Abstract
Proline residues in collagens are extensively hydroxylated post-translationally. A rare form of this modification, 3S, 2S-L-hydroxyproline (3Hyp), remains without a clear function. Disruption of the enzyme complex responsible for prolyl 3-hydroxylation results in severe forms of recessive osteogenesis imperfecta (OI). These OI types exhibit a loss or reduction of 3-hydroxylation at two proline residues, α1(I) Pro986 and α2(I) Pro707. Whether the resulting brittle bone phenotype is caused by the lack of the 3-hydroxyl addition or by another function of the enzyme complex is unknown. We have speculated that the most efficient mechanism to explain the chemistry of collagen intermolecular cross-linking is for pairs of collagen molecules in register to be the subunit that assembles into fibrils. In this concept the exposed hydroxyls from 3Hyp are positioned within mutually interactive binding motifs on adjacent collagen molecules that contribute through hydrogen bonding to the process of fibril supramolecular assembly. Here we report observations on the physical binding properties of 3Hyp in collagen chains from experiments designed to explore the potential for interaction using synthetic collagen-like peptides containing 3Hyp. Evidence of self-association was observed between a synthetic peptide containing 3Hyp and the CB6 domain of the α1(I) chain, which contains the single fully 3-hydroxylated proline. Using collagen from a case of severe recessive OI with a CRTAP defect, in which Pro986 was minimally 3-hydroxylated, such binding was not observed. Further study on the role of 3Hyp in supramolecular assembly is warranted for understanding the evolution of tissue-specific variations in collagen fibril organization.
Keywords: collagen, 3-hydroxyproline, bone, supramolecular assembly, osteogenesis imperfecta
Introduction
Mineralization and the unique material properties of bone require a highly ordered collagenous matrix (1). Collagens undergo many post-translational modifications prior to secretion into the extracellular matrix, most of which are known to function in conferring strength and stability. About half the proline residues in mammalian fibrillar collagens are hydroxylated to 4Hyp1, residues that stabilize the triple helix through water-bridged hydrogen bonding (2). The much less frequent 3Hyp is still without a clear function. The single 3Hyp residue per chain of type I collagen is formed by an enzyme complex of three proteins encoded by LEPRE1 (P3H1), CRTAP (CRTAP) and PPIB (PPIB also known as cyclophilin B). Mutations that disrupt expression of any protein in this complex have been shown to cause recessive OI (variably assigned as clinical types II and III, or genetic types VII, VIII or IX) characterized by bone fragility (3–8). Analysis of collagen from tissue and cultured fibroblasts from these OI cases revealed a lack or decrease in 3-hydroxylation at proline residues α1(I) Pro986 and α2(I) Pro707.
The pathobiology became more complicated with the discovery that further recessive OI sub-variants are caused by mutations in genes that encode other endoplasmic reticulum chaperones. Mutations in SERPINH1 (HSP47) and FKBP10 (FKBP65) cause OI types X and XI, neither of which affect 3-hydroxylation at Pro986 (9–11). Adding to the complexity was the recent discovery that mutations in SERPINF1, a gene encoding pigment epithelium-derived factor, cause recessive OI type VI (12–13). Also, COL1 C-propeptide cleavage site mutations (14) and a BMP-1 (C-propeptidase) homozygous mutation (15) have been shown to cause unusual forms of OI. Thus, whether the brittle bone phenotype of those recessive OI variants with reduced prolyl 3-hydroxylation is to any extent a direct result of the missing modification itself or another consequence of the disrupted enzyme complex has yet to be determined. The discovery of several additional 3Hyp sites in fibrillar collagens, which exhibit tissue specificity in their degree of 3-hydroxylation (16, 17), further complicates understanding whether there is a common underlying pathogenic mechanism. Moreover, individual enzymes in the P3H family (P3H1, P3H2, and P3H3) have evolved differing collagen substrate site and tissue specificities in their activities and probably therefore functions (18).
Evidence for selective evolutionary pressure on 3Hyp site occupancy implies a fundamental functional role for prolyl 3-hydroxylation rather than being simply a coincidental mark of the hydroxylase complex having acted as a chaperone during assembly, folding and transport of collagen molecules in the endoplasmic reticulum (19). We proposed that 3Hyp formation impacted collagen fibril assembly at the threshold of vertebrate evolution in a way that benefited the properties of skeletal tissues and their development in particular. In addition to a role in collagen assembly, the 3Hyp motifs may provide sites of interaction for fibril-binding non-collagenous proteins, for example those involved in mineralization (20). The function of 3Hyp is clearly important to understand given its potential role in recessive OI and most recently in heritable high myopia (21).
We speculated that the known sites of mature trivalent cross-linking in collagen would form most efficiently if pairs of collagen molecules in register were the subunits that assembled into fibrils (22, 16). The identification of several D-periodically-spaced collagen 3Hyp sites further prompted us to investigate the potential role for aligned 3Hyp domains to bridge between adjacent molecules by hydrogen bonding. Specifically, the externally directed 3-hydroxyls of aligned like sequences have potential to form hydrogen bonds to their neighboring peptide backbones and so establish short range lateral order in the fibrillar supramolecular arrangement of the collagen triple helices. Using synthetic collagen-based peptides synthesized to match the 3Hyp locus at Pro986, we designed experiments to test such a possibility.
