Abstract
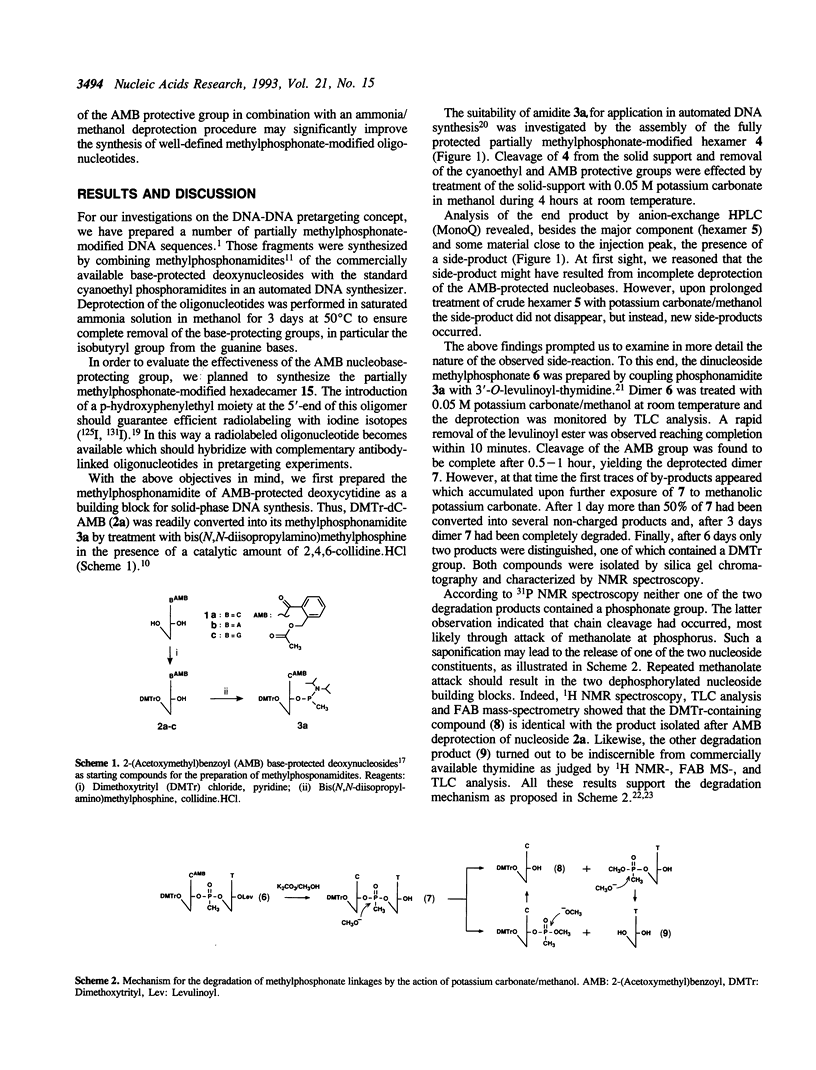
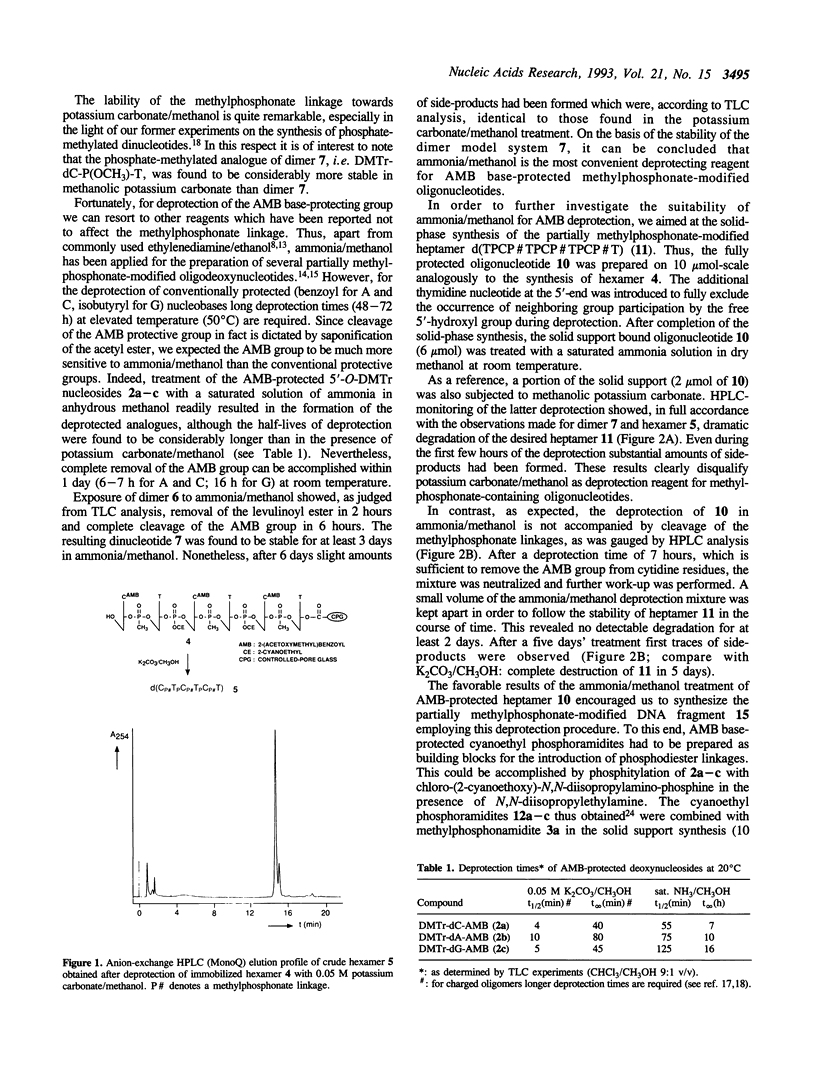
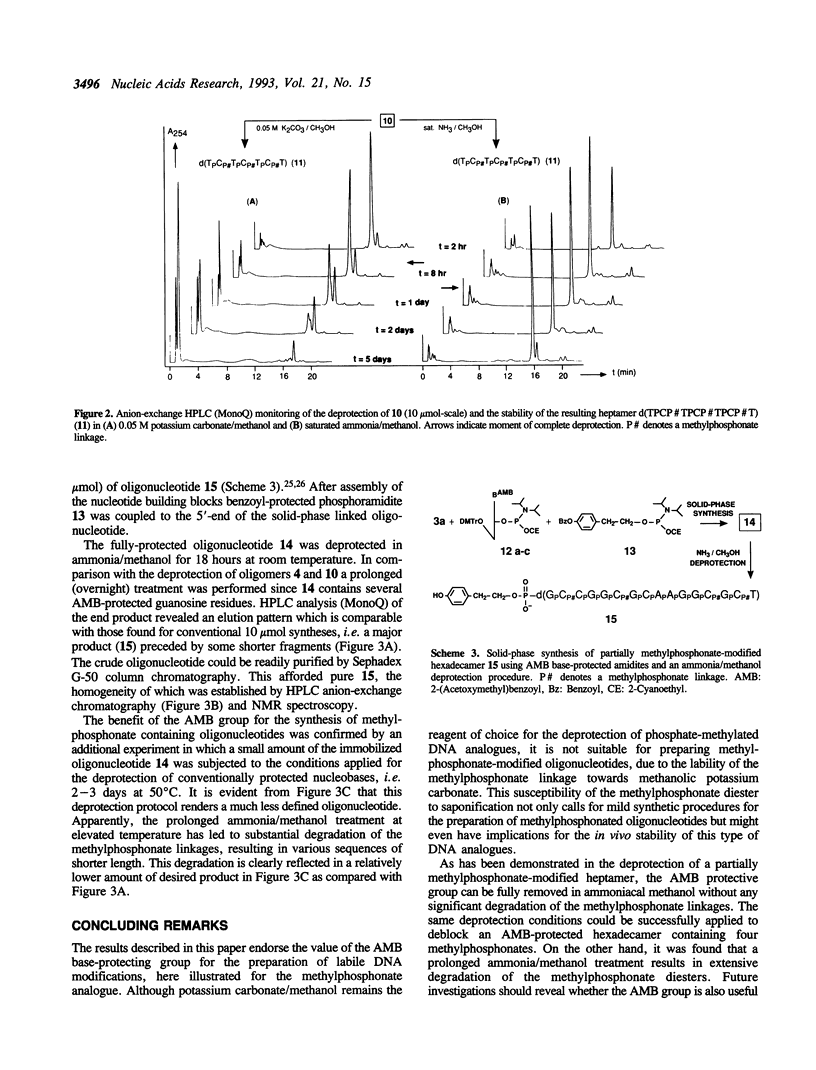
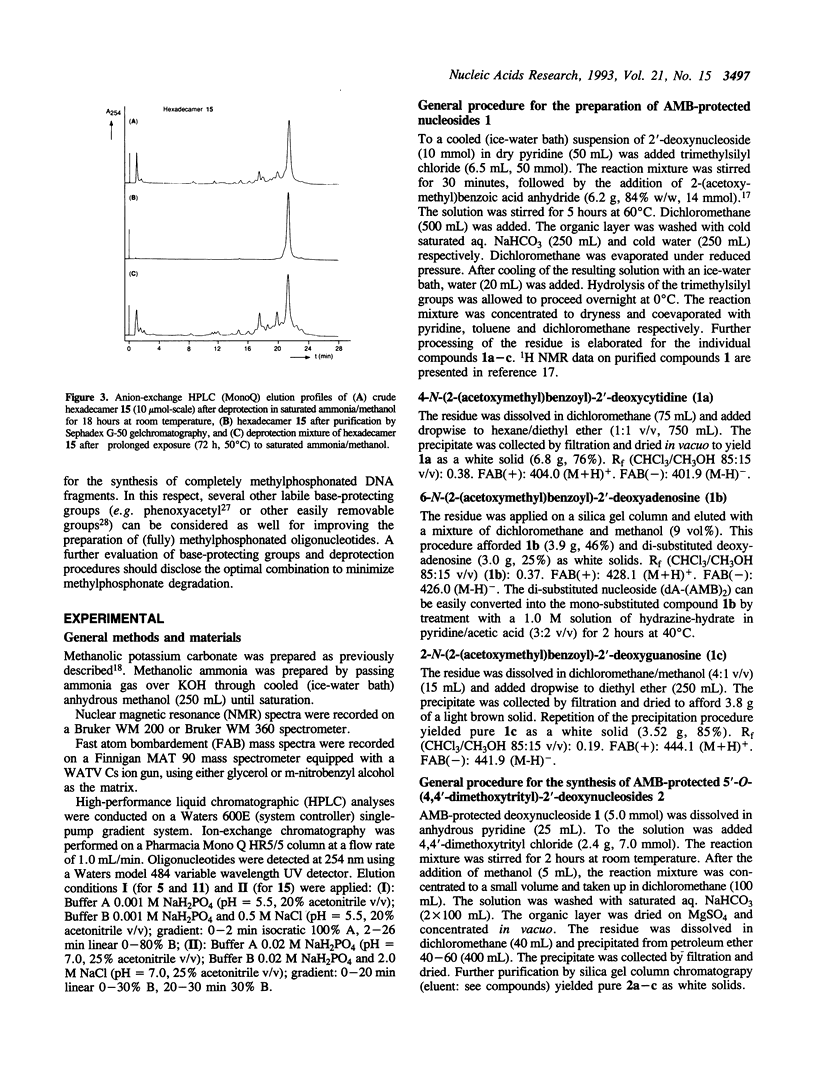
Partially methylphosphonate-modified oligodeoxynucleotides were synthesized on solid-phase by employing the easily removable 2-(acetoxymethyl)benzoyl (AMB) group as base-protecting group. Although a rapid AMB deprotection can be accomplished in methanolic potassium carbonate, the lability of the methylphosphonate linkage towards potassium carbonate/methanol excludes the use of this deprotection reagent. Thus, saturated ammonia solution in methanol was investigated as an alternative reagent for AMB removal. It is demonstrated that the combination of the AMB protective group and ammonia/methanol as deprotection reagent significantly improves the synthesis of methylphosphonate-modified DNA fragments. A mild overnight treatment at room temperature is sufficient for complete removal of the AMB group, whereas deprotection of conventionally protected oligonucleotides requires much longer exposure to basic conditions