moreover, C-peptide replacement in diabetic BB/Wor rats limited hippocampal neuronal loss (Fig. 7A) and prevented upregulation of receptor for advanced glycosylation end product and NF-κB (10), while the diabetes-induced downregulation of trophic factors, such as IGF-I, IGF-II, and NGF, was diminished or prevented (74). In keeping with these findings, C-peptide was found to limit the diabetes-induced rise in Bcl2-associated X protein (Bax) (Fig. 7B) and caspase expression (74) and diminish the fall in synaptophysin expression (Fig. 7C) (10). Moreover, C-peptide replacement partially normalizes the inflammatory cascade in hippocampus of diabetic rats (10). The innate inflammatory cascade may elicit oxidative DNA damage, activation of proapoptotic stressors, and, eventually, apoptotic neuronal death. C-peptide replacement from onset of diabetes in the BB/Wor rat corrects neurotrophic factors, ameliorates presynaptic loss, restores the expression of the postsynaptic excitatory GluR2 (10), and prevents to a significant degree oxidative DNA damage, apoptosis, and the neuronal loss in chronically diabetic rats (10,74). Available preclinical evidence thus suggests that replacement of C-peptide may exert significant protective effects on this newly recognized and potentially devastating complication.
FIG. 7.
A: Neuronal densities in CA1–CA4 in hippocampi from control, BB/Wor, and C-peptide–replaced BB/Wor rats (n = 4 per group). In CA1 and CA2 of BB/Wor rats, neuronal densities were significantly decreased compared with controls. C-peptide replacement resulted in a significant protection against neuronal loss in CA1. No significant differences were seen for the groups in CA3 and CA4. *P < 0.05 vs. BB/Wor rats. Data are from Ref. 74. B: The protein expression of Bax in hippocampi in four individual animals per group. Bax was significantly increased in BB/Wor rats. C-peptide replacement significantly (*P < 0.05 vs. untreated BB/Wor rats) prevented this increase. Data are from Ref. 74. C: Expression of hippocampal presynaptic synaptophysin was significantly decreased in 4-month diabetic rats and was prevented by C-peptide replacement from onset of diabetes (***P < 0.001 vs. untreated BB/Wor rats). Data are from Ref. 10.
CONCLUSIONS AND FUTURE OUTLOOK
From the multifaceted effects of C-peptide discussed above, it should be clear that the peptide can no longer be considered an irrelevant by-product of insulin biosynthesis. Its specific binding to cell membranes, its particular intracellular signaling pattern with end effects involving activation and enhanced expression of eNOS and Na+,K+-ATPase, and its activation of several important transcription factors all attest to the peptide being a bioactive endogenous peptide in its own right. Extensive studies in animal models of diabetes and early clinical trials in type 1 diabetic patients demonstrate that replacement of C-peptide results in beneficial effects on the diabetes-induced functional and structural abnormalities of peripheral nerves, the kidneys, and the brain. Much remains to be learned about C-peptide physiology, but even a cautious evaluation of the available evidence presents in plain sight the picture of a previously unrecognized endogenous peptide with therapeutic potential. Since no disease-modifying therapy is available for patients with microvascular complications of type 1 diabetes, it can be hoped that the ongoing development of a long-acting C-peptide (75) will facilitate further clinical trials and allow definition of C-peptide’s potential role in the therapy of type 1 diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (TID RAID NIH-NIDDK-05-05 and R01-DK-081470-01) and the Thomas Foundation to A.A.F.S.
J.W. and Å.K. are employees of Cebix AB, Stockholm, Sweden. No other potential conflicts of interest relevant to this article were reported.
J.W., Å.K., and A.A.F.S. surveyed the literature and wrote, reviewed, and edited the manuscript. J.W. and A.A.F.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Excellent work with the illustrations by Gunilla Elam Design, Stockholm, is gratefully acknowledged. The authors apologize to those colleagues whose work could not be cited because of space limitations.