Materials and Methods
Peptide synthesis and protein preparation
Peptides were synthesized to mimic the Pro986 domain of type I collagen with either Pro or 3Hyp at position 986 (New England Peptides). The following sequences were chosen as probes: the 3Hyp probe (α1(I) residues 974-994; KDGLNGLP*GPIGP#P*GPRGRTG) and the Pro probe (α1(I) residues 974-994; KDGLNGLP*GPIGPP*GPRGRTG); where P* is 4Hyp and P# is 3Hyp. The ε-amine of the N-terminal Lys was biotinylated to enable detection. Control peptides were designed with the amino-terminal group biotinylated (Genescript): the A2 probe (α1(I) residues 934-948; GFSGLQGPP*GPP*GSP*) and the CB8 probe (α1(I) residues 295-309; GEP*GPTGLP*GPP*GER). All synthesized peptides were random coils in the incubation solution used to test binding (see below) as confirmed on a Jasco 720 circular dichroism spectrophotometer. The purity and concentration of each synthetic peptide was determined by RP-HPLC (C8 RP-300, 25 cm × 4.6 mm, Brownlee columns, Applied Biosystems), using the peak area at 220 nm as a measure of quantity. The lyophilized synthetic peptides were dissolved in 50% methanol (5 mg/mL) and stored at −20°C.
Type I collagen was prepared from adult human bone (purchased from the Northwest Tissue Center, Seattle, WA). Human fetal OI bone from a case of severe recessive OI with a CRTAP mutation was obtained from the International Skeletal Dysplasia Registry at Cedars-Sinai Medical Center, Los Angeles. Human fetal control bone tissue was obtained from an NIH-sponsored institutional resource (Birth Defects Research Laboratory, University of Washington). Bone was powdered and defatted in (3:1, v/v) chloroform: methanol and demineralized in 0.5 M EDTA, 0.05 M Tris-HCl, pH 7.5, at 4°C. Demineralized bone collagen was digested with CNBr in 70% formic acid at room temperature for 24 hr and freeze-dried. Human bone matrix samples were treated with NaBH4 (0.02 mg/mL) in 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.0, to reduce collagen divalent ketoamine cross-links as described previously (23). After 60 min at room temperature the reaction was acidified and dialyzed against 0.1 M (v/v) acetic acid. Whole collagen chains were solubilized from each tissue matrix (bone, skin and tendon) by heat denaturation for 2 min at 100°C in SDS-PAGE sample buffer. Chicken tendon and skin samples were also defatted and treated with NaBH4 prior to collagen extraction.
Far Western analysis
Pepsin-solubilized collagen and CNBr-digested collagen peptides (CNBr-peptides) were resolved on 6% and 12% SDS-PAGE. Proteins were transblotted to 0.2 μm nitrocellulose and blocked for 2 hr with 1% BSA in 0.05 M Tris-HCl, 0.15 M NaCl, pH 7.5, 0.05% Tween. Blots were probed with a biotinylated synthetic probe (0.2 μg/mL, unless specified otherwise) in 0.1% BSA in 0.05 M Tris-HCl, 0.15 M NaCl, pH 7.5, 0.05% Tween for 16 hours at 4°C. Sites of interaction between Type I collagen and the probes were detected with extravidin-conjugated horseradish peroxidase (HRP) (0.15 μg/mL) in 0.1% BSA in 0.05 M Tris-HCl, 0.15 M NaCl, pH 7.5, 0.05% Tween for 60 minutes at room temperature. Between steps, blots were washed three times for 20 min in 0.05 M Tris-HCl, 0.15 M NaCl, pH 7.5, 0.05% Tween. Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposed on autoradiography film. In a separate experiment to study the role of hydrogen bonds in the probe interaction, far Western blots were probed with the biotinylated synthetic peptides in the presence of 2 M glucose (all other steps were identical).
Size exclusion chromatography
CNBr-peptides were resolved by size exclusion chromatography on an agarose A1.5m column (170 × 1.5 cm, 200–400 mesh, Bio-Rad), eluted by 2 M guanidine HCl, 0.05 M Tris-HCl, pH 7.5). Lyophilized samples were dissolved in 4 M guanidine HCl, 0.05 M Tris-HCl, pH 7.5 and run at a flow rate of 6 mL/hr. Peptide bond absorbance was monitored at 230 nm. Selected fractions were pooled, dialyzed against 0.1 M (v/v) acetic acid and freeze dried.
Mass spectrometry
Protein bands from SDS polyacrylamide gels were excised and subjected to in-gel trypsin digestion and resulting peptides were analyzed by electrospray mass spectrometric analysis as previously described (16). Briefly, peptides were subjected to analysis using an LCQ Deca XP ion trap mass spectrometer equipped with in-line liquid chromatography (ThermoFinnigan) and using a C8 reverse-phase capillary column (300 μm × 150 mm; Grace Vydac 208MS5.315). An electrospray ionization source introduced the liquid chromatography sample stream into the mass spectrometer. Hydroxyl differences were identified by scrolling to average the full scan over several minutes, enabling combination of all the post-translational variations of a given peptide.
Characterization of collagen cross-links
The pyridinoline cross-link content of collagen preparations was determined by HPLC after acid hydrolysis in 6 N HCl for 24 hrs at 108°C. Dried samples were dissolved in 1% (v/v) n-heptafluorobutyric acid and their reverse-phase HPLC HP and LP contents quantified by fluorescence monitoring as previously described (24).