at elevated temperatures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hassner A., Strand G., Rubenstein M., Patchornik A. Letter: Levulinic esters. An alcohol protecting group applicable to some nucleosides. J Am Chem Soc. 1975 Mar 19;97(6):1614–1615. doi: 10.1021/ja00839a077. [DOI] [PubMed] [Google Scholar]
- Hogrefe R. I., Vaghefi M. M., Reynolds M. A., Young K. M., Arnold L. J., Jr Deprotection of methylphosphonate oligonucleotides using a novel one-pot procedure. Nucleic Acids Res. 1993 May 11;21(9):2031–2038. doi: 10.1093/nar/21.9.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers W. H., Bos E. S., Kaspersen F. M., Veeneman G. H., van Boeckel C. A. Specific recognition of antibody-oligonucleotide conjugates by radiolabeled antisense nucleotides: a novel approach for two-step radioimmunotherapy of cancer. Bioconjug Chem. 1993 Jan-Feb;4(1):94–102. doi: 10.1021/bc00019a013. [DOI] [PubMed] [Google Scholar]
- Kuijpers W. H., Huskens J., Koole L. H., van Boeckel C. A. Synthesis of well-defined phosphate-methylated DNA fragments: the application of potassium carbonate in methanol as deprotecting reagent. Nucleic Acids Res. 1990 Sep 11;18(17):5197–5205. doi: 10.1093/nar/18.17.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. E., de Vroom E., Dreef C. E., Tromp M., van der Marel G. A., van Boom J. H. Synthesis of nucleic acid methylphosphonates via the 1-hydroxybenzotriazole phosphotriester approach. Nucleic Acids Res. 1986 Mar 11;14(5):2171–2185. doi: 10.1093/nar/14.5.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Preparation of oligodeoxyribonucleoside methylphosphonates on a polystyrene support. Nucleic Acids Res. 1983 Sep 24;11(18):6225–6242. doi: 10.1093/nar/11.18.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Reddy M. P., Murakami A., Blake K. R., Lin S. B., Agris C. H. Solid-phase syntheses of oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Sep 9;25(18):5092–5097. doi: 10.1021/bi00366a017. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Ts'o P. O. A new approach to chemotherapy based on molecular biology and nucleic acid chemistry: Matagen (masking tape for gene expression). Anticancer Drug Des. 1987 Oct;2(2):117–128. [PubMed] [Google Scholar]
- Miller P. S., Yano J., Yano E., Carroll C., Jayaraman K., Ts'o P. O. Nonionic nucleic acid analogues. Synthesis and characterization of dideoxyribonucleoside methylphosphonates. Biochemistry. 1979 Nov 13;18(23):5134–5143. doi: 10.1021/bi00590a017. [DOI] [PubMed] [Google Scholar]
- Quartin R. S., Brakel C. L., Wetmur J. G. Number and distribution of methylphosphonate linkages in oligodeoxynucleotides affect exo- and endonuclease sensitivity and ability to form RNase H substrates. Nucleic Acids Res. 1989 Sep 25;17(18):7253–7262. doi: 10.1093/nar/17.18.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin P. S., Agrawal S., Civeira M. P., Goodchild J., Ikeuchi T., Zamecnik P. C. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7448–7451. doi: 10.1073/pnas.85.20.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur D. S. Radiohalogenation of proteins: an overview of radionuclides, labeling methods, and reagents for conjugate labeling. Bioconjug Chem. 1992 Nov-Dec;3(6):433–470. doi: 10.1021/bc00018a001. [DOI] [PubMed] [Google Scholar]