REFERENCES
- 1.Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science 1967;157:697–700 [DOI] [PubMed] [Google Scholar]
- 2.Panero F, Novelli G, Zucco C, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 2009;32:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson BL, Sjöberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 1992;35:121–128 [DOI] [PubMed] [Google Scholar]
- 4.Johansson BL, Linde B, Wahren J. Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of type 1 (insulin-dependent) diabetic patients. Diabetologia 1992;35:1151–1158 [DOI] [PubMed] [Google Scholar]
- 5.Johansson BL, Kernell A, Sjöberg S, Wahren J. Influence of combined C-peptide and insulin administration on renal function and metabolic control in diabetes type 1. J Clin Endocrinol Metab 1993;77:976–981 [DOI] [PubMed] [Google Scholar]
- 6.Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with type 1 diabetes mellitus. Diabet Med 2000;17:181–189 [DOI] [PubMed] [Google Scholar]
- 7.Johansson BL, Borg K, Fernqvist-Forbes E, Odergren T, Remahl S, Wahren J. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia 1996;39:687–695 [DOI] [PubMed] [Google Scholar]
- 8.Hills CE, Brunskill NJ. C-peptide and its intracellular signaling. Rev Diabet Stud 2009;6:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luppi P, Cifarelli V, Wahren J. C-peptide and long-term complications of diabetes. Pediatr Diabetes 2011;12:276–292 [DOI] [PubMed] [Google Scholar]
- 10.Sima AA, Zhang W, Muzik O, Kreipke CW, Rafols JA, Hoffman WH. Sequential abnormalities in type 1 diabetic encephalopathy and the effects of C-peptide. Rev Diabet Stud 2009;6:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia 2007;50:503–509 [DOI] [PubMed] [Google Scholar]
- 12.Flatt PR, Swanston-Flatt SK, Hampton SM, Bailey CJ, Marks V. Specific binding of the C-peptide of proinsulin to cultured B-cells from a transplantable rat islet cell tumor. Biosci Rep 1986;6:193–199 [DOI] [PubMed] [Google Scholar]
- 13.Rigler R, Pramanik A, Jonasson P, et al. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci U S A 1999;96:13318–13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafqat J, Juntti-Berggren L, Zhong Z, et al. Proinsulin C-peptide and its analogues induce intracellular Ca2+ increases in human renal tubular cells. Cell Mol Life Sci 2002;59:1185–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong Z, Davidescu A, Ehrén I, et al. C-peptide stimulates ERK1/2 and JNK MAP kinases via activation of protein kinase C in human renal tubular cells. Diabetologia 2005;48:187–197 [DOI] [PubMed] [Google Scholar]
- 16.Al-Rasheed NM, Willars GB, Brunskill NJ. C-peptide signals via Galpha i to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol 2006;17:986–995 [DOI] [PubMed] [Google Scholar]
- 17.Ido Y, Vindigni A, Chang K, et al. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 1997;277:563–566 [DOI] [PubMed] [Google Scholar]
- 18.Henriksson M, Shafqat J, Liepinsh E, et al. Unordered structured of proinsulin C-peptide in aqueous solution and in the presence of lipid vesicles. Cell Mol Life Sci 2000;57:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindahl E, Nyman U, Melles E, et al. Cellular internalization of proinsulin C-peptide. Cell Mol Life Sci 2007;64:479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luppi P, Geng X, Cifarelli V, Drain P, Trucco M. C-peptide is internalised in human endothelial and vascular smooth muscle cells via early endosomes. Diabetologia 2009;52:2218–2228 [DOI] [PubMed] [Google Scholar]
- 21.Lindahl E, Nyman U, Zaman F, et al. Proinsulin C-peptide regulates ribosomal RNA expression. J Biol Chem 2010;285:3462–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierson CR, Zhang W, Sima AA. Proinsulin C-peptide replacement in type 1 diabetic BB/Wor-rats prevents deficits in nerve fiber regeneration. J Neuropathol Exp Neurol 2003;62:765–779 [DOI] [PubMed] [Google Scholar]