Results
Far Western analyses revealed that the biotinylated 3Hyp-containing probe recognized and bound to intact α-chains of type I collagen from multiple tissue sources, such as bone, skin and tendon (Figure 1). Binding to α1(I) is consistent with the hypothesis being tested since the chain contains a fully hydroxylated 3Hyp986 site in a local sequence that matches that of the synthetic peptide probe. We anticipate that amide bonds of the peptide backbone are the hydrogen-bond binding partners for the 3-hydroxyl side chains. The observed binding to α2(I) presumably occurs between the local sequence containing 3Hyp707 on the α2-chain and that in the 3Hyp probe. This is supported by our findings that a decreased but still detectable 3Hyp probe-binding signal was observed on far Western analysis of chicken skin α1(III) CB6B (3Hyp primary site sequence: KDGRGGYP*GPIGP#P*GPRGNRG) and bovine articular cartilage α1(II) CB9,7 (3Hyp primary site sequence: KDGANGIP*GPIGP#P*GPRGRSG), despite some differences in these sequences from the immediate sequence of the human α1(I) 3Hyp986 site (3Hyp primary site sequence: KDGLNGLP*GPIGP#P*GPRGRTG) (data not shown). The extravidin-conjugated HRP alone showed no binding (Figure 1, HRP).
Figure 1. The 3Hyp probe recognizes and binds type I collagen from multiple tissue sources.
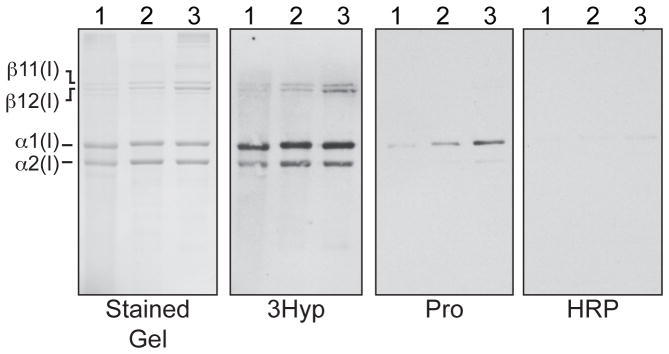
Intact type I collagen chains were separated on 6% SDS-PAGE and either stained with Coomassie blue (stained gel) or transblotted to nitrocellulose for far Western analysis. Blots were probed using 3Hyp probe (3Hyp), Pro probe (Pro), or extravidin-HRP without prior incubation with a primary probe (HRP). Lanes 1, human bone collagen; lanes 2, chicken skin collagen; lanes 3, chicken tendon collagen.
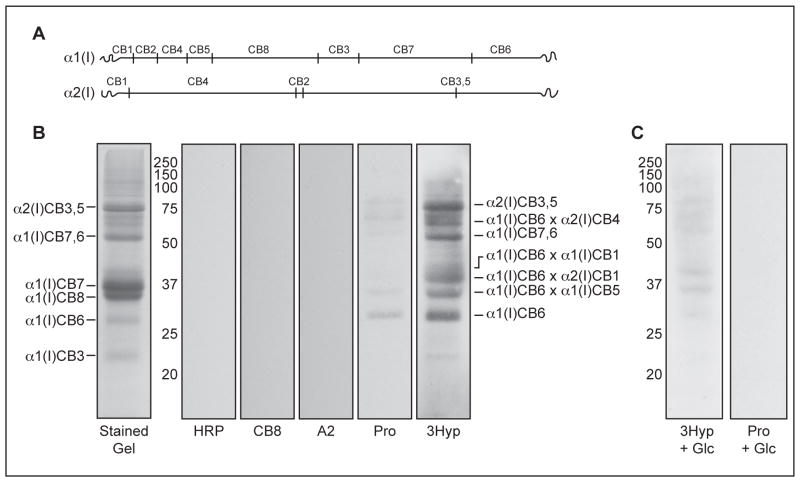
In order to locate the site of interaction, human bone matrix was digested with CNBr, which specifically cleaves peptide bonds at the C-terminus of methionine residues, giving a characteristic collagen type I peptide map when separated by SDS-PAGE (Figure 2A and B). The far Western analysis of CNBr-digested bone collagen at first impression appeared to show that the 3Hyp probe had bound to multiple sites on the collagen α-chains (Figure 2B, 3Hyp). However, the reactive bands did not all coincide with the linear chain fragments detected in the stained gel. The specific sites of interaction were therefore sought by peptide mass spectrometry following in-gel trypsin digestion of the reactive bands excised from a representative SDS-PAGE. Interestingly, when analyzed by mass spectrometry all but one band prominently revealed trypsin digestion products from CB6 (Figure 3A and B). This may be explained in part by incomplete CNBr cleavage, resulting in some higher molecular weight partial cleavage products, but the dominant reason is that much of the CB6 is present in cross-linked forms. CB6 contains two separate sites of cross-linking, a Lys in the C-telopeptide (that can cross-link to helix Lys87 of α1(I) CB5 or α2(I) CB4) and helix Lys930 (that can cross-link to an N-telopeptide Lys in α1(I) or α2(I)). The predicted cross-linked forms of CB6 are indicated in Figure 2B. It is notable that high mass poly-α1CB6 cross-linked multimers were reported to be prominent in digests of bone and tendon collagens (25, 26). The reactive band lacking any form of CB6 was determined to be CB3,5 from the α2(I)-chain, which contains the single 3Hyp site of α2 at Pro707.
Figure 2. Interaction with CNBr-digested collagen peptides is specific for the 3Hyp probe and is enhanced by the 3-hydroxyl modification.
Peptide map of CNBr-digested type I collagen chains (A). Replicate sample lanes of CNBr-digested human bone matrix were separated on 12% SDS-PAGE (B) and stained with Coomassie blue (stained gel), or transblotted to nitrocellulose and probed individually using the following synthetic peptides: extravidin-HRP without prior incubation with a primary probe (HRP), CB8 probe (CB8), A2 probe (A2), Pro probe (Pro), or 3Hyp probe (3Hyp). The predominant CNBr-digested collagen peptides are indicated on the far left. The predicted CB6-containing cross-linked peptides are indicated on the far right (cross-links are depicted as ×), see text for details. The N-terminal telopeptides of α1(I) and α2(I) are indicated as α1(I)CB1 and α2(I)CB1, respectively. Replicate sample lanes of CNBr-digested human bone matrix were analyzed with Pro probe (Pro) or 3Hyp probe (3Hyp) in the presence of 2 M glucose (Glc) to inhibit hydrogen bond formation (C). The protein standard sizes are shown in kDa.
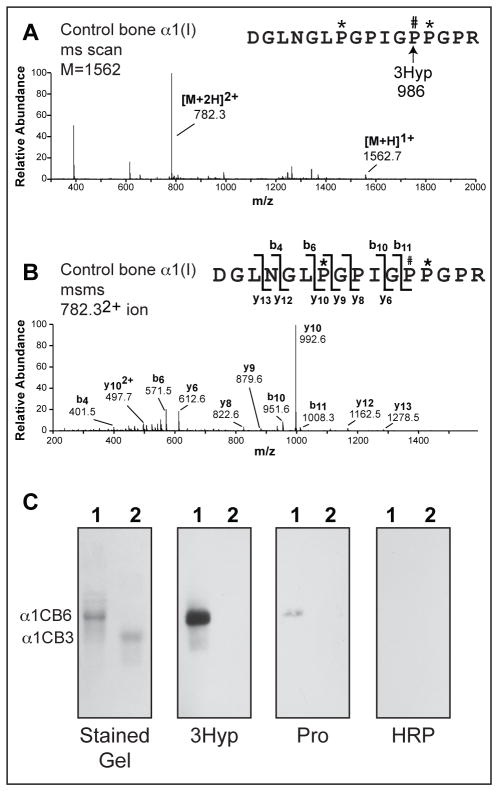
Figure 3. Binding epitope for 3Hyp probe is located within the CB6 region of collagen α1(I).
Full scan spectra from LC-MS profile (A) and MS/MS fragmentation profile (B) of in-gel trypsin digest of a representative reactive band from far Western analysis (Figure 2B) reveals the presence of the 3Hyp-containing CB6 peptide at multiple sites on the 12% SDS-PAGE. Far Western blots of isolated CB6 (lanes 1) and CB3 (lanes 2) from α1(I) collagen of human bone were probed using 3Hyp probe (3Hyp), Pro probe (Pro), or extravidin-HRP without prior incubation with a primary probe (HRP) (C).
The 3Hyp and Pro peptide probes had indistinguishable secondary structure (random coil) by circular dichroism spectroscopy (data not shown), consistent with the observed differences in binding being due exclusively to the presence or absence of a 3-hydroxyl in the synthetic peptides. The Pro probe exhibited some binding to the same sites as the 3Hyp probe, albeit with lower affinity (Figure 2B, Pro). We postulate that the binding is due to hydrogen bond formation by 3Hyp at Pro986. The role of hydrogen bonds in the oligomeric self-assembly of collagen-like peptides has been demonstrated by employing sugar molecules to disrupt the hydrogen bonding necessary for fibril formation (27, 28). When solid-phase binding assays were performed in the presence of 2 M glucose, peptide binding was significantly reduced (Figure 2C). Neither control peptide, the A2 probe and the CB8 probe, synthesized to match distinct regions of α1(I) that lack 3Hyp, nor the extravidin-conjugated HRP alone showed any interaction with type I collagen (Figure 2B). Thus, the biotinylated collagen-like peptides are not simply interacting with their matching sequences in the α1(I)-chain, even though such sequences do become aligned intramolecularly when α1(I)-chains fold into a natural triple helix.
The α1(I) CNBr-peptides CB6 and CB3 were isolated by molecular sieve chromatography and analyzed by far Western to verify that the 3Hyp probe was specifically binding to CB6 (Figure 3C). The 3Hyp probe bound only to the CB6 peptide, confirming the requirement for a specific sequence within this region of the collagen α-chain. The Pro probe bound significantly less than the 3Hyp probe and the extravidin-HRP secondary did not interact with either CNBr-peptide. From these observations we conclude that CB6 (i.e., the C-terminal domain of α1(I) from residue 822) contains the epitope responsible for synthetic peptide binding and that the 3-hydroxylation of Pro986 plays a significant role in the interaction.