- 23.Al-Rasheed NM, Chana RS, Baines RJ, Willars GB, Brunskill NJ. Ligand-independent activation of peroxisome proliferator-activated receptor-gamma by insulin and C-peptide in kidney proximal tubular cells: dependent on phosphatidylinositol 3-kinase activity. J Biol Chem 2004;279:49747–49754 [DOI] [PubMed] [Google Scholar]
- 24.Kitamura T, Kimura K, Jung BD, et al. Proinsulin C-peptide rapidly stimulates mitogen-activated protein kinases in Swiss 3T3 fibroblasts: requirement of protein kinase C, phosphoinositide 3-kinase and pertussis toxin-sensitive G-protein. Biochem J 2001;355:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong Z, Kotova O, Davidescu A, et al. C-peptide stimulates Na+, K+-ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci 2004;61:2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsimaratos M, Roger F, Chabardès D, et al. C-peptide stimulates Na+,K+-ATPase activity via PKC alpha in rat medullary thick ascending limb. Diabetologia 2003;46:124–131 [DOI] [PubMed] [Google Scholar]
- 27.Galuska D, Pirkmajer S, Barrès R, Ekberg K, Wahren J, Chibalin AV. C-peptide increases Na,K-ATPase expression via PKC- and MAP kinase-dependent activation of transcription factor ZEB in human renal tubular cells. PLoS ONE 2011;6:e28294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallerath T, Kunt T, Forst T, et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 2003;9:95–102 [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, Kimura K, Makondo K, et al. Proinsulin C-peptide increases nitric oxide production by enhancing mitogen-activated protein-kinase-dependent transcription of endothelial nitric oxide synthase in aortic endothelial cells of Wistar rats. Diabetologia 2003;46:1698–1705 [DOI] [PubMed] [Google Scholar]
- 30.Grunberger G, Qiang X, Li Z, et al. Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia 2001;44:1247–1257 [DOI] [PubMed] [Google Scholar]
- 31.Li ZG, Qiang X, Sima AA, Grunberger G. C-peptide attenuates protein tyrosine phosphatase activity and enhances glycogen synthesis in L6 myoblasts. Biochem Biophys Res Commun 2001;280:615–619 [DOI] [PubMed] [Google Scholar]
- 32.Schram MT, Chaturvedi N, Schalkwijk C, et al. ; EURODIAB Prospective Complications Study Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2003;26:2165–2173 [DOI] [PubMed] [Google Scholar]
- 33.Devaraj S, Cheung AT, Jialal I, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 2007;56:2790–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scalia R, Coyle KM, Levine BJ, Booth G, Lefer AM. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J 2000;14:2357–2364 [DOI] [PubMed] [Google Scholar]
- 35.Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M. Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappaB pathway. Diabetologia 2008;51:1534–1543 [DOI] [PubMed] [Google Scholar]
- 36.Haidet J, Cifarelli V, Trucco M, et al. C-peptide reduces pro-inflammatory cytokine secretion in LPS-stimulated U937 monocytes in condition of hyperglycemia. Inflamm Res 2012;61:27–35 [DOI] [PubMed]
- 37.Cifarelli V, Luppi P, Tse HM, He J, Piganelli J, Trucco M. Human proinsulin C-peptide reduces high glucose-induced proliferation and NF-kappaB activation in vascular smooth muscle cells. Atherosclerosis 2008;201:248–257 [DOI] [PubMed] [Google Scholar]
- 38.Mughal RS, Scragg JL, Lister P, et al. Cellular mechanisms by which proinsulin C-peptide prevents insulin-induced neointima formation in human saphenous vein. Diabetologia 2010;53:1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi Y, Naruse K, Hamada Y, et al. Human proinsulin C-peptide prevents proliferation of rat aortic smooth muscle cells cultured in high-glucose conditions. Diabetologia 2005;48:2396–2401 [DOI] [PubMed] [Google Scholar]