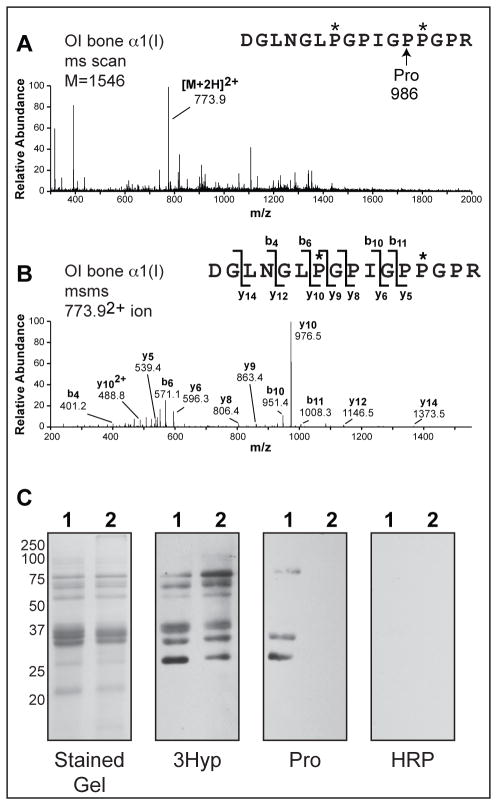
To further assess the significance of 3Hyp in type I collagen, we repeated the synthetic probe binding assays using fetal bone collagen from a human case of severe recessive OI caused by a CRTAP mutation, in which α1(I) was minimally 3-hydroxylated at Pro986. Mass spectrometry showed that the OI bone collagen had 3-hydroxyl occupancies of 10% at Pro986 in α1(I) (Figure 4A and B) and 18% at Pro707 in α2(I) (data not shown). No qualitative difference was detected between the band patterns given by control and OI bone using the 3Hyp probe on far Western analysis (Figure 4C, 3Hyp). However, this was not unexpected as the Pro probe was shown to bind to control bone collagen, albeit with a lower affinity (Figure 2B, Pro and Figure 3C, Pro); thus suggesting that the minimum requirement for binding is a single 3Hyp in one binding partner. The difference in Pro probe signal between control and OI bone collagen on far Western analysis was made clear by increasing the amount of the Pro probe 2.5 × in the binding assay (Figure 4C, Pro). At this concentration difference, the interaction profiles with control bone for the 3Hyp and Pro probes were of similar intensity (Figure 4C, 3Hyp and Pro). Differences in the specific signal pattern and intensity between the two probes (3Hyp and Pro) may reflect the inherent limitations of an artificial solid-phase system. No interaction was observed between the Pro probe and OI bone even at elevated concentrations (Figure 4C, Pro). Mass spectrometric analysis of the fetal OI bone collagen revealed that the CB6 peptide contained no glycosylated lysines, only hydroxylated lysines as in control bone collagen. This is consistent with the non-retarded mobility of OI bone CB-peptides on SDS-PAGE (Fig 4). The possibility that overmodification had extended to this end of the molecule and was responsible for preventing probe binding was therefore ruled out. The requirement for at least one 3Hyp residue (either in the probe or collagen chain or chain fragment) for the binding to be observed underscores the potential significance of this modification at α1(I) Pro986 for inducing collagen short-range molecular self-association and hence order in the fibril.
Figure 4. Self-association is not observed from OI bone in which Pro986 is not 3-hydroxylated.
Full scan spectra from LC-MS profile (A) and MS/MS fragmentation profile (B) of in-gel trypsin digests confirm that Pro986 is not hydroxylated in the OI bone. Far Western blots of CNBr-digested fetal control (lanes 1) and OI (lanes 2) bone were probed using 0.2 μg/mL 3Hyp probe (3Hyp ), 0.5 μg/mL Pro probe (Pro), or extravidin-HRP without incubation with a primary probe (HRP) (C). These data are consistent with our hypothesis that intermolecular recognition resulting in self-association is specific to collagen peptides containing 3Hyp at position 986.
Potential differences in covalent cross-linking between adult and fetal control bone and fetal OI bone were assessed as an index of altered fibrillar assembly (Table 1). Lower levels of LP, evidenced by a consistently higher HP:LP ratio, were found in the fetal OI bone compared to fetal control bone (7.0:1 vs. 2.4:1). The altered cross-linking profiles indicate post-translational over-hydroxylation of helical domain cross-linking lysines in the OI versus control tissue. The total concentration of mature pyridinoline cross-links is lower in fetal bone (control and OI) compared to adult bone. It has been shown in one study that the content of pyridinoline cross-links is lower and the content of divalent cross-links is higher in human fetal versus adult bone (23). CRTAP mutations have previously been associated with delayed collagen triple helix folding, which in turn can result in higher modification by lysyl hydroxylase and P4H at least in cultured cells (4). As mentioned above, mass spectrometric analysis of the fetal OI bone collagen revealed complete hydroxylation but no glycosylation of both cross-linking lysines in CB6 (data not shown).
Table 1.
Concentration of 3-hydroxypyridinium cross-linking residues in fetal and adult bone collagen samples (control and severe recessive OI) expressed as moles/mole of collagen. (HP, hydroxylysylpyridinoline; LP, lysylpyridinoline).
| HP+LP (moles/mole) | HP:LP (ratio) | |
|---|---|---|
| Adult bone (control)a | 0.29 | 3.6 : 1 |
| Fetal bone (control, n = 3) | 0.17 | 2.4 : 1 |
| Fetal bone (OI) | 0.16 | 7.0 : 1 |
adult male, aged 25 years (42)
Discussion
The presumed evolutionary advantage conferred by 3Hyp986 site in the α1(I) collagen chain is still not clear. However, this study provides strong preliminary evidence for a functional role for 3Hyp during the molecular assembly of collagen triple helices into fibrils. The findings show an interaction localized to the CB6 domain of α1(I), the strength of which is dependent on 3-hydroxylation of Pro986 and of the equivalent Pro in the peptide probe. Indeed, in far Westerns using the Pro probe against bone collagen from a case of recessive OI, in which Pro986 is only partially 3-hydroxylated, significantly reduced interchain binding was observed. As a result, we suggest that intermolecular recognition, resulting in self-association, is specific to collagen sequences containing 3Hyp at α1(I) Pro986. To our knowledge, this is the first study providing evidence for a mechanism by which the brittle bone phenotype observed in forms of OI with reduced prolyl 3-hydroxylation could be contributed to by the decrease in modification and not just a consequence of the defective enzyme complex failing to act as a chaperone in the endoplasmic reticulum and, for example, driving cellular stress through an unfolded protein response.
A limitation of the present findings is the reliance on an artificial system that uses denatured collagen chain fragments to seek binding to non-triple-helical peptides. Though much more complicated to manage and control, experiments designed to test triple-helical interactions would be more convincing of physiological significance in vivo. Nevertheless, studies employing synthetic collagen-like peptides have revealed the importance of proline modifications and conformation in determining collagen structure and stability. Indeed, the stabilizing effect of 4Hyp on the triple helix was originally revealed by experiments with collagen-like peptides (29). Subtle variations in local proline hydroxylation chemistry have considerable effects on collagen peptide stability. For example, 4Hyp can both stabilize and destabilize the triple helix depending on its location in the Gly-Xaa-Yaa motif (30, 31), whereas the diastereoisomer 4S-hydroxy-2S-proline has a destabilizing effect regardless of position (30). Many stabilizing/destabilizing effects brought about by prolyl hydroxyl moieties are thought to result from either favorable electrostatic dipole-dipole interactions or steric clashes within the collagen triple helix (32).
We propose that the binding interactions observed in this study are representative of an interaction between in-register dimers of two collagen triple-helical molecules. Such an interaction is suggested from the crystal structure of a 3Hyp-containing collagen-like synthetic polypeptide (33), in which the 3-hydroxyl extended outward from the triple helix axis, thereby allowing potential intermolecular interactions to occur. These interactions could be driven by hydrogen bonding between the 3-hydroxyl and a peptide backbone carbonyl of an adjacent triple helix perhaps via one or more water molecules analogous to the mechanism of triple helix stabilization by 4Hyp (34, 35). Collagen supramolecular assembly is thought to be entropy driven, i.e., requiring energy to disrupt pre-existing triple helix to water bonds in order to form the new interactions necessary for higher order structures (36–38). A telling artifact from collagen-like peptide crystal structures is the association of triple-helical molecules through hydrogen bonding interactions of exposed 4Hyp side chains (34, 35). To what extent these hydrogen bonds influence collagen fibril formation in vivo is unknown. Notably, in the crystal structure of the 3Hyp-containing peptide these direct interhelical hydrogen bonds between 4Hyp side chains are absent (33). Indeed, the only inter-helical hydrogen bonds identified in their peptide model are between the hydroxyls of two 3Hyp side chains (33). This is in agreement with our model concept in which 3Hyp aids in the short range alignment and lateral association of collagen triple-helical dimers through hydrogen bonds. Such interactions have the potential to fine-tune short-range relationships both axially, laterally and, in the case of a heterotrimer such as type I collagen, azimuthally, so inducing local order in the packing structure and in the placement of neighboring covalent cross-links.
In this concept of fibril molecular packing, the formation of parallel in-register molecular dimers staggered axially by D-periods in a square lattice arrangement would ensure the necessary alignment of collagen molecules to maximize the content and placement of known mature cross-linking bonds (16). Trivalent cross-links that are prominent in skeletal tissue collagens are best accommodated at sites between three collagen molecules, two in-register and a third shifted by a 4D period (22). Early studies on non-native collagen fibrils formed in vitro proposed that the subunits were antiparallel molecular dimers (39). Woodhead-Galloway later proposed altering the then-current model of native collagen molecular packing by replacing single molecules of collagen with in-register collagen dimers in a tetragonal lattice to account better for details in the observed diffraction patterns (40). This would also result in a regular array of larger holes in the gap region of fibrils, which could theoretically provide the added spacing required for bone fibril mineralization (40).
The observed differences in HP/LP ratios between control and OI bone may reflect cross-linking differences that affect the quality of bone mineralization, which is believed to depend on both the manner of molecular packing and the chemistry and placement of cross-links. In our model, the lack of 3-hydroxylation at Pro986 may affect the nearest neighbor relationships required for the optimum cross-linking arrangement and supramolecular assembly to enable mineral crystal deposition together with retained fibril strength. The brittle bone phenotype could be in part the direct result of disordered mineralization within an effectively weakened collagenous matrix, i.e., a matrix defect causing brittle bone. The general post-translational disturbance in the endoplasmic reticulum that leads to over-hydroxylation of lysines in OI bone collagen can account for the altered cross-linking profiles. For example, higher levels of chemically stable pyridinoline cross-links, hydroxylysine and glucosyl galactosyl hydroxylysine, have been reported in the P3H1 null mouse bone, tendon and skin collagens (41). Further study is warranted of the relationship between 3Hyp content and quality of collagen cross-linking and their relationship with the unique properties of bone and other skeletal tissues, normally and in OI and other disorders.
Acknowledgments
We thank Russell Fernandes for his critical review of the manuscript. We also thank James Wu and Geoff Traeger for their helpful comments and suggestions.
Funding information
This work was supported, in whole or in part, by National Institutes of Health grants AR37694 and AR37318 from NIAMS (D.R.E.) and HD22657 and HD070394 (D.H.C. and D.R.E.) from NICHD. This work was also supported by funds from the Ernest M. Burgess Endowed Chair research program of the University of Washington.
Footnotes
Abbreviations: 4Hyp, 4R, 2S-L-hydroxyproline; 3Hyp, 3S, 2S-L-hydroxyproline; P3H, prolyl 3-hydroxylase; CRTAP, cartilage associated protein; PPIB, peptidyl prolyl cis-trans isomerase B (cyclophilin B); OI, osteogenesis imperfecta; CNBr, cyanogen bromide; LP, lysyl pyridinoline; HP, hydroxylysyl pyridinoline.