- 40.Walcher D, Babiak C, Poletek P, et al. C-peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res 2006;99:1181–1187 [DOI] [PubMed] [Google Scholar]
- 41.Marx N, Walcher D, Raichle C, et al. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol 2004;24:540–545 [DOI] [PubMed] [Google Scholar]
- 42.Cifarelli V, Geng X, Styche A, Lakomy R, Trucco M, Luppi P. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia 2011;54:2702–2712 [DOI] [PubMed] [Google Scholar]
- 43.Kunt T, Schneider S, Pfützner A, et al. The effect of human proinsulin C-peptide on erythrocyte deformability in patients with type I diabetes mellitus. Diabetologia 1999;42:465–471 [DOI] [PubMed] [Google Scholar]
- 44.Hach T, Forst T, Kunt T, et al. C-peptide and its C-terminal fragments improve erythrocyte deformability in type 1 diabetes patients. Exp Diabetes Res 2008;2008:730594 [DOI] [PMC free article] [PubMed]
- 45.Medawala W, McCahill P, Giebink A, Meyer J, Ku CJ, Spence DM. A molecular level understanding of zinc activation of C-peptide and its effects on cellular communication in the bloodstream. Rev Diabet Stud 2009;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer JA, Froelich JM, Reid GE, Karunarathne WK, Spence DM. Metal-activated C-peptide facilitates glucose clearance and the release of a nitric oxide stimulus via the GLUT1 transporter. Diabetologia 2008;51:175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cotter MA, Ekberg K, Wahren J, Cameron NE. Effects of proinsulin C-peptide in experimental diabetic neuropathy: vascular actions and modulation by nitric oxide synthase inhibition. Diabetes 2003;52:1812–1817 [DOI] [PubMed] [Google Scholar]
- 48.Johansson BL, Wahren J, Pernow J. C-peptide increases forearm blood flow in patients with type 1 diabetes via a nitric oxide-dependent mechanism. Am J Physiol Endocrinol Metab 2003;285:E864–E870 [DOI] [PubMed] [Google Scholar]
- 49.Ekberg K, Johansson B-L, Wahren J. Stimulation of blood flow by C-peptide in patients with type 1 diabetes. Diabetologia 2001;44(Suppl. 1):A323 [Google Scholar]
- 50.Hansen A, Johansson BL, Wahren J, von Bibra H. C-peptide exerts beneficial effects on myocardial blood flow and function in patients with type 1 diabetes. Diabetes 2002;51:3077–3082 [DOI] [PubMed] [Google Scholar]
- 51.Johansson BL, Sundell J, Ekberg K, et al. C-peptide improves adenosine-induced myocardial vasodilation in type 1 diabetes patients. Am J Physiol Endocrinol Metab 2004;286:E14–E19 [DOI] [PubMed] [Google Scholar]
- 52.Forst T, Kunt T, Pohlmann T, et al. Biological activity of C-peptide on the skin microcirculation in patients with insulin-dependent diabetes mellitus. J Clin Invest 1998;101:2036–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjöquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int 1998;54:758–764 [DOI] [PubMed] [Google Scholar]
- 54.Nordquist L, Lai EY, Sjöquist M, Patzak A, Persson AE. Proinsulin C-peptide constricts glomerular afferent arterioles in diabetic mice. A potential renoprotective mechanism. Am J Physiol Regul Integr Comp Physiol 2008;294:R836–R841 [DOI] [PubMed] [Google Scholar]
- 55.Kamikawa A, Ishii T, Shimada K, et al. Proinsulin C-peptide abrogates type-1 diabetes-induced increase of renal endothelial nitric oxide synthase in rats. Diabetes Metab Res Rev 2008;24:331–338 [DOI] [PubMed] [Google Scholar]
- 56.Nordquist L, Brown R, Fasching A, Persson P, Palm F. Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. Am J Physiol Renal Physiol 2009;297:F1265–F1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samnegård B, Jacobson SH, Jaremko G, et al. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol Dial Transplant 2005;20:532–538 [DOI] [PubMed] [Google Scholar]