References
- 1.Glimcher MJ, Krane SM. The organization and structure of bone, and the mechanism of calcification. In: Ramachandran GN, Gould BS, editors. Treatise on Collagen. II. Academic Press; New York: 1968. pp. 68–251. [Google Scholar]
- 2.Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple helix of collagen. Biochem Biophys Res Commun. 1973;52:115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- 3.Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bächinger HP, Pace JM, Schwarze U, Byers PH, Weis M, Fernandes RJ, Eyre DR, Yao Z, Boyce BF, Lee B. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127:291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Barnes AM, Chang W, Morello R, Cabral WA, Weis MA, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral WA, Chang W, Barnes AM, Weis MA, Scott MA, Leikin S, Makareeva E, Kuznetsova NV, Rosenbaum KN, Tifft CJ, Bulas DI, Kozma C, Smith PA, Eyre DR, Marini JC. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldridge D, Schwarze U, Morello R, Lennington J, Bertin TK, Pace JM, Pepin MG, Weis M, Eyre DR, Walsh J, Lambert D, Green A, Robinson H, Michelson M, Houge G, Lindman C, Martin J, Ward J, Lemyre E, Mitchell JJ, Krakow D, Rimoin DL, Cohn DH, Byers PH, Lee B. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008;29:1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk FS, Nesbitt IM, Zwikstra EH, Nikkels PG, Piersma SR, Fratantoni SA, Jimenez CR, Huizer M, Morsman AC, Cobben JM, van Roij MH, Elting MW, Verbeke JI, Wijnaendts LC, Shaw NJ, Högler W, McKeown C, Sistermans EA, Dalton A, Meijers-Heijboer H, Pals G. PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet. 2009;85:521–527. doi: 10.1016/j.ajhg.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes AM, Carter EM, Cabral WA, Weis M, Chang W, Makareeva E, Leikin S, Rotimi CN, Eyre DR, Raggio CL, Marini JC. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, Fehr M, Baumann U, Lindblad-Toh K, Leeb T. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet. 2009;5:e1000579. doi: 10.1371/journal.pgen.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alanay Y, Avaygan H, Camacho N, Utine G, Boduroglu K, Aktas D, Alikasifoglu M, Tuncbilek E, Orhan D, Bakar F, Zabel B, Superti-Furga A, Bruckner-Tuderman L, Curry CJ, Pyott S, Byers PH, Eyre DR, Baldridge D, Lee B, Merrill AE, Davis EC, Cohn DH, Akarsu N, Krakow D. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:551–559. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley BP, Malfait F, Bonafe L, Baldridge D, Homan E, Symoens S, Willaert A, Elcioglu N, Van Maldergem L, Verellen-Dumoulin C, Gillerot Y, Napierala D, Krakow D, Beighton P, Superti-Furga A, De Paepe A, Lee B. Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J Bone Miner Res. 2011;26:666–672. doi: 10.1002/jbmr.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, Bergmann C, Rohrbach M, Koerber F, Zimmermann K, de Vries P, Wirth B, Schoenau E, Wollnik B, Veltman JA, Hoischen A, Netzer C. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–371. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homan EP, Rauch F, Grafe I, Lietman C, Doll JA, Dawson B, Bertin T, Napierala D, Morello R, Gibbs R, White L, Miki R, Cohn DH, Crawford S, Travers R, Glorieux FH, Lee B. Mutations in SERPINF1 cause Osteogenesis imperfecta Type VI. J Bone Miner Res. 2011;26:2798–2803. doi: 10.1002/jbmr.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindahl K, Barnes AM, Fratzl-Zelman N, Whyte MP, Hefferan TE, Makareeva E, Brusel M, Yaszemski MJ, Rubin CJ, Kindmark A, Roschger P, Klaushofer K, McAlister WH, Mumm S, Leikin S, Kessler E, Boskey AL, Ljunggren O, Marini JC. COL1 C-propeptide cleavage site mutations cause high bone mass osteogenesis imperfecta. Hum Mutat. 2011;32:598–609. doi: 10.1002/humu.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Glez V, Valencia M, Caparrós-Martín JA, Aglan M, Temtamy S, Tenorio J, Pulido V, Lindert U, Rohrbach M, Eyre D, Giunta C, Lapunzina P, Ruiz-Perez VL. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2011;33:343–350. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weis MA, Hudson DM, Kim L, Scott M, Wu JJ, Eyre DR. Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J Biol Chem. 2010;285:2580–2590. doi: 10.1074/jbc.M109.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyre DR, Weis M, Hudson DM, Wu JJ, Kim L. A novel 3-hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen. J Biol Chem. 2011;286:7732–7736. doi: 10.1074/jbc.C110.195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes RJ, Farnand AW, Traeger GR, Weis MA, Eyre DR. A role for prolyl 3-hydroxylase 2 in the post-translational modification of fibril-forming collagens. J Biol Chem. 2011;286:30662–30669. doi: 10.1074/jbc.M111.267906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson DM, Weis MA, Eyre DR. Insights on the evolution of prolyl 3-hydroxylation sites from comparative analysis of chicken and Xenopus fibrillar collagens. PLoS One. 2011;6:e19336. doi: 10.1371/journal.pone.0019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno K, Peyton DH, Hayashi T, Engel J, Bächinger HP. Effect of the –Gly-3(S)-hydroxyprolyl-4(R)-hydroxyprolyl-tripeptide unit on the stability of collagen model peptides. FEBS J. 2008;275:5830–5840. doi: 10.1111/j.1742-4658.2008.06704.x. [DOI] [PubMed] [Google Scholar]
- 21.Mordechai S, Gradstein L, Pasanen A, Ofir R, El Amour K, Levy J, Belfair N, Lifshitz T, Joshua S, Narkis G, Elbedour K, Myllyharju J, Birk OS. High myopia caused by a mutation in LEPREL1, encoding prolyl 3-hydroxylase 2. Am J Hum Genet. 2011;89:438–445. doi: 10.1016/j.ajhg.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 23.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyre DR. Collagen cross-linking amino acids. Methods Enzymol. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- 25.Light ND, Bailey AJ. The chemistry of the collagen cross-links. Purification and characterization of cross-linked polymeric peptide material from mature collagen containing unknown amino acids. Biochem J. 1980;185:373–381. doi: 10.1042/bj1850373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Light ND, Bailey AJ. Polymeric C-terminal cross-linked material from type-I collagen. A modified method for purification, anomalous behaviour on gel filtration, molecular weight estimation, carbohydrate content and lipid content. Biochem J. 1980;189:111–124. doi: 10.1042/bj1890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznetsova N, Chi SL, Leikin S. Sugars and polyols inhibit fibrillogenesis of type I collagen by disrupting hydrogen-bonded water bridges between the helices. Biochemistry. 1998;37:11888–11895. doi: 10.1021/bi980089+. [DOI] [PubMed] [Google Scholar]
- 28.Kar K, Amin P, Bryan MA, Persikov AV, Mohs A, Wang YH, Brodsky B. Self-association of collagen triple helical peptides into higher order structures. J Biol Chem. 2006;281:33283–33290. doi: 10.1074/jbc.M605747200. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran GN, Bansal M, Bhatnagar RS. A hypothesis on the role of hydroxyproline in stabilizing collagen structure. Biochim Biophys Acta. 1973;322:166–171. doi: 10.1016/0005-2795(73)90187-6. [DOI] [PubMed] [Google Scholar]
- 30.Inouye K, Sakakibara S, Prockop DJ. Effects of the stereoconfiguration of the hydroxyl group in 4-hydroxyproline on the triple-helical structures formed by homogenous peptides resembling collagen. Biochim Biophys Acta. 1976;420:133–141. doi: 10.1016/0005-2795(76)90352-4. [DOI] [PubMed] [Google Scholar]
- 31.Inouye K, Kobayashi Y, Kyogoku Y, Kishida Y, Sakakibara S, Prockop DJ. Synthesis and physical properties of (hydroxyproline-proline-glycine)10: Hydroxyproline in the X-position decreases the melting temperature of the collagen triple helix. Arch Biochem Biophys. 1982;219:198–203. doi: 10.1016/0003-9861(82)90149-7. [DOI] [PubMed] [Google Scholar]
- 32.Vitagliano L, Berisio R, Mazzarella L, Zagari A. Structural bases of collagen stabilization induced by proline hydroxylation. Biopolymers. 2001;58:459–464. doi: 10.1002/1097-0282(20010415)58:5<459::AID-BIP1021>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher MA, Mizuno K, Bachinger HP. The crystal structure of a collagen-like polypeptide with 3(S)-Hydroxyproline residues in the Xaa position forms a standard 7/2 collagen triple helix. J Biol Chem. 2006;281:27566–27574. doi: 10.1074/jbc.M602797200. [DOI] [PubMed] [Google Scholar]
- 34.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 35.Kramer RZ, Venugopal MG, Bella J, Mayville P, Brodsky B, Berman HM. Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J Mol Biol. 2000;301:1191–1205. doi: 10.1006/jmbi.2000.4017. [DOI] [PubMed] [Google Scholar]
- 36.Kadler KE, Hojima Y, Prockop DJ. Assembly of type I collagen fibrils de novo. Between 37 and 41 degrees C the process is limited by micro-unfolding of monomers. J Biol Chem. 1988;263:10517–10523. [PubMed] [Google Scholar]
- 37.Na GC, Butz LJ, Carroll RJ. Mechanism of in vitro collagen fibril assembly. Kinetic and morphological studies. J Biol Chem. 1986;261:12290–12299. [PubMed] [Google Scholar]
- 38.Na GC, Phillips LJ, Freire EI. In vitro collagen fibril assembly: thermodynamic studies. Biochemistry. 1989;28:7153–7161. doi: 10.1021/bi00444a004. [DOI] [PubMed] [Google Scholar]
- 39.Doyle BB, Hukins DW, Hulmes DJ, Miller A, Woodhead-Galloway J. Collagen polymorphism: its origins in the amino acid sequence. J Mol Biol. 1975;91:79–99. doi: 10.1016/0022-2836(75)90373-3. [DOI] [PubMed] [Google Scholar]
- 40.Woodhead-Galloway J. Structure of the collagen fibril: some variations on a theme of tetragonally packed dimers. Proc R Soc Lond B. 1980;209:275–297. [Google Scholar]
- 41.Vranka JA, Pokidysheva E, Hayashi L, Zientek K, Mizuno K, Ishikawa Y, Maddox K, Tufa S, Keene DR, Klein R, Bächinger HP. Prolyl 3-hydroxylase 1 null mice display abnormalities in fibrillar collagen-rich tissues such as tendons, skin, and bones. J Biol Chem. 2010;285:17253–17262. doi: 10.1074/jbc.M110.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson DA, Eyre DR. Molecular site specificity of pyridinoline and pyrrole cross-links in type I collagen of human bone. J Biol Chem. 1996;271:26508–26516. doi: 10.1074/jbc.271.43.26508. [DOI] [PubMed] [Google Scholar]