- 58.Hills CE, Al-Rasheed N, Al-Rasheed N, Willars GB, Brunskill NJ. C-peptide reverses TGF-beta1-induced changes in renal proximal tubular cells: implications for treatment of diabetic nephropathy. Am J Physiol Renal Physiol 2009;296:F614–F621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maezawa Y, Yokote K, Sonezaki K, et al. Influence of C-peptide on early glomerular changes in diabetic mice. Diabetes Metab Res Rev 2006;22:313–322 [DOI] [PubMed] [Google Scholar]
- 60.Sima AA, Zhang W, Sugimoto K, et al. C-peptide prevents and improves chronic type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia 2001;44:889–897 [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Kamiya H, Ekberg K, Wahren J, Sima AA. C-peptide improves neuropathy in type 1 diabetic BB/Wor-rats. Diabetes Metab Res Rev 2007;23:63–70 [DOI] [PubMed] [Google Scholar]
- 62.Cherian PV, Kamijo M, Angelides KJ, Sima AA. Nodal Na(+)-channel displacement is associated with nerve-conduction slowing in the chronically diabetic BB/W rat: prevention by aldose reductase inhibition. J Diabetes Complications 1996;10:192–200 [DOI] [PubMed] [Google Scholar]
- 63.Brismar T, Sima AA. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: potential clamp analysis. Acta Physiol Scand 1981;113:499–506 [DOI] [PubMed] [Google Scholar]
- 64.Stevens MJ, Dananberg J, Feldman EL, et al. The linked roles of nitric oxide, aldose reductase and, (Na+,K+)-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J Clin Invest 1994;94:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevens MJ, Zhang W, Li F, Sima AA. C-peptide corrects endoneurial blood flow but not oxidative stress in type 1 BB/Wor rats. Am J Physiol Endocrinol Metab 2004;287:E497–E505 [DOI] [PubMed] [Google Scholar]
- 66.Li ZG, Zhang W, Sima AA. C-peptide enhances insulin-mediated cell growth and protection against high glucose-induced apoptosis in SH-SY5Y cells. Diabetes Metab Res Rev 2003;19:375–385 [DOI] [PubMed] [Google Scholar]
- 67.Kamiya H, Zhang W, Sima AA. Dynamic changes of neuroskeletal proteins in DRGs underlie impaired axonal maturation and progressive axonal degeneration in type 1 diabetes. Exp Diabetes Res 2009;2009:793281 [DOI] [PMC free article] [PubMed]
- 68.Sima AA, Nathaniel V, Bril V, McEwen TA, Greene DA. Histopathological heterogeneity of neuropathy in insulin-dependent and non-insulin-dependent diabetes, and demonstration of axo-glial dysjunction in human diabetic neuropathy. J Clin Invest 1988;81:349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sima AA, Zhang W, Li ZG, Murakawa Y, Pierson CR. Molecular alterations underlie nodal and paranodal degeneration in type 1 diabetic neuropathy and are prevented by C-peptide. Diabetes 2004;53:1556–1563 [DOI] [PubMed] [Google Scholar]
- 70.Ekberg K, Brismar T, Johansson BL, Jonsson B, Lindström P, Wahren J. Amelioration of sensory nerve dysfunction by C-peptide in patients with type 1 diabetes. Diabetes 2003;52:536–541 [DOI] [PubMed] [Google Scholar]
- 71.Ekberg K, Brismar T, Johansson BL, et al. C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care 2007;30:71–76 [DOI] [PubMed] [Google Scholar]
- 72.Wahren J, Ekström U, Ekberg K. C-peptide improves erectile function in type 1 diabetes. Diabetes 2011;60(Suppl. 1):A285 [Google Scholar]
- 73.Northam EA, Rankins D, Lin A, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care 2009;32:445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sima AA, Li ZG. The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes 2005;54:1497–1505 [DOI] [PubMed] [Google Scholar]
- 75.Callaway J, Mårtensson A, Mazzoni M, et al. Development of a long-acting C-peptide. Diabetes 2011;60(Suppl. 1):A288 [Google Scholar